Abstract
Objective
Prior research has demonstrated that dieting, or the restriction of caloric intake, does not lead to long-term weight loss. This study tested the hypothesis that dieting is ineffective because it increases chronic psychological stress and cortisol production – two factors that are known to cause weight gain. Further, this study examined the respective roles of the two main behaviors that comprise dieting – monitoring one’s caloric intake and restricting one’s caloric intake – on psychological and biological stress indicators.
Methods
In a 2 (monitoring vs. not) × 2 (restricting vs. not) fully crossed, controlled experiment, 121 female participants were randomly assigned to one of four dietary interventions for three weeks. The monitoring + restricting condition tracked their caloric intake and restricted their caloric intake (1200 kcal/day); the monitoring only condition tracked their caloric intake but ate normally; the restricting only condition was provided 1200 kcal/day of food but did not track their calories, and the control group ate normally and did not track their intake. Before and after the interventions, participants completed measures of perceived stress and two days of diurnal saliva sampling to test for cortisol.
Results
Restricting calories increased the total output of cortisol, and monitoring calories increased perceived stress.
Conclusions
Dieting may be deleterious to psychological well-being and biological functioning, and changes in clinical recommendations may be in order.
Keywords: Dieting, stress, cortisol
Introduction
Obesity is among the most significant health problems facing the United States, and levels of obesity are increasing throughout the world. One-third of U.S. adults are estimated to be obese (1), and obesity is beginning to replace malnutrition and infectious diseases as the most significant contributor to poor health worldwide (2). In light of these trends, much research is being conducted on intentional weight loss and weight maintenance. Although intentional weight loss efforts may be effective in the short-term, these losses are often not maintained over the long-term, and it is essential that researchers identify the mechanisms that lead to weight regain after intentional weight loss to inform interventions that can reverse, circumvent, or alter them.
One of the most common methods of intentional weight loss is the restriction of caloric intake, commonly known as dieting (3). Nationally representative numbers from 2005 indicate that approximately 47% of adults in the United States are trying to lose weight at any given time (4). Despite the high prevalence of dieting, research finds that dieting leads to successful weight loss in the short-term but not the long-term (5, 6). A recent review article found that 30–64% of diet study participants gain back more weight than they lost on the diet (5). Further, having dieted in the past was a predictor of weight gain, and the longer participants were followed up, the more weight they had regained (5).1
To fully understand the mechanisms of diet failure, it is necessary to consider the biological and psychological processes that occur when individuals go on diets. We began with the broad hypothesis that diets fail because they increase stress. In this study, we specifically tested the latter half of this hypothesis, namely that dieting causes increases in indicators of stress. Stress is defined as a negative emotional experience that is accompanied by predictable biochemical, physiological, cognitive, and behavioral changes (11), and it is a prime suspect as a potential cause of weight regain after dieting. First, stress can increase weight through the stress-responsive hypothalamic-pituitary-adrenocortical (HPA) pathway that affects energy metabolism – the focus of the current study. HPA-mediated weight gain has been studied extensively (12). Research suggests strong connections between stress and weight gain through elevations of cortisol regulated by the prolonged activation of the HPA axis and resulting insulin resistance (12–16).
Dieting is likely psychologically stressful. As dieting, by definition, is an act of restriction of eating, this deprivation elicits negative emotion. Dieting involves not merely resisting temptation, but also a physically aversive feeling of being hungry. Reviews of dieting studies have documented negative emotional consequences of dieting such as depression, anxiety, decreased self-esteem, nervousness, and irritability (17).
In addition to restricting one’s intake, there is another main task in dieting: monitoring one’s caloric intake. Research indicates that frequent, repetitive hassles can accumulate over time to comprise a chronic stressor (18) that has negative health consequences. Monitoring food and calorie intake during a diet may be one of these daily hassles. Consistent with this point, in the original sample used in the development of the Hassles Scale, “concerns about weight” were among the five most frequently cited hassles (18).
The relationship between dieting and both perceived stress and cortisol has been investigated in cross-sectional and longitudinal studies. For example, in a study of 17,159 adolescent females, French and colleagues found that dieting five or more times in the past year was correlated with emotional stress in Whites, Blacks, and Asians (17). Researchers have long known that fasting and starvation are associated with an elevation of cortisol or failure to suppress cortisol after a dexamethasone suppression test (19–21). In addition, studies have found that higher dietary restraint (a measure of dieting2) is associated with higher 24-hour urinary free cortisol concentrations, and cortisol-creatinine ratios, salivary cortisol, and cortisol awakening response (22–24). To date, however, no study has used an experimental design to test the causal effects of dieting on stress in humans.
The present research tested the following two hypotheses: (1) The monitoring aspect of dieting causes increases in psychological stress and cortisol; and (2) The restricting aspect of dieting causes increases in psychological stress and cortisol. These hypotheses were tested using a fully-crossed, randomized, controlled experiment with monitoring and restricting as two factors.
