Summary
Most studies on the stress-responsiveness of the hypothalamic-pituitary-adrenal (HPA) axis have focused on glucocorticoids, while few studies have investigated the adrenal secretion of dehydroepiandrosterone-sulfate (DHEAS), which is unique to primates. Monkeys were chair-restrained for two hours per day for seven consecutive days, and blood samples were collected upon placement in the chair, and at 15, 30, 60 and 120 minutes later. Like cortisol, DHEAS concentrations increased throughout the initial session of chair restraint (acute stress). Unlike the cortisol response, which decreased after repeated exposure to the stressor, the DHEAS response was sustained throughout the seventh session of restraint (chronic stress) and response to the seventh session of restraint did not differ from the DHEAS response to the initial session. Like cortisol, DHEAS concentrations showed a diurnal rhythm with higher concentrations in the morning compared to the evening and a decrease in response to dexamethasone (DEX) administration. After repeated exposure to the stressor, the suppression of DHEAS in response to dexamethasone was more complete, suggesting an increase in negative feedback sensitivity. These data show that DHEAS concentrations increase in response to both acute and chronic (repeated) stress and provide another measure of HPA activity that parallels cortisol during acute responses to stress but diverges in chronic or repeated stress.
Keywords: hypothalamic-pituitary-adrenal (HPA) axis, dehydroepiandrosterone sulfate, DHEAS, cortisol, stress, rhesus monkey, dexamethasone
1. Introduction
While most studies on the stress responsiveness of the hypothalamic-pituitary-adrenal (HPA) axis have focused on adrenal secretion of glucocorticoids, little research has been done on the secretion of the adrenal androgen dehydroepiandrosterone (DHEA) and its more stable sulfate ester DHEA-sulfate (DHEAS). In part, this is because androgen secretion by the adrenal cortex is restricted to primate (human and non-human) species due to a unique three-layered cortex. While both primates and rodents (rats and mice) have a zona glomerulosa layer that secretes mineralocorticoids (e.g., aldosterone) and a zona fasciculata layer that secretes glucocorticoids (e.g., cortisol), only primates have a zona reticularis layer that expresses cytochrome P450c17 (the enzyme responsible for the synthesis of DHEA from pregnenolone) and secretes DHEA and DHEAS (Conley et al., 2004; Nguyen and Conley, 2008). DHEAS is the major secretory steroid of the adrenal gland (Vaitukaitis et al., 1969) and the most abundant steroid hormone in the human body (Orentreich et al., 1984). Despite high circulating concentrations, DHEAS’ mechanisms of action are not completely understood as no unique steroid hormone receptor for DHEAS (or DHEA) has been found (Rehman and Carr, 2004; Maninger et al., 2009). We do know that DHEA and DHEAS (jointly referred to in this article as “DHEA(S)”) have neuroprotective actions, and are precursors to more potent androgens and estrogens, such as testosterone and estradiol (Kurata et al., 2004; Labrie, 2004; Maninger et al., 2009). Exogenous administration of DHEA (which gets converted to DHEAS in vivo) has been found to improve symptoms of depression in patients with major depression, as well as improve negative symptoms in patients with schizophrenia (Wolkowitz et al., 1999; Strous et al., 2007; Wolkowitz et al., 2008; Maninger et al., 2009).
Like humans, rhesus macaque monkeys show an increase in plasma DHEAS in response to corticotropin releasing hormone (CRH) administration (Goncharova and Lapin, 2002). Cortisol and DHEAS are secreted from the adrenal cortex in response to corticotropin (ACTH) stimulation in both humans (Nieschlag et al., 1973) and rhesus monkeys (Koritnik et al., 1983). Therefore, given the similar mechanism of secretion, it is reasonable to predict that both hormones would increase in response to a stressor. A concomitant increase in serum DHEAS and salivary cortisol concentrations has been demonstrated in military personnel experiencing the stressors associated with mock prisoner of war camp, including food and sleep deprivation (Morgan et al., 2004). In a study of hormonal response to blood sampling procedures in animals’ individual home cages (with squeeze mechanisms), female rhesus monkeys showed an increase in serum cortisol and DHEAS concentrations over time (Fuller et al., 1984). In this study, animals were restrained for 2-4 min with the squeeze mechanism of their cages and bled at 30-min intervals for two hours. Although serum concentrations of both hormones increased after animals were restrained, cortisol concentrations were significantly elevated from baseline at 30 min while DHEAS concentrations were significantly increased from baseline at 90 min (Fuller et al., 1984). While these results suggest similar regulation in healthy individuals (i.e., both cortisol and DHEAS concentrations increase in response to a stressor), the difference in time course suggests a more complex picture; in fact, other data also indicate differences in the regulation of these two adrenal steroids.
Under conditions of chronic medical illness, levels of DHEA(S) and cortisol have been observed to become dissociated (Parker et al., 1985); for example, cortisol concentrations either increase or do not change, and DHEAS concentrations tend to decrease (Semple et al., 1987; Wade et al., 1988). This has also been observed in people with acquired immunodeficiency syndrome (AIDS) (Villette et al., 1990), rheumatoid arthritis (Masi et al., 2006), type 2 diabetes mellitus (Yamauchi et al., 1996), and other medical illnesses (for a review, see (Kroboth et al., 1999)). Although these “conditions” are sometimes referred to in the literature as “chronic stress” – these refer to the chronic stress of medical illness and not other types of psychological chronic stress, such as unemployment, which are not associated with a disease process. The “imbalance” of levels of cortisol and DHEA(S) in medical illness is thought to be important since the actions of cortisol and DHEA(S) can be antagonistic. For example, while cortisol is a catabolic hormone, DHEA(S) is a precursor to more potent anabolic steroids, such as testosterone (Labrie, 2004). DHEA has also been described as having anti-glucocorticoid properties because it buffers or antagonizes the effects of cortisol (Kalimi et al., 1994; Hennebold et al., 1995; Maninger et al., 2009). Because of these properties, DHEA(S) has been proposed to play a role in modulating the vulnerability of an organism to the negative consequences of stress (Charney, 2004; Morgan et al., 2004).
