Abstract
Prehypertension is associated with significant damage to the coronary vasculature and increased rates of adverse cardiovascular events. Circulating endothelial progenitor cells (EPCs) are critical to vascular repair and the formation of new blood vessels. We tested the hypothesis that prehypertension is associated with EPC dysfunction. Peripheral blood samples were collected from 83 middle-aged and older adults (51 M/32 F): 40 normotensive (age 53±2 yr; BP 111/74±1/1 mmHg) and 43 prehypertensive (54±2; 128/77±1/1 mmHg). EPCs were isolated from peripheral blood and EPC colony-forming capacity (colony-forming unit assay), migratory activity (Boyden chamber) and apoptotic susceptibility (active caspase-3 concentrations) were determined. There were no significant differences in either the number of EPC CFUs (10±2 vs. 9±1), EPC migration (1165±82 vs. 1120±84 fluorescent units), or active intracellular caspase-3 concentrations (2.7±0.3 vs. 2.3±0.2 ng/mL) between the normotensive and prehypertensive groups. When groups were stratified into low prehypertension (n=27; systolic BP: 120–129 mmHg) and high prehypertension (n=16; 130–139 mmHg), it was found that EPCs from the high prehypertensive group produced fewer (~65%, P<0.05) CFUs compared with the low prehypertensive (4±1 vs. 12±2) and normotensive adults. In conclusion, EPC colonyforming capacity is impaired only in prehypertensive adults with systolic BP greater than 130 mmHg. Prehypertension is not associated with migratory dysfunction or enhanced apoptosis of EPCs.
Keywords: endothelial progenitor cells, prehypertension
INTRODUCTION
Approximately 30% of the adult population in the United States is prehypertensive, defined as systolic blood pressure (BP) between 120–139 mmHg or diastolic BP between 80–89 mmHg (1). A primary concern in adults with prehypertension is a heightened cardiovascular disease risk. Indeed, the multivariate adjusted relative risk of an adverse cardiovascular event, such as myocardial infarction, is 3.5 times higher in individuals with prehypertension compared with their normotensive counterparts of similar age (2).
Although the precise mechanisms underlying the elevated cardiovascular risk associated with prehypertension are not fully understood, moderate elevations in blood pressure may damage vascular endothelium and disrupt endothelial cell repair. Prehypertensive individuals demonstrate significant target-organ damage of the coronary (3) and renal circulation (4). Circulating endothelial progenitor cells (EPCs) have the capacity to migrate to local sites of ischemia and endothelial damage, resist cellular apoptosis, secrete potent angiogenic growth factors, and participate in vascular repair and neovascularization (5;6). Moreover, systemic transplantation of ex vivo expanded EPCs into the coronary artery can rescue ischemic tissue and significantly improve coronary function in patients with myocardial infarction (7). Declines in EPC bioavailability and function, including reduced colony-forming capacity and migratory activity, have been linked with accelerated atherosclerotic disease progression, poor cardiovascular disease prognosis and adverse outcomes (8–10). Thus, a potential mechanism contributing to the heightened cardiovascular risk with prehypertension may be abnormalities in EPC function.
Accordingly, the experimental aim of the present study was to determine whether prehypertension is associated with EPC dysfunction. To address this aim, we isolated EPCs from healthy normotensive and prehypertensive adults and assessed EPC colony-forming capacity, migratory activity and apoptotic susceptibility.
MATERIALS AND METHODS
Subjects
Eighty-three subjects across the adult age range were studied: 40 normotensive [systolic BP < 120 mmHg and diastolic BP < 80 mmHg] and 43 prehypertensive (systolic BP 120–139 mmHg and/or diastolic BP 80–89 mmHg). Subjects reported for testing procedures between 8 and 11 am following an overnight fast. Prehypertension was defined according to JNC-7 guidelines (11). All subjects were non-obese and free of overt cardiovascular and metabolic disease as assessed by medical history, physical examination, and fasting blood chemistries. Men over the age of 40 years were further evaluated for clinical evidence of coronary artery disease with electrocardiograms and blood pressure at rest and during incremental exercise performed to exhaustion. All of the women were at least 1 year postmenopausal (mean: 7 ± 1 years) and had never taken or had discontinued use of hormone replacement therapy at least 1 year before the start of the study. None of the subjects smoked, were taking medications, or performed regular physical exercise for at least 6 months before the start of the study. Before participation, all of the subjects had the research study and its potential risks and benefits explained fully before providing written informed consent according to the guidelines of the University of Colorado at Boulder. All experiments conformed to the Declaration of Helsinki.