Methods
Participants were randomly assigned to one of four diet conditions, and self-reported stress and diurnal cortisol were measured at baseline and immediately following the three-week manipulation. The short-term nature of the study allowed the investigation of the effects of the diet at its most potent point, as participants in diet studies tend to adhere closely to their diets during the early weeks of the diet, with non-adherence becoming more likely after the initial months of the diet (26). To test whether restricting intake, monitoring intake, or a combination of the two leads to stress and elevations in cortisol, a fully-crossed 2 (monitoring diet or not) × 2 (restricting diet or not) between-groups longitudinal design with repeated measures compared participants at baseline and post-manipulation.
Sample
Because dieting can have deleterious health consequences, the sample consisted of individuals from the UCLA and University of Minnesota communities who were seeking to go on a diet and would have done so regardless of participation in the study. We focused on females because the prevalence of restrained eating is higher in this population (27). One hundred and fifteen participants were randomized; complete follow-up data was available for 99 participants (86% retention rate).
Inclusion/Exclusion Criteria
To ensure that weight was measured with high validity, participants were required to have a history of stable weight (no weight change of more than 5 kg or 11 lbs in the past three months) and not taking weight-altering medications. To ensure that cortisol was measured with high validity and for participant safety, participants were excluded if they were smokers or had recent or current history of cardiovascular disease, diabetes, endocrine disorder, substance abuse, eating disorders, or any other self-reported significant disease. For safety reasons pertaining to caloric restriction and potential weight loss, participants were also excluded if their body mass index (height in meters/weight in kilograms squared) was in the underweight category (below 18.5).
Procedures
This study was conducted in compliance with American Psychological Association ethical standards for the treatment of human subjects and was fully approved by the UCLA and University of Minnesota Institutional Review Boards. Data collection occurred between September 2007 and January 2009. At baseline, all participants provided informed consent, underwent screening for inclusion and exclusion criteria, were weighed and measured, and completed baseline questionnaires. Cortisol follows a diurnal rhythm, with highest levels at around 30–45 minutes after waking and a gradual decline throughout the day (28). Following the recommendations of the MacArthur Research Network on Socioeconomic Status and Health (29), diurnal saliva sampling was used to capture both the cortisol awakening response as well as the total daily output of cortisol. Participants’ salivary cortisol levels were measured at three time points for two days: immediately after wake-up, 45 minutes after wake-up, and 12 hours after wake-up. Cortisol was assessed with commercially available salivettes (Salimetrics, State College, PA). Participants were thoroughly trained on diurnal saliva sampling and were instructed to begin sampling the following day and continue for two days total. Participants were also instructed to check in via phone, email, or text message after each sample was taken to ensure compliance. At the end of each sampling day, participants also completed a questionnaire to measure potential confounders of cortisol: physical activity, stressful events, general health, pain, and alcohol and caffeine consumption.
Participants were then randomly assigned to one of four study conditions (discussed below). All participants returned the day after their saliva sampling days to return the samples and were trained on the condition to which they had been randomized. Participants followed the instructions of their assigned condition for three weeks.
Participants assigned to the Monitoring and Restricting condition received training on how to follow a classic low-calorie diet consisting of 1200 kilocalories per day with no more than 50% energy from carbohydrates, 30% energy from total fat, and 20% energy from protein. Participants also received instructions on how to complete a daily food diary, so that they monitored their caloric intake. Participants in the Monitoring Only condition were not placed on a low-calorie diet, but were instructed on how to complete a daily food diary, so that they monitored their intake. Participants in Restricting Only condition were provided all the food that they ate over the course of the study. The food was prepackaged prepared food from one of two diet food companies and was prepared and eaten by participants in the same manner that participants in this type of diet plan typically prepare and consume these foods. The daily menus varied, but calorie intake was restricted to 1200 calories per day, with the same percentage of calories from carbohydrates, fats, and protein as in the full monitoring + restricting diet. Thus this condition served as a control for the full monitoring + restricting diet, in terms of the amount of daily caloric intake; however, participants did not engage in any monitoring of their intake, since all food was eaten in prescribed amounts given to them. Participants in the Control condition were not placed on a low-calorie diet or instructed to complete a daily food diary.
Each condition was, to the greatest extent possible, modeled after what people do in real life when they go on diets. Thus the Monitoring & Restricting and Monitoring Only condition materials were designed by a registered dietitian to be representative of what dietitians usually use when advising their clients. The Restricting Only condition utilized an actual diet food company and is analogous to many similar types of diet plans such as Jenny Craig.
On the day following the conclusion of the three weeks, participants completed follow-up questionnaires and conducted two final days of diurnal saliva sampling. Finally, participants returned to the lab for follow-up measurements and returned the samples. Participants were paid $40 for participation.
Materials
All measures were taken at both baseline and follow-up.
Perceived Stress
Stress was assessed via the widely-used 10-item Perceived Stress Scale (PSS, Cronbach’s α in this sample = .90) (30). A sample item is, “How often have you felt nervous and stressed?” Respondents are asked to rate how often they experienced stress in the past month (baseline) or past 3 weeks (post manipulation) on 5-point Likert-type scales from Never = 0 to Very Often = 4. The PSS was modified at the post-intervention follow-up to say “In the past three weeks while you were in the study…” to attempt to capture more specifically dieting-related stress.
