Abstract
Although the amygdala has been repeatedly implicated in normal primate social behavior, great variability exists in the specific social and nonsocial behavioral changes observed after bilateral amygdala lesions in nonhuman primates. One plausible explanation pertains to differences in social context. To investigate this idea, we measured the social behavior of amygdala-lesioned and unoperated rhesus monkeys (Macaca mulatta) in two contexts. Animals interacted in four-member social groups over 32 test days. These animals were previously assessed in pairs (Emery et al., 2001), and were, therefore, familiar with each other at the beginning of this study. Across the two contexts, amygdala lesions produced a highly consistent pattern of social behavior. Operated animals engaged in more affiliative social interactions with control group partners than did control animals. In the course of their interactions, amygdala-lesioned animals also displayed an earlier decrease in nervous and fearful personality qualities than controls. The increased exploration and sexual behavior recorded for amygdala-lesioned animals in pairs was not found in the four-member groups. We conclude that the amygdala contributes to social inhibition and this function transcends various social contexts.
Keywords: amygdala, social behavior, nonhuman primate, affiliation, personality
Introduction
Deficits in social behavior are prominent symptoms of many human psychiatric disorders (DSM-IV-TR, 2000), but the neural substrates of such deficits remain largely unknown. Research with both human and nonhuman primates has demonstrated that the neural network responsible for normal social behavior, while still largely unidentified, is likely to be complex and widely distributed (Adolphs, 2001; Bachevalier & Meunier, 2005). One brain structure that has drawn particular interest is the amygdala, a collection of thirteen subnuclei buried deep within the temporal lobes. Neuroanatomical evidence indicates that the amygdala receives sensory information from all modalities and is heavily interconnected with other brain regions thought to participate in memory, emotional expression and reward assessment (Amaral, Price, Pitkänen, Carmichael, & Aggleton, 1992; Barbas, 1995; Cavada, Company, Tejedor, Cruz-Rizzolo, & Reinoso-Suarez, 2000; Ghashghaei, Hilgetag, & Barbas, 2007; Suzuki, 1996; Suzuki & Amaral, 1994; Witter & Amaral, 1991).
Early studies of nonhuman primates with large lesions that included the amygdala demonstrated profound changes in behavior. In a laboratory setting, operated animals displayed hyper-exploration, hyper-sexuality and hypo-emotionality; a spectrum dubbed the Klüver-Bucy Syndrome (Brown & Schäfer, 1888; Klüver & Bucy, 1939). Social interactions within pairs, small groups or in the wild were also profoundly disturbed. The most common alterations included decreased dominance, decreased initiation of affiliative interactions, and increased aggression from others, with the operated animals sometimes being ostracized from their social group (Dicks, Myers, & Kling, 1968; Kling, 1968; Kling & Cornell, 1971; Kling, Lancaster, & Benitone, 1970; Rosvold, Mirsky, & Pribram, 1954). Interestingly, observations in social environments typically did not detect the profound Klüver-Bucy characteristics (Kling, 1972; Kling & Brothers, 1992; Kling & Steklis, 1976).
These early studies provided an important framework for the design and interpretation of social behavior studies. First, behavioral impairments appear to be highly dependent upon species, gender, age at time of lesion, previous experience with social partners and especially the complexity of the social environment. Second, lesion specificity is important for conclusions regarding how individual neural regions contribute to social behavior. Third, the lack of precisely defined and quantifiable behavioral categories complicates the comparison of deficits across studies.
Recent advancements in selective lesioning procedures as well as techniques to reliably classify and record nonhuman primate behavior have rekindled interest in studying the neural network underlying nonhuman primate social behavior. Emery and colleagues (2001) studied the social behavior of adult male, rhesus macaques (Macaca mulatta) following bilateral neurotoxic amygdala lesions. Social behavior was assessed while animals interacted in pairs. In two experiments, unoperated control and amygdala-lesioned animals interacted with a common group of age-matched male or female monkeys. In another context, each control or amygdala-lesioned animal received one 20-minute paired interaction with each of the other eleven experimental animals. A common theme developed from these three experiments. Relative to controls, and contrary to expectations, amygdala-lesioned animals initiated and received more positive or affiliative social behaviors (e.g., grooming, physical contact, sexual behavior etc.), engaged in less aggression (e.g., contact aggression, chasing, etc.) and displayed fewer tension-related behaviors (e.g., tooth grinding, dominance displays, yawning, etc.). These differences were most prominent during the earliest encounters with unfamiliar partners. This was especially surprising since normal adult male rhesus monkeys typically fight or temporarily refrain from interacting with unfamiliar partners, presumably to ascertain each other’s abilities, intentions and threat level (Mendoza, 1993). In terms of nonsocial behavioral abnormalities, amygdala-lesioned animals also consistently displayed increases in locomotion, autoerotic behavior, and oral and tactile cage exploration.
Complementary findings have been provided by Málková and colleagues (2003) who studied social interactions between pairs of pigtail macaques (Macaca nemestrina) following unilateral infusions of the GABAA antagonist, bicuculine methiodide. By blocking the inhibitory influence of GABA, this drug effectively disinhibits or hyperactivates the amygdala. Relative to untreated controls, animals receiving the drug displayed less physical contact with social partners, a complete loss of play and increased incidence of active avoidance by partners. Infused animals also received more aggression from untreated partners. Thus, the reports by both Emery and colleagues (2001) and Málková and colleagues (2003) support the view that an active amygdala inhibits social interaction in rhesus monkeys.
An example of how social context may alter behavioral outcomes was reported by Machado and Bachevalier (2006). Rhesus macaques with bilateral neurotoxic amygdala lesions were evaluated in four-member social groups both pre- and post-surgery. The four-member groups consisted of a sham-operated animal, an animal with an amygdala lesion, an animal with a hippocampal lesion and an animal with an orbital frontal cortex lesion. In this case, amygdala-lesioned animals did not show the social disinhibition, hyper-sexuality or environmental exploration demonstrated by Emery and colleagues (2001). The current study sought to address the issue of how social context might modify the social behavior of animals with amygdala lesions. Observations reported here for four-member groups were made on the same animals that were studied in two-member groups by Emery and colleagues (2001). On the basis of that previous study, we predicted that amygdala-lesioned monkeys would continue to produce greater frequencies and durations of affiliative social behaviors; they would thus potentially be preferred as social partners.
Materials and methods
All experimental procedures were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and developed through consultation with the veterinary staff at the California National Primate Research Center (CNPRC). All protocols were also approved by the University of California, Davis, Institutional Animal Care and Use Committee.
Subjects & housing
Twelve adult male, rhesus monkeys (Macaca mulatta) were randomly assigned to receive either bilateral ibotenic acid lesions of the amygdala (A-IBO; n = 6) or to act as unoperated controls (CON; n = 6). Each was born and raised at the CNPRC in one of twelve half-acre enclosures containing approximately seventy animals each. The animals were chosen after trained behavioral observers watched each animal for two 30-minute sessions over a two-week period. The observers ascertained that all animals displayed a moderate level of social behavior and no animals displayed unusual or inappropriate aggressive behavior or motor stereotypies. All selected animals were of mid-dominance rank. Each animal was raised in a different corral from all other animals, but had previously encountered the other experimental animals during one 20-min interaction in a Round Robin Dyad study (Emery et al., 2001). Prior to the current experiment, these animals also participated in studies of emotional reactivity to potentially dangerous objects (Mason, Capitanio, Machado, Mendoza, & Amaral, 2006), social behavior in three paired settings (Emery et al., 2001), resting hormonal stress response (Emery et al., 1998), as well as emotional reactivity to video presentations of social stimuli. Approximately four months intervened between the end of the paired social behavior experiments (Emery et al., 2001) and the beginning of the current experiment, during which no other behavioral testing took place.
Animals were relocated to indoor housing at the CNPRC when they were between 5 and 8 years old. The current study did not begin until the animals ranged in age between 7 and 10 years and weighed 10 – 15 kg. For complete housing details, refer to Emery and colleagues (2001). Briefly, animals were housed in individual cages (66 cm width × 61 cm length × 81 cm height) and allowed visual access to other male macaques participating in a separate study. The housing room was maintained on a twelve-hour light/dark cycle. All animals were maintained on a diet of fresh fruit, vegetables and monkey chow (Lab Diet #5047, PMI Nutrition International Inc., Brentwood, MO), with water available ad libitum.
Neuroimaging, surgeries and histological lesion assessment
Neuroimaging, surgical and histological lesion assessment procedures have been reported in detail previously (Emery et al., 2001). Briefly, each animal assigned to the A-IBO group underwent an initial aseptic surgical procedure. Animals were initially sedated with ketamine hydrochloride (8 mg/kg), had their heads shaved, were intubated with an endotracheal cannula and brought to a surgical level of anesthesia using isoflurane (1-2%). The animal’s vital signs were monitored by CNPRC veterinarians throughout the procedure. Following a midline scalp incision and reflection of the underlying fascia, two small, copper sulphate-filled glass beads were cemented to the skull at known stereotaxic coordinates. These beads were visible on magnetic resonance images (MRI) and were used as landmarks to create individualized stereotaxic coordinates for ibotenic acid injections into the amygdala (Saunders, Aigner, & Frank, 1990).