To our knowledge, no study has examined the DHEAS response to an acute novel stressor and to repeated exposure to the same stressor (laboratory model of chronic stress). Rhesus monkeys are an ideal animal model to examine this question. In addition to the advantage of increased experimental control (compared to human participants), rhesus monkeys are similar to humans in their zona reticularis zone of the adrenal cortex, which mice and rats do not have (Conley et al., 2004; Nguyen and Conley, 2008). Like humans, rhesus monkeys show a decrease in DHEAS concentrations in response to the synthetic glucocorticoid dexamethasone (Wickings and Nieschlag, 1978; Koritnik et al., 1983), presumably due to its negative feedback effect on pituitary secretion of ACTH, which in turn suppresses adrenal output of both cortisol and DHEAS. While ACTH administration following dexamethasone (DEX) suppression results in an increase in DHEAS concentrations in rhesus monkeys (Koritnik et al., 1983), administration of human chorionic gonadotropin (hCG) following DEX suppression does not (Wickings and Nieschlag, 1978). This evidence, along with the localization of DHEA sulfotransferase (DST, the enzyme that sulfonates DHEA) to the zona reticularis layer of the adrenal cortex but not the ovary or testis in rhesus monkeys (Parker et al., 2000), suggests that DHEAS is primarily of adrenal origin in rhesus monkeys. Because DHEA can be secreted by both the testis and the adrenal gland (Wickings and Nieschlag, 1978) and we are interested in HPA activity, this study focuses on DHEAS.
In order to better understand the stress responsive nature of DHEAS, the current investigation takes advantage of banked blood samples from a previous study in our laboratory that examined changes in plasma glucocorticoid levels and behavior in adult male rhesus monkeys exposed to repeated physical restraint (Ruys et al., 2004). The animals were restrained in primate chairs for two hours each day for seven consecutive days, and blood samples were collected at regular intervals during each two hour chairing session. Animals showed a sustained increase in cortisol concentrations throughout their first consecutive session of chair restraint, but the cortisol response to the seventh session of restraint was substantially reduced (Ruys et al., 2004). Chair restraint sessions in which DEX was administered prior to restraint revealed that animals had an increase in glucocorticoid negative feedback sensitivity after seven days of consecutive chair restraint (Ruys et al., 2004). Morning basal cortisol concentrations on the day after the initial session of restraint were increased in comparison to pre-restraint samples. After seven consecutive sessions of chair restraint, morning basal cortisol concentrations were no different from pre-restraint samples, further demonstrating that the HPA response to chair restraint had changed (Ruys et al., 2004). The current study will examine the DHEAS concentrations in each of these blood samples and address the following questions: (1) Does the initial chair restraint session (acute stress) lead to an increase in DHEAS as it does in cortisol?; (2) How does DHEAS respond after repeated exposure to the stressor, and does DHEAS respond in the same manner as cortisol?; (3) How does DHEAS respond to chair restraint after pre-treatment with DEX, and does repeated chair restraint change the DHEAS response to DEX and chair restraint as it changes the cortisol response?; (4) Does DHEAS show a diurnal rhythm like cortisol, and if so, is the rhythm altered by repeated chair restraint?
2. Methods
2.1. Subjects and Housing
The subjects of the current investigation were 36 adult male rhesus macaque monkeys (Macaca mulatta). These animals were selected from a larger sample of 88 males that were born and raised in outdoor half-acre enclosures each containing approximately 70-100 animals of mixed sex and age at the California National Primate Research Center (CNPRC). Selection criteria for this project included low-to-intermediate social rank in natal groups, sex (male), age, personality ratings, good physical health, and negative serostatus for simian immunodeficiency virus, simian retrovirus D, and simian T-lymphotropic virus 1. At a mean age of 7.3 (range 5.4-9.4) years, subjects were relocated from their natal enclosures to indoor individual housing with automatically regulated lighting (12 h light/12 h dark cycle, with lights on at 0600h). Animals were fed monkey chow twice daily at 0700h and 1200h supplemented with fresh fruits and vegetables and water was available ad libitum. These subjects were part of a larger project designed to explore biobehavioral differences in High- and Low-Sociable animals (for details of personality rating procedures see (Capitanio, 1999); for details of rating procedures on the current group of animals see (Maninger et al., 2003)). Preliminary analyses revealed that the personality dimension of Sociability did not impact the results reported herein and will not be discussed further.
2.2. Training
Prior to data collection for this study, all subjects were trained to extend their arm through a small opening in the front of the cage for blood sample collection from an antecubital vein. Approximately two weeks before the study began, subjects were fitted with metal collars specially designed to allow two poles to be attached, permitting the monkeys to be removed from their home cage while their movement was guided by technicians (Anderson and Houghton, 1983). Each subject received five training sessions with the pole and collar method before data collection began, so that subjects were familiar with all of the procedures leading up to chair restraint, but had no experience being restrained in a primate restraint chair (Primate Products, Redwood City, CA; Anderson and Houghton, 1983) nor in the testing environment. Further details about procedures have been published in Ruys et al. (2004). All procedures were approved by the University of California, Davis, Institutional Animal Care and Use Committee. CNPRC is accredited by the Association and Accreditation of Laboratory Animal Care International.
2.3. Design and Procedure
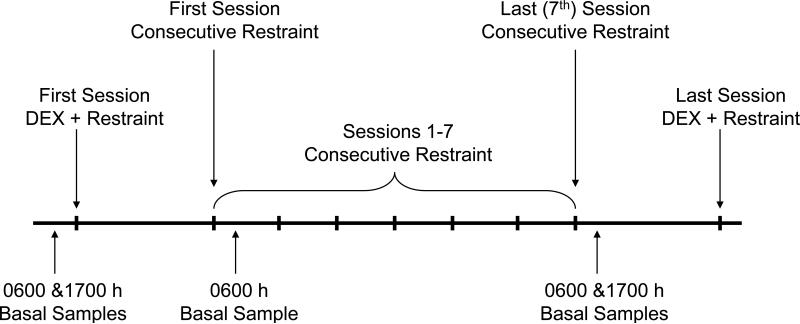
Figure 1 shows the experimental design.