Cortisol
Total cortisol output was calculated by calculating the area-under-the-curve (AUC) for the two pre- and post-intervention days using the formula (with respect to ground) outlined by Pruessner, Kirschbaum, Meinlschmid, and Hellhammer (28). The cortisol awakening response (CAR) refers to the normative increase between waking up and 30–45 minutes after waking up. The meaning of high versus low awakening responses has been debated in the literature, but has been characterized as indexing the robustness of the HPA axis as well as tonic stress levels in some studies (31), and was therefore used as an outcome measure here. The cortisol awakening response was calculated by subtracting the wakeup cortisol (natural log) value from the wakeup + 45 minute sample value.
Anthropometry
A portable adult measuring stadiometer was used to measure height. Subjects were measured at least twice, in their stocking feet with head positioned in the Frankfort plane. Body weight was measured using a physician’s scale (Detecto, Webb City, MO).
Results
Preliminary analyses
Descriptive statistics for demographic characteristics of the study sample measured at baseline are pictured in Table 1. Table 2 depicts descriptive statistics for the major outcome variables stratified by group assignment and time of assessment. Values beyond three standard deviations on key variables were considered outliers, and all analyses were re-run without the inclusion of these participants. Notably, the results were the same whether outliers were included or excluded.
Table 1.
Descriptive statistics for key variables at baseline and post-manipulation. Cortisol values are natural log values.
| Variable | Pre | Post | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | St. Dev. | Min | Max | n | Mean | St. Dev. | Min | Max | n | |
| Weight (lb) | 147.38 | 28.8 | 101 | 250 | 101 | 148.02 | 28.79 | 100 | 246 | 99 |
| BMI | 24.79 | 3.85 | 18.6 | 38.2 | 101 | 24.85 | 3.76 | 18.4 | 38 | 99 |
| PSS | 2.68 | 0.52 | 1.62 | 3.92 | 101 | 2.82 | 0.59 | 1.14 | 4.29 | 98 |
| Cort AUC | 1674.27 | 337.07 | 362.79 | 2882.13 | 99 | 1651.8 | 375.3 | 147.92 | 2717.36 | 95 |
| CAR | 0.44 | 0.59 | −1.6 | 2.37 | 100 | 0.34 | 0.66 | −2.35 | 2.6 | 95 |
Lb = pounds; BMI = Body Mass Index; PSS = Perceived Stress Scale; AUC = Area-uner-the-curve with respect to ground; CAR = Cortisol awakening response.
Table 2.
Descriptive statistics for major outcome variables stratified by group assignment and time of assessment.
| Variable | Pre-intervention Mean (SD) [minimum-maximum] | n | Post-intervention Mean (SD) [minimum-maximum] | n |
|---|---|---|---|---|
| Monitoring & Restricting (Full Diet) | ||||
| Weight (lb) | 155.88(27.53) [113–250] | 24 | 153.23(23.26) [117–224] | 22 |
| Body Mass Index | 25.77(3.63) [19.39–35.9] | 24 | 25.19(3.02) [20.08–32.1] | 22 |
| Perceived Stress Scale | 2.67(0.58) [1.69–3.85] | 23 | 2.97(0.73) [1.57–4.29] | 21 |
| Total Cortisol Output | 1800.45(374.45) [1230.82–2882.13] | 24 | 1703.42(411.12) [968.86–2717.36] | 21 |
| Cortisol Awakening Response | 0.60(0.49) [−0.14–1.68] | 24 | 0.51(0.64) [−0.14–2.22] | 21 |
| Kilocalories | 26449.89(5483.57) [19467–43042.1] | 20 | ||
| (1259.52 per day) | ||||
| Monitoring (Food Diary) | ||||
| Weight (lb) | 147.65(30.71) [113–247.5] | 28 | 147.88(31.05) [111–246] | 28 |
| Body Mass Index | 24.42(4.01) [18.6–38.2] | 28 | 24.56(4.12) [18.4–38] | 28 |
| Perceived Stress Scale | 2.61(0.38) [1.69–3.23] | 28 | 2.83(0.51) [1.71–3.93] | 28 |
| Total Cortisol Output | 1719.14(235.13) [1233.58–2251.06] | 27 | 1541.67(426.76) [147.92–2174.63] | 26 |
| Cortisol Awakening Response | 0.58(0.42) [−0.18–1.48] | 27 | 0.21(0.74) [−2.35–1.45] | 26 |
| Kilocalories | 35715.5(9717.21) [14420–63153] | 27 | ||
| (1700.74 per day) | ||||
| Restricting (Food Provided) | ||||
| Weight (lb) | 140.2(30.93) [101–209] | 20 | 139.62(30.59) [100–204] | 20 |
| Body Mass Index | 24.9(4.48) [18.8–38.2] | 20 | 24.71(4.07) [18.6–35.57] | 20 |
| Perceived Stress Scale | 2.79(0.53) [2–3.54] | 20 | 2.79(0.62) [1.14–3.86] | 20 |
| Total Cortisol Output | 1545.21(453.96) [362.79–2289.68] | 19 | 1807.93(304.56) [1441.14–2478.92] | 20 |
| Cortisol Awakening Response | 0.32(0.94) [−1.60–2.37] | 20 | 0.37(0.56) [−1.02–1.68] | 20 |
| Control | ||||
| Weight (lb) | 145.04(26.01) [112.25–239] | 29 | 149.98(29.22) [108.75–234] | 29 |
| Body Mass Index | 24.12(3.41) [19.8–37.4] | 29 | 24.98(3.86) [19.73–36.6] | 29 |
| Perceived Stress Scale | 2.69(0.57) [1.62–3.92] | 29 | 2.72(0.53) [1.79–3.93] | 29 |
| Total Cortisol Output | 1612.64(260.46) [1168.89–2370.50] | 29 | 1603.94(311.58) [1023.99–2386.70] | 28 |
| Cortisol Awakening Response | 0.27(0.45) [−0.74–1.46] | 29 | 0.30(0.67) [−1.12–2.60] | 28 |
| Key: | ||||
| Cortisol-ln(nmol/liter) | ||||
Randomization check
Participants in the four conditions did not differ significantly in age, day in menstrual cycle, number of total lifetime diets, and baseline: weight, BMI, perceived stress, cortisol awakening response, or cortisol slope (all p values nonsignificant).
