Abstract
Background
Infection with Mycoplasma pneumoniae in asthma can occur both acutely and chronically with an associated Th2 inflammatory response and/or increased numbers of bronchial mast cells. Mast cells have previously been shown to promote mycoplasma clearance in mice; however, it is unknown whether mast cells would aid M. pneumoniae clearance under allergic conditions.
Objective
Our aim was to determine the impact of allergic inflammation on mast cell-mediated lung M. pneumoniae clearance. Furthermore, as we have previously demonstrated an essential role for IL-6 in lung M. pneumoniae clearance we also investigated the role of mast cell-derived IL-6.
Methods
Mast cell deficient WBB6F1/J-KitW/KitW-v mice were challenged with ovalbumin to induce airway inflammation prior to M. pneumoniae infection. The role of mast cell-derived IL-6 in bacterial clearance was further investigated by reconstitution of mast cell deficient mice with IL-6-/- mast cells.
Results
Allergic mast cell deficient mice exhibited increased lung M. pneumoniae burden compared to control littermates. Intravenous adoptive transfer of wild type and IL-6-/- mast cells significantly improved M. pneumoniae clearance in mast cell deficient mice. Acutely after M. pneumoniae infection, allergen-challenged mast cell deficient mice had increased levels of the pro-inflammatory cytokines IL-6 and TNF-α in the BAL fluid. The total number of neutrophils was also increased in mast cell deficient mice.
Conclusions
Our results establish that mast cells aid host defense against M. pneumoniae in an allergic setting and that while IL-6 is necessary for lung M. pneumoniae clearance, mast cell-derived IL-6 is not required.
Keywords: Asthma, host defense, innate immunity, mast cells, Mycoplasma pneumoniae
Introduction
Mycoplasma pneumoniae (Mp) is an atypical bacterium commonly recognized to cause community acquired pneumonia. However, evidence has documented Mp infection in individuals presenting with acute asthma exacerbations as well as stable chronic asthmatics [1-6]. Mast cells have long been implicated in the pathogenesis of asthma [7, 8] and acute asthma exacerbations and/or asthma onset associated with Mp are characterized by mast cell related events such as atopy, IgE, and Th2 cytokine responses [1-5]. Furthermore in chronic asthma, in which we have previously reported that 42% of stable asthmatics are positive for Mp by PCR detection from bronchial biopsy [6], the number of mast cells was significantly increased in airway tissue. However, this increase was observed in Mp positive asthmatics compared to Mp negative asthmatics. As mast cells have been demonstrated with increasing frequency to be involved in host defense responses, the question raised is whether mast cells serve in a bactericidal role in an allergic milieu?
Evidence for the role of mast cells in bacterial clearance was first reported by Echtenacher et. al. and Malaviya et. al. in models of ceacal ligation and puncture and acute septic peritonitis respectively [9, 10]. In subsequent studies mast cells were further demonstrated to play a role in bacterial clearance from the skin [11] and lungs [12], including clearance of lung Mycoplasma pulmonis [13]. In the case of peritoneal infection, neutrophil influx driven by mast cell-derived TNF-α was demonstrated to mediate bacterial clearance [9, 10], and a host of cell surface receptors including Toll-like receptor 4 and complement receptors were shown to play a role [14, 15]. However, no mechanism for mast cell-mediated bacterial clearance has been proven in the lung. We have previously shown that IL-6 is necessary for Mp clearance in non-allergic mice and that IL-6 is increased in response to Mp [16]. Mp was further demonstrated to induce IL-6 expression in mast cell cultures [17].
While it was demonstrated that mast cells promote bacterial clearance in an allergen naïve environment, it was not clear if mast cells would function similarly under allergic conditions from the following evidence. First, lung allergic inflammation suppresses bacterial clearance in vivo [16, 18], and mast cells contribute to allergic inflammation in response to allergen challenge [19], specifically through the release of TNF-α [20, 21]. Second, mast cells were shown to enhance eosinophilic inflammation when the bacterial toxin LPS was co-administered with allergen [22]. Finally, simultaneous treatment of mast cells with IgE/allergen and agonists for Toll-like receptors, a class of pattern recognition molecules involved in host defense, synergistically enhanced cytokine release, including IL-13 [23, 24] which has been demonstrated to play a central role in the pathogenesis of asthma [25, 26]. Therefore, to investigate our clinical question pertaining to the interaction of mast cells and Mp in asthmatics, it was essential to examine Mp clearance in a mouse model of allergic inflammation.