Figure 1.
Diagram of experimental design, adapted from Ruys et al (2004).
2.3.1. Consecutive Restraint Sessions
Each subject was placed into a primate restraint chair for 2 h per day on each of seven consecutive days. Placing an animal into a restraint chair involved a technician attaching a pole to the animal's collar, opening the cage door, coaxing the animal from the cage, guiding the animal to the chair, fitting the collar to the chair, and attaching the shoulder straps. On each of the first and seventh day of consecutive chairing, a blood sample was collected as soon as possible following the animal being restrained in the chair (Time 0). The chaired animal was then moved, in the restraint chair, to an adjacent testing room, and subsequent blood samples were collected 15, 30, 60, and 120 min after initial placement in the chair (i.e., Time 0). The animals were assigned to a morning or afternoon cohort, and 4-6 animals were tested concurrently. There were barriers set up in the testing room so that animals did not have visual access but could hear each other. Morning cohorts were tested from 0800h to 1030h, and afternoon cohorts were tested from 1300h to 1500h.
2.3.2. Dexamethasone (DEX) Restraint Sessions
There were two chair restraint sessions where animals were treated with the synthetic glucocorticoid dexamethasone (DEX) prior to the restraint session (DEX restraint sessions). The first DEX restraint session occurred 3-5 days prior to the consecutive restraint sessions, and thus was the first experience each subject had in a primate restraint chair. The second DEX restraint session occurred two days after the final consecutive restraint session. For each DEX restraint session, subjects were administered a low dose of dexamethasone (50 μg/kg IM) five hours prior to restraint. As in the consecutive restraint sessions, blood samples were collected during the DEX restraint sessions immediately upon placement in the chair (Time 0) and after 15, 30, 60, 90, and 120 min of restraint.
2.3.3. Diurnal Rhythm
Basal blood samples were collected via armpulls from unanesthetized animals at 0600h (near the diurnal peak) and 1700h (near the diurnal trough) at the beginning of the study (before any of the animals were chaired) and the day following the last day of consecutive chair restraint. An additional morning sample was collected after the first day of consecutive chair restraint at 0600h.
2.3.4. Sample Collection and Hormone Assessment
Blood samples (1 ml) were collected using 3 ml syringes and were immediately transferred to tubes containing EDTA and put on wet ice. Within 1 h of collection, blood samples were spun at 3000 rpm for 10 min and the plasma was removed and stored at -70° C until assay. Concentrations of DHEAS were assessed from banked plasma samples previously assayed for cortisol concentrations (Ruys et al., 2004). All of a given subject's samples were run in the same assay. Plasma samples were assayed in duplicate using commercial direct radioimmunoassay kits (DHEAS Coat-a-Count kit, Diagnostics Products Corporation, Los Angeles, CA); this assay has been used by another group to measure DHEAS in rhesus monkeys (Kemnitz et al., 2000). The DPC DHEAS assay is highly specific for DHEAS; its antiserum has a high (100%) cross-reactivity with DHEAS and a very low (0.57%) cross-reactivity with DHEA. The sensitivity (detectability) of the DHEAS assay was 2.423 μg/dl (65.76 nmol/l). Intra- and inter-assay coefficients of variation (CV) were 6.17% and 6.56%, respectively.
2.4. Statistical Analysis
The effect of chair restraint on DHEAS concentrations over time was assessed with repeated measures analysis of variance (ANOVA) with Cohort chairing time (morning vs. afternoon) as a between-subjects measure and Session (first vs. last) as a within-subjects measure. Repeated-measures contrasts were used to compare each time point to the next (e.g., 0 vs. 15, 15 vs. 30, etc). Separate ANOVAs were run for consecutive restraint sessions and DEX restraint sessions. DEX suppression was tested by comparing Time 0 of the first DEX session to the first consecutive chair restraint session (which occurred at the same time of day for each animal) using a paired t-test.
Diurnal rhythm (prior to any chair restraint) was examined by comparing the 0600h and 1700h basal samples using a paired t-test. The difference in 0600h morning DHEAS concentrations between the pre-chair basal (Pre) sample and the first day after consecutive chair restraint (Post) was tested using repeated-measures ANOVA with Pre-Post as a within-subjects measure and Cohort (morning vs. afternoon) as a between-subjects measure. Changes in basal morning and evening DHEAS concentrations prior to (Pre) and following all sessions of consecutive chair restraint (Last) were evaluated using repeated measures ANOVA with Pre-Last and Time of Day (morning vs. evening) as within-subjects measures and Cohort (morning vs. afternoon) as a between-subjects variable.
The sample size was n=36 for all measures with the exception of the DEX restraint sessions. There were two subjects who did not receive DEX pre-treatment in the first DEX restraint session and one subject who did not receive DEX prior to the last DEX restraint session, resulting in n=33 subjects with complete data for both DEX restraint sessions. All variables met homogeneity of variance assumptions based on Levene's test and a sample size greater than 30. Because sphericity assumptions were not met, the Huynh-Feldt adjusted degrees of freedom are reported for the repeated-measures ANOVA results. Statistical significance was assessed at the alpha = 0.05 level. Analyses were run with the Statistical Package for Social Sciences (SPSS, version 16, Chicago, IL).