Manipulation check
The participants in the Monitoring + Restricting and Monitoring Only conditions significantly differed in the number of calories they consumed, such that Monitoring Only participants, who were asked to not restrict their calories, ate more (grand mean = 35715.5, SD = 9717.21, daily mean = 1700.74) than did Monitoring + Restricting condition participants who were instructed to keep their consumption to 1200 kcal per day (grand mean = 26449.89, SD = 5483.57, daily mean = 1259.52).
Weight change
To test whether the groups differed in the amount of weight they lost or gained, a repeated measures ANOVA was conducted, revealing a main effect of restricting (but not monitoring) on weight change (F(97,1) = 4.67, p = .03). The restricting groups lost significantly more weight (mean weight loss of 1.9 pounds) than the groups who did not restrict (mean weight gain of 2.6 pounds), and therefore weight change was used in all analyses as a covariate.
Main Results
To test the two hypotheses, three ANOVA analyses were conducted using the following post-intervention variables as outcomes: (1) psychological stress (indexed by the Perceived Stress Scale); (2) total cortisol output; (3) cortisol awakening response. Restricting and monitoring were dummy-coded and entered as fixed factors. Baseline values of each of the three outcome variables were included in each respective analysis as covariates. The respective main effects of monitoring and restricting were examined to determine whether these factors increased stress. Significance was set at p = .05 and all tests were two-tailed. Physical activity, stressful events unrelated to the diet, general health, pain, alcohol consumption, and caffeine consumption were tested one at a time to see if they were significantly related to each relevant outcome measure. Including these covariates did not change the pattern of results in any of the reported analyses.
Psychological Stress
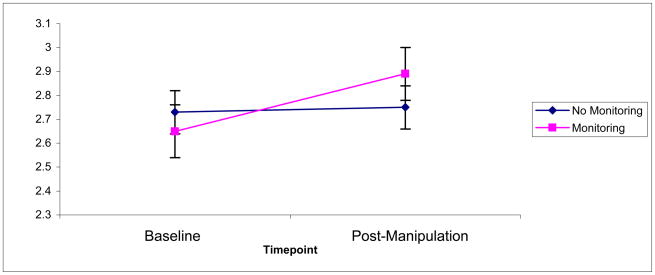
Results indicated that participants who monitored their caloric intake reported increased perceived stress (F(97,1) = 5.45, p = .02, partial η2 = .06; see Figure 1). The effect size was small to medium, with a Cohen’s d of 0.38. Restricting, on the other hand, did not increase perceived stress (F(97,1) = .92, p = .34), and there was no interaction between the two factors (F(97,1) = .07, p = .80).
Figure 1.
Main effect of monitoring diet on perceived stress as measured by the Perceived Stress Scale.
Salivary Cortisol
Q-Q plots indicated that the values for salivary cortisol were non-normally distributed. A natural-log transformation corrected this skew, and thus natural log values were used in the following analyses.