Body Composition
Body mass was measured to the nearest 0.1 kg using a medical beam balance. Percent body fat was determined by dual-energy X-ray absorptiometry (Lunar, Madison, WI). Body mass index was calculated as weight (kilograms) divided by height (meters) squared. Minimal waist circumference was measured according to published guidelines (12).
Treadmill Exercise Test
To assess aerobic fitness, subjects performed incremental treadmill exercise using a modified Balke protocol. Maximal oxygen consumption (VO2 max) was measured using online computer-assisted open-circuit spirometry as previously described (13).
Metabolic Measurements
Fasting plasma lipid and lipoprotein, glucose, and insulin concentrations were determined using conventional methods by the clinical laboratory affiliated with the University of Colorado Clinical and Translational Research Center.
EPC Isolation and Characterization
As described previously (14), circulating putative EPCs were isolated from peripheral blood by Ficoll density-gradient centrifugation (Histopaque 1077, Sigma Aldrich, St Louis, MO, USA), washed and resuspended in growth medium (Medium 199; Gibco, Grand Island, NY, USA) supplemented with 20% fetal calf serum (Gibco), penicillin (100 U/ml, Gibco), and streptomycin (100 μg/ml, Gibco). Endothelial phenotype of theses cells was confirmed by immunofluorescent staining for the uptake of DiI-ac-LDL (Biomedical Technologies Inc. Stoughton, MA, USA) and expression of VEcadherin, von Willebrand factor, CD31, and vascular endothelial growth factor receptor-2 (VEGFR-2, Invitron, Carlsbad, CA, USA).
EPC Colony-Forming Assay
Endothelial progenitor cell colony-forming capacity was determined as previously described by our laboratory (15). Briefly, freshly isolated mononuclear cells were plated on six-well plates coated with human fibronectin (BD Biosciences, San Jose, CA, USA) for 48 hours at 37° C. Thereafter, non-adherent cells were collected, and 5 × 105 cells were replated onto 24-well fibronectin-coated plates (BD Biosciences). Growth medium was changed every 3 days, and the EPC colony-forming units (CFUs) were counted manually in four random wells on the 7th day by two independent investigators blinded to sample identification. Only CFUs consisting of multiple thin, flat cells emanating from a central cluster of rounded cells were counted.
Migration Assay
EPC migratory capacity was measured using a modified Boyden chamber (15). Non-adherent cells (4 × 105) were resuspended in serum-free culture medium (Medium 199, Gibco) and loaded in the upper compartment of a 24-well modified Boyden chamber coated with fibronectin (FluoroBlok, BD Biosciences). The upper chamber was placed in the lower chamber containing culture medium supplemented with vascular endothelial growth factor (2 ng/ml) and incubated for 22 hours at 37°C. Following incubation, cells were labeled with calcein AM (Molecular Probes, Eugene, Oregon), and relative fluorescent units (RFU) of migrated cells were determined in triplicate.
Determination of Caspase-3 Activation
Activation of caspase-3 was induced in EPCs by incubating with staurosporine (1 μmol/L) for 3 h at 37°C. Following staurosporine stimulation, cells were washed in PBS and incubated with 10 μmol biotin-ZVKD-fmk inhibitor for 1 h at 37°C. Thereafter, cells were washed and lysed with extraction buffer at a concentration of 1 × 107 cells/mL. The concentration of active caspase-3 in the supernatant was determined by enzyme immunoassay (R&D Systems, Minneapolis, MN).
Statistical Analysis
Differences in subject baseline characteristics and the primary outcome variables were determined by analysis of variance (ANOVA). Relations between variables of interest were determined by linear regression analysis. There were no significant gender interactions in any of the primary outcome variables; therefore, the data were pooled and presented together. Data are reported as mean ± SEM. Statistical significance was set at P<0.05.