3. Results
3.1. Time to Collect Blood Samples
Analyses of the time taken to collect blood samples revealed no impact on DHEAS concentrations. Basal morning and evening blood samples were collected in an average of 9.9 (± 0.45) min from room entry (basal disturbance time) and a mean of 1.61 (± 0.45) min from the initial disturbance of each monkey. For the restraint sessions, Time 0 blood was taken within a mean of 14.12 (± 0.56) min from the initial disturbance of the room. There were no significant correlations (p > 0.10) between DHEAS concentrations and time to collect either basal morning and evening samples, or Time 0 blood samples from the chair restraint sessions (with and without dexamethasone).
3.2. Consecutive Restraint Sessions
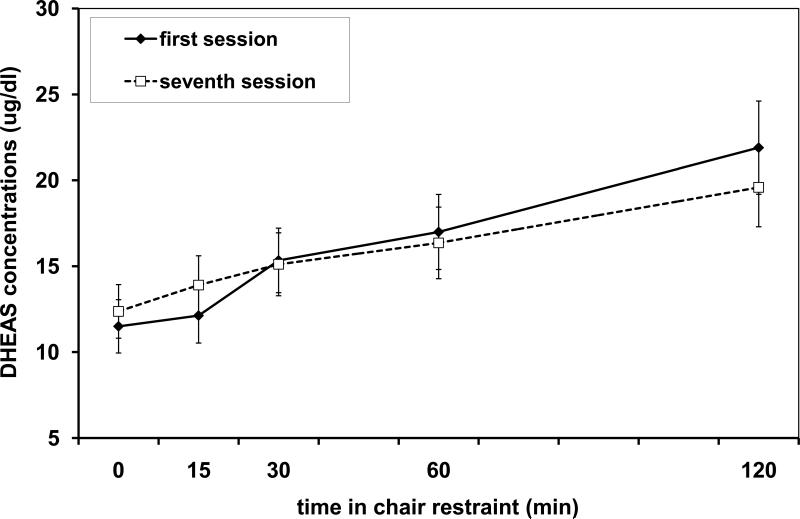
DHEAS concentrations increased during the 2 h restraint in both the first and last session of consecutive chair restraint [main effect of Time: F(1.46,75.04)=42.174, p<0.001; see Figure 2]. There were also significant Session × Cohort [F(1, 75.04)=5.65, p=0.023] and Session × Time [F(2.21, 75.04)=41.76, p<0.001] interactions. As expected, post hoc analysis revealed that subjects in the morning cohort had higher DHEAS concentrations than subjects in the afternoon cohort, and this overall difference was more pronounced in the last session of consecutive chair restraint than the first. Post hoc analysis of the Session × Time interaction revealed that DHEAS concentrations were higher at the 15 min time point during the last consecutive restraint session compared to the first session, while the rest of the time points revealed no significant differences between the first and last consecutive restraint session. The comparison of each time point to the next indicated that DHEAS concentrations increased significantly after 15 min during the first session and at each time point during the last session of consecutive chair restraint (all Ps <0.03).
Figure 2.
Mean (with standard error bars) DHEAS concentrations over time during the first consecutive restraint session (diamond points, solid line) and during the last (seventh) consecutive restraint session (open squares, dotted line) for N=36 adult male rhesus monkeys. Note that 1 ug/dl = 27.138 nmol/l.
3.3. Dexamethasone (DEX) Restraint Sessions
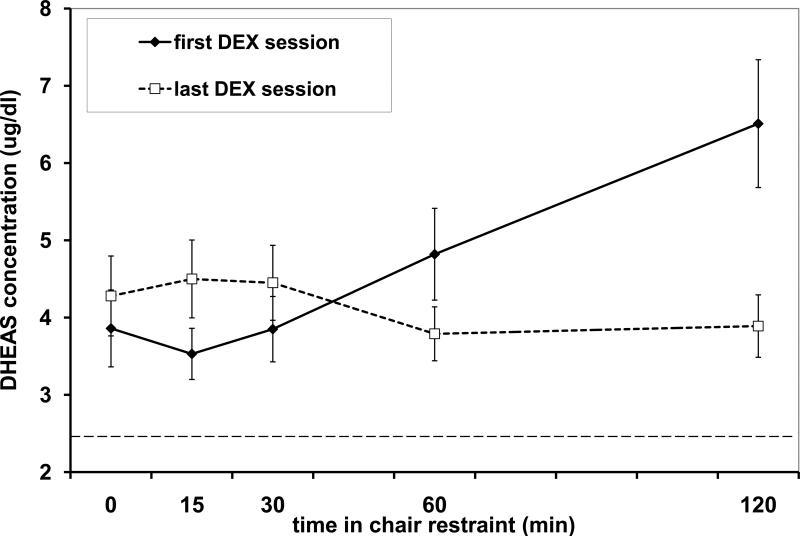
Pretreatment with 50 μg/kg dexamethasone (DEX) resulted in lower Time 0 DHEAS concentrations compared to the first consecutive restraint session (where DEX was not given) [DHEAS with DEX= 3.93 μg/dl (106.65 nmol/l), DHEAS without DEX=11.63 μg/dl (315.61 nmol/l), t(33)=6.345, p<0.001]. DHEAS concentrations were reduced in 31 of the 34 subjects administered DEX, and 3 subjects had DHEAS concentrations that remained at the sensitivity level of the assay [2.423 μg/dl (65.76 nmol/l) both prior to and in response to DEX]. When the first DEX restraint session was compared to the last DEX restraint session, there was no difference in mean DHEAS concentration [no main effect of Session, p>0.304], but DHEAS concentrations were found to change over time [main effect of Time, F(1.67,48.45)=6.835, p=0.004], and the pattern of change was different across each session [Session × Time interaction, F(1.56, 48.45)=14.556, p<0.001; see Figure 3]. There was no difference between animals chaired in the morning vs. the afternoon [no main effect or interactions with Cohort, Ps >0.10]. Post hoc analysis of the Session × Time interaction using paired t-tests revealed that DHEAS concentrations were lower at the 15 min time point (t(32)=-3.346, p=0.002) and higher at the 60 min (t(32)=2.091, p=0.045) and 120 min (t(32)=3.602, p<0.001) time points in the first DEX session compared to the last DEX session. During the first DEX restraint session, a comparison of each time point to the next indicated that DHEAS concentrations increased significantly at 60 and 120 min after the animals were placed in restraint chairs (Ps <0.02). During the last DEX restraint session, a comparison of each time point to the next indicated that DHEAS concentrations had small but statistically significant fluctuations around a low mean value (4.20 μg/dl or 114 nmol/l), with an increase between the 0 and 15 min time points and a decrease between 30 and 60 min (Ps <0.04).
