Abstract
Background & Aims
Abscisic acid (ABA) has shown effectiveness in ameliorating inflammation in obesity, diabetes and cardiovascular disease models. The objective of this study was to determine whether ABA prevents or ameliorates experimental inflammatory bowel disease (IBD).
Methods
C57BL/6J mice were fed diets with or without ABA (100 mg/kg) for 35 days prior to challenge with 2.5% dextran sodium sulfate (DSS). The severity of clinical disease was assessed daily. Colonic mucosal lesions were evaluated by histopathology, and cellular adhesion molecular and inflammatory markers were assayed by real-time quantitative PCR. Flow cytometry was used to quantify leukocyte populations in the blood, spleen, and mesenteric lymph nodes (MLN). The effect of ABA on cytotoxic T-lymphocyte antigen 4 (CTLA-4) expression in splenocytes was also investigated.
Results
ABA significantly ameliorated disease activity, colitis and reduced colonic leukocyte infiltration and inflammation. These improvements were associated with down-regulation in vascular cell adhesion marker-1 (VCAM-1), E-selectin, and mucosal addressin adhesion marker-1 (MAdCAM-1) expression. ABA also increased CD4+ and CD8+ T-lymphocytes in blood and MLN and regulatory T-cells in blood. In vitro, ABA increased CTLA-4 expression through a PPAR γ-dependent mechanism.
Conclusions
We conclude that ABA ameliorates gut inflammation by modulating T cell distribution and adhesion molecule expression.
Keywords: Inflammatory bowel disease, abscisic acid, T cells, immune modulation, cellular adhesion molecules
1. Introduction
Inflammatory bowel disease (IBD), with its two clinical manifestations Crohn’s Disease (CD) and Ulcerative Colitis (UC), is characterized with significant inflammation and immune cell infiltration into gastrointestinal (GI) tract, resulting in incredible pain and discomfort for those afflicted [1]. IBD is often managed by an array of treatments, which traditionally include aminosalicylates, corticosteroids, immunosuppressors, and anti-tumor necrosis factor α (TNF-α) therapy. The type of treatment used depends on the clinical goal (induction or remission-maintenance), the severity of inflammation, the response to current or prior treatment, and the presence of complications [2]. Current IBD treatments are associated with significant side-effects, such as weight gain, cushingoid appearance, osteopenia/osteoporosis, pancreatitis, and systemic immune suppression [3], and there is an overall need for more safe and effective treatments.
In this regard, our laboratory has been recently investigating the anti-inflammatory effects of the isoprenoid phytohormone abscisic acid (ABA) [4]. ABA functions as an important regulator of stress tolerance and defense response in plants [5, 6], and only within the past 3 years has its role in mammals been extensively examined. We have demonstrated that ABA intake improves glucose tolerance and reduces white adipose tissue inflammation and immune cell infiltration in genetically obese db/db mice without side-effects [7, 8]. This mechanism was shown to be partially dependent on the nuclear receptor peroxisome proliferator-activated receptor γ (PPAR γ) [9]. We have also recently shown that ABA inhibits CD4+ lymphocyte and F4/80+CD11b+ macrophage infiltration, lowers vascular adhesion molecule 1 (VCAM-1) expression, and reduces atherosclerotic lesion size in aortic roots of ApoE -/- mice [10]. In this mouse model of atherosclerosis the mechanism of ABA was more consistent with intracellular accumulation of messenger cAMP [10]. Other recent studies have shown that cAMP upregulation is important process in ABA’s mechanism of action [11, 12].
Because ABA has shown effectiveness in reducing inflammation in animals models of obesity/diabetes where treatment with PPAR γ agonists (i.e., Avandia) is very popular, and given that PPAR γ agonists have shown promising results in IBD therapy [13, 14], the objective of this study was to assess the anti-inflammatory efficacy of ABA against colitis and immune cell infiltration during experimental IBD. Our findings show that ABA significantly lowers inflammation in colons of DSS-challenged mice and inhibits adhesion molecule and inflammatory cytokine expression. These improvements occurred alongside significant increases CD4+ and CD8+ lymphocytes and CD4+FoxP3+ regulatory T-cells in the blood of ABA-fed mice. Treatment with ABA in vitro also significantly increased expression of lymphocyte regulatory protein cytotoxic T-cell lymphocyte antigen 4 (CTLA-4) in CD3/CD28-stimulated splenocytes. Overall, these findings indicate that ABA modulates T cell responses and it may be a therapeutic option for individuals with IBD.
2. Research Design and Methods
2.1 Animal Procedures
Six to eight week old C57BL/6J mice (n=20) and MMTV-PPAR γflfl Cre+ and PPAR γflfl Cre- mice (n=4) were housed at the animal facilities at Virginia Polytechnic Institute and State University in a room maintained at 75° F, with a 12:12 h light-dark cycle starting from 6:00 AM. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Virginia Polytechnic Institute and State University and met or exceeded requirements of the Public Health Service/National Institutes of Health and the Animal Welfare Act. C57BL/6J mice were fed AIN-93G-based diets with or without 100 mg/kg of a racemic ABA mixture for 35 days prior to induction of colitis with water containing 2.5% dextran sodium sulfate (DSS), 36,000–44,000 mol wt (ICN Biomedicals, Aurora, OH). After the DSS challenge mice were weighed on a daily basis and examined for clinical signs of disease associated with colitis (i.e., perianal soiling, rectal bleeding, diarrhea, and piloerection). For the DSS challenge, the disease activity indices and rectal bleeding scores were calculated using a modification of a previously published compounded clinical score [15]. Briefly, disease activity index consisted of a scoring for diarrhea and lethargy (0–3), whereas rectal bleeding consisted of a visual observation of blood in feces and the perianal area (0–4). Mice in the DSS study were euthanized on day 7 of the DSS challenge. On day 7 blood mice were euthanized by CO2 narcosis followed by secondary thoracotomy and blood was withdrawn from the heart. Spleen and mesenteric lymph nodes (MLN) were scored based on size and macroscopic inflammatory lesions (0-3), excised, and then crushed to produce single-cell suspensions for flow cytomerty.