RESULTS
Selected subject characteristics are presented in the table. Although most anthropometric characteristics were similar between the groups, percent body fat was significantly lower in the prehypertensive adults. By design, both systolic and diastolic BP were higher (P<0.05) in the prehypertensive group compared with the normotensive controls. There were no differences between the groups in plasma lipid and lipoprotein, glucose, and insulin concentrations.
Table.
Selected Subject Characteristics
| Variable | Normotensive (n=40) |
Prehypertensive (n=43) |
|---|---|---|
| Age, yr | 53±2 | 54±2 |
| Gender, M/F | 19/21 | 32/11 |
| Body mass, kg | 76.1±2.5 | 78.6±1.7 |
| BMI, kg/m2 | 25.7±0.6 | 25.9±0.4 |
| Body fat, % | 33.5±1.6 | 28.2±1.3* |
| Waist circumference, cm | 88.3±2.2 | 89.7±1.4 |
| Systolic BP, mmHg | 111±1 | 128±1* |
| Diastolic BP, mmHg | 74±1 | 77±1* |
| V̇O2max, L/min | 2.6±0.2 | 2.7±0.2 |
| Total cholesterol, mmol/L | 5.2±0.1 | 5.2±0.1 |
| LDL-cholesterol, mmol/L | 3.2±0.1 | 3.4±0.1 |
| HDL-cholesterol, mmol/L | 1.4±0.1 | 1.3±0.1 |
| Triglycerides, mmol/L | 1.2±0.1 | 1.3±0.1 |
| Glucose, mmol/L | 5.0±0.1 | 5.2±0.1 |
| Insulin, pmol/L | 31.5±3.7 | 34.0±3.7 |
| HOMA-IR | 1.1±0.1 | 1.3±0.2 |
Values are mean±SEM. BMI: body mass index; BP: blood pressure; LDL: low-density lipoprotein; HDL: high-density lipoprotein; HOMA-IR: homeostasis model of insulin resistance.