For the MRI scans, A-IBO animals were anaesthetized with Telazol (10 mg/kg) and placed in an MRI-compatible stereotaxic apparatus (Crist Instruments Co., Inc., Damascus, MD). A Phillips 1.5T Gyroscan magnet was used for imaging, with 3.0 mm spaced coronal and 3.0 mm spaced sagittal sections taken using a T1-weighted Inversion Recovery pulse sequence (TR = 2084, TI = 708, TE = 20, NEX 2, FOV = 18cm, Matrix 154 × 256). Once acquired, these images were overlaid with a 1 mm × 1 mm square matrix and the stereotaxic locations of intended ibotenic acid injections throughout the amygdala were determined.
For the neurotoxic lesion surgeries, A-IBO animals were anesthetized and monitored by CNPRC veterinarians as described above for the bead-implantation surgeries. Five A-IBO animals underwent a two-stage lesion of the amygdala, with each stage separated by one week. One animal (case 26085) underwent a single-stage bilateral lesion surgery, and all animals recovered similarly. A midline incision was made, followed by reflection of the periosteum and temporalis muscles. Craniotomies were made over the amygdala. The dorsoventral location of the amygdala was verified electrophysiologically. Once the dorsal and ventral borders of the amygdala were verified, the electrode was withdrawn and a 10 μl Hamilton syringe (26 gauge beveled needle) was lowered to the appropriate coordinates. At each injection site (20 – 24 per hemisphere), 0.5 – 1.5 μl of ibotenic acid (Biosearch Technologies Inc., 10 mg/ml in 0.1 M phosphate buffered saline) was injected at a rate of 0.2 μl/minute. The single-stage bilateral lesion was carried out using two identical Hamilton syringes to simultaneously inject ibotenic acid into each amygdala. After all injections were completed, the dura was closed, the craniotomy was filled with Gelfoam (Pharmacia & Upjohn, Peapack, N.J.) and the temporalis muscles were replaced and sutured. The wound was then closed in layers. The animal was removed from anesthesia and monitored continuously for 24 hours by veterinary staff. All A-IBO animals received prophylactic doses of the antibiotics Cefazolan (20 mg/kg t.i.d.) and Baytril (BVP, 5 mg/kg s.i.d) and the analgesic Oxymorphone (0.15 mg/kg t.i.d.) as needed and were allowed to recover for four weeks before any behavioral testing commenced.
Animals assigned to the CON group did not receive any invasive surgical procedures (bead implantation or neurotoxin injections), nor did they receive MRI scans. These animals were, however, sedated as described above for the bead implantation surgeries, brought to the surgical suite, had their head shaved, were intubated and brought to a surgical level of anesthesia with isoflurane gas. These animals remained sedated for the average length of the neurotoxic lesion surgeries experienced by A-IBO animals. After recovery, the CON animals were returned to their home cages.
At the completion of all behavioral testing, the A-IBO animals were immobilized with ketamine hydrochloride (8 mg/kg), deeply anesthetized with Nembutal (50-100 mg/kg i.v.) and perfused intracardially. Perfusates were 4% paraformaldehyde in 0.1 M sodium phosphate buffer (pH 7.2 at 40° C), 250 ml/minute for 10 minutes and 100 ml/minute for 50 min. The brain was then blocked stereotaxically, removed from the skull and post-fixed in the same 4% paraformaldehyde solution for six hours. The brain was then cryoprotected in a solution containing 10% glycerol and 2% dimethylsulfoxide (DMSO) overnight, followed by submersion in a solution containing 20% glycerol and 2% DMSO for three days. Brains were frozen using an isopentane method (Rosene, Roy, & Davis, 1986) and stored at −70°C until sectioned. Frozen sections were cut on a sliding microtome in the coronal plane at a thickness of 30 μm. The sections were then placed in a cryoprotectant tissue collecting solution (TCS; 30% ethylene glycol, 25% glycerin in 0.005 M sodium-phosphate buffer). The sections were stored at −20°C until every eighth section was mounted onto glass slides. These sections were subsequently stained for Nissl with 0.25% thionin solution.
The volumes of the entire amygdala, as well as the lateral, basal, accessory basal and central nuclei were measured in the left hemisphere of five normal age and weight matched rhesus monkeys (kindly provided by Dr Peter Rapp, Mount Sinai School of Medicine, New York). For each of these control cases and the six A-IBO animals, the amygdala and the four amygdaloid nuclei noted above were drawn on a template rhesus monkey atlas using a Leica stereomicroscope and camera lucida (Wetzlar, Germany). Drawings were digitized using a Summasketch II digitizing tablet connected to a PC running Sigma Scan software. The volume of the remaining amygdaloid tissue was computed for each case by multiplying surface area by the distance between each coronal section (240 μm for the A-IBO animals and 400 μm for the control animals). The volume of remaining amygdala and the four subnuclei were then compared to the normal volume of the amygdala and these subnuclei to calculate the percentage of the total volume damaged in the left and right hemispheres, as well as the arithmetic mean across hemispheres. Similar percent unintended damage measurements were made for the subjacent entorhinal cortex.
Apparatus
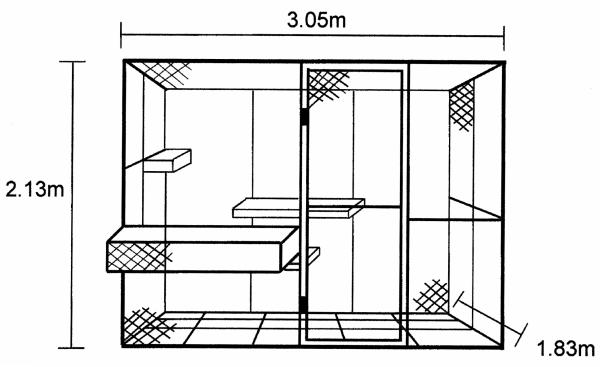
Behavioral measures were collected while animals interacted in a large indoor enclosure (3.05 m × 2.13 m × 1.83 m; Figure 1), constructed from galvanized steel pipe and chain link fencing. The floor was constructed from heavy gauge (2.5 cm × 2.5 cm) galvanized steel mesh, which was suspended 15 cm above the concrete laboratory floor. A chain link door (0.81 m × 1.89m) was located at the front of the cage, 0.81 m from the right side. The monkeys entered the enclosure via a rectangular chute (1.2 m × 0.45 m × 0.39 m) attached to the front of the cage. This chute was constructed from 2.5 cm × 2.5 cm steel mesh and the bottom was 54 cm above the floor of the enclosure. Animals entered the main enclosure through a 30 cm × 45 cm door at the far-left end of the chute, which could be raised and lowered by the experimenter via a pulley system. Three perches were located inside the enclosure, each constructed from 2.5 cm diameter, PVC-coated galvanized steel pipes. The perches were 0.9 m, 1.5 m and 1.29 m in length and positioned 0.425 m, 0.8 m and 1.08 m respectively above the mesh floor as shown in Figure 1. Before the current experiment began, all animals were trained to enter and exit the chute and main enclosure to reduce the inter-session interval and minimize each animal’s stress resulting from handling procedures.
Figure 1.
Diagram with dimensions of the testing enclosure where all social interactions took place. The cage was located indoors and raised 15 cm from the concrete floor. A 3 × 5 square matrix was painted on the floor for the collection of inter-animal distance data.
Experimental design
The twelve experimental animals were randomly divided into three, four-member social groups, with the only constraint being that each group consisted of two A-IBO and two CON animals. Each group was allowed to freely interact in the testing enclosure for two hours on 32 test days (four days per week; Tuesday – Friday). To control for circadian effects on social behavior, observations were conducted during three sessions throughout the day (8:05 – 10:10, 10:45 – 12:50 and 13:15 – 15:20) and testing time was balanced for all groups.
At the beginning of each interaction session, the experimenter released the four members of a group into the testing enclosure in reverse dominance order (lowest ranked monkey entered first) based on the experimenter’s experience with the animals. The experimenter remained in the room during the entire interaction session and sat behind a rolling computer cart approximately 2 meters from the front-left corner of the interaction cage. The experimenter carried out behavioral assessments during the two-hour interaction session (Figure 2).
Figure 2.
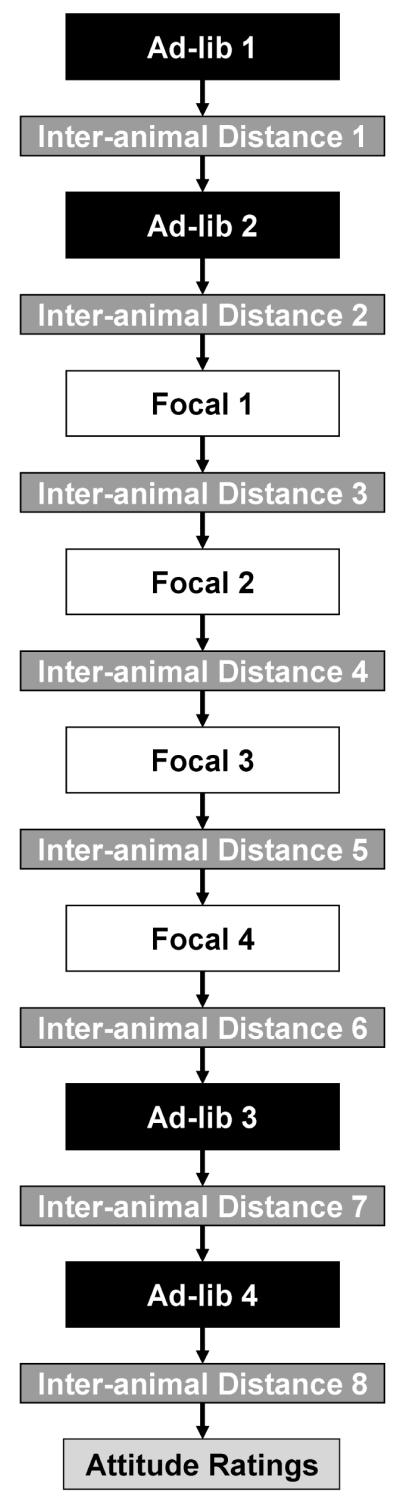
Schematic representation of the structure of each testing session. A total of four ad-lib and four focal sampling sessions were used to capture social and nonsocial behaviors. Eight sampling sessions were made to measure mean inter-animal distance. The final measurement each day captured aspects of each animal’s attitude or personality.