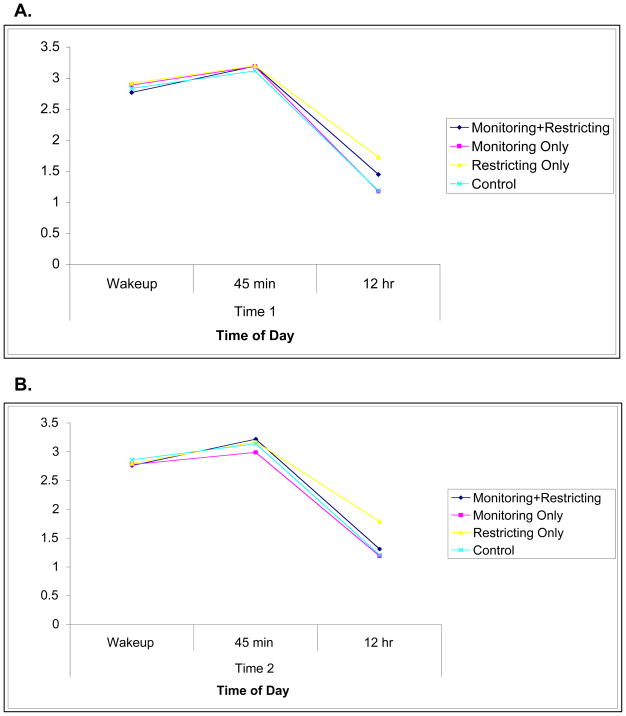
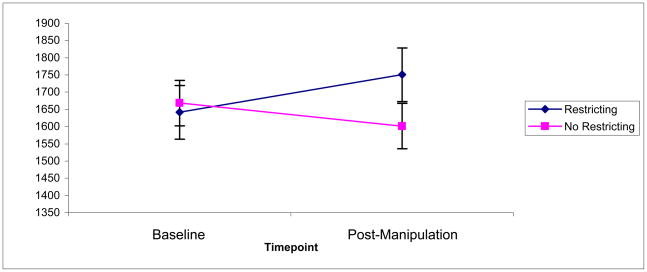
Results indicated a main effect of restricting (F(92,1) = 8.77, p = .004, partial η2 = .05), such that restricting increased the total output of cortisol (Figure 2). The effect size was medium, with a Cohen’s d of 0.63. There was no main effect of monitoring (F(92,1) = 3.71, p = .07) and no interaction (F(92,1) = 0.27, p = .60). The difference in cortisol output seems to have been driven by the evening cortisol level, as pictured in Figure 3.
Figure 2.
Main effect of restricting caloric intake on total cortisol output (AUC), nmol/liter
Figure 3.
A. Cortisol (natural log) values for each diurnal timepoint at baseline. B. Cortisol (natural log) values for each diurnal timepoint post-manipulation.
For cortisol awakening response, there were no main effects (monitoring: F(93,1) = 0.88, p = .63; restricting: F(93,1) = 0.10, p = .35) and there was no interaction (F(93,1) = 0.70, p = .41).
Discussion
Dieting is one of the most common behaviors used to control weight. This study suggests that dieting may, however, potentially be deleterious to psychological well-being and biological functioning. Specifically, this study found that monitoring one’s diet increased perceived psychological stress, and restricting one’s caloric intake increased total daily cortisol. These findings lend support to the idea that stress may be a mechanism of diet failure.
Monitoring one’s diet involved continuously recording consumed food. Like the stressors characterized in the daily hassles literature (“irritating, frustrating demands that occur during everyday transactions with the environment,”) (32), monitoring via the use of food diaries likely increased perceived stress by creating repeated stressors throughout the day.
Restricting, on the other hand, increased the total cortisol output among the participants, consistent with previous research (23, 33). This finding may seem unexpected, as restricting caloric intake can trigger mechanisms to reduce energy expenditure, including reduced corticotrophin-releasing hormone output, which, in turn, may reduce cortisol downstream (34). However, restricting caloric intake may be a biological stressor because one of the main functions of cortisol is to increase the availability of energy in the body. The stress resulting from restricting one’s caloric intake to a mere 1200 kilocalories, therefore, may have reduced the absolute amount of energy available to the body, therefore leading to increased cortisol output to release energy stores. Alternatively, the increase in cortisol under conditions of caloric restriction may simply reflect a biological freeing of energy that is entirely not stress-mediated.
A critical and related question is whether the cortisol increase is likely to translate into weight gain. No studies to our knowledge have examined basal cortisol and weight gain longitudinally in humans but experimental studies show a dose/response relationship between glucocorticoids and weight in rodents (35).
We found no change in the cortisol awakening response. However, as noted, the “healthy” versus “unhealthy” cortisol awakening response is unclear, with some studies documenting a higher response in stressed populations, whereas others find the opposite (36). It is therefore difficult to speculate on the meaning of not finding a change in awakening response.
In this study, there was a disconnect between psychological and biological stress responses, whereby monitoring increased perceived stress whereas restricting increased cortisol output. This may have been due simply to a potential inadequacy of the PSS to capture the dieting-related stress in the restricting conditions only (despite the fact that the prompt was changed to say “In the past three weeks while you were in the study…”). A more fine-grained measurement of perceived stress in future studies would also allow for an untangling of whether the effect is stress-mediated or not. Alternatively, the prolonged increase in perceived stress in the monitoring conditions may yet translate, over a longer timeframe, into increased cortisol for this group as well. Differences between psychological and biological stress responses are, however, commonly found throughout the stress literature (37). For example, Fischer and colleagues examined cortisol and psychological stress responses over 7,145 hours in physicians and nurses and found that cortisol and psychological stress did not overlap 71.3% of the time (38).
In fact, dieters may not even realize that restricting their caloric intake produces a physiological stress response, as it does not lead to a perceived stress response. As a result, dieters may assume that their restriction is not harmful, and in fact persevere in what may be a physiologically stressful diet because they do not feel psychologically stressed.