P < 0.05 vs. normotensive
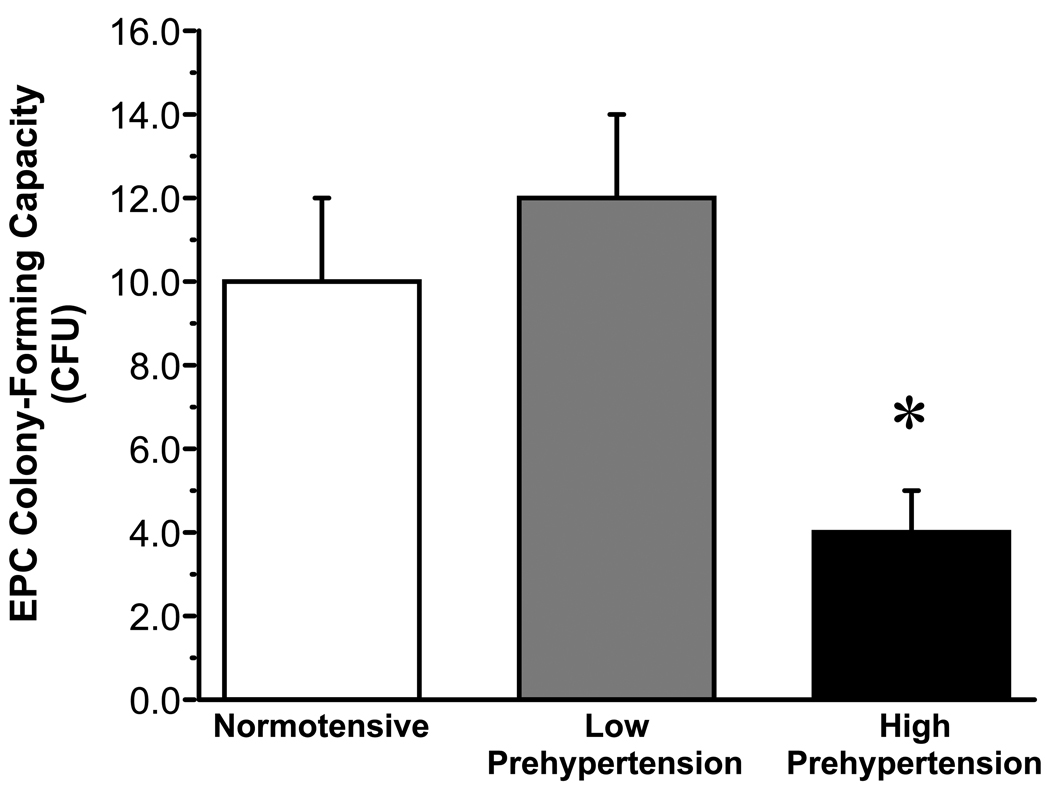
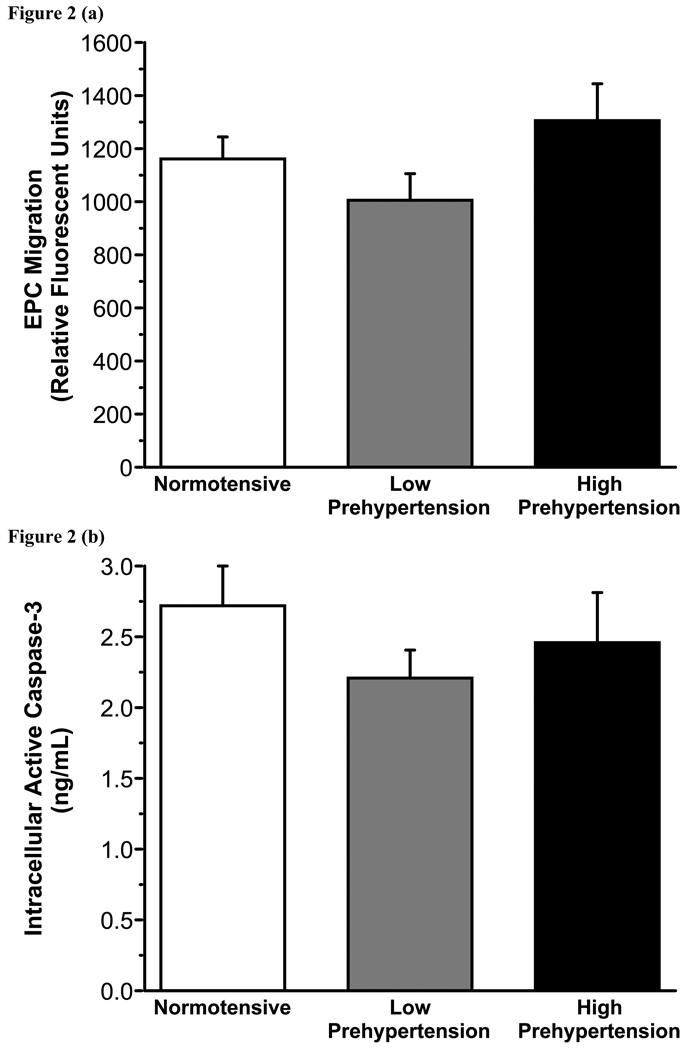
There were no significant differences in the number of EPC CFUs between the normotensive (10±2) and prehypertensive (9±1) groups. EPC migratory activity was also similar between the normotensive (1165±82 RFU) and prehypertensive (1120±84 RFU) adults. In addition, basal (0.3±0.04 vs. 0.3 ± 0.03 ng/mL) and stimulated (2.7±0.3 vs. 2.3±0.2 ng/mL) intracellular active caspase-3 concentrations were not different between the normotensive and prehypertensive adults. However, when the prehypertensive group was stratified into low prehypertension (n = 27; 120–129 mmHg systolic BP or 80–84 mmHg diastolic BP) and high prehypertension (n = 16; 130–139 mmHg systolic BP or 85–89 mmHg diastolic BP), differences in EPC colony-forming capacity emerged. EPCs isolated from adults with high prehypertension produced ~65% (4±1; P<0.05) fewer CFUs compared with both low prehypertensive (12±2) and normotensive subjects (Figure 1). There was a significant correlation (r = − 0.22) between the number of EPC CFUs and systolic BP. There were no significant differences in EPC migratory activity (1010±100 vs. 1307±139 RFU) or staurosporine-induced active intracellular caspase-3 concentrations (2.2±0.2 vs. 2.5±0.4 ng/mL) between the low and high prehypertensiv groups (Figure 2).