2.2 Histopathology
Colonic sections were fixed in 10% buffered neutral formalin, later embedded in paraffin, and then sectioned (5 μm) and stained with H&E stain for histologic examination. Colons were blindly graded with a compounded histologic score including the extent of (1) lymphocyte infiltration, (2) mucosal thickening, and (3) epithelial cell erosion. The sections were graded with a score of 0–4 for each of the previous categories and data were analyzed as a normalized compounded score.
2.3 Quantitative Real-Time PCR
Total RNA was isolated from colons using the RNA isolation Minikit (Qiagen) according to the manufacturer’s instructions. Total RNA (1 μg) was used to generate complementary DNA (cDNA) template using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). The total reaction volume was 20 μL with the reaction incubated as follows in an MJ MiniCycler: 5 minutes at 25°C, 30 minutes at 52°, 5 minutes at 85°C, and hold at 4°C. PCR was performed on the cDNA using Taq DNA polymerase (Invitrogen, Carlsbad, CA) and using previously described conditions [15]. Each gene amplicon was purified with the MiniElute PCR Purification Kit (Qiagen) and quantitated on an agarose gel by using a DNA mass ladder (Promega). These purified amplicons were used to optimize real-time PCR conditions and to generate standard curves in the real-time PCR assay. Primer concentrations and annealing temperatures were optimized for the iCycler iQ system (Bio-Rad) for each set of primers using the system’s gradient protocol. PCR efficiencies were maintained between 92 and 105% and correlation coefficients above 0.98 for each primer set during optimization and also during the real-time PCR of sample DNA.
Complementary DNA (cDNA) concentrations for genes of interest were examined by real-time quantitative PCR using an iCycler IQ System and the iQ SYBR green supermix (Bio-Rad). A standard curve was generated for each gene using 10-fold dilutions of purified amplicons starting at 5 pg of cDNA and used later to calculate the starting amount of target cDNA in the unknown samples. SYBR green I is a general double-stranded DNA intercalating dye and may therefore detect nonspecific products and primer/dimers in addition to the amplicon of interest. In order to determine the number of products synthesized during the real-time PCR, a melting curve analysis was performed on each product. Real-time PCR was used to measure the starting amount of nucleic acid of each unknown sample of cDNA on the same 96-well plate. Results are presented as starting quantity of target cDNA (picograms) per microgram of total RNA. Primer sequences, the length of the PCR product, and gene accession numbers are outlined in Table 1.
Table 1.
| Primer | Sequence | Length | Accession Number |
|---|---|---|---|
| β-actinF | 5’CCCAGGCATTGCTGACAGG3’ | 141 | X03672 |
| β-actinR | 5’TGGAAGGTGGACAGTGAGGC3’ | ||
| PPAR γF | 5’CAGGCTTGCTGAACGTGAAG3’ | 117 | NM_011146 |
| PPAR γR | 5’GGAGCACCTTGGCGAACA3’ | ||
| CD36F | 5’CCGGGCCACGTAGAAAACA3’ | 156 | NM_007643 |
| CD36R | 5’CCTCCAAACACAGCCAGGAC3’ | ||
| FABP4F | 5’TCTCTTATCAAAGGCTCTACTTCC3’ | 78 | BC054426 |
| FABP4R | 5’CAAAATTCCATCCAGGCCTCT3’ | ||
| E-selectinF | 5’CAGCTTTGCATGATGGCGTCT3’ | 83 | NM_011345 |
| E-selectinR | 5’GAAGGGTACAGGCGAGTTGG3’ | ||
| VCAM-1F | 5’TCTCCCAGGAATACAACGAT3’ | 75 | NM_011693 |
| VCAM-1R | 5’ACAGGTCATTGTCACAGCAC3’ | ||
| MAdCAM-1F | 5’CCCATGGCCACAGCTACCTCA3’ | 85 | NM_013591 |
| MAdCAM-1R | 5’CCCTGGCCCTAGTACCCTAC3’ | ||
| PECAM-1F | 5’AGAGACGGTCTTGTCGCAGTA3’ | 131 | NM_001032378 |
| PECAM-1R | 5’CGCACACCTGGATCGG3’ | ||
| ICAM-1F | 5’AAGGAGATCACATTCACGGT3’ | 119 | NM_010493.2 |
| ICAM-1R | 5’GCCTCGGAGACATTAGAGAA3’ | ||
| MMP-9F | 5’GGTGGCAGCGCACGAGTT3’ | 128 | NM_013599 |
| MMP-9R | 5’GGATGCCGTCTATGTCGTCTT3’ | ||
| iNOSF | 5’GCTTTGTGCGAAGTGTCAGT3’ | 147 | NM_010927 |
| iNOSR | 5’CTCCTTTGAGCCCTTTGTG3’ | ||
| IL-6F | 5’TTTCCTCTGGTCTTCTGGAG3’ | 92 | NM_031168 |
| IL6-R | 5’CTGAAGGACTCTGGCTTTGT3’ | ||
| MCP-1F | 5’CTGCCTAATCCACAGACTG3’ | 142 | AJ238892 |
| MCP-1R | 5’GCCTGAACAGCACCACTA3’ |
F, forward; R, reverse. PCR primer pairs were designed for an optimal annealing temperature of 57°C and product lengths between 78 and 157 base pairs.
When plotting threshold cycle versus log starting quantity (pg), standard curves had slopes between -3.1 and -3.7; PCR efficiencies between 92 and 105 and R2 above 0.98.