Figure 3.
Mean (with standard error bars) DHEAS concentrations over time during the first DEX restraint session (diamond points, solid line) and during the last DEX restraint session (open squares, dotted line) for N=33 adult male rhesus monkeys. The dotted horizontal line at 2.423 μg/dl represents the sensitivity (lowest detectable dose) of the DHEAS assay. Note that 1 ug/dl = 27.138 nmol/l.
Due to the difference in time course between the first and last DEX restraint sessions, area under the curve (AUC) was calculated to determine if overall concentrations of DHEAS differed between the two conditions. Comparison of AUC for the first and last DEX restraint session revealed that there was no difference in the volume of DHEAS secreted between the first and last session (t(32)=1.301, p>0.20).
3.4. Diurnal Rhythm
Prior to any chair restraint, DHEAS concentrations showed a diurnal rhythm with morning (0600h) basal samples having higher concentrations than evening (1700h) samples [mean pre-chair AM = 14.39 μg/dl (390.52 nmol/l), PM = 6.82 μg/dl (185.08 nmol/l), t(35)=6.725, p<0.001]. Following the first day of consecutive chair restraint (Post), morning basal DHEAS concentrations were no different from the morning samples collected prior to chair restraint [mean post-chair day 1 AM = 15.84 μg/dl (429.87 nmol/l); no main effect of Pre-Post, F(1,34)=2.342, p=0.135]. There were no differences between animals chaired in the morning vs. the afternoon [no main effect or interaction with Cohort, Ps >0.10].
When the morning and evening samples on the day after the last consecutive restraint session (Last) were compared to the diurnal samples prior to any chair restraint (Pre), DHEAS concentrations were found to be higher in the morning than in the evening overall [main effect of Time of Day, F(1,34)=71.581, p<0.001]. There were also significant Pre-Last × Cohort [F(1, 34)=6.038, p=0.019] and Pre-Last × Time of Day [F(1,34)=11.649, p=0.002] interactions. Post hoc analysis of the Pre-Last × Cohort interaction revealed that animals chaired in the morning had higher mean DHEAS concentrations than animals chaired in the afternoon after seven sessions of consecutive chair restraint. Post hoc analysis of the Pre-Last × Time of Day interaction revealed that morning (but not evening) DHEAS concentrations were higher after consecutive chair restraint for all subjects compared to prior to restraint [mean last consecutive restraint AM = 17.38 μg/dl (483.87 nmol/l), PM = 6.42 μg/dl, (174.23 nmol/l)].
4. Discussion
To our knowledge, this is the first study to examine the adrenocortical response of plasma DHEAS to a novel stressor in rhesus monkeys. As predicted, we found that DHEAS concentrations increased in response to the initial session of chair restraint (acute stressor) in adult male rhesus monkeys. Unlike the DHEAS response of female rhesus monkeys to blood sampling procedures where cortisol concentrations were increased from baseline at 30 min and DHEAS concentrations were increased at 90 min (Fuller et al., 1984), our animals showed significant increases in both cortisol and DHEAS concentrations from baseline at 30 min (cortisol data, Ruys et al, 2004). Thus, it appears that the adrenocortical response of cortisol and DHEAS to acute stress is similar, at least in this situation.
In the examination of other aspects of HPA activity, we found that DHEAS concentrations (like cortisol concentrations) showed a diurnal rhythm with higher concentrations in the morning than in the evening. Variation in plasma DHEAS concentrations across the day has been confirmed in another study of rhesus monkeys (Goncharova et al., 2006). Also consistent with other studies in rhesus monkeys (Wickings and Nieschlag, 1978; Koritnik et al., 1983), we found that DHEAS concentrations decreased in response to dexamethasone (DEX) administration. During the first chair restraint session that followed the DEX administration (first DEX restraint session), the low dose of DEX did not fully block DHEAS secretion. DHEAS concentrations increased and “broke out” of the DEX suppression 60 min after the animals were placed in chair restraint for the first time. During the second DEX restraint session, which was each animal's ninth time in the restraint chair, the standard dose of DEX was more effective in keeping DHEAS from “breaking out” from DEX suppression compared to the first DEX restraint session. The DHEAS response to the DEX restraint sessions was similar to the cortisol response in these animals. Both cortisol and DHEAS “broke out” of DEX suppression during the first DEX restraint session and both hormones remained low in the last DEX restraint session (Ruys et al., 2004). The lack of a large hormonal response (for DHEAS in addition to cortisol) during the last DEX restraint session (compared to the first) provides further support for the Ruys et al. (2004) conclusion that the HPA axis was more sensitive to the negative feedback effects of glucocorticoids after a week of consecutive chair restraint.
Although the adrenocortical response of cortisol and DHEAS were similar with regard to diurnal rhythm, DEX suppression, and response to acute stress, the DHEAS response was different from the cortisol response following repeated exposure to chair restraint stress in our group of adult male rhesus monkeys. Unlike the reduced cortisol response that was observed after seven days of two hour consecutive chair restraint (Ruys et al., 2004), the animals’ DHEAS response to the last consecutive restraint session was as strong as the DHEAS response to the initial restraint session. This result was surprising given that repeated exposure to a stressor typically results in a decreased glucocorticoid response, which is what we previously observed in this group of animals where cortisol concentrations increased for the first 30 min and then did not change (overall reduction of 29.8%; Ruys et al., 2004).