Figure 1.
EPC colony forming units in normotensive, low prehypertensive and high prehypertensive groups.
Values are mean±SEM. *P< 0.05 vs. normotensive and low prehypertensive.
Figure 2.
EPC migration (a) and intracellular concentrations of active caspase-3 in response to staurosporine (b) in normotensive, low prehypertensive and high prehypertensive groups.
Values are mean±SEM.
DISCUSSION
The primary new findings of the present study are that: 1) the ability of EPCs to form colonies is impaired in prehypertensive adults with systolic BP greater than 130 mmHg; and 2) prehypertension is not associated with diminished EPC migratory capacity or increased apoptotic susceptibility. To our knowledge, this is the first study to determine the influence of prehypertension on important functional characteristics of EPCs.
Numerous epidemiological studies have reported that the risk of coronary artery disease and major vascular events progressively increases with incremental elevations in blood pressure, independent of traditional risk factors, even before the onset of overt hypertension (blood pressure ≥ 140/90 mmHg) (16;17). For example, in a large cohort of the Framingham Heart Study, Vasan et al. (18) demonstrated a significant stepwise increase in the relative risk of myocardial infarction, stroke, and heart failure in subjects with high prehypertension (systolic BP > 130 mmHg) compared with low prehypertensive adults. Unfortunately, limited information is available concerning the pathophysiology contributing to cardiovascular disease with blood pressure in the high prehypertensive range.
Impairments in EPC function have been reported in hypertensive adults and may contribute etiologically to the increased cardiovascular risk in this group (9;19). EPC colony formation is a distinctive pattern of cell arrangement that manifests after approximately one week of culture. EPC colony-forming capacity is related to flow-mediated brachial artery vasodilation and cardiovascular risk, and is a better predictor of endothelial dysfunction than conventional cardiovascular risk factors (20). In addition, the number of colony-forming units can predict the extent of multivessel coronary artery disease (CAD) in middle-aged adults. For example, it has been suggested that for every 10 EPC colony-forming unit increase, the risk of multivessel CAD declines by 20% (8).
In the present study, EPC colony-forming capacity was significantly lower (~65%) in prehypertensive adults with systolic BP ≥ 130 mmHg. In contrast, no differences in the number of EPC CFUs were found between the low prehypertensive (systolic BP 120–129 mmHg) and normotensive adults. In addition, we observed a modest, but significant, inverse relation (r = − 0.22) between the number of EPC CFUs and systolic BP. Although a previous investigation found no difference in EPC colony-formation between patients with essential hypertension and non hypertensive subjects, the control group included prehypertensive individuals (21), meaning potential differences in EPC function between true normotensive controls and prehypertensive/hypertensive subjects may have been obscured. Indeed, the mean colony number of our high-prehypertensive subjects is similar to the control subjects in that study (4±1 vs. 3±1). Of note, our findings support the notion that the negative influence of prehypertension on EPC function is a consequence of elevated systolic rather than diastolic blood pressure. Moreover, it is consistent with many prospective observational studies demonstrating that the deleterious cardiovascular consequences associated with prehypertension, e.g. increased risk of myocardial infarction and ischemic stroke, are attributable to elevations in systolic BP (22;23).
In contrast to colony-formation, EPC migration was remarkably similar in the normotensive and prehypertensive groups, even when stratified into low and high prehypertension. The ability of EPCs to migrate is essential for homing to local sites of vascular injury and promoting revascularization and new blood vessel formation (24). For example, in patients with myocardial infarction, the reduction in infarct size following transplantation of ex vivo expanded progenitor cells into the coronary artery is determined by their ability to migrate to ischemic tissue (25). At sites of vascular trauma and injury, VEGF and other angiogenic cytokines such as stromal cell derived factor-1 and granulocyte colony stimulating factor are released to recruit circulating EPCs to areas of tissue repair (26). Impaired migration of EPCs may limit the number of cells available at injured areas of vascular tissue, thus attenuating their regenerative capacity. The results of the present study indicate that prehypertension does not negatively influence the capacity of EPCs to migrate. Interestingly, Vasa et al. demonstrated that essential hypertension is an independent predictor of impaired EPC migration (9). Thus, it appears that a blood pressure threshold exists with respect to impaired migratory function of EPCs.