2.4 Immunophenoptying of blood, mesenteric lymph nodes, and spleen
Spleens and MLNs were excised and crushed with frosted slides. Splenocytes were lysed of red blood cells with erythrocyte lysis buffer, and spleen and MLN were resuspended in PBS and enumerated with a Coulter Counter (Beckman Coulter, Fullerton, CA). Spleen and MLN-derived cells (2 × 105 cells/well) or whole blood (10 μL/well) were seeded onto 96-well plates, centrifuged at 4°C at 3000 rpm for 4 minutes, and washed with PBS containing 5% serum and 0.09% sodium azide (FACS buffer). To assess differential monocyte/macrophage infiltration, the cells were then incubated in the dark at 4°C for 20 minutes in FcBlock (20 μg/ml; BD Pharmingen) for macrophage assessment, and then for an additional 20 minutes with fluorochrome-conjugated primary antibodies anti-F4/80-PE-Cy5 (5 μg/mL, ebioscience) and anti-CD11b-Alexa Fluor 700 (2 μg/mL, eBioscience). For lymphocyte assessment, cells were incubated with anti-CD4-Alexa Fluor 700 (2 μg/mL; BD Pharmingen), anti-CD8-PerCp-Cy5.5 (2 μg/mL, eBioscience), CD3 PE-Cy5 (2 μg/mL; BD Pharmingen), or anti-FoxP3-PE (2 μg/mL, eBioscience) as previously shown [16]. Flow results were computed with a BD LSR II flow cytometer and data analyses was performed with FACS Diva software (BD).
2.5 CTLA-4 assessment in cultured splenocytes
Splenocytes from MMTV-PPAR γflfl Cre+ and PPAR γflfl Cre- mice (n=4) were isolated under sterile conditions and seeded into 96-well plates as described above. Cells were stimulated with anti-CD3 (5 μg/mL; BD Pharmingen) and anti-CD28 (1 μg/mL; BD Pharmingen) to induce proliferation and then treated with DMSO or ABA (10 μM, Sigma) with and without 2’5’ dideoxyadenosine (cAMPi, 10 μM; Sigma). Cells were then placed in a 37 °C incubator with 5% CO2 for 20 hours and immunophenotyped with anti-CD4 Alexa Fluor 700 (BD Pharmingen), anti-CD3-PE-Cy5 (BD Pharmingen), and anti-CD152 (CTLA-4)-biotin (BD). Streptavidin PE-Texas Red (1 μg/mL; BD Pharmingen) was used as a secondary antibody. Flow results were computed with a BD LSR II flow cytometer and data analyses was performed with FACS Diva software (BD).
2.6 Statistics
Data were analyzed as a completely randomized design. To determine the statistical significance of the model, analysis of variance (ANOVA) was performed using the general linear model procedure of Statistical Analysis Software (SAS), and probability value (P) < 0.05 was considered to be significant. When the model was significant, ANOVA was followed by Fisher’s Protected Least Significant Difference multiple comparison method.
3. Results
3.1 ABA reduces disease activity and colonic inflammation
To determine the effect of ABA on colonic inflammation, mice were fed diets with or without ABA (100 mg/kg) for 35 days prior to DSS challenge. After 7 days, mice fed ABA had a significantly reduced disease activity index (DAI) compared to control-fed mice (Figure 1A). There were no significant differences in body weight loss between the two groups although ABA intake numerically decreased weight loss (Figure 1B). The colon, spleen, and mesenteric lymph nodes (MLN) from each mouse were scored based on gross anatomical signs of inflammation. The DSS challenge increased the macroscopic score of all three tissues (Figures 1C-1E). Based on the anatomical observation, ABA significantly reduced colonic inflammation but had no significant effect on the spleen or MLN.
Figure 1.
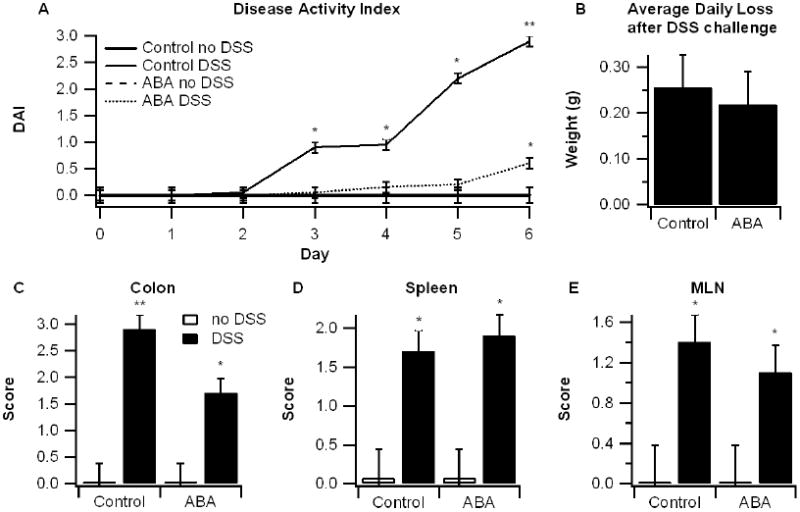
Effect of dietary abscisic acid (ABA) supplementation on disease severity. C57BL/6J mice were fed ABA-supplemented (100 mg/kg) or control diets for 35 days and challenged with 2.5% dextran sodium sulfate (DSS) or water (no DSS) for 7 days. The disease activity index (DAI), a composite score reflecting clinical signs of the disease (i.e. perianal soiling, rectal bleeding, diarrhea, and piloerection) was assessed daily (A) and the average daily loss in body weights (B) were calculated for mice undergoing the DSS challenge. On day 7 mice were euthanized and the colon, spleen, and mesenteric lymph nodes (MLN) (C-E) were macroscopically scored for inflammation. Data are represented as mean ± standard error. Points with an asterisk are significantly different from control, non-DSS treatment (P<0.05).