Social and nonsocial behavioral assessments
With The Observer software package (Noldus, Trienes, Hendriksen, Jansen, & Jansen, 2000) and a desktop PC, the experimenter used ad-lib and focal sampling techniques (Altmann, 1974) to record social and nonsocial behaviors during eight 10-minute collection periods. The software also allowed the experimenter to record the specific animal that initiated or received a social behavior, as well as specify the identity of their social partner. Table 1 describes all behaviors that the experimenter could record during these eight collection periods. As shown in Figure 2, the data collection began with two ad-lib samplings, a technique that is particularly useful for collecting behavioral data during periods of extremely high or low activity (i.e., the beginning and ending of an interaction session). With this technique, the observer continuously scans the enclosure in a clockwise motion and records behaviors produced by all animals as soon as they are seen. After the two ad-lib samplings, four focal samples were collected. This technique requires the observer to record the behavior of one, “focal” animal per collection period, along with the identity of all interaction partners. Only behaviors that the focal animal produces or receives are recorded. The focal sampling technique is ideal for recording behavior in social groups once activity levels have stabilized. Each member of the group served as the focal animal once during these four collection periods, and the temporal sequence of focal observations across animals was balanced by Latin Square. Following the four focal samplings, two additional ad-lib samplings were conducted as described above.
Table 1.
Social and nonsocial behavior ethogram
| Behavior category & specific behavior |
Brief Definition |
|---|---|
| Affiliative social behaviors | |
| Approachb | Movement to within arm’s reach of partner for more than 3 seconds. |
| Proximitya,b | Remaining within arm’s reach for more than 3 seconds. |
| Contacta,b | Direct physical contact with partner for more than 3 seconds. |
| Grooma,b | Picking through or licking partner’s fur for more than 3 seconds. |
| Playb | Rough-and-tumble play or grappling, with play face. |
| Mountb | Hands on partner’s hips, double foot clasp and thrusting. |
| Incomplete mountb | Missing hands on partner’s hip, double foot clasp or thrusting. |
| Anogenital exploreb | Sniffing, touching, or licking anogenital area of partner. |
| Grunt vocalization | Soft, bubbly, guttural sound, made in affiliative situations. |
| Affiliative social signals | |
| Lipsmackb | Rhythmic lip movements, often with pursed lips. |
| Groom solicitationb | Rigid posture with presentation of body part for grooming. |
| Mount solicitationb | Stiff four-point stance, rump oriented toward partner with tail up. |
|
Dominance-related
behaviors |
|
| Cage aggression | Rapid shaking of enclosure walls or perch, body slams against walls. |
| Crooktail | Animal struts with tail held up in a “?” shape. |
| Aggressive behaviors | |
| Chaseb | Hostile, rapid movement after another animal. |
| Aggressionb | Physical contact with intent to harm: grabbing, hitting, biting or slapping. |
| Threatb | Two or more of open-mouthed stare, head bobbing, ear flaps and lunges. |
| Displaceb | Take over another animal’s position in the enclosure for more than 3 seconds. |
| Bark Vocalization | High-intensity, low-pitch, guttural bark vocalization. |
| Self-directed behaviors | |
| Self-bite | Hair-plucking, self-biting, or other self-mutilation. |
| Self-clasp | Abnormal grasping of the torso. |
| Self-groom | Picking or licking at one’s own fur or nonfur body part. |
| Self-sex | Manual or oral manipulation of one’s own genitals. |
| Urine drinking | Drinking of one’s own urine from penis, hand, or ground. |
| Anxious behaviors | |
| Tooth grinding | Audible rubbing of lower premolars on upper canines. |
| Scratch | Crude, rapid, hand movements, using fingers to scratch. |
| Fear grimaceb | Large grin, exposing teeth. |
| Yawn | Fully open mouth, with lips fully retracted and teeth showing. |
| Motor stereotypy | Abnormal and repetitive motor behaviors: bucking, bouncing, circling, etc. |
| Scream vocalization | High-pitched, high-intensity vocalization or alarm call. |
| Exploratory behaviors | |
| Tactile exploration | Use of hands to explore the physical environment. |
| Oral exploration | Use of the mouth to explore the physical environment. |
|
Avoidant/solitary
behaviors |
|
| Withdrawb | Animal moves out of arm’s reach of another animal for more than 3 seconds. |
| Nonsociala,b | Animal remains out of arm’s reach for more than 3 seconds. |
| Other behaviors | |
| Extended Sociala,b | Describes a mount, chase, aggressive episode or play bout lasting more than 3 seconds. |
| Coo vocalization | High-pitched, soft “oooo” vocalization. |
| Walk byb | Movement into and out of proximity in less than 3 seconds. |
List of all social and nonsocial behaviors recorded during live behavioral observations. All behaviors were analyzed for frequency (total number of occurrences).
Behavior for which total duration was also measured during focal observations.
Behavior for which a specific partner was recorded.
Inter-animal distance
As shown in Figure 2, following each ad-lib or focal behavior collection period, the experimenter recorded the spatial location of all animals in the group on paper forms. Fifteen squares were painted on the cage floor in a 3 × 5 matrix (Figure 1) to allow for quick and reliable spatial localization in the horizontal plane. The three rows were designated as A, B and C (back to front, respectively) and the five columns were designated as 1 – 5 (left to right, respectively). Location of all group members was noted four times during each one-minute sampling interval using notation based on the specific square where an animal’s head was located. The letter and number designation of the square (e.g., A3) was recorded, and the animal’s position in the vertical plane was noted as floor, perch, wall or ceiling. These spatial coordinates were then transformed into linear distances (in meters) between individuals with in-house software programmed in Q-Basic to serve as an indirect measure of sociability.
Attitude ratings
At the end of every two-hour social interaction session (Figure 2), the experimenter subjectively rated each individual monkey on seven adjectives describing various aspects of macaque social and nonsocial personality or attitude, using a five-point Likert-type scale (Capitanio, 1999; Stevenson-Hinde & Zunz, 1980). Table 2 describes each of these attitude assessment categories. The definition for each rating point was as follows: 1 = definition not at all descriptive, 2 = definition slightly descriptive, 3 = definition moderately descriptive, 4 = definition mostly descriptive, and 5 = definition completely descriptive. All ratings were made solely on the basis of the interactions observed on a given day. Observers were explicitly instructed not to use prior knowledge of the monkeys to influence how each session was scored.
Table 2.
Attitude assessment ethogram
| General category & individual adjectives |
Brief Definition |
|---|---|
| Sociable | |
| Confident | Animal freely moves within the cage. Movements are fluid, not furtive and the animal may strut with a crooktail posture. |
| Affiliative | Animal actively seeks to be near and/or in friendly contact with another animal or facilitates contact by the other animal. |
| Interaction-inhibiting | |
| Avoidant | Animal actively refrains from engaging in positive or negative social interactions. |
| Aggressive | Animal attempts to or actually causes physical harm to several other group members. |
| Nervous | Animal’s behavior is characterized by fidgeting, picking at the cage or perches, motor stereotypies, fear grimacing, yawing, or furtive movements. |
| Fearful | Animal is anxious in the presence of others; readily fear grimaces, and appears overly vigilant to the movements of others. |
| Other | |
| Active | Animal is moves around the enclosure excessively and remains stationary only for short periods of time. |
Data analysis
Data from the three measures above were divided into four 8-test-day blocks to capture changes in the groups’ behavior over time. The frequency and duration of discrete social and nonsocial behaviors collected during ad-lib and focal samplings were summed within each block, whereas the inter-animal distance and attitude assessment data were averaged within each block. Attitude rating data were normally distributed for both groups, as determined with the Shapiro-Wilk test and by inspecting the skewness and kurtosis ratios. However, these normality assessments indicated that the inter-animal distance dataset and a majority of behavioral frequency and duration measures were not normally distributed. These data were therefore log10(x+1) transformed prior to statistical analyses (Sokal & Rohlf, 1995), but non-transformed values were used for illustration purposes. All data were analyzed using General Linear Model ANOVAs with Group (2) as a between subjects factor and Block (4) as a within subjects factor with repeated measures using the SPSS 12.0 statistical analyses package. For analysis of inter-animal distances and behavioral frequencies and durations, a second within-subjects factor of Partner (2) was also included to differentiate between interactions with lesioned or control animals. A Huynh-Feldt correction was used to adjust the degrees of freedom if group variances did not remain equal across the four testing blocks. Alpha was set at p < .05. However, given the low number of animals in each experimental group and the variation of lesion extent in Group A-IBO, we occasionally report results for which p values fall above this threshold. Results are identified as “marginally significant” if their p value is greater than .05 but less than .08.