The aim of our study was to determine the impact of mast cells on Mp burden after the establishment of airway inflammation. Utilizing mast cell deficient WBB6F1/J-KitW/KitW-v (W/Wv) mice and their wild type littermates (WBB6F1-+/+), we demonstrate that mast cells promote Mp clearance under allergic conditions. Furthermore, although Mp infection in bone marrow-derived cultured mast cells prompted rapid release of IL-6, adoptive transfer of IL-6-/- bone marrow-derived cultured mast cells significantly reduced bacterial burden in mast cell deficient mice.
Methods
Mice
Mast cell deficient WBB6F1/J-KitW/KitW-v (W/Wv), congenic WBB6F1-+/+ littermates, C57BL/6, and B6.129S2-Il6tm1Kopf/J mice were obtained from Jackson Laboratory (Bar Harbor, ME, USA). Male mice ages 6-8 weeks were used for experiments. Mice were maintained in a pathogen free facility and serology reports during the time period of all of our experiments were negative for the presence of the mouse pathogen Mycoplasma pulmonis. All experiments were approved by the Institutional Animal Care and Use Committee at National Jewish Health.
Mouse model of allergic inflammation and Mp infection
The establishment of allergic inflammation and Mp preparation has been previously described [16]. Briefly, mice were sensitized though two i.p. injections of 20 μg of ovalbumin (Sigma-Aldrich, St. Louis, MO, USA) emulsified in 2.25 mg aluminum hydroxide (Pierce, Rockford, IL, USA) on days one and 14. Twenty eight days after the last OVA sensitization, mice were placed in a closed chamber and challenged once daily for three consecutive days with 1% aerosolized OVA for 20 minutes using an ultrasonic nebulizer (DeVilbiss). Mice were rested one day and infected intranasally with Mp (Stain FH 15531 ATCC, Manassas, VA, USA) at a dose 108 CFU/mouse, unless otherwise noted. Avertine (synthesized from 2,2,2-tribromoethanol and tert-amyl-alcohol, Sigma-Aldrich, St. Louis, MO, USA) was given at a dose of 0.25 g/kg mouse to anesthetize the mouse during infection. Non-infected control mice were also anesthetized and given an equal volume of saline intranasally. One day or seven days after infection mice were sacrificed by a single i.p. injection of 200 mg/kg pentobarbital.
Lung and BAL collection
The left lung was excised and homogenized in PBS for Mp culture and CFU counting. Lung homogenate was diluted and directly plated onto PPLO plates (Remel, Lenexa, KS, USA). Plates were incubated at 37°C for 7 days prior to Mp quantification. One ml of saline was used to lavage the lung for each mouse, and BAL fluid was collected and cell free supernatants were stored at -80°C for ELISA analysis. Total number of white blood cells in the lavage fluid was determined for each individual mouse and cytospins were prepared from the cells isolated from the BAL fluid. Cell differentials were determined by microscopic examination via cytospin preparation after staining. Hema 3 Stain Set (Fisher Scientific) was used to stain all cytospins and has staining characteristics similar to Wright and Wright-Giemsa stains. Macrophages, neutrophils, eosinophils, and lymphocytes were distinguished based on staining characteristics.
Bone marrow-derived cultured mast cells
Bone marrow-derived cultured mast cells (BMCMCs) were generated from wild type C57BL/6 mice. Mice were sacrificed with a lethal dose of pentobarbital (200 mg/kg) and sterilized with 70% ethanol. Femurs were extracted and the bone marrow flushed with FBS followed by RPMI with 10% FBS. Cells were centrifuged and resuspended at a density of 1 × 106 cells/ml in RPMI supplemented with 10% FBS, 1% penicillin/streptomycin, and 5 ng/ml recombinant mouse IL-3 (R&D Systems, Minneapolis, MN, USA). Half of the media was changed twice a week. After 4 to 6 weeks in culture, the purity of mast cells was determined by toluidine blue staining. Cultures were used when mast cell purity was greater than 98%. Prior to treatment and infection, mast cells were washed twice in PBS and resuspended in antibiotic free media. 5 × 105 cells were treated in triplicate for all experiments. IL-4 (10 ng/ml) and IL-13 (10 ng/ml) were included in mast cell media 2 hours prior to infection. Cells were infected with Mp at a dose of 10 CFU/cell and cells and supernatants were collected 2 hours after infection.