3.2 ABA improves colon histopatholgy and reduces leukocyte infiltration
Macroscopic and observational data indicated that ABA-supplementation ameliorated IBD severity. To more closely examine the effect of ABA, colon specimens from mice were examined histologically. In line with reduced DAI, mice fed ABA showed significant histological improvements in the colon (Figure 2). Epithelial erosion, mucosal thickening, and leukocyte infiltration were significantly reduced in ABA-fed mice with DSS colitis (Figure 2).
Figure 2.
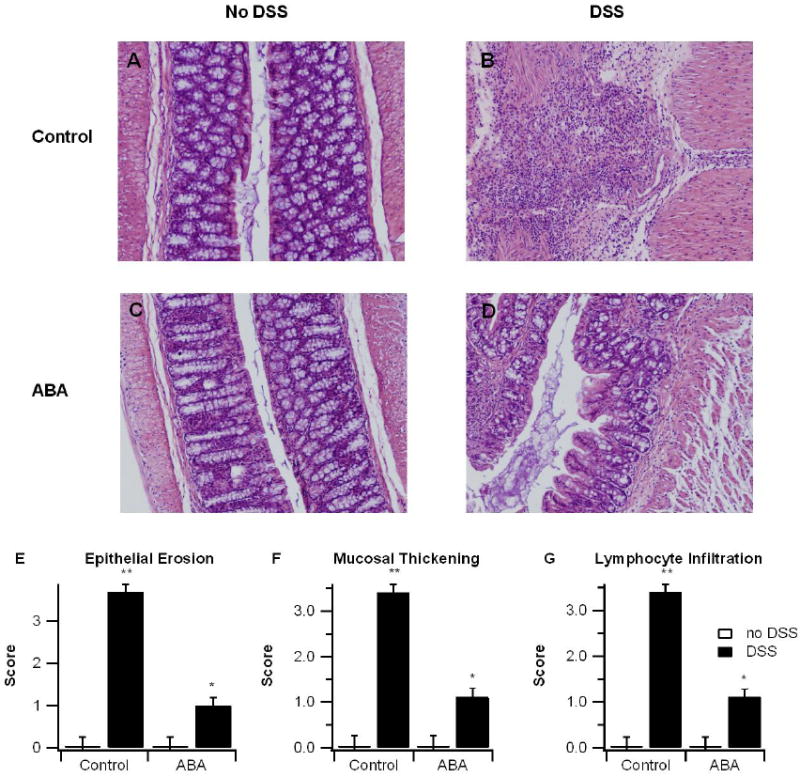
Effect of dietary abscisic acid (ABA) supplementation on colon histopathology. C57BL/6J mice were fed ABA-supplemented (100 mg/kg) or control diets for 35 days and challenged with 2.5% dextran sodium sulfate (DSS) or water (no DSS) for 7 days. Representative photomicrographs from control, no-DSS (A), control, DSS (B), ABA, no-DSS (C), and ABA, DSS (D) groups are depicted. All specimens underwent blinded histological examination and were scored (1-4) on epithelial erosion (E), mucosal wall thickening (F), and leukocyte infiltration (G). Data are represented as mean ± standard error. Points with an asterisk are significantly different from control, non-DSS treatment (P<0.05).
3.3 ABA suppresses colonic expression of adhesion molecules
We have previously shown that ABA activates PPAR γ, and PPAR γ agonists have been successfully used in the treatment of IBD [9]. Thus, we sought to determine whether ABA modulates gene expression in a manner that resembled established agonists of PPAR γ such as rosiglitazone or conjugated linoleic acid. Here, we found no evidence of PPAR γ-mediated affect in colons of ABA-fed mice. Neither colonic PPAR γ nor its responsive genes CD36, or FABP4 were significantly affected by ABA.
Despite no increase in PPAR γ, ABA-supplementation significantly lowered the colonic expression of adhesion molecules VCAM-1, muscosal vascular addressin cell adhesion molecule 1 (MAdCAM-1), and E-selectin and had no affect on intracellular adhesion molecule 1 (ICAM-1) or platelet endothelial cell adhesion molecule 1 (PECAM-1). Colons from ABA-treated mice also had significantly attenuated levels of inflammatory proteins IL-6, iNOS, and MMP-9. IL-6 and iNOS are proteins secreted mainly by M1 activated macrophages, while MMP-9 is secreted predominantly by neutrophils and is a key mediator of tissue damage in IBD [17].
3.4 ABA increase CTLA-4 expression in cultured lymphocytes
To more closely examine the immunomodulating effects of ABA, splenocytes from PPAR γ-expressing (MMTV-Cre-) and PPAR γ-deficient (MMTV-Cre+) mice were stimulated with CD3/CD28 and treated overnight with ABA with or without the cAMP inhibitor 2’5’-dideoxyadenosine (cAMPi). Lymphocytes were then immunophenotyped and CTLA-4 expression was assessed. CTLA-4 is an immunomodulating protein on lymphocytes whose genetic polymorphisms have been linked to IBD in humans [18]. CTLA-4 levels can also be modulated by changes in intracellular cAMP [19]. We found that ABA significantly increased the percentage of CTLA-4 expressing cells and enhanced CTLA-4 median fluorescence intensity (MFI) on CD4+ T cells expressing PPAR γ (Figure 5). There were no significant differences observed in PPAR γ-deficient cells. The cAMP inhibitor modestly decreased ABA’s influence on CTLA-4, thought the effect was not significant.
Figure 5.