Because main effects of Block do not provide any specific information regarding amygdala function, these effects were omitted from the Results section below. Post-hoc examination of interactions between Group and Block or between Group and Partner were accomplished with separate independent-samples t-tests for each Block or Partner, respectively. Interactions between Partner and Block and changes in behavior between Blocks were examined using paired-sample t-tests. If any variable under post-hoc evaluation had a group mean equal to zero, nonparametric versions of the preceding tests (such as the Wilcoxon signed-rank test and Mann-Whitney U test) were used.
Finally, for A-IBO animals, Pearson product moment correlation matrices were generated to determine if the extent of intended damage to the amygdala (as a whole or separate subnuclei) or unintended damage to the entorhinal cortex significantly influenced any behavioral parameters measured.
Inter-observer Reliability Assessments
The two behavioral observers (CJM and NJE) for this study had extensive previous experience observing adult male and female rhesus macaques, collecting inter-animal distance data and rating attitude or personality qualities. These observers were aware of the lesion status of the animals in the current study and had observed them previously while interacting in pairs (Emery et al., 2001), but never in four-member groups. Therefore, before the current study began, the two observers were tested for inter-observer reliability on all behavioral measures described above using non-experimental adult male rhesus monkeys. The observers simultaneously recorded the behavior of these animals using the ad-lib sampling technique (33 trials total) and the mean percent agreement across these trials was 89.5 +/− 1% (Range: 74.2-100%). The observers also simultaneously recorded the behavior of these animals using the focal sampling technique (9 trials total) and the mean percent agreement was 94.8 +/− 1.6% (Range: 83.1-100%). The two observers were also tested until their inter-observer reliability for collecting inter-animal distance data was greater than 90% agreement and for making attitude assessment ratings was greater than 85% agreement for all adjectives. To further counteract any biases, the observers were pseudo-randomly assigned to observe different social groups on different days, and the total number of observations per group for the two observers was counter-balanced.
Results
Lesion extents
A detailed description of each A-IBO animal’s lesion has been provided before (Emery et al., 2001). However, a brief description of this group as a whole will be given here and a summary of the histological lesion assessment for each animal is provided in Table 3. Ibotenic acid injections were intended to damage the entire amygdaloid complex but primarily targeted the lateral, basal and accessory basal nuclei, since these nuclei have the most substantial direct connections with the neocortex. For the most part, the lesions across the A-IBO group were quite successful in achieving their goal, with average bilateral damage to the amygdala as a whole ranging from 66.5% to 84.0%. Damage to the lateral and basal nuclei was even more complete, ranging from 84.2% to 99.3% in the lateral nucleus bilaterally and from 82.2 % to 98.3% in the basal nucleus bilaterally. Average bilateral damage was slightly less in the accessory basal nucleus, ranging from 55.7% - 93.8%. The central nucleus was damaged extensively but less than the deep nuclei (range: 55.8% - 83.4%). The more superficial areas of the amygdala, such as the medial nucleus and the periamygdaloid cortex, were typically spared. Nevertheless, it is likely that these superficial nuclei were heavily denervated since a majority of their input arises from the lateral, basal and accessory basal nuclei (Pitkänen & Amaral, 1998).
Table 3.
Lesion extents for animals with bilateral ibotenic acid amygdala lesions
| Amygdala |
Lateral Nucleus |
Basal Nucleus |
Accessory Basal Nucleus |
Central Nucleus |
Entorhinal Cortex |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | L | R | Avg | L | R | Avg | L | R | Avg | L | R | Avg | L | R | Avg | L | R | Avg |
| 24349 | 61.2 | 89.7 | 75.4 | 79.1 | 100.0 | 89.5 | 77.9 | 100.0 | 88.9 | 49.6 | 100.0 | 74.8 | 46.1 | 68.0 | 57.1 | 49.3 | 40.7 | 45.0 |
| 25468 | 75.6 | 87.7 | 81.7 | 93.2 | 99.5 | 96.3 | 95.2 | 100.0 | 97.6 | 81.0 | 99.6 | 90.3 | 59.5 | 84.4 | 72.0 | 83.6 | 83.5 | 83.6 |
| 25571 | 95.5 | 70.4 | 82.9 | 100.0 | 89.2 | 94.6 | 100.0 | 90.3 | 95.2 | 100.0 | 70.2 | 85.1 | 90.9 | 75.9 | 83.4 | 49.9 | 78.0 | 64.0 |
| 25627 | 71.9 | 80.3 | 76.1 | 98.5 | 92.5 | 95.5 | 97.2 | 96.8 | 97.0 | 58.3 | 89.1 | 73.7 | 47.9 | 63.7 | 55.8 | 57.3 | 66.6 | 62.0 |
| 25942 | 73.6 | 59.3 | 66.5 | 94.5 | 73.9 | 84.2 | 94.4 | 70.0 | 82.2 | 66.0 | 45.5 | 55.7 | 60.2 | 61.3 | 60.8 | 60.2 | 77.9 | 69.1 |
| 26085 | 83.3 | 84.7 | 84.0 | 98.5 | 100.0 | 99.3 | 97.2 | 99.4 | 98.3 | 93.0 | 94.6 | 93.8 | 59.6 | 65.4 | 62.5 | 33.1 | 57.7 | 45.4 |
|
|
|
|
|
|
|
|||||||||||||
| Mean | 76.8 | 78.7 | 77.8 | 94.0 | 92.5 | 93.2 | 93.6 | 92.7 | 93.2 | 74.6 | 83.2 | 78.9 | 60.7 | 69.8 | 65.2 | 55.6 | 67.4 | 61.5 |
Data are the percentage of normal volume damaged within the amygdala as a whole, the lateral, basal, accessory basal and central nuclei, as well as the entorhinal cortex. L – percentage of damage to the left hemisphere; R – percentage of damage to the right hemisphere; Avg – average of L and R.
Unintended damage to adjacent areas was mild in most cases. Five of six cases showed minor cell loss in the rostral hippocampus, but more caudal portions of this structure were completely undamaged. All animals received minor to extensive cell loss in the piriform cortex and ventral claustrum, and all cases received extensive damage to the rostral field of the entorhinal cortex that is located ventral to the amygdala.
Inter-animal distance
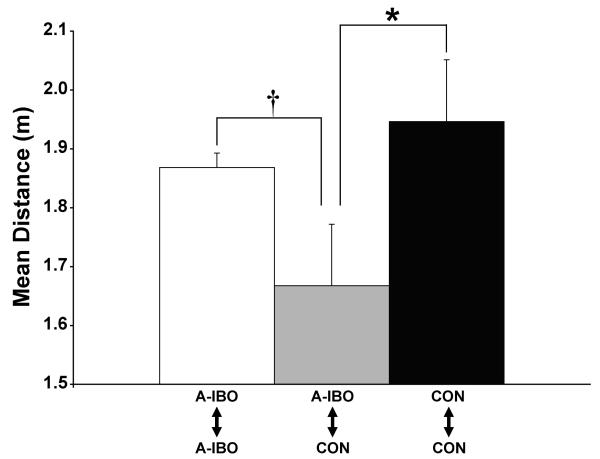
Inter-animal distances within each social group were averaged into four consecutive eight-day blocks to investigate changes over time depending on lesion condition. Between groups comparisons were made with partner (amygdala-lesioned or control) and block as within-subjects factors. Only the Group × Partner interaction attained significance [F(1,10) = 9.015; p = .013]. Post-hoc examination revealed that mean distance between CON and A-IBO animals was significantly lower than the distance between two CON animals (t = 2.20; p = .05, Figure 3). Distance between two A-IBO animals was also typically greater than CON/A-IBO pairs, but this difference was only marginally significant (t = 2.31; p = .07). These results indicate that control animals tended to be closer to amygdala-lesioned animals than other control animals, whereas amygdala-lesioned animals were typically closer to control animals than to other lesioned animals.
Figure 3.
Mean inter-animal distance for each of the three possible animal pairs within a social group. Vertical bars indicate the Standard Errors of the Mean. * p ≤ .05, † p = .07.
Total frequency and duration of behaviors
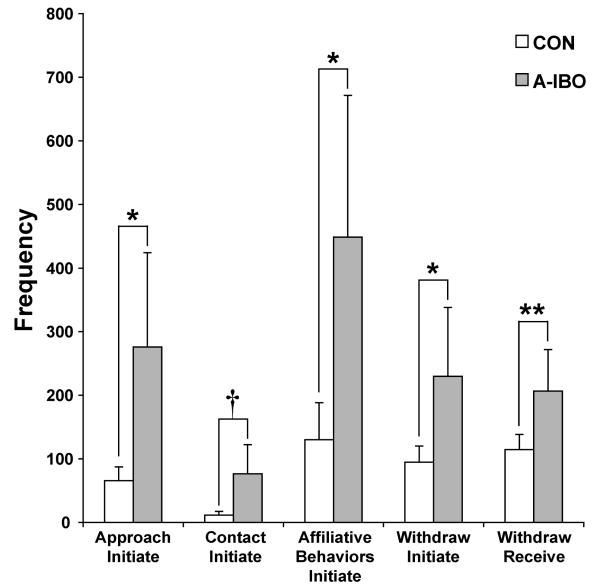
There were several differences between the groups related to affiliative social interactions, regardless of Block or Partner (Figure 4). A-IBO animals approached other group mates, to within arm’s reach, significantly more than CON [F(1,10) = 5.231, p < .05]. The same pattern emerged for the initiation of physical contact with others, but the difference between groups was only marginally significant [F(1,10) = 3.685, p = .08]. If the frequencies of all affiliative social behaviors (approach, contact, groom, play, mount, incomplete mount, anogenital explore and grunt vocalizations; see Table 1) were summed into a general category, A-IBO animals again initiated significantly more than CON [F(1,10) = 4.826, p = .05]. Consistent with these examples of heightened social interaction, A-IBO animals also initiated and received more withdrawals from arm’s reach by other group mates than did CON animals [withdraw initiate: F(1,10) = 7.132, p < .05; withdraw receive: F(1,10) = 11.698, p < .01].