The findings from this study provide preliminary evidence that dieting may not lead to positive outcomes in terms of stress indicators. This does not mean, however, that the implication is that individuals engaged in weight management efforts should disregard self-awareness of eating patterns or give up on the practice of portion control. Lifestyle modifications that include exercise and avoiding overeating (39) may be the most prudent approach for improving weight-related health.
There were several limitations to this study. A number of participants (n = 15) dropped out of the study after undergoing the first round of saliva sampling, which may have led to a biased sample. Indeed, the participants who dropped out at this point were experiencing significantly more perceived stress than those who continued, and as such the results of the current study may not be generalizable to people most likely to be stressed by the procedures. Note, however, that this bias in the sample results in an underestimation of the effect. Further, perhaps monitoring and restricting have effects only on compliant participants. If this were the case, however, the results of this study may still be informative, as compliant participants are likely to be compliant dieters who are most likely to monitor and restrict their intake successfully. Finally, as dropouts ocurred before randomization, the validity of the results are not in question.
It should also be noted that the manipulations in this study altered more than simply restricting and monitoring. The participants in the different groups may have been eating different types (and even quantities) of food with different macronutrient compositions. Because we could not have the control group tell us what they were eating (because that would have constituted a monitoring manipulation), we must consider an alternative explanation that some differences, for example in carbohydrate composition of participants’ diets, may have differentially altered cortisol production (40, 41).
Finally, we note that the saliva sampling protocol in this study was suboptimal. In particular, basing the CAR on only two timepoints was problematic, with at least three needed to calculate a reliable CAR (36). Further, afternoon values and at least one or two additional days of sampling may have been necessary to assess a true tonic measure of cortisol (42). Funding limitations precluded such gold-standard measurement of cortisol in this study, and therefore the null results of (a) the outcome measure of CAR and (b) monitoring on cortisol may have been due to unreliable measurement of cortisol.
The broad hypothesis framing this study is that dieting is ineffective because it is a stressor. This study, however, did not assess actual dieting outcomes, and thus the full hypothesis remains to be tested. At the moment, this study has provided initial evidence of the two manipulations, monitoring and restricting, on the presumptive mediators of psychological stress and cortisol, respectively. Future research must examine the effects of monitoring and restricting over longer terms and in relation to the outcomes of the diets themselves, namely whether or not participants actually lose weight and whether or not they keep it off. The relationship between dieting and stress over the long term may in fact be curvilinear, such that the initial weeks of monitoring and restricting are stressful, becoming less so as individuals actually lose weight, usually through the first six months (5), and then perhaps more so as the weight returns. This needs to be studied further.
The results of this study have a number of important implications. Regardless of diet success or failure, if dieting is shown in future studies to reliably increase stress and cortisol, clinicians may need to rethink recommending dieting to their patients to improve health. Chronic stress, in addition to promoting weight gain, has been linked with a host of negative health outcomes such as atherosclerosis, coronary heart disease, hypertension, diabetes, cancer, and impaired immune functioning (43). To the extent that dieting might potentially add to this stress burden, its psychological and biological consequences would best not be ignored.
Acknowledgments
This research was supported by the following: National Science Foundation Graduate Fellowship, American Psychological Association Dissertation Research Award, and NIMH Training Grant T32MH15750-29 to AJT; National Institute of Mental Health Grant MH63795 and National Heart, Blood, and Lung Institute Grant NHLBI grant HL088887 to TM; and National Institute of Aging Grant AG030309 to SET.
The authors also would like to thank the following individuals for their essential support on this project: Dr. Theodore Robles, Dr. Christine Dunkel Schetter, Dr. Sarosh Motivala, Dr. Baldwin Way, Dr. Andrew Ward, Dr. Richard Slatcher, and Ashley Moskovich.
Abbreviations
- HPA
Hypothalamic-pituitary-adrenocortical
- PSS
Perceived Stress Scale
- AUC
Area-under-the-curve
- CAR
Cortisol awakening response
- ANOVA
Analysis of Variance
Footnotes
We note that some studies such as the Finnish Diabetes Prevention Study (7), Diabetes Prevention Program (8), Diabetes Prevention Program Outcomes Study (9), and Look AHEAD (10) have indeed demonstrated some positive weight (and health) outcomes. These interventions, however, were lifestyle interventions that confound physical activity with pure caloric restriction, and we therefore focus on caloric restriction interventions only in our discussion.
The construct of restrained eating overlaps, but is not entirely analogous to, simple caloric restriction. While a complete discussion of the restrained eating construct is outside the scope of this paper, we acknowledge that restrained eating refers to a constellation of behaviors that include but are not limited to caloric restriction, such as concerns about being overweight, weight fluctuation, and disinhibition (25).