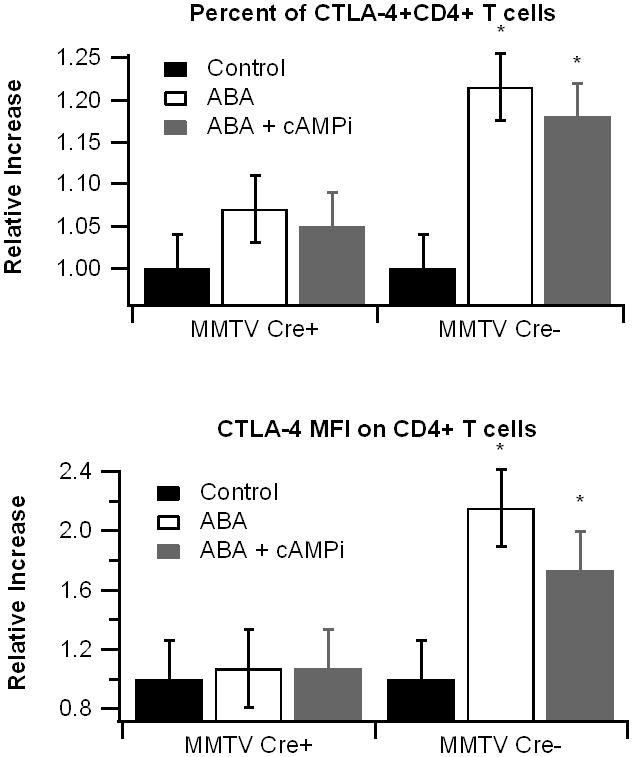
Effect of abscisic acid (ABA) treatment on cytotoxic T-lymphocyte antigen 4(CTLA-4) expression. Splenocytes were isolated from peroxisome proliferator-activated receptor γ (PPAR γ)-flfl; MMTV-Cre+ mice, who lack PPAR γ in hematopoietic cells, and control MMTV-Cre- littermates. Cells were stimulated with anti-CD3 (5 μg/mL)/CD28 (1 μg/mL) and treated with ABA (10 μM with or without the cAMP inhibitor 2’5’-dideoxyadenosine (icAMP, 10 μM) or 14-22 myristolated PKA inhibitor fragment (iPKA, 30 μM), a PKA-specific inhibitor, for 20 hours. Expression of CTLA-4 on CD3+, CD4+, and CD8+ lymphocytes was assessed with flow cytometry. Representative histograms (A-D) of CTLA-4 expression on CD3+ cells in MMTV, Cre- cells are depicted. The percentage of CTLA-4+ cells and CTLA-4 median fluorescence intensity (MFI) in CD8+ (E-F) and CD4+ (G-H) in PPAR γ expressing (black bars) and non-expressing cells (white bars) were assessed. Data are represented as mean ± standard error. Points with an asterisk indicate a significant effect of the treatment in comparison to the DMSO control (P<0.05).
4. Discussion
Our findings show that dietary ABA-supplementation significantly reduces colonic inflammation and reduces the severity of DSS-induced colitis. The model of colitis used in this study does carry with it limitations. DSS is a non-specific promoter of colonic inflammation that can induce colitis independent of T cells and B cells [20, 21]. It is also an acute model of inflammation, whereas CD and UC are chronic conditions. This model has been used successfully to screen therapeutic agents for IBD and is a tool for assessing a treatment’s ability to modulate inflammation.
We found that treatment with ABA significantly decreased adhesion molecule expression and pro-inflammatory cytokine expression in the colonic mucosa. The strategy to block adhesion molecule expression has been recognized as a promising strategy to treat IBD, though currently it does not play an important role in the therapy of IBD sufferers [22]. The specific cellular adhesion molecules which we found were decreased by ABA, VCAM-1, MAdCAM-1, and E-selectin, have been correlated to IBD disease severity [23-25]. In particular, the expression of E-selectin on endothelial cells of venules is thought to be an initial sign indicating relapse from remissive IBD [26, 27]. It is unclear whether the effect of ABA is mediated directly on adhesion molecules or whether the decrease we observed was secondary to ABA’s inhibition of inflammation.
We have reported that ABA-dependent effects on white adipose tissue inflammation are partially dependent on the nuclear receptor PPAR γ [8], a protein that increases regulatory function in immune cells [28-30]. The mechanism underlying the ABA-induced downregulation in adhesion molecules and inflammation does not appear to involve colonic PPAR γ, as neither colonic PPAR γ nor its responsive genes CD36 and FABP4 were significantly affected by ABA-treatment. The mechanism underlying ABA’s anti-inflammatory effects in the colon may involve cAMP, which has been shown to mediate ABA-induced effects in a number of mammalian cell types [10, 11, 31]. Agonists of cAMP often inhibit NF-κB-related gene expression [32], and in particular cAMP activation has been shown to inhibit TNF-α-induced upregulation in E-selectin and VCAM-1 in endothelial cells [33]. Activation of cAMP/PKA also inhibits MMP-9 production in neutrophils [34]. MMP-9 is a key promoter of tissue injury in IBD patients and in the DSS model [17].
At the cellular level, we observed that ABA increased T-regs in the blood and CD4+ and CD8+ T lymphocytes in blood and spleen. The increase in CD4+ and CD8+ T cells is unexpected considering their possible role as effector cells and the anti-inflammatory effects observed in the colon. However, a possible explanation for this paradox is that ABA increases the regulatory capacity of these lymphocyte subsets. We examined this possibility in vitro by assessing the affect of ABA on the lymphocyte regulatory marker CTLA-4 on T cells. ABA treatment significantly increased CTLA-4 protein content on CD4+ lymphocytes in PPAR γ-dependent manner. CTLA-4 is a protein expressed on the surface of T-cells that plays an important role in downregulating the immune response [35]. Polymorphisms of CTLA-4 have been implicated as a genetic factor leading to IBD [18]. Interestingly, there are no studies that have investigated the role of CTLA-4 in DSS-induced colitis, perhaps because colonic inflammation in this model is largely considered to be mediated by epithelial cell apoptosis and macrophage recruitment and activation [21]. While mice deficient in T cells still develop the colitis in the DSS model, it is important to note that these immunodeficient mice lack both T-regs and effecter cells. Thus, T cells may play an important regulatory capacity in DSS model.