Another piece of evidence that there was a change in the HPA axis across the week of consecutive chair restraint comes from morning basal samples collected prior to chair restraint and two more times following the first and last day of consecutive chair restraint. DHEAS concentrations on the morning after the first day of restraint were no different from pre-restraint samples. After a week of chair restraint, DHEAS concentrations on the morning after the last day of restraint were higher than pre-restraint samples. Unlike DHEAS, basal cortisol concentrations were higher the morning after the first consecutive session of chair restraint but no different from pre-restraint morning samples on the morning following the last session of restraint (Ruys et al, 2004). It is unclear why an increase in DHEAS concentrations was not observed on the morning after the initial chair restraint session as it was for cortisol. DHEAS concentrations increased in response to both the first and the last chair restraint sessions, but basal DHEAS concentrations were only higher on the morning after the last day of consecutive chair restraint, suggesting that the DHEAS response (unlike the cortisol response) was still activated after a week of chair restraint.
Physical restraint of monkeys in specially designed chairs is a routine procedure in many laboratories where experimental protocols require animals to sit in place for sustained periods or require close contact between animals and humans. Animals quickly submit to the procedure of being placed in restraint and show substantial reductions in behavioral agitation. Most investigators have relied on such behavioral changes as indicators of habituation to the chairing procedure. However, behavior is not necessarily an indicator of underlying physiological processes (Ruys et al., 2004). In Ruys et al (2004), we argued that the reduction in cortisol could be due to either (1) psychological habituation to the stressor or (2) physiological adaptation to avoid sustained high levels of circulating glucocorticoids. In psychological habituation, the cortisol response is reduced when chair restraint is no longer perceived as a “stressor” and subsequently, the HPA response is reduced. In physiological adaptation, the animal still perceives chair restraint as a stressor, but the body has adapted physiologically in order to avoid sustained high levels of circulating glucocorticoids, which can be cytotoxic. When the animals were re-exposed to the chair restraint procedure following a six-month absence, they showed a full glucocorticoid response (and remained behaviorally similar to the last session of consecutive chair restraint). Given these findings, the diminished glucocorticoid response observed on the last day of consecutive chair restraint was reasoned to be due to physiological adaptation, rather than psychological habituation (Ruys et al., 2004). Our current investigation examining the adrenocortical response of DHEAS demonstrates that part of the HPA axis still responding to the chair restraint and, therefore, chairing is still perceived by the animal as “stressful.”
The dissociation between cortisol and DHEAS concentrations has been documented under conditions of chronic illness (Parker et al., 1985), as well as during aging, surgery and fasting (Maninger et al., 2009). However, this pattern is typically characterized by an increase in cortisol and a decrease in DHEAS concentrations. In contrast to this pattern shown in chronic illness, we found that the cortisol response was reduced following a chronic laboratory stressor (repeated chair restraint), while the DHEAS response was sustained after repeated exposure to the stressor. Thus, we observed a decrease in cortisol responsiveness and a sustained increase in DHEAS responsiveness.
Scientists have been searching for decades for another pituitary secretagogue for DHEAS and other adrenal androgens, but so far, none has been found, although many modulators have been proposed (Parker and Odell, 1980). More recently, there has been research investigating interactions between the adrenal cortex and adrenal medulla (Ehrhart-Bornstein and Bornstein, 2008). A local CRH/ACTH system has been found in the adrenal medulla (Vrezas et al., 2003), which may help explain why an increase in DHEAS is observed in response to acute and chronic stress, since the zona reticularis is closest in proximity to the adrenal medulla.
Unlike the high sustained glucocorticoid output that is often predicted for individuals experiencing chronic stress, our animals showed a reduced cortisol response following repeated exposure to the stressor (Ruys et al., 2004). A reduction in cortisol concentrations also has been observed in other studies from our laboratory on chronic social stress in squirrel monkeys and rhesus monkeys (Mendoza et al., 2000). Because one of the primary functions of cortisol is to terminate the stress response (Munck et al., 1984), the negative effects of chronic stress may be due to cortisol levels that are too low and unable to suppress and terminate the stress response.
While DHEA(S) may buffer the effects of high cortisol, it is unclear if DHEA(S) modulates instances where chronic stress is accompanied by low basal cortisol concentrations or a lack of cortisol responsiveness. This cortisol response was observed in our monkeys exposed to repeated chair restraint (Ruys et al., 2004), and has been observed in people with post-traumatic stress disorder (PTSD) (Yehuda, 2001). High DHEA(S) responses to ACTH have been speculated to be salutary in people with PTSD (Rasmusson et al., 2004), yet little research has been done on the stress-responsiveness of DHEAS. Our animals showed a sustained increase in DHEAS in response to both acute and chronic stress. Interestingly, the physiology of our monkeys exposed to repeated chair restraint is similar to people with PTSD, which is characterized by low cortisol concentrations, increased DHEA or DHEAS concentrations, and increased sensitivity to glucocorticoid feedback (Yehuda, 2001; Yehuda et al., 2006). What role DHEA(S) is playing with regard to PTSD is still unclear, but repeated stress studies in rhesus monkeys may be helpful in understanding whether alterations in DHEA(S) are associated with pathology or adaptation.