Cytokine determination
Cytokine levels were determined in cell-free supernatants of bone marrow-derived cultured mast cells or bronchoalveolar lavage (BAL) supernatant by ELISA analysis. For the cell culture supernatants, IL-6 and TNF-α levels were determined by standard sandwich ELISA (ELISATech, Aurora, CO, USA).
Adoptive Transfer
BMCMCs were cultured from wild type and IL-6-/- C57BL/6 donors as described above. W/Wv mice were reconstituted with 5 × 106 mast cells through i.v. injection. Mice were infected with Mp 12 weeks after i.v. reconstitution based on previous reports that demonstrated mast cell engraftment of organs 12 weeks after reconstitution [28]. OVA sensitizations were initiated one month prior to Mp infection (8 weeks after reconstitution) according to our standard protocol. It has been previously reported that W/Wv mice can be reconstituted with C57BL/6 mast cells without rejection [29].
Toluidine Blue Staining
Lung tissue was fixed in 10% neutral buffered formalin and embedded in paraffin. Mast cells were identified in lung tissue by toluidine blue staining. Sections were stained with a working solution of 1% (w/v) toluidine blue (in 70% ethanol) in a 1:10 ratio with 1% sodium chloride (pH 2.5) for 3 minutes. Sections were dehydrated and coversliped prior to analysis.
Statistical Analysis
Lung Mp CFU counts were log transformed to obtain normally distributed data and means between two groups were compared using the Student's t-test. Significance between more than two groups was determined using one-way ANOVA. Non-parametric data, consisting of BAL cytokine levels and neutrophils numbers, were analyzed using the Mann-Whitney U test to determine significance between two groups or the Kruskal-Wallis test to determine significance between more than two groups. Non-parametric data are presented as medians with interquartile (25-75%) ranges. A p-value of < 0.05 was considered significant and GraphPad Prism 5.0 was used for all statistical analysis.
Results
Mast cells promote lung Mp clearance under allergic conditions
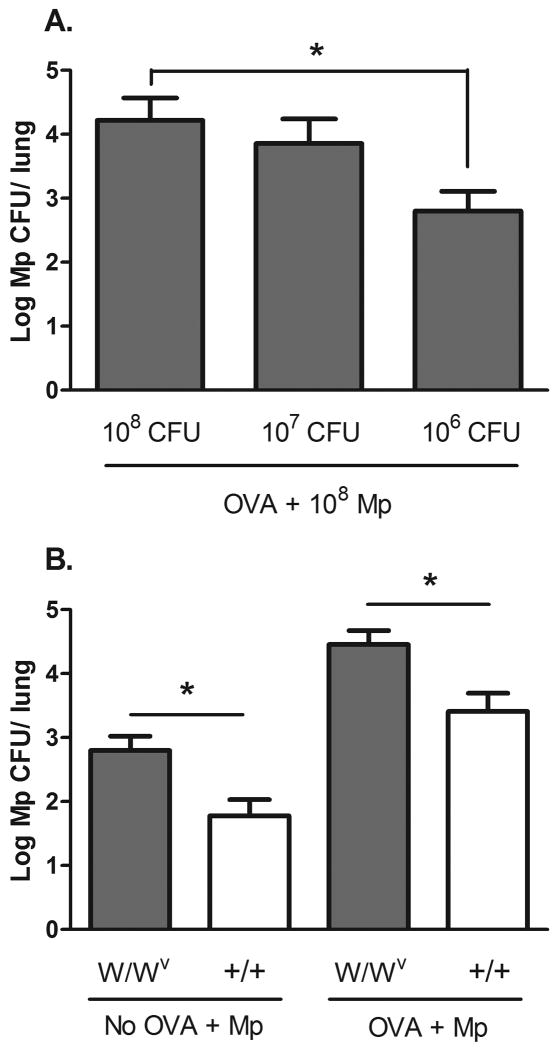
We have previously reported allergic mice have impaired Mp clearance, with the most significant difference 7 days post infection with 108 CFU of Mp [16]. Mp burden is the highest in infected mice 24 hours after infection and thereafter declines in both allergen-challenged and allergen-naïve models. In the current study, we further determined if bacterial load in allergic W/Wv mice was dose dependent by infecting mice with 108, 107, and 106 CFU (Fig. 1 A). As we have consistently observed a difference in bacterial burden between allergic and non-allergic mice at a dose of 108 CFU of Mp [16], we choose to use this dose for our in vivo experiments. To determine the impact of mast cells on Mp clearance in an allergic environment we examined lung bacterial burden in OVA-challenged mast cell deficient WBB6F1/J-KitW/KitW-v (W/Wv) mice 7 days after infection. Allergic W/Wv mice exhibited a significantly higher lung Mp burden than the WBB6F1-+/+ littermate controls (p < 0.05) 7 days post Mp infection (Fig. 1 B), establishing that mast cells aid Mp clearance in an allergic environment. Non-allergic W/Wv mice also had a higher lung burden of Mp than non-allergic wild type controls, agreeing with a previous report that demonstrated mast cell deficient mice are more susceptible to infection with Mycoplasma pulmonis [13]. Analysis of the cell profile in the bronchoalveolar lavage at this time showed no differences between W/Wv and control mice in either the allergen naïve or allergen-challenged groups (Table 1).