In conclusion, we find that ABA intake significantly ameliorates DSS-induced colitis, reduces colonic inflammation, and inhibits leukocyte infiltration into the colon. These changes were associated with a significant inhibition in adhesion molecule expression and an increase in T-regs in the blood. This is the first study that demonstrates ABA’s potential as a therapeutic agent for IBD.
Figure 3.
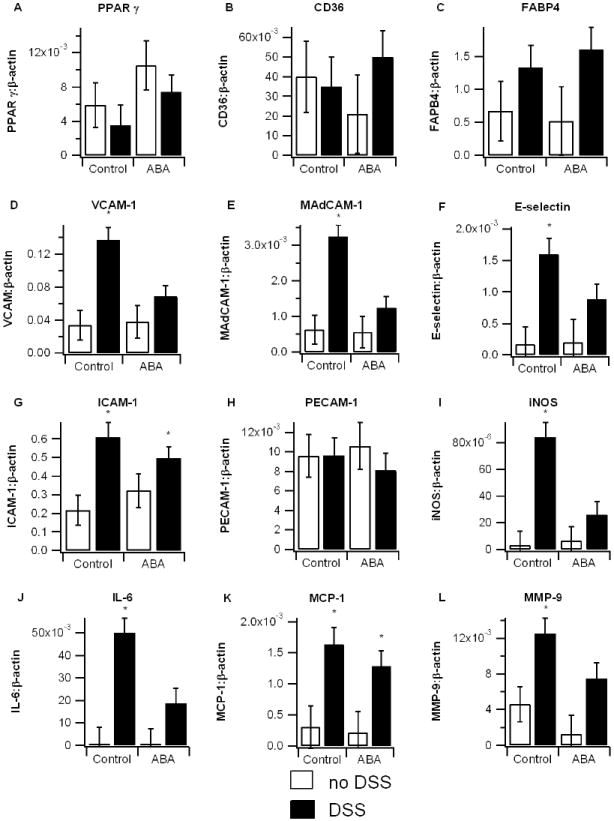
Effect of dietary abscisic acid (ABA) supplementation on colon gene expression. C57BL/6J mice were fed ABA-supplemented (100 mg/kg) or control diets for 35 days and challenged with 2.5% dextran sodium sulfate (DSS) or water (no DSS) for 7 days. Expressions of peroxisome proliferator-activated receptor γ (PPAR γ) and PPAR γ-responsive genes CD36, fatty acid binding protein 4 (FABP-4) (A-C), adhesion molecules vascular adhesion molecule 1 (VCAM-1), muscosal vascular addressin cell adhesion molecule 1 (MAdCAM-1), E-selectin, intracellular adhesion molecule 1 (ICAM-1), and platelet/endothelial cell adhesion molecule 1 (PECAM-1) (D-H), and pro-inflammatory proteins interleukin-6 (IL-6), inducible nitric oxide synthase (iNOS), monocyte chemoattractant protein-1 (MCP-1), and matrix metalloproteinase 9 (MMP-9) (I-L) were assessed by real-time quantitative PCR. Data are represented as mean ± standard error. Points with an asterisk are significantly different from control, non-DSS treatment (P<0.05).
Figure 4.
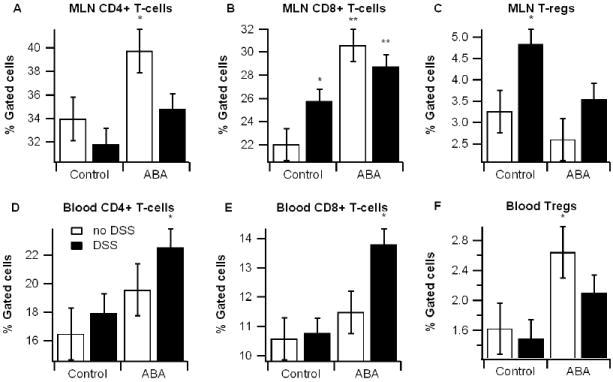
Effect of dietary abscisic acid (ABA) supplementation on lymphocyte subsets in mesenteric lymph nodes (MLN) and blood. MLN (A-C) and blood (D-E) were immunophenotyped with anti-CD3, anti-CD4, anti-CD8, and anti-FoxP3 mouse monoclonal antibodies and the lymphocyte populations were analyzed with flow cytometry with FACS diva software. Data are represented as mean ± standard error. Points with an asterisk are significantly different from control, non-DSS treatment (P<0.05).
Acknowledgments
Supported by a grant Supported by award number 5R01AT4308 of the National Center for Complementary and Alternative Medicine at the National Institutes of Health awarded to J.B.-R., European Commission grant number 224836, the Ramon y Cajal Program and funds from the Nutritional Immunology and Molecular Nutrition Laboratory.