Figure 4.
Total frequency of approach initiate, contact initiate, affiliative behaviors initiate, withdraw initiate and withdraw receive (left to right, respectively) for CON and A-IBO, regardless of partner or testing block. Vertical bars indicate the Standard Errors of the Mean. ** p ≤ .01, * p ≤ .05, † p = .08
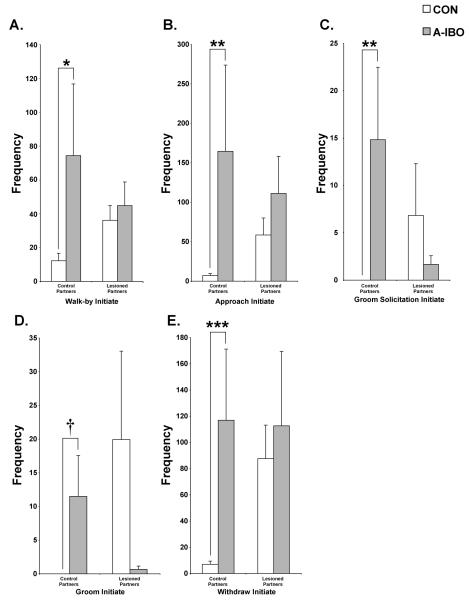
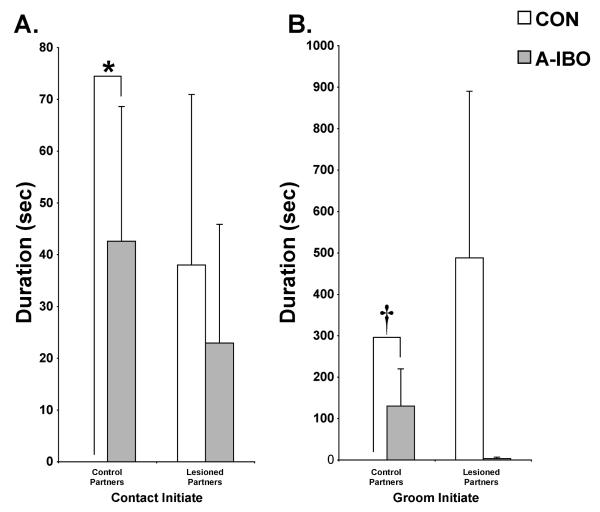
Far more differences between the groups emerged when data were analyzed with regard to the type of partner involved (i.e., Group × Partner interactions). A-IBO again appeared to engage in more affiliative social interactions than CON, and predominantly directed those interactions toward control partners rather than amygdala-lesioned partners (Figure 5). Specifically, A-IBO initiated more walk-bys [F(1,10) = 11.434, p < .01, post-hoc: t = −2.69, p < .05], approaches to within arm’s reach [F(1,10) = 4.446, p = .06, post-hoc: t = −3.438, p < .01], groom solicitations [F(1,10) = 5.794, p < .05, post-hoc: z = −3.083, p < .01], grooming [F(1,10) = 7.635, p < .05, post-hoc: z = −1.892, p = .06] and withdrawals from arm’s reach [F(1,10) = 12.037, p < .01, post-hoc: t = −4.801, p = .001] with control partners than did CON (left panels). As shown in Figure 6, the same pattern also held for the duration of physical contact [F(1,10) = 7.502, p < .05, post-hoc: z = −2.286, p < .05] and grooming [F(1,10) = 4.786, p = .05, post-hoc: z = −1.892, p = .06]. For each of these frequency and duration measures, A-IBO and CON animals did not differ in their interactions with amygdala-lesioned partners (right panels, all p > .10).
Figure 5.
Total frequency across all testing blocks of walk-by initiate (A), approach initiate (B), groom solicitation initiate (C), groom initiate (D) and withdraw initiate (E) with both control and amygdala-lesioned partners for CON and A-IBO. Vertical bars indicate the Standard Errors of the Mean. ** p ≤ .01, * p ≤ .05, † p = .06
Figure 6.
Total duration across all testing blocks of contact initiate (A) and groom initiate (B) with both control and amygdala-lesioned partners for CON and A-IBO. Vertical bars indicate the Standard Errors of the Mean. * p ≤ .05, † p = .06
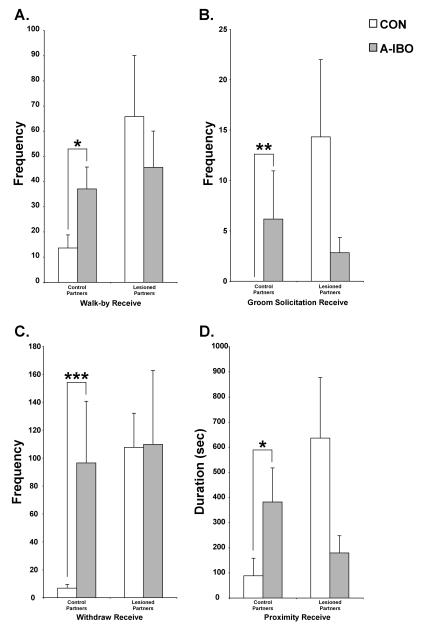
A similar pattern of results was also detected for affiliative social behaviors received (Figure 7). Specifically, A-IBO animals received more walk-bys [F(1,10) = 6.86, p < .05, post-hoc: t = −2.655, p < .05], groom solicitations [F(1,10) = 4.213, p = .07, post-hoc: z = −2.69, p < .01] and withdrawals from arm’s reach [F(1,10) = 6.86, p < .05, post-hoc: t = −4.762, p = .001] from control partners than did CON. A-IBO also received a greater duration of proximity [F(1,10) = 6.894, p < .05, post-hoc: t = −3.023, p < .05] from control partners than did CON. Again, for each of these frequency and duration measures, interactions with amygdala-lesioned partners did not differ significantly between the groups (all p > .10).
Figure 7.
Total frequency across all testing blocks of walk-by receive (A), groom solicitation receive (B), and withdraw initiate (C), as well as total duration of proximity receive (D) with both control and amygdala-lesioned partners for CON and A-IBO. Vertical bars indicate the Standard Errors of the Mean. *** p ≤ .001, ** p ≤ .01, * p ≤ .05
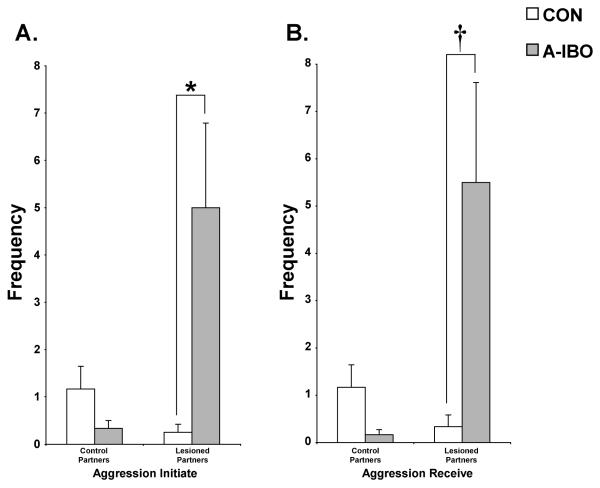
The incidence of aggressive behavior was very low within each social group. However, as shown in Figure 8, the frequency of physical forms of aggression (i.e., grabbing, hitting, biting or slapping) initiated and received demonstrated significant Group × Partner interactions [F(1,10) = 9.753, p = .01 and F(1,10) = 8.55, p < .05, respectively]. Post-hoc analyses indicated that A-IBO animals initiated more aggression to amygdala-lesioned partners than did CON [t = −2.575, p < .05]. Likewise, A-IBO received more aggression from amygdala-lesioned partners than did CON [t = 2.076, p = .07]. Aggressive interactions with control animals were low and did not differ appreciably between the groups (both p > .10).
Figure 8.
Total frequency across all testing blocks of aggression initiate (A) and aggression receive (B) with both control and amygdala-lesioned partners for CON and A-IBO. Vertical bars indicate the Standard Errors of the Mean. * p ≤ .05, † p = .07
Finally, there were no appreciable differences between the groups in terms of dominance-related behaviors, self-directed behaviors, anxious behaviors or exploratory behaviors, regardless of whether or not Block or Partner were included in the analysis.
Attitude ratings
Table 4 provides the mean ratings for the two general attitude assessment categories (sociable and interaction-inhibiting) as well as the individual adjectives within each of these general categories. Qualities such as “confident” and “affiliative” were placed into the “sociable” category because they tend to promote strong, positive social relations between male macaques. By contrast, qualities such as “avoidant,” “aggressive,” “nervous” and “fearful” inhibit strong positive social interactions and therefore were categorized under the general label of “interaction-inhibiting.” The adjective “active” does not fit into either of these general categories because it refers more to how animals interact with their environment than with their group mates. Therefore, this adjective was analyzed separately.