The ability of EPCs to resist apoptosis is also an important functional characteristic as continual activation and recruitment of EPCs to sites of vascular damage can lead to enhanced apoptosis and attrition (5). Greater apoptosis and enhanced susceptibility to apoptotic factors could attenuate vascular repair and contribute to endothelial dysfunction and atherogenesis. Activation of caspase-3, the so-called ‘executioner molecule’ of apoptosis, represents cellular commitment to programmed cell death. However, we did not observe any differences in intracellular caspase-3 concentrations stimulated by staurosporine between the prehypertensive and normotensive adults.
A potential mechanism underlying reduced EPC colony formation with prehypertension is oxidative stress and/or increased angiotensin II activity. Prehypertension is associated with a pro-oxidant environment and elevations in angiotensin II (27;28). Furthermore, both oxidative stress and angiotensin II have been shown to impair EPC function (29–31). For example, treatment of ex vivo expanded EPCs with angiotensin II was shown to accelerate cellular senescence, inactivate telomerase, and impair EPC proliferation through increased intracellular oxidative damage, while pre-treatment with the angiotensin type 1 receptor antagonist valsartan inhibited these effects (30). Thus, it is possible that elevated angiotensin II activity with prehypertension may increase intracellular oxidative stress and accelerate telomerase inactivation, which may in turn reduce EPC colony formation. This possibility, however, requires further investigation.
There are a number of experimental considerations of the present study that should be mentioned. Firstly, there is currently no clear consensus on the most appropriate criteria for the isolation, culture or quantification of EPCs, creating ambiguity when comparing and interpreting studies involving EPCs. In the present investigation, we isolated a putative EPC cell population from circulating peripheral blood mononuclear cells and then employed a short culture period to eliminate mature, adherent cell types. The remaining cells exhibit several endothelial phenotypic characteristics, including LDL uptake and expression of lectin, VEGFR-2 and CD31 (32;33). It has been consistently documented that these cells participate in vasculogenesis and vascular repair (33–35), and are related to both endothelial dysfunction and cardiovascular risk (10;20). Secondly, as with all cross-sectional experimental designs, we must acknowledge the possibility that genetic and/or lifestyle behaviors influenced our results. Similarly, numerous pathologic, pharmacologic and physiologic factors have been reported to influence EPC number and function. We attempted to minimize these potential confounds by studying thoroughly screened adults who were free of overt disease, non-medicated, nonsmokers, and were not physically active.
In conclusion, the results of the present study suggest that, by and large, EPC dysfunction commonly observed with hypertension is not present in the prehypertensive state. Prehypertension is associated with reduced EPC colony forming capacity but this impairment is limited to the prehypertensive state with systolic BP ≥ 130 mmHg. However, prehypertension does not exert a negative influence on EPC migration or susceptibility to apoptosis. Thus, EPC dysfunction does not appear to be a major contributor to the increased cardiovascular risk associated with prehypertension.
Summary Table.