Footnotes
Conflict of interest statement No conflicts to report
Statement of authorship JBR and RH designed the studies, interpreted the results, contributed to write the paper. AJG performed the animal and in vitro studies, run the statistical analyses and wrote the first draft of the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis. 2006;12(Suppl 1):S3–9. doi: 10.1097/01.mib.0000195385.19268.68. [DOI] [PubMed] [Google Scholar]
- 2.Hanauer SB, Present DH. The state of the art in the management of inflammatory bowel disease. Rev Gastroenterol Disord. 2003;3(2):81–92. [PubMed] [Google Scholar]
- 3.Mackner LM, Sisson DP, Crandall WV. Review: psychosocial issues in pediatric inflammatory bowel disease. J Pediatr Psychol. 2004;29(4):243–257. doi: 10.1093/jpepsy/jsh027. [DOI] [PubMed] [Google Scholar]
- 4.Bassaganya-Riera J, Skoneczka J, Kingston DGJ, Krishnan A, Misyak SA, Carter A, Pereira A, Guri AJ, Minorsky P, Turmakin R, et al. Mechanisms of action and medicinal applications of abscisic acid. Current Medicinal Chemistry. 2009 doi: 10.2174/092986710790226110. In press. [DOI] [PubMed] [Google Scholar]
- 5.Fan J, Hill L, Crooks C, Doerner P, Lamb C. Abscisic acid has a key role in modulating diverse plant-pathogen interactions. Plant Physiol. 2009;150(4):1750–1761. doi: 10.1104/pp.109.137943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seo PJ, Park CM. MYB96-mediated abscisic acid signals induce pathogen resistance response by promoting salicylic acid biosynthesis in Arabidopsis. New Phytol. doi: 10.1111/j.1469-8137.2010.03183.x. [DOI] [PubMed] [Google Scholar]
- 7.Guri AJ, Hontecillas R, Si H, Liu D, Bassaganya-Riera J. Dietary abscisic acid ameliorates glucose tolerance and obesity-related inflammation in db/db mice fed high-fat diets. Clin Nutr. 2007;26(1):107–116. doi: 10.1016/j.clnu.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Guri AJ, Hontecillas R, Ferrer G, Casagran O, Wankhade U, Noble AM, Bassaganya-Riera J. The loss of PPAR gamma in immune cells abrogates the ability of abscisic acid to improve insulin sensitivity through a mechanism involving suppression of MCP-1 expression and macrophage infiltration into white adipose tissue. Faseb Journal. 2007;21(5):A64–A64. [Google Scholar]
- 9.Guri AJ, Hontecillas R, Ferrer G, Casagran O, Wankhade U, Noble AM, Eizirik DL, Ortis F, Cnop M, Liu D, et al. Loss of PPAR gamma in immune cells impairs the ability of abscisic acid to improve insulin sensitivity by suppressing monocyte chemoattractant protein-1 expression and macrophage infiltration into white adipose tissue. J Nutr Biochem. 2008;19(4):216–228. doi: 10.1016/j.jnutbio.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Guri AJ, Misyak S, Hontecillas R, Hasty A, Liu D, Si H, Bassaganya-Riera J. Abscisic acid ameliorates atherosclerosis by suppressing macrophage and CD4+ T cell recruitment into the aortic wall. Journal of Nutritional Biochemistry. 2009 doi: 10.1016/j.jnutbio.2009.10.003. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruzzone S, Bodrato N, Usai C, Guida L, Moreschi I, Nano R, Antonioli B, Fruscione F, Magnone M, Scarfi S, et al. Abscisic acid is an endogenous stimulator of insulin release from human pancreatic islets with cyclic ADP ribose as second messenger. J Biol Chem. 2008;283(47):32188–32197. doi: 10.1074/jbc.M802603200. [DOI] [PubMed] [Google Scholar]
- 12.Magnone M, Bruzzone S, Guida L, Damonte G, Millo E, Scarfi S, Usai C, Sturla L, Palombo D, De Flora A, et al. Abscisic acid released by human monocytes activates monocytes and vascular smooth muscle cell responses involved in atherogenesis. J Biol Chem. 2009 doi: 10.1074/jbc.M809546200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desreumaux P, Dubuquoy L. PPARgamma agonists as a new class of effective treatment for ulcerative colitis. Inflamm Bowel Dis. 2009;15(6):959–960. doi: 10.1002/ibd.20765. [DOI] [PubMed] [Google Scholar]
- 14.Lewis JD, Lichtenstein GR, Deren JJ, Sands BE, Hanauer SB, Katz JA, Lashner B, Present DH, Chuai S, Ellenberg JH, et al. Rosiglitazone for active ulcerative colitis: a randomized placebo-controlled trial. Gastroenterology. 2008;134(3):688–695. doi: 10.1053/j.gastro.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bassaganya-Riera J, Reynolds K, Martino-Catt S, Cui Y, Hennighausen L, Gonzalez F, Rohrer J, Benninghoff AU, Hontecillas R. Activation of PPAR gamma and delta by conjugated linoleic acid mediates protection from experimental inflammatory bowel disease. Gastroenterology. 2004;127(3):777–791. doi: 10.1053/j.gastro.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 16.Bassaganya-Riera J, Misyak S, Guri AJ, Hontecillas R. PPAR gamma is highly expressed in F4/80(hi) adipose tissue macrophages and dampens adipose-tissue inflammation. Cell Immunol. 2009;258(2):138–146. doi: 10.1016/j.cellimm.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garg P, Vijay-Kumar M, Wang L, Gewirtz AT, Merlin D, Sitaraman SV. Matrix metalloproteinase-9-mediated tissue injury overrides the protective effect of matrix metalloproteinase-2 during colitis. Am J Physiol Gastrointest Liver Physiol. 2009;296(2):G175–184. doi: 10.1152/ajpgi.90454.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo Y, Xia B, Li C, Chen Z, Ge L, Jiang T, Zhou F, Zhao Y. Cytotoxic T lymphocyte antigen-4 promoter -658CT gene polymorphism is associated with ulcerative colitis in Chinese patients. Int J Colorectal Dis. 2009;24(5):489–493. doi: 10.1007/s00384-008-0626-6. [DOI] [PubMed] [Google Scholar]