Fig. 1.
Mp burden is increased in allergic W/Wv mice. Mice were challenged with OVA or saline prior to infection with 108 CFU Mp/mouse. The left lobe of the lung was collected 7 days after infection and homogenized for Mp culture. (A) Mp burden in allergic W/Wv mice is dose-dependent; n = 9-10 and data presented was collected from one replicate. (B) Lung Mp burden is increased in allergic W/Wv mice compared to littermate controls (+/+) and in non-allergic W/Wv mice compared to controls; n = 17-18 and data are pooled from the results of two independent replicates. Mp burden is presented as the number of live colonies (log10 of CFU ± SEM). Comparison between groups was performed using a Student's t-test, * p < 0.05.
Table 1. Bronchoalveolar lavage cell profiles in mice 7 days after Mp infection.
| Groups | Macrophages (× 104/ml) |
Neutrophils (× 104/ml) |
Eosinophils (× 104/ml) |
Lymphocytes (× 104/ml) |
|---|---|---|---|---|
| W/Wv (Mp) | 6.2 (2.9-6.8) | 0.06 (0.05-0.16) | 0 | 0.87 (0.43-1.19) |
| WBB6F1-+/+ (Mp) | 5.8 (2.3-7.9) | 0.04 (0.01-0.007) | 0 | 0.22 (0.03-0.40)* |
| W/Wv (OVA + Mp) | 6.7 (5.0-9.2) | 1.45 (0.66-5.92)† | 22.7 (2.9-48.2)† | 1.16 (0.57-2.11) |
| WBB6F1-+/+ (OVA + Mp) | 5.5 (1.9-8.2) | 0.32 (0.09-0.59)‡ | 22.7 (16.7-21.3)† | 1.21 (0.23-3.18) |
P < 0.05 compared to non-OVA groups by Kruskal-Wallis test
P < 0.05 compared to non-OVA, +/+ group by Kruskal-Wallis test
P < 0.05, compared to all other groups by Kruskal-Wallis test
OVA, ovalbumin; Mp, Mycoplasma pneumoniae, 108 CFU
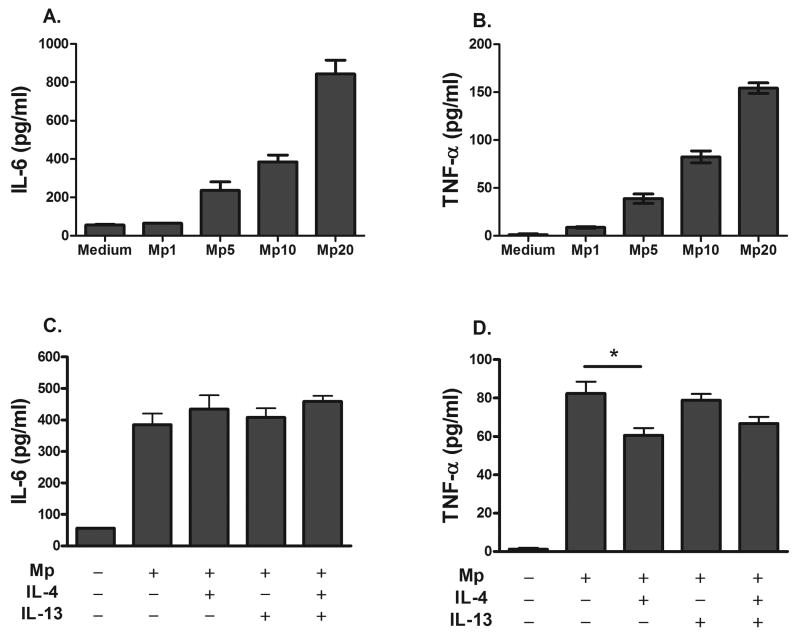
Mp induces the release of IL-6 and TNF-α in cultured bone marrow-derived cultured mast cells (BMCMCs)
From the evidence that IL-6-/- allergen-naïve mice have a higher lung Mp burden than wild type controls [16], and that mast cell produce IL-6 in response to Mp infection in the absence of allergic inflammation [17], we determined whether pre-treatment of bone marrow-derived cultured mast cells (BMCMCs) with IL-4 and IL-13 would affect IL-6 production after infection. We further examined TNF-α production because of its role in mast cell-mediated host defense in the peritoneal cavity [9, 10]. Mp infection alone induced IL-6 and TNF-α release by BMCMCs in a dose-dependent manner (Fig 2 A, B). We utilized pre-treatment with 10 ng/ml of IL-4, IL-13 or both for 2 hours to recapitulate an allergic environment prior to infection. We found neither IL-4 nor IL-13 altered IL-6 production, and only IL-4 treatment slightly suppressed TNF-α production (Fig. 2 C, D). We expected BMCMCs to be insensitive to IL-13 treatment as a previous report demonstrated BMCMCs lack expression of the IL-13Rα1 and are unresponsive to IL-13 [30]. We further determined TNF-α production, but not IL-6, by BMCMCs is suppressed by IL-4 in a dose-dependent manner, however we found prolonged treatment with IL-4 up to two weeks does not change the cytokine expression patterns.