| What is known about the topic: | |
| - | Prehypertension is associated with an increased risk of coronary artery disease and acute vascular events, such as myocardial infarction and stroke. |
| - | Endothelial injury and dysfunction are thought to contribute to heightened the cardiovascular risk in individuals with prehypertension. |
| - | Impairments in endothelial progenitor cell (EPC) function, including reduced colony- forming capacity and migratory activity, have been linked to endothelial dysfunction, atherosclerotic disease progression, and cardiovascular events. |
| What this study adds: | |
| - | The capacity of EPCs to form colonies is impaired in prehypertensive adults with SBP greater than 130 mmHg. |
| - | Prehypertension is not associated with diminished EPC migratory activity or enhanced susceptibility to apoptotis. |
ACKNOWLEDGEMENTS
We would like to thank all of the subjects who participated in the study as well as Yoli Casas and Jeremy Stoner for their technical assistance. This study was supported by National Institutes of Health Awards HL068030, DK062061, HL076434, and MO1 #RR00051.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
REFERENCE LIST
- 1.Wang Y, Wang QJ. The prevalence of prehypertension and hypertension among US adults according to the new joint national committee guidelines: new challenges of the old problem. Arch Intern Med. 2004;164(19):2126–2134. doi: 10.1001/archinte.164.19.2126. [DOI] [PubMed] [Google Scholar]
- 2.Qureshi AI, Suri MF, Kirmani JF, Divani AA, Mohammad Y. Is prehypertension a risk factor for cardiovascular diseases? Stroke. 2005;36(9):1859–1863. doi: 10.1161/01.STR.0000177495.45580.f1. [DOI] [PubMed] [Google Scholar]
- 3.Erdogan D, Caliskan M, Yildirim I, Gullu H, Baycan S, Ciftci O, et al. Effects of normal blood pressure, prehypertension and hypertension on left ventricular diastolic function and aortic elastic properties. Blood Press. 2007;16(2):114–121. doi: 10.1080/08037050701395910. [DOI] [PubMed] [Google Scholar]
- 4.Knight EL, Kramer HM, Curhan GC. High-normal blood pressure and microalbuminuria. Am J Kidney Dis. 2003;41(3):588–595. doi: 10.1053/ajkd.2003.50120. [DOI] [PubMed] [Google Scholar]
- 5.Dimmeler S, Zeiher AM. Vascular repair by circulating endothelial progenitor cells: the missing link in atherosclerosis? J Mol Med. 2004;82(10):671–677. doi: 10.1007/s00109-004-0580-x. [DOI] [PubMed] [Google Scholar]
- 6.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85(3):221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 7.Assmus B, Schachinger V, Teupe C, Britten M, Lehmann R, Dobert N, et al. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI) Circulation. 2002;106(24):3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 8.Kunz GA, Liang G, Cuculi F, Gregg D, Vata KC, Shaw LK, et al. Circulating endothelial progenitor cells predict coronary artery disease severity. Am Heart J. 2006;152(1):190–195. doi: 10.1016/j.ahj.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89(1):E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 10.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353(10):999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 11.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 12.Lohman T, Roche A, Mortorell R. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics; 1988. [Google Scholar]
- 13.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, et al. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 14.MacEneaney OJ, Kushner EJ, Van Guilder GP, Greiner JJ, Stauffer BL, DeSouza CA. Endothelial progenitor cell number and colony-forming capacity in overweight and obese adults. Int J Obes (Lond) 2009;33(2):219–225. doi: 10.1038/ijo.2008.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoetzer GL, MacEneaney OJ, Irmiger HM, Keith R, Van Guilder GP, Stauffer BL, et al. Gender differences in circulating endothelial progenitor cell colony-forming capacity and migratory activity in middle-aged adults. Am J Cardiol. 2007;99(1):46–48. doi: 10.1016/j.amjcard.2006.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chobanian AV. Prehypertension revisited. Hypertension. 2006;48(5):812–814. doi: 10.1161/01.HYP.0000241684.29799.14. [DOI] [PubMed] [Google Scholar]