Table 4.
Attitude assessment data
| Category & adjectives |
CON |
A-IBO |
||||||
|---|---|---|---|---|---|---|---|---|
| Block 1 | Block 2 | Block 3 | Block 4 | Block 1 | Block 2 | Block 3 | Block 4 | |
| Sociable | 2.6 ± 0.2 | 2.6 ± 0.4 | 2.8 ± 0.3 | 2.8 ± 0.3 | 2.8 ± 0.3 | 3.3 ± 0.5 | 3.0 ± 0.3 | 3.1 ± 0.3 |
| Confident | 3.0 ± 0.2 | 2.9 ± 0.4 | 3.1 ± 0.4 | 3.1 ± 0.3 | 2.8 ± 0.3 | 3.6 ± 0.5 | 3.0 ± 0.3 | 3.3 ± 0.4 |
| Affiliative | 2.2 ± 0.2 | 2.3 ± 0.4 | 2.5 ± 0.3 | 2.5 ± 0.3 | 2.8 ± 0.2 | 3.1 ± 0.5 | 3.1 ± 0.4 | 3.0 ± 0.3 |
| Interaction-inhibiting | 2.5 ± 0.1 | 2.5 ± 0.3 | 2.1 ± 0.2 † | 2.2 ± 0.2 | 2.5 ± 0.2 | 1.8 ± 0.3 * | 1.8 ± 0.2 | 2.0 ± 0.2 |
| Avoidant | 3.0 ± 0.2 | 3.0 ± 0.4 | 2.7 ± 0.3 | 2.7 ± 0.2 | 2.7 ± 0.2 | 2.1 ± 0.4 | 2.3 ± 0.3 | 2.2 ± 0.2 |
| Aggressive | 2.0 ± 0.2 | 1.7 ± 0.1 | 1.3 ± 0.1 | 1.4 ± 0.2 | 1.6 ± 0.2 | 1.4 ± 0.1 | 1.1 ± 0.1 | 1.4 ± 0.2 |
| Nervous | 2.7 ± 0.2 | 2.7 ± 0.5 | 2.3 ± 0.3 † | 2.3 ± 0.3 | 2.9 ± 0.2 | 2.0 ± 0.5 * | 2.0 ± 0.4 | 2.1 ± 0.3 |
| Fearful | 2.4 ± 0.2 | 2.6 ± 0.5 | 2.0 ± 0.3 # | 2.2 ± 0.3 | 2.7 ± 0.3 | 1.9 ± 0.5 * | 1.8 ± 0.4 | 2.3 ± 0.4 |
| Other | ||||||||
| Active | 2.7 ± 0.1 | 2.8 ± 0.2 | 2.8 ± 0.2 | 2.5 ± 0.1 | 3.1 ± 0.3 | 3.2 ± 0.3 | 2.8 ± 0.3 | 2.6 ± 0.3 |
Data are the mean attitude assessment data (±SEM) for the two experimental groups. CON – control monkeys; A-IBO – animals with ibotenic acid amygdala lesions. Block 1, Block 2, etc. – sequential testing blocks, each representing an average over eight test days.
p = .05, change relative to Block 1.
p = .07, change relative to Block 2.
p = .08, change relative to Block 2.
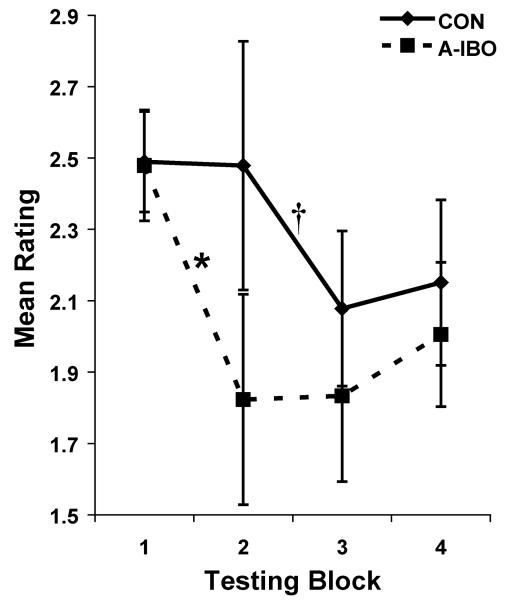
The two general categories (sociable and interaction-inhibiting) and the active adjective were first analyzed over the four testing blocks. For active and the sociable category, there were no significant main effects of Group, nor interactions between Group and Block (all ps > .10), indicating that amygdala lesions do not appreciably impact on such qualities. However, for interaction-inhibiting qualities, a significant interaction between Group and Block [F(3,30) = 2.865, p = .05] was detected, but the main effect of Group was not significant. Post-hoc analyses of this interaction indicated that while the groups did not differ in interaction-inhibiting ratings during any Block, A-IBO showed a significant decline in ratings between Blocks 1 and 2 (t = 3.087; p = .05), but ratings for CON remained unchanged during this time (Figure 9). By contrast, CON showed decreased interaction-inhibiting ratings between Blocks 2 and 3 to levels commensurate with A-IBO, but this change was marginally significant (t = 2.917; p = .07).
Figure 9.
Mean rating of interaction-inhibiting qualities for CON and A-IBO groups in each of the four testing blocks (average of eight test days). The interaction-inhibiting category is a composite of other more discrete qualities that do not promote strong, positive social relationships, such as aggressive, nervous, fearful and avoidant. Vertical bars indicate the Standard Errors of the Mean. * p ≤ .05, † p = .07.
To further investigate this general difference between the groups, similar analyses were conducted with the individual adjectives that make up the interaction-inhibiting category (fearful, nervous, avoidant and aggressive). None of these individual adjectives displayed any significant main effects of Group (all ps > .10), but significant Group × Block interactions were detected for fearful [F(3,30) = 3.275, p < .05] and nervous [F(3,30) = 2.994, p < .05]. Post-hoc analyses of both interactions did not reveal any robust differences between the groups in any Block. However, similar to the interaction-inhibiting category, A-IBO displayed decreased ratings of fearful (t = 2.526, p = .05) and nervous (t = 2.823, p < .05) between Blocks 1 and 2, but CON did not show similar decreases in ratings for these adjectives until between Blocks 2 and 3 (t = 2.360, p = .07 and t = 2.158, p = .08, respectively). These results indicate that amygdala-lesioned animals displayed earlier decreases in fearful and nervous attributes than did control animals during the 32 testing days.
Correlations
No significant correlations were found between any of the behavioral measures and intended damage to the amygdala (as a whole or individual subnuclei) or unintended damage to the entorhinal cortex.
Discussion
The main goal of this study was to determine if the pattern of social and nonsocial behavior observed for amygdala-lesioned monkeys during paired social interactions (Emery et al., 2001) would also be observed in four-member groups. Similar to our previous study, the average distance between control and amygdala-lesioned animals was less than between two lesioned or two control animals. Amygdala-lesioned animals also continued to initiate more affiliative behaviors (i.e., approaching to within arm’s reach, physical contact and grooming) than controls. Amygdala-lesioned animals directed far more of these affiliative behaviors towards control partners than did other control animals. By contrast, amygdala-lesioned animals initiated more aggression towards lesioned partners than control animals. Behaviors related to dominance displays, generalized anxiety and cage exploration did not differentiate the two groups. Finally, amygdala-lesioned animals also displayed an earlier decrease in “interaction-inhibiting” personality qualities (i.e., nervous and fearful) than control animals.
Social disinhibition persists across contexts
Across multiple measures, amygdala-lesioned animals in the present study demonstrated a pattern of behavior we collectively refer to as social disinhibition. Measures of frequency and duration demonstrated that regardless of social partner, amygdala-lesioned animals initiated more affiliative social behaviors, especially approaching to within arm’s reach, physical contact and withdrawals from close proximity. Amygdala-lesioned animals also predominantly directed their heightened affiliative social behaviors towards normal partners in their groups, particularly in the form of approaches, withdrawals, solicitations for grooming, physical contact and grooming. These affiliative overtures seemed to be reciprocated by control animals, since amygdala-lesioned animals also received more groom solicitations, walk-bys (transient proximity) and extended time in close proximity from control animals than did other controls. Heightened affiliative interactions between control and amygdala-lesioned animals were also apparent in their consistently shorter inter-animal distance relative to pairs of control or pairs of lesioned animals. Each of these findings is highly consistent with the heightened sociality demonstrated during our previous study of these same animals when they interacted in pairs (Emery et al., 2001). These findings are also in line with several previous nonhuman primate lesion studies of similar group-sizes (Kling & Brothers, 1992) and one experiment that used GABAA antagonists to stimulate the nonhuman primate amygdala (Málková et al., 2003). We believe this evidence suggests that heightened affiliative social interactions following amygdala lesions stems from a more general inability to properly perceive danger or threat in the environment and use such information to modulate social behavior adaptively. In line with this view, deficits in threat detection or fear reactivity have been specifically demonstrated for monkeys with bilateral neurotoxic amygdala lesions in both social (Machado & Bachevalier, 2006) and nonsocial settings (Izquierdo, Suda, & Murray, 2005; Kalin, Shelton, & Davidson, 2004; Kalin, Shelton, Davidson, & Kelley, 2001; Mason et al., 2006; Meunier, Bachevalier, Murray, Málková, & Mishkin, 1999). These abnormalities are not restricted to nonhuman primates, since humans with amygdala lesions also demonstrate specific deficits in identifying fearful facial expressions (Adolphs et al., 1999), rating the magnitude of fearful expressions (Adolphs, Tranel, Damasio, & Damasio, 1995) and assessing the approachability or trustworthiness of unfamiliar individuals (Adolphs, Tranel, & Damasio, 1998). It is also interesting that recent functional neuroimaging (Hoffman, Gothard, Schmid, & Logothetis, 2007) and electrophysiological recording (Gothard, Battaglia, Erickson, Spitler, & Amaral, 2007) studies with nonhuman primates have demonstrated heightened activity in the amygdala when viewing threatening facial expressions relative to appeasement gestures. Similar results have been present in the human neuroimaging literature for some time (Blair, Morris, Frith, Perrett, & Dolan, 1999; Gur et al., 2002; LaBar, Crupain, Voyvodic, & McCarthy, 2003; Morris, deBonis, & Dolan, 2002; Morris et al., 1996; Phillips et al., 1997; Whalen et al., 2001) and reinforce the similarities between human and nonhuman primate amygdala function.