Acknowledgements
We would like to acknowledge the assistance of Christine Brennan, Norman Brule, Laura Del Rosso, Steve Kinsey, Edna Lowe, Erna Tarara, Greg Vicino, and Robert Vogt in data collection. This project was supported by Grant No. MH49033 (to J.P.C.) and Grant No. RR00169 to the California National Primate Research Center (CNPRC). This material is based upon work supported under a National Science Foundation Graduate Fellowship (to N.M.), and N.M. was funded by an NIMH T32 training grant (MH09391). All procedures were approved by the University of California, Davis, Institutional Animal Care and Use Committee. CNPRC is accredited by the Association and Accreditation of Laboratory Animal Care International.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson JH, Houghton P. The pole and collar system. A technique for handling and training nonhuman primates. Lab Animal. 1983;12:47–49. [Google Scholar]
- Capitanio JP. Personality dimensions in adult male rhesus macaques: prediction of behaviors across time and situation. Am J Primatol. 1999;47:299–320. doi: 10.1002/(SICI)1098-2345(1999)47:4<299::AID-AJP3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- Conley AJ, Pattison JC, Bird IM. Variations in adrenal androgen production among (nonhuman) primates. Semin Reprod Med. 2004;22:311–326. doi: 10.1055/s-2004-861548. [DOI] [PubMed] [Google Scholar]
- Ehrhart-Bornstein M, Bornstein SR. Cross-talk between adrenal medulla and adrenal cortex in stress. Ann N Y Acad Sci. 2008;1148:112–117. doi: 10.1196/annals.1410.053. [DOI] [PubMed] [Google Scholar]
- Fuller GB, Hobson WC, Reyes FI, Winter JS, Faiman C. Influence of restraint and ketamine anesthesia on adrenal steroids, progesterone, and gonadotropins in rhesus monkeys. Proc Soc Exp Biol Med. 1984;175:487–490. doi: 10.3181/00379727-175-41825. [DOI] [PubMed] [Google Scholar]
- Goncharova ND, Lapin BA. Effects of aging on hypothalamic-pituitary-adrenal system function in non-human primates. Mech Ageing Dev. 2002;123:1191–1201. doi: 10.1016/s0047-6374(02)00012-x. [DOI] [PubMed] [Google Scholar]
- Goncharova ND, Shmaliy AV, Bogatyrenko TN, Koltover VK. Correlation between activity of antioxidant enzymes and circadian rhythms of corticosteroids in Macaca mulatta monkeys of different age. Exp Gerontol. 2006;41:778–783. doi: 10.1016/j.exger.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Hennebold JD, Poynter ME, Daynes RA. DHEA and immune function: Activities and mechanism of action. Semin Reprod Endocrinol. 1995;13:257–269. [Google Scholar]
- Kalimi M, Shafagoj Y, Loria R, Padgett D, Regelson W. Anti-glucocorticoid effects of dehydroepiandrosterone (DHEA). Mol Cell Biochem. 1994;131:99–104. doi: 10.1007/BF00925945. [DOI] [PubMed] [Google Scholar]
- Kemnitz JW, Roecker EB, Haffa AL, Pinheiro J, Kurzman I, Ramsey JJ, MacEwen EG. Serum dehydroepiandrosterone sulfate concentrations across the life span of laboratory-housed rhesus monkeys. J Med Primatol. 2000;29:330–337. doi: 10.1034/j.1600-0684.2000.290504.x. [DOI] [PubMed] [Google Scholar]
- Koritnik DR, Laherty RF, Rotten D, Jaffe RB. A radioimmunoassay for dehydroepiandrosterone sulfate in the circulation of rhesus monkeys. Steroids. 1983;42:653–667. doi: 10.1016/0039-128x(83)90129-0. [DOI] [PubMed] [Google Scholar]
- Kroboth PD, Salek FS, Pittenger AL, Fabian TJ, Frye RF. DHEA and DHEA-S: a review. J Clin Pharmacol. 1999;39:327–348. doi: 10.1177/00912709922007903. [DOI] [PubMed] [Google Scholar]
- Kurata K, Takebayashi M, Morinobu S, Yamawaki S. beta-estradiol, dehydroepiandrosterone, and dehydroepiandrosterone sulfate protect against N-methyl-D-aspartate-induced neurotoxicity in rat hippocampal neurons by different mechanisms. J Pharmacol Exp Ther. 2004;311:237–245. doi: 10.1124/jpet.104.067629. [DOI] [PubMed] [Google Scholar]
- Labrie F. Adrenal androgens and intracrinology. Semin Reprod Med. 2004;22:299–309. doi: 10.1055/s-2004-861547. [DOI] [PubMed] [Google Scholar]
- Maninger N, Capitanio JP, Mendoza SP, Mason WA. Personality influences tetanus-specific antibody response in adult male rhesus macaques after removal from natal group and housing relocation. Am J Primatol. 2003;61:73–83. doi: 10.1002/ajp.10111. [DOI] [PubMed] [Google Scholar]
- Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front Neuroendocrinol. 2009;30:65–91. doi: 10.1016/j.yfrne.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masi AT, Aldag JC, Chatterton RT. Sex hormones and risks of rheumatoid arthritis and developmental or environmental influences. Ann N Y Acad Sci. 2006;1069:223–235. doi: 10.1196/annals.1351.020. [DOI] [PubMed] [Google Scholar]
- Mendoza SP, Capitanio JP, Mason WA. Chronic social stress: Studies in non-human primates. In: Moberg GP, Mench JA, editors. Biology of Animal Stress. Basic Principles and Implications for Animal Welfare. CABI Publishing New York; NY: 2000. pp. 227–247. [Google Scholar]
- Morgan CA, 3rd, Southwick S, Hazlett G, Rasmusson A, Hoyt G, Zimolo Z, Charney D. Relationships among plasma dehydroepiandrosterone sulfate and cortisol levels, symptoms of dissociation, and objective performance in humans exposed to acute stress. Arch Gen Psychiatry. 2004;61:819–825. doi: 10.1001/archpsyc.61.8.819. [DOI] [PubMed] [Google Scholar]
- Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 1984;5:25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- Nguyen AD, Conley AJ. Adrenal androgens in humans and nonhuman primates: production, zonation and regulation. Endocr Dev. 2008;13:33–54. doi: 10.1159/000134765. [DOI] [PubMed] [Google Scholar]
- Nieschlag E, Loriaux DL, Ruder HJ, Zucker IR, Kirschner MA, Lipsett MB. The secretion of dehydroepiandrosterone and dehydroepiandrosterone sulphate in man. J Endocrinol. 1973;57:123–134. doi: 10.1677/joe.0.0570123. [DOI] [PubMed] [Google Scholar]
- Orentreich N, Brind JL, Rizer RL, Vogelman JH. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab. 1984;59:551–555. doi: 10.1210/jcem-59-3-551. [DOI] [PubMed] [Google Scholar]
- Parker CR, Jr., Jian M, Conley AJ. The localization of DHEA sulfotransferase in steroidogenic and steroid metabolizing tissues of the adult rhesus macaque monkey. Endocr Res. 2000;26:517–522. doi: 10.3109/07435800009048564. [DOI] [PubMed] [Google Scholar]
- Parker LN, Odell WD. Control of adrenal androgen secretion. Endocr Rev. 1980;1:392–410. doi: 10.1210/edrv-1-4-392. [DOI] [PubMed] [Google Scholar]
- Parker LN, Levin ER, Lifrak ET. Evidence for adrenocortical adaptation to severe illness. J Clin Endocrinol Metab. 1985;60:947–952. doi: 10.1210/jcem-60-5-947. [DOI] [PubMed] [Google Scholar]
- Rasmusson AM, Vasek J, Lipschitz DS, Vojvoda D, Mustone ME, Shi Q, Gudmundsen G, Morgan CA, Wolfe J, Charney DS. An increased capacity for adrenal DHEA release is associated with decreased avoidance and negative mood symptoms in women with PTSD. Neuropsychopharmacology. 2004;29:1546–1557. doi: 10.1038/sj.npp.1300432. [DOI] [PubMed] [Google Scholar]
- Rehman KS, Carr BR. Sex differences in adrenal androgens. Semin Reprod Med. 2004;22:349–360. doi: 10.1055/s-2004-861551. [DOI] [PubMed] [Google Scholar]
- Ruys JD, Mendoza SP, Capitanio JP, Mason WA. Behavioral and physiological adaptation to repeated chair restraint in rhesus macaques. Physiol Behav. 2004;82:205–213. doi: 10.1016/j.physbeh.2004.02.031. [DOI] [PubMed] [Google Scholar]
- Semple CG, Gray CE, Beastall GH. Adrenal androgens and illness. Acta Endocrinol (Copenh) 1987;116:155–160. doi: 10.1530/acta.0.1160155. [DOI] [PubMed] [Google Scholar]
- Strous RD, Stryjer R, Maayan R, Gal G, Viglin D, Katz E, Eisner D, Weizman A. Analysis of clinical symptomatology, extrapyramidal symptoms and neurocognitive dysfunction following dehydroepiandrosterone (DHEA) administration in olanzapine treated schizophrenia patients: A randomized, double-blind placebo controlled trial. Psychoneuroendocrinology. 2007;32:96–105. doi: 10.1016/j.psyneuen.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Vaitukaitis JL, Dale SL, Melby JC. Role of ACTH in the secretion of free dehydroepiandrosterone and its sulfate ester in man. J Clin Endocrinol Metab. 1969;29:1443–1447. doi: 10.1210/jcem-29-11-1443. [DOI] [PubMed] [Google Scholar]
- Villette JM, Bourin P, Doinel C, Mansour I, Fiet J, Boudou P, Dreux C, Roue R, Debord M, Levi F. Circadian variations in plasma levels of hypophyseal, adrenocortical and testicular hormones in men infected with human immunodeficiency virus. J Clin Endocrinol Metab. 1990;70:572–577. doi: 10.1210/jcem-70-3-572. [DOI] [PubMed] [Google Scholar]
- Vrezas I, Willenberg HS, Mansmann G, Hiroi N, Fritzen R, Bornstein SR. Ectopic adrenocorticotropin (ACTH) and corticotropin-releasing hormone (CRH) production in the adrenal gland: basic and clinical aspects. Microsc Res Tech. 2003;61:308–314. doi: 10.1002/jemt.10340. [DOI] [PubMed] [Google Scholar]
- Wade CE, Lindberg JS, Cockrell JL, Lamiell JM, Hunt MM, Ducey J, Jurney TH. Upon-admission adrenal steroidogenesis is adapted to the degree of illness in intensive care unit patients. J Clin Endocrinol Metab. 1988;67:223–227. doi: 10.1210/jcem-67-2-223. [DOI] [PubMed] [Google Scholar]
- Wickings EJ, Nieschlag E. Serum levels of testicular and adrenal steroids after dexamethasone and HCG-administration in the laboratory-maintained Rhesus monkey. Acta Endocrinol (Copenh) 1978;87:650–658. doi: 10.1530/acta.0.0870650. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Reus VI, Keebler A, Nelson N, Friedland M, Brizendine L, Roberts E. Double-blind treatment of major depression with dehydroepiandrosterone (DHEA). Am J Psychiatry. 1999;156:646–649. doi: 10.1176/ajp.156.4.646. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Reus VI, Maninger N, Mellon SH. Dehydroepiandrosterone in the Treatment of Neuropsychiatric Conditions. Second Edition Lippincott Williams & Wilkins; Philadelphia, PA: 2008. [Google Scholar]
- Yamauchi A, Takei I, Kasuga A, Kitamura Y, Ohashi N, Nakano S, Takayama S, Nakamoto S, Katsukawa F, Saruta T. Depression of dehydroepiandrosterone in Japanese diabetic men--comparison between non-insulin-dependent diabetes mellitus and impaired glucose tolerance. Eur J Endocrinol. 1996;135:101–104. doi: 10.1530/eje.0.1350101. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Biology of posttraumatic stress disorder. J Clin Psychiatry. 2001;62(Suppl 17):41–46. [PubMed] [Google Scholar]
- Yehuda R, Brand SR, Golier JA, Yang RK. Clinical correlates of DHEA associated with post-traumatic stress disorder. Acta Psychiatr Scand. 2006;114:187–193. doi: 10.1111/j.1600-0447.2006.00801.x. [DOI] [PubMed] [Google Scholar]