- 19.Vendetti S, Riccomi A, Sacchi A, Gatta L, Pioli C, De Magistris MT. Cyclic adenosine 5’-monophosphate and calcium induce CD152 (CTLA-4) upregulation in resting CD4+ T lymphocytes. J Immunol. 2002;169(11):6231–6235. doi: 10.4049/jimmunol.169.11.6231. [DOI] [PubMed] [Google Scholar]
- 20.Axelsson LG, Landstrom E, Goldschmidt TJ, Gronberg A, Bylund-Fellenius AC. Dextran sulfate sodium (DSS) induced experimental colitis in immunodeficient mice: effects in CD4(+) -cell depleted, athymic and NK-cell depleted SCID mice. Inflamm Res. 1996;45(4):181–191. doi: 10.1007/BF02285159. [DOI] [PubMed] [Google Scholar]
- 21.Dieleman LA, Ridwan BU, Tennyson GS, Beagley KW, Bucy RP, Elson CO. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology. 1994;107(6):1643–1652. doi: 10.1016/0016-5085(94)90803-6. [DOI] [PubMed] [Google Scholar]
- 22.Bosani M, Ardizzone S, Porro GB. Biologic targeting in the treatment of inflammatory bowel diseases. Biologics. 2009;3:77–97. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Sans M, Panes J, Ardite E, Elizalde JI, Arce Y, Elena M, Palacin A, Fernandez-Checa JC, Anderson DC, Lobb R, et al. VCAM-1 and ICAM-1 mediate leukocyte-endothelial cell adhesion in rat experimental colitis. Gastroenterology. 1999;116(4):874–883. doi: 10.1016/s0016-5085(99)70070-3. [DOI] [PubMed] [Google Scholar]
- 24.Soriano A, Salas A, Sans M, Gironella M, Elena M, Anderson DC, Pique JM, Panes J. VCAM-1, but not ICAM-1 or MAdCAM-1, immunoblockade ameliorates DSS-induced colitis in mice. Lab Invest. 2000;80(10):1541–1551. doi: 10.1038/labinvest.3780164. [DOI] [PubMed] [Google Scholar]
- 25.Farkas S, Hornung M, Sattler C, Edtinger K, Steinbauer M, Anthuber M, Schlitt HJ, Herfarth H, Geissler EK. Blocking MAdCAM-1 in vivo reduces leukocyte extravasation and reverses chronic inflammation in experimental colitis. Int J Colorectal Dis. 2006;21(1):71–78. doi: 10.1007/s00384-004-0709-y. [DOI] [PubMed] [Google Scholar]
- 26.Cellier C, Patey N, Fromont-Hankard G, Cervoni JP, Leborgne M, Chaussade S, Barbier JP, Brousse N. In-situ endothelial cell adhesion molecule expression in ulcerative colitis. E-selectin in-situ expression correlates with clinical, endoscopic and histological activity and outcome. Eur J Gastroenterol Hepatol. 1997;9(12):1197–1203. [PubMed] [Google Scholar]
- 27.Gulubova MV, Manolova IM, Vlaykova TI, Prodanova M, Jovchev JP. Adhesion molecules in chronic ulcerative colitis. Int J Colorectal Dis. 2007;22(6):581–589. doi: 10.1007/s00384-006-0236-0. [DOI] [PubMed] [Google Scholar]
- 28.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AW, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447(7148):1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klotz L, Burgdorf S, Dani I, Saijo K, Flossdorf J, Hucke S, Alferink J, Novak N, Beyer M, Mayer G, et al. The nuclear receptor PPAR gamma selectively inhibits Th17 differentiation in a T cell-intrinsic fashion and suppresses CNS autoimmunity. J Exp Med. 2009;206(10):2079–2089. doi: 10.1084/jem.20082771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hontecillas R, Bassaganya-Riera J. Peroxisome proliferator-activated receptor gamma is required for regulatory CD4+ T cell-mediated protection against colitis. J Immunol. 2007;178(5):2940–2949. doi: 10.4049/jimmunol.178.5.2940. [DOI] [PubMed] [Google Scholar]
- 31.Bruzzone S, Moreschi I, Usai C, Guida L, Damonte G, Salis A, Scarfi S, Millo E, De Flora A, Zocchi E. Abscisic acid is an endogenous cytokine in human granulocytes with cyclic ADP-ribose as second messenger. Proc Natl Acad Sci U S A. 2007;104(14):5759–5764. doi: 10.1073/pnas.0609379104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hattori Y, Suzuki K, Tomizawa A, Hirama N, Okayasu T, Hattori S, Satoh H, Akimoto K, Kasai K. Cilostazol inhibits cytokine-induced nuclear factor-kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells. Cardiovasc Res. 2009;81(1):133–139. doi: 10.1093/cvr/cvn226. [DOI] [PubMed] [Google Scholar]
- 33.Pober JS, Slowik MR, De Luca LG, Ritchie AJ. Elevated cyclic AMP inhibits endothelial cell synthesis and expression of TNF-induced endothelial leukocyte adhesion molecule-1, and vascular cell adhesion molecule-1, but not intercellular adhesion molecule-1. J Immunol. 1993;150(11):5114–5123. [PubMed] [Google Scholar]
- 34.Ernens I, Rouy D, Velot E, Devaux Y, Wagner DR. Adenosine inhibits matrix metalloproteinase-9 secretion by neutrophils: implication of A2a receptor and cAMP/PKA/Ca2+ pathway. Circ Res. 2006;99(6):590–597. doi: 10.1161/01.RES.0000241428.82502.d4. [DOI] [PubMed] [Google Scholar]
- 35.Thompson CB, Allison JP. The emerging role of CTLA-4 as an immune attenuator. Immunity. 1997;7(4):445–450. doi: 10.1016/s1074-7613(00)80366-0. [DOI] [PubMed] [Google Scholar]