The heightened sociality of the amygdala-lesioned animals in the current study appeared to make them the preferred social partners within each group. Animals with amygdala lesions received more affiliative social behaviors (such as groom solicitations and time spent in close proximity) from control partners than did other controls animals (Figure 7). In fact, control animals hardly initiated any affiliative social behaviors (such as approaches, groom solicitations, contact and grooming) to other control animals (Figures 5 and 6). The inter-animal distance was also less for control and amygdala-lesioned pairs than pairs of control animals (Figure 3). These findings are again consistent with our previous study of these animals in a paired context. This continuity across contexts suggests that the amygdala plays a highly selective and modulatory role in normal primate social behavior; one highly specialized for proper reactivity to threat as opposed to other facets of social behavior.
The present experiment also demonstrated three surprising results in terms of social behavior. First, our previous study of paired social interactions demonstrated that amygdala-lesioned animals engaged in more autoerotic behavior and mounted male and female partners with a greater frequency than control animals (Emery et al., 2001). Although both behaviors remained common in the current study, neither differentiated the groups. One explanation for these incongruent effects of lesion could be the difference in social context afforded by the two experiments. Some support for this has been provided by the extensive work of Kling and colleagues (1992). In laboratory-based assessments of social behavior, amygdala-lesioned animals demonstrated hyper-sexuality, but this was typically not observed in free-ranging social contexts. Similarly, Machado and Bachevalier (2006) did not observe heightened mounting or autoerotic behavior in rhesus monkeys with neurotoxic amygdala lesions in four-member social groups.
A second unexpected result concerned changes in behavior over time. When observed in pairs, our amygdala-lesioned animals displayed heightened affiliative social interactions predominantly during the earliest interactions with unfamiliar partners (Emery et al., 2001). Although the current study included more social interaction sessions (maximum of six between partners for Emery et al., 2001, a total of 32 presently), only one significant change over time was noted for the two experimental groups. The amygdala-lesioned animals demonstrated decreased ratings of fearful and nervous personality qualities after Block 1 (first 2 weeks of interactions), but control animals did not show a commensurate drop in these ratings until after Block 2 (after week 4). While not identical, these results are similar to those generated by the same animals in social pairs. However, the lack of other group differences related to time again indicate that the one 20-minute interaction that animals received with all other possible partners in our previous study largely established the social rules and hierarchy that would later govern the four-member social groups. This is plausible since the formation of dominance hierarchies within groups of unfamiliar macaques occurs after only a few minutes of interactions and remains extremely stable over time regardless of the outcome of subsequent social interactions (Barchas & Mendoza, 1984). Machado and Bachevalier (2006) also did not find any profound changes in behavior across testing sessions for amygdala-lesioned monkeys when observed in four-member social groups established prior to surgery.
One final unexpected result from the current study concerned contact aggression. Although the total number of aggressive encounters was low across all groups, amygdala-lesioned animals initiated and received more aggression with other amygdala-lesioned animals than controls (Figure 8). Although this result seems to fit with amygdala-lesioned animals directing more affiliative behaviors towards control partners rather than other lesioned animals (above), this result was not found in our previous observations of these animals in social pairs (Emery et al., 2001). In fact, control animals displayed more aggression than amygdala-lesioned animals in our previous experiments. Heightened aggression has not typically been observed in earlier studies of amygdala lesions in nonhuman primates, whether in small or large social groups (Kling & Brothers, 1992). There is no clear reason for this discrepancy between studies, but at least two possibilities exist. First, since behavioral data were collected over such a protracted time frame (32 testing sessions, 2 hours each), it is possible that amygdala-lesioned animals were able to demonstrate a more generalized pattern of social disinhibition; one that includes inappropriate aggression along with the more overt affiliative disinhibition reported currently and in our previous study (Emery et al., 2001). A second explanation could also be rooted in the different social contexts of each study. Some support for this idea has been provided recently when amygdala-lesioned animals were also rated as more aggressive post-surgery in four-member social groups (Machado & Bachevalier, 2006).
Nonsocial abnormalities appear to be context specific
Heightened environmental exploration (both tactile and oral) was found for amygdala-lesioned animals in our previous study (Emery et al., 2001). The current study did not detect any profound differences in cage exploration between amygdala-lesioned and control animals. Likewise, earlier studies of amygdala-lesioned animals in social groups comprised of four animals or more have not demonstrated heightened frequencies of oral or tactile exploration (Kling & Brothers, 1992; Machado & Bachevalier, 2006). These findings are consistent with the idea that reduced opportunities for social interaction and/or restrictive testing environments promote hyper-exploration following brain lesions in monkeys. Further support for this idea has come from investigations of food preferences in amygdala-lesioned monkeys. When tested in highly restrictive environments (i.e., a Wisconsin General Testing Apparatus), amygdala lesioned monkeys demonstrate heightened preference for inedible nonfoods (Machado & Bachevalier, 2007a; Murray, Gaffan, & Flint, 1996; Stefanacci, Clark, & Zola, 2003). By contrast, in a more naturalistic setting, amygdala lesions do not produce heightened nonfood selection (Machado & Bachevalier, 2007b).
Acknowledgments
This research was supported by grants from the National Institute of Mental Health (R37 MH57502 and R01 MH41479) and was conducted at the California National Primate Research Center (RR00169). We thank Pamela Tennant, Jeff Bennett, Caroline Chen, John Ruys and Noah Merin for assistance with histology, and the technical, animal care and veterinary staff at the California National Primate Research Center for supporting this research. We would also like to thank Dr Peter Rapp for providing the control brain sections used for histological lesion analysis.