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–43. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 3.Price JH, Desmond SM, Krol RA, Snyder FF, O’Connell JK. Family practice physicians’ beliefs, attitudes, and practices regarding obesity. Am J Prev Med. 1987;3:339–45. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey Data. Hyattsville, MD: National Center for Health Statistics; 2009. [Google Scholar]
- 5.Mann T, Tomiyama AJ, Lew AM, Westling E, Chatman J, Samuels B. The search for effective obesity treatments: Should Medicare fund diets? American Psychologist. 2007;62:220–33. doi: 10.1037/0003-066X.62.3.220. [DOI] [PubMed] [Google Scholar]
- 6.Korkeila M, Rissanen A, Kaprio J, Sorensen TI, Koskenvuo M. Weight-loss attempts and risk of major weight gain: a prospective study in Finnish adults. American Journal of Clinical Nutrition. 1999;70:965–75. doi: 10.1093/ajcn/70.6.965. [DOI] [PubMed] [Google Scholar]
- 7.Lindstrom J, Eriksson JG, Valle TT, Aunola S, Cepaitis Z, Hakumaki M, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Mannelin M, Martikkala V, Moltchanov V, Rastas M, Salminen V, Sundvall J, Uusitupa M, Tuomilehto J. Prevention of diabetes mellitus in subjects with impaired glucose tolerance in the Finnish Diabetes Prevention Study: results from a randomized clinical trial. J Am Soc Nephrol. 2003;14:S108–13. doi: 10.1097/01.asn.0000070157.96264.13. [DOI] [PubMed] [Google Scholar]
- 8.West DS, Elaine Prewitt T, Bursac Z, Felix HC. Weight loss of black, white, and Hispanic men and women in the Diabetes Prevention Program. Obesity (Silver Spring) 2008;16:1413–20. doi: 10.1038/oby.2008.224. [DOI] [PubMed] [Google Scholar]
- 9.Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, Brenneman AT, Brown-Friday JO, Goldberg R, Venditti E, Nathan DM. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:1677–86. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wadden TA, West DS, Neiberg RH, Wing RR, Ryan DH, Johnson KC, Foreyt JP, Hill JO, Trence DL, Vitolins MZ. One-year weight losses in the Look AHEAD study: factors associated with success. Obesity (Silver Spring) 2009;17:713–22. doi: 10.1038/oby.2008.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baum A. Stress, intrusive imagery, and chronic distress. Health Psychology. 1990;9:653–75. doi: 10.1037//0278-6133.9.6.653. [DOI] [PubMed] [Google Scholar]
- 12.Peeke PM, Chrousos GP. Hypercortisolism and obesity. Ann N Y Acad Sci. 1995;771:665–76. doi: 10.1111/j.1749-6632.1995.tb44719.x. [DOI] [PubMed] [Google Scholar]
- 13.Bjorntorp P. Do stress reactions cause abdominal obesity and comorbidities? Obes Rev. 2001;2:73–86. doi: 10.1046/j.1467-789x.2001.00027.x. [DOI] [PubMed] [Google Scholar]
- 14.Bjorntorp P, Rosmond R. Obesity and cortisol. Nutrition. 2000;16:924–36. doi: 10.1016/s0899-9007(00)00422-6. [DOI] [PubMed] [Google Scholar]
- 15.Epel ES, McEwen B, Seeman T, Matthews K, Castellazzo G, Brownell KD, Bell J, Ickovics JR. Stress and body shape: stress-induced cortisol secretion is consistently greater among women with central fat. Psychosom Med. 2000;62:623–32. doi: 10.1097/00006842-200009000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Raber J. Detrimental effects of chronic hypothalamic-pituitary-adrenal axis activation. From obesity to memory deficits. Mol Neurobiol. 1998;18:1–22. doi: 10.1007/BF02741457. [DOI] [PubMed] [Google Scholar]
- 17.French SA, Jeffery RW. Consequences of dieting to lose weight: Effects on physical and mental health. Health Psychology. 1994;13:195–212. doi: 10.1037//0278-6133.13.3.195. [DOI] [PubMed] [Google Scholar]
- 18.DeLongis A, Coyne JC, Dakof G, Folkman S, Lazarus RS. Relationship of daily hassles, uplifts, and major life events to health status. Health Psychology. 1982:1. [Google Scholar]
- 19.Galvao-Teles A, Graves L, Burke CW, Fotherby K, Fraser R. Free cortisol in obesity; effect of fasting. Acta Endocrinol (Copenh) 1976;81:321–9. doi: 10.1530/acta.0.0810321. [DOI] [PubMed] [Google Scholar]
- 20.Edelstein CK, Roy-Byrne P, Fawzy FI, Dornfeld L. Effects of weight loss on the dexamethasone suppression test. Am J Psychiatry. 1983;140:338–41. doi: 10.1176/ajp.140.3.338. [DOI] [PubMed] [Google Scholar]
- 21.Berger M, MPK, Doerr P, Krieg C, von Zersseen D. Influence of weight loss on the dexamethasone suppression test. Archives of General Psychiatry. 1983:40. doi: 10.1001/archpsyc.1983.01790050111015. [DOI] [PubMed] [Google Scholar]
- 22.Anderson DA, Shapiro JR, Lundgren JD, Spataro LE, Frye CA. Self-reported dietary restraint is associated with elevated levels of salivary cortisol. Appetite. 2002;38:13–7. doi: 10.1006/appe.2001.0459. [DOI] [PubMed] [Google Scholar]