- 17.Gu Q, Burt VL, Paulose-Ram R, Yoon S, Gillum RF. High blood pressure and cardiovascular disease mortality risk among U.S. adults: the third National Health and Nutrition Examination Survey mortality follow-up study. Ann Epidemiol. 2008;18(4):302–309. doi: 10.1016/j.annepidem.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Vasan RS, Larson MG, Leip EP, Evans JC, O'Donnell CJ, Kannel WB, et al. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345(18):1291–1297. doi: 10.1056/NEJMoa003417. [DOI] [PubMed] [Google Scholar]
- 19.Imanishi T, Moriwaki C, Hano T, Nishio I. Endothelial progenitor cell senescence is accelerated in both experimental hypertensive rats and patients with essential l hypertension. J Hypertens. 2005;23(10):1831–1837. doi: 10.1097/01.hjh.0000183524.73746.1b. [DOI] [PubMed] [Google Scholar]
- 20.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348(7):593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 21.Delva P, Degan M, Vallerio P, Arosio E, Minuz P, Amen G, et al. Endothelial progenitor cells in patients with essential hypertension. J Hypertens. 2007;25(1):127–132. doi: 10.1097/HJH.0b013e3280109271. [DOI] [PubMed] [Google Scholar]
- 22.Stamler J, Stamler R, Neaton JD. Blood pressure, systolic and diastolic, and cardiovascular risks. US population data. Arch Intern Med. 1993;153(5):598–615. doi: 10.1001/archinte.153.5.598. [DOI] [PubMed] [Google Scholar]
- 23.He J, Whelton PK. Elevated systolic blood pressure and risk of cardiovascular and renal disease: overview of evidence from observational epidemiologic studies and randomized controlled trials. Am Heart J. 1999;138((3) Pt 2):211–219. doi: 10.1016/s0002-8703(99)70312-1. [DOI] [PubMed] [Google Scholar]
- 24.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95(4):343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 25.Britten MB, Abolmaali ND, Assmus B, Lehmann R, Honold J, Schmitt J, et al. Infarct remodeling after intracoronary progenitor cell treatment in patients with acute myocardial infarction (TOPCARE-AMI): mechanistic insights from serial contrast-enhanced magnetic resonance imaging. Circulation. 2003;108(18):2212–2218. doi: 10.1161/01.CIR.0000095788.78169.AF. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, et al. Ischemiaand cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5(4):434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 27.Chrysohoou C, Panagiotakos DB, Pitsavos C, Skoumas J, Economou M, Papadimitriou L, et al. The association between pre-hypertension status and oxidative stress markers related to atherosclerotic disease: the ATTICA study. Atherosclerosis. 2007;192(1):169–176. doi: 10.1016/j.atherosclerosis.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 28.Julius S, Nesbitt S, Egan B, Kaciroti N, Schork MA, Grozinski M, et al. Trial of preventing hypertension: design and 2-year progress report. Hypertension. 2004;44(2):146–151. doi: 10.1161/01.HYP.0000130174.70055.ca. [DOI] [PubMed] [Google Scholar]
- 29.Imanishi T, Hano T, Sawamura T, Nishio I. Oxidized low-density lipoprotein induces endothelial progenitor cell senescence, leading to cellular dysfunction. Clin Exp Pharmacol Physiol. 2004;31(7):407–413. doi: 10.1111/j.1440-1681.2004.04022.x. [DOI] [PubMed] [Google Scholar]
- 30.Imanishi T, Hano T, Nishio I. Angiotensin II accelerates endothelial progenitor cell senescence through induction of oxidative stress. J Hypertens. 2005;23(1):97–104. doi: 10.1097/00004872-200501000-00018. [DOI] [PubMed] [Google Scholar]
- 31.Yu Y, Fukuda N, Yao EH, Matsumoto T, Kobayashi N, Suzuki R, et al. Effects of an ARB on endothelial progenitor cell function and cardiovascular oxidation in hypertension. Am J Hypertens. 2008;21(1):72–77. doi: 10.1038/ajh.2007.5. [DOI] [PubMed] [Google Scholar]
- 32.Ito H, Rovira II, Bloom ML, Takeda K, Ferrans VJ, Quyyumi AA, et al. Endothelial progenitor cells as putative targets for angiostatin. Cancer Res. 1999;59(23):5875–5877. [PubMed] [Google Scholar]
- 33.Hur J, Yang HM, Yoon CH, Lee CS, Park KW, Kim JH, et al. Identification of a novel role of T cells in postnatal vasculogenesis: characterization of endothelial progenitor cell colonies. Circulation. 2007;116(15):1671–1682. doi: 10.1161/CIRCULATIONAHA.107.694778. [DOI] [PubMed] [Google Scholar]
- 34.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, et al. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18(14):3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chade AR, Zhu X, Lavi R, Krier JD, Pislaru S, Simari RD, et al. Endothelial progenitor cells restore renal function in chronic experimental renovascular disease. Circulation. 2009;119(4):547–557. doi: 10.1161/CIRCULATIONAHA.108.788653. [DOI] [PMC free article] [PubMed] [Google Scholar]