References
- Adolphs R. The neurobiology of social cognition. Current Opinion In Neurobiology. 2001;11(2):231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393(6684):470–474. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio AR. Fear and the human amygdala. Journal of Neuroscience. 1995;15(9):5879–5891. doi: 10.1523/JNEUROSCI.15-09-05879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Hamann S, Young AW, Calder AJ, Phelps EA, et al. Recognition of facial emotion in nine individuals with bilateral amygdala damage. Neuropsychologia. 1999;37(10):1111–1117. doi: 10.1016/s0028-3932(99)00039-1. [DOI] [PubMed] [Google Scholar]
- Altmann J. Observational study of behavior: sampling methods. Behaviour. 1974;49(3):227–267. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL, Pitkänen A, Carmichael ST, Aggleton JP. The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. John Wiley & Sons, Inc.; New York: 1992. Anatomical organization of the primate amygdaloid complex; pp. 1–66. [Google Scholar]
- Bachevalier J, Meunier M. The neurobiology of social-emotional cognition in nonhuman primates. In: Easton A, Emery NJ, editors. The Cognitive Neuroscience of Social Behaviour. Psychology Press; London: 2005. pp. 19–57. [Google Scholar]
- Barbas H. Anatomic basis of cognitive-emotional interactions in the primate prefrontal cortex. Neuroscience and Biobehavioral Reviews. 1995;19:499–510. doi: 10.1016/0149-7634(94)00053-4. [DOI] [PubMed] [Google Scholar]
- Barchas PR, Mendoza SP. Emergent hierarchical relationships in rhesus macaques: An application of Chase’s Model. In: Barchas PR, editor. Social Hierarchies - Essays Toward a Sociophysiological Perspective. Greenwood Press; Westport: 1984. pp. 81–95. [Google Scholar]
- Blair RJ, Morris JS, Frith CD, Perrett DI, Dolan RJ. Dissociable neural responses to facial expressions of sadness and anger. Brain. 1999;122(Pt 5):883–893. doi: 10.1093/brain/122.5.883. [DOI] [PubMed] [Google Scholar]
- Brown S, Schäfer EA. An investigation into the functions of the occipital and temporal lobes of the monkey’s brain. Philos Trans R Soc Lond B Biol Sci. 1888;179:303–327. [Google Scholar]
- Capitanio JP. Personality dimensions in adult male rhesus macaques: prediction of behaviors across time and situation. Am.J.Primatol. 1999;47:299–320. doi: 10.1002/(SICI)1098-2345(1999)47:4<299::AID-AJP3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cerebral Cortex. 2000;10(3):220–242. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- Dicks D, Myers RE, Kling A. Uncus and amygdala lesions: effects on social behavior in the free- ranging rhesus monkey. Science. 1968;165(888):69–71. [PubMed] [Google Scholar]
- DSM-IV-TR . Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. American Psychiatric Association; Arlington, VA: 2000. Text Revision. [Google Scholar]
- Emery NJ, Capitanio JP, Mason WA, Machado CJ, Mendoza SP, Amaral DG. The effects of bilateral lesions of the amygdala on dyadic social interactions in rhesus monkeys (Macaca mulatta) Behavioral Neuroscience. 2001;115(3):515–544. [PubMed] [Google Scholar]
- Emery NJ, Machado CJ, Mendoza SP, Capitanio JP, Mason WA, Amaral DG. Role of the amygdala in dyadic social interactions & the stress response in monkeys: 28th Annual Meeting; Los Angeles, CA: Society for Neuroscience; 1998. p. 780. [Google Scholar]
- Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothard KM, Battaglia FP, Erickson CA, Spitler KM, Amaral DG. Neural responses to facial expression and face identity in the monkey amygdala. J Neurophysiol. 2007;97(2):1671–1683. doi: 10.1152/jn.00714.2006. [DOI] [PubMed] [Google Scholar]
- Gur RC, Schroeder L, Turner T, McGrath C, Chan RM, Turetsky BI, et al. Brain activation during facial emotion processing. Neuroimage. 2002;16(3 Pt 1):651–662. doi: 10.1006/nimg.2002.1097. [DOI] [PubMed] [Google Scholar]
- Hoffman KL, Gothard KM, Schmid MC, Logothetis NK. Facial-Expression and Gaze-Selective Responses in the Monkey Amygdala. Curr Biol. 2007;17(9):766–772. doi: 10.1016/j.cub.2007.03.040. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Suda RK, Murray EA. Comparison of the effects of bilateral orbital prefrontal cortex lesions and amygdala lesions on emotional responses in rhesus monkeys. Journal of Neuroscience. 2005;25(37):8534–8542. doi: 10.1523/JNEUROSCI.1232-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. Journal of Neuroscience. 2004;24(24):5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ, Kelley AE. The primate amygdala mediates acute fear but not the behavioral and physiological components of anxious temperament. Journal of Neuroscience. 2001;21(6):2067–2074. doi: 10.1523/JNEUROSCI.21-06-02067.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kling A. Effects of amygdalectomy and testosterone on sexual behavior of male juvenile macaques. Journal of Comparative & Physiological Psychology. 1968;65:466–471. doi: 10.1037/h0025804. [DOI] [PubMed] [Google Scholar]
- Kling A. Effects of amygdalectomy on socio-affective behavior in non-human primates. In: Eleftheriou BE, editor. Neurobiology of the Amygdala. Plenum; New York: 1972. pp. 511–536. [Google Scholar]
- Kling A, Brothers L. The amygdala and social behavior. In: Aggleton JP, editor. The Amygdala: Neurobiological Aspects of Emotion. Wiley-Liss; New York: 1992. pp. 353–377. [Google Scholar]
- Kling A, Cornell R. Amygdalectomy and social behaviour in the caged stump-tailed macaque. Folia Primatol. 1971;14:91–103. doi: 10.1159/000155350. [DOI] [PubMed] [Google Scholar]
- Kling A, Lancaster J, Benitone J. Amygdalectomy in the free-ranging vervet (Cercopithecus aethiops) Journal of Psychiatric Research. 1970;7(3):191–199. doi: 10.1016/0022-3956(70)90006-3. [DOI] [PubMed] [Google Scholar]
- Kling A, Steklis HD. A neural substrate for affiliative behavior in nonhuman primates. Brain, Behavior and Evolution. 1976;13(2-3):216–238. doi: 10.1159/000123811. [DOI] [PubMed] [Google Scholar]
- Klüver H, Bucy PC. Preliminary analysis of functions of the temporal lobes in monkeys. Archives of Neurology and Psychiatry. 1939;42(6):979–1000. [Google Scholar]
- LaBar KS, Crupain MJ, Voyvodic JT, McCarthy G. Dynamic perception of facial affect and identity in the human brain. Cerebral Cortex. 2003;13(10):1023–1033. doi: 10.1093/cercor/13.10.1023. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. The impact of selective amygdala, orbital frontal cortex or hippocampal formation lesions on established social relationships in monkeys. Behavioral Neuroscience. 2006;120(4):761–786. doi: 10.1037/0735-7044.120.4.761. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. The effects of selective amygdala, orbital frontal cortex or hippocampal formation lesions on reward assessment in nonhuman primates. European Journal of Neuroscience. 2007a;25(9):2885–2904. doi: 10.1111/j.1460-9568.2007.05525.x. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. Measuring reward assessment in a semi-naturalistic context: The effects of selective amygdala, orbital frontal or hippocampal lesions. Neuroscience. 2007b;148:599–611. doi: 10.1016/j.neuroscience.2007.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Málková L, Barrow KV, Lower LL, Gale K. Decreased social interactions in monkeys after unilateral blockade of GABA-A receptors in the basolateral amygdala. Annals of the New York Academy of Sciences. 2003;985:540–541. [Google Scholar]
- Mason WA, Capitanio JP, Machado CJ, Mendoza SP, Amaral DG. Amygdalectomy and responsiveness to novelty in rhesus monkeys: Generality and individual consistency of effects. Emotion. 2006;6(2):73–81. doi: 10.1037/1528-3542.6.1.73. [DOI] [PubMed] [Google Scholar]
- Mendoza SP. Social conflict on first encounters. In: Mason WA, Mendoza SP, editors. Primate Social Conflict. University of New York Press; New York: 1993. pp. 85–110. [Google Scholar]
- Meunier M, Bachevalier J, Murray EA, Málková L, Mishkin M. Effects of aspiration versus neurotoxic lesions of the amygdala on emotional responses in monkeys. European Journal of Neuroscience. 1999;11(12):4403–4418. doi: 10.1046/j.1460-9568.1999.00854.x. [DOI] [PubMed] [Google Scholar]
- Morris JS, deBonis M, Dolan RJ. Human amygdala responses to fearful eyes. Neuroimage. 2002;17(1):214–222. doi: 10.1006/nimg.2002.1220. [DOI] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, et al. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383(6603):812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Murray EA, Gaffan EA, Flint RW., Jr. Anterior rhinal cortex and amygdala: dissociation of their contributions to memory and food preference in rhesus monkeys. Behavioral Neuroscience. 1996;110(1):30–42. [PubMed] [Google Scholar]
- Noldus LP, Trienes RJ, Hendriksen AH, Jansen H, Jansen RG. The Observer Video-Pro: new software for the collection, management, and presentation of time-structured data from videotapes and digital media files. Behavior Research Methods, Instruments, & Computers. 2000;32(1):197–206. doi: 10.3758/bf03200802. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, Brammer M, Andrew C, Calder AJ, et al. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389(6650):495–498. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Amaral DG. Organization of the intrinsic connections of the monkey amygdaloid complex: projections originating in the lateral nucleus. Journal of Comparative Neurology. 1998;398(3):431–458. doi: 10.1002/(sici)1096-9861(19980831)398:3<431::aid-cne9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Rosene DL, Roy NJ, Davis BJ. A cryoprotection method that facilitates cutting frozen sections of whole monkey brains for histological and histochemical processing without freezing artifact. J Histochem Cytochem. 1986;34(10):1301–1315. doi: 10.1177/34.10.3745909. [DOI] [PubMed] [Google Scholar]
- Rosvold HE, Mirsky AF, Pribram KH. Influence of amygdalectomy on social behavior in monkeys. Journal of Comparative and Physiological Psychology. 1954;47:173–178. doi: 10.1037/h0058870. [DOI] [PubMed] [Google Scholar]
- Saunders RC, Aigner TG, Frank JA. Magnetic resonance imaging of the rhesus monkey brain: use for stereotactic neurosurgery. Experimental Brain Research. 1990;81(2):443–446. doi: 10.1007/BF00228139. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry: The principles and practice of statistics in biological research. 3 ed Freman; New York: 1995. [Google Scholar]
- Stefanacci L, Clark RE, Zola SM. Selective neurotoxic amygdala lesions in monkeys disrupt reactivity to food and object stimuli and have limited effects on memory. Behavioral Neuroscience. 2003;117(5):1029–1043. doi: 10.1037/0735-7044.117.5.1029. [DOI] [PubMed] [Google Scholar]
- Stevenson-Hinde J, Zunz M. Subjective assessment of individual rhesus monkeys. Primates. 1980;19(3):473–482. [Google Scholar]
- Suzuki WA. Neuroanatomy of the monkey entorhinal, perirhinal and parahippocampal cortices: Organization of cortical inputs and interconnections with amygdala and striatum. Seminars in the Neurosciences. 1996;8:3–12. [Google Scholar]
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. Journal of Comparative Neurology. 1994;350(4):497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL. A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion. 2001;1(1):70–83. doi: 10.1037/1528-3542.1.1.70. [DOI] [PubMed] [Google Scholar]
- Witter MP, Amaral DG. Entorhinal cortex of the monkey: V. Projections to the dentate gyrus, hippocampus, and subicular complex. J.Comp Neurol. 1991;307(3):437–459. doi: 10.1002/cne.903070308. [DOI] [PubMed] [Google Scholar]