- 23.McLean JA, Barr SI, Prior JC. Cognitive dietary restraint is associated with higher urinary cortisol excretion in healthy premenopausal women. Am J Clin Nutr. 2001;73:7–12. doi: 10.1093/ajcn/73.1.7. [DOI] [PubMed] [Google Scholar]
- 24.Goodwin GM, Fairburn CG, Keenan JC, Cowen PJ. The effects of dieting and weight loss upon the stimulation of thyrotropin (TSH) by thyrotropin-releasing hormone (TRH) and suppression of cortisol secretion by dexamethasone in men and women. J Affective Disorders. 1988;14:137–44. doi: 10.1016/0165-0327(88)90056-0. [DOI] [PubMed] [Google Scholar]
- 25.Polivy J, Herman CP, Howard KI. Restraint scale: Assessment of dieting. In: Hersen M, Bellack AS, editors. Dictionary of behavioral assessment techniques. New York: Pergamon Press; 1988. pp. 377–80. [Google Scholar]
- 26.Jeffery RW, Drewnowski A, Epstein LH, Stunkard AJ, Wilson GT, Wing RR, Hill DR. Long-term maintenance of weight loss: current status. Health Psychol. 2000:19S. doi: 10.1037/0278-6133.19.suppl1.5. [DOI] [PubMed] [Google Scholar]
- 27.Phillips EL, Pratt HD. Eating disorders in college. Pediatr Clin North Am. 2005;52:85–96. viii. doi: 10.1016/j.pcl.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Pruessner J, Kirschbaum C, Meinlschmidt G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–31. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- 29.MacArthur Research Network on Socioeconomic Status and Health. Salivary cortisol measurement. 2000 http://wwwmacsesucsfedu/Research/Allostatic/notebook/salivarycorthtml.
- 30.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health & Social Behavior. 1983;24:385–96. [PubMed] [Google Scholar]
- 31.Schulz P, Kirschbaum C, Pruessner JC, Hellhammer DH. Increased free cortisol secretion after awakening in chronically stressed individuals due to work overload. Stress Medicine. 1998;14:91–7. [Google Scholar]
- 32.Holm JE, Holroyd KA. The Daily Hassles Scale (Revised): Does it measure stress or symptoms? Behavioral Assessment. 1993;14:465–82. [Google Scholar]
- 33.Rideout CA, Linden W, Barr SI. High cognitive dietary restraint is associated with increased cortisol excretion in postmenopausal women. J Gerontology A. 2006;62:628–33. doi: 10.1093/gerona/61.6.628. [DOI] [PubMed] [Google Scholar]
- 34.Lightman SL. The neuroendocrinology of stress: A never ending story. J Neuroendocrinology. 2008;20:880–4. doi: 10.1111/j.1365-2826.2008.01711.x. [DOI] [PubMed] [Google Scholar]
- 35.Pecoraro N, Gomez F, Dallman MF. Glucocorticoids dose-dependently remodel energy stores and amplify incentive relativity effects. Psychoneuroendocrinology. 2005;30:815–25. doi: 10.1016/j.psyneuen.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 36.Clow A, Thorn L, Evans P, Hucklebridge F. The awakening cortisol response: Methodological issues and significance. Stress. 2004:7. doi: 10.1080/10253890410001667205. [DOI] [PubMed] [Google Scholar]
- 37.Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–91. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- 38.Fischer JE, Calame A, Dettling AC, Zeier H, Fanconi S. Experience and endocrine stress responses in neonatal and pediatric critical care nurses and physicians. Critical Care. 2000;28:3281–8. doi: 10.1097/00003246-200009000-00027. [DOI] [PubMed] [Google Scholar]
- 39.Ouwens MA, van Strien T, van der Staak CP. Tendency toward overeating and restraint as predictors of food consumption. Appetite. 2003;40:291–8. doi: 10.1016/s0195-6663(03)00006-0. [DOI] [PubMed] [Google Scholar]
- 40.Rohleder N, Kirschbaum C. Effects of nutrition on neuro-endocrine stress responses. Curr Opin Clin Nutr Metab Care. 2007;10:504–10. doi: 10.1097/MCO.0b013e3281e38808. [DOI] [PubMed] [Google Scholar]
- 41.Stimson RH, Johnstone AM, Homer NZ, Wake DJ, Morton NM, Andrew R, Lobley GE, Walker BR. Dietary macronutrient content alters cortisol metabolism independently of body weight changes in obese men. J Clin Endocrinol Metab. 2007;92:4480–4. doi: 10.1210/jc.2007-0692. [DOI] [PubMed] [Google Scholar]
- 42.Hellhammer J, Fries E, Schweisthal OW, Schlotz W, Stone AA, Hagemann D. Several daily measurements are necessary to reliably assess the cortisol rise after awakening: state- and trait components. Psychoneuroendocrinology. 2007;32:80–6. doi: 10.1016/j.psyneuen.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 43.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–9. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]