Fig. 2.
Mp infection of bone marrow-derived cultured mast cells (BMCMCs) induces production of IL-6 and TNF-α. BMCMCs were infected with 10 CFU of Mp per mast cell 2 hours after pretreatment with 10 ng/ml of IL-4 or 10 ng/ml IL-13. Cytokine levels were determined 2 hours after infection. (A, B) The production of IL-6 and TNF-α in response to Mp is dose-dependent. (C) Pre-treatment of BMCMCs with IL-4, IL-13, or both did not reduce levels of IL-6 induced by Mp infection. (D) Pre-treatment of BMCMCs with IL-4 slightly decreased TNF-α production in response to Mp although levels remained significantly higher than non-infected controls. Data are representative of 3 independent replicates and is presented as the mean ± SEM, * p < 0.05 using one-way ANOVA.
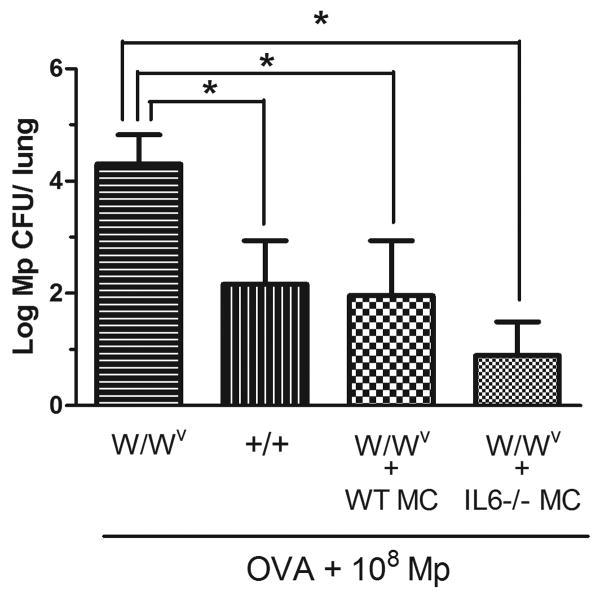
Adoptive transfer of IL-6-/- mast cells improves Mp burden in W/Wv mice
To confirm whether mast cell-derived IL-6 was involved in Mp host defense, we used adoptive transfer to reconstitute W/Wv mice with IL-6-/- mast cells. Wild type C57BL/6 mast cells served as a control. W/Wv mice reconstituted with both IL-6-/- and wild type mast cells showed significantly improved Mp clearance 7 days after infection over W/Wv mice and CFU levels were comparable to +/+ control mice (Fig. 3). We verified by toluidine blue staining that mast cells were present in the lung parenchyma of W/Wv mice after adoptive transfer which is consistent with a previous report [29].
Fig. 3.
Adoptive transfer of wild type and IL-6-/- mast cells to W/Wv mice reduces Mp burden 7 days after infection. 5 × 106 mast cells were used to reconstitute mice and Mp burden was determined 12 weeks after reconstitution. Mice were challenged with OVA to induce airway inflammation prior to Mp infection; n = 7-9. Mp burden is presented as the number of live colonies (log10 of means ± SEM). Comparison between groups was performed using a Student's t-test, * p < 0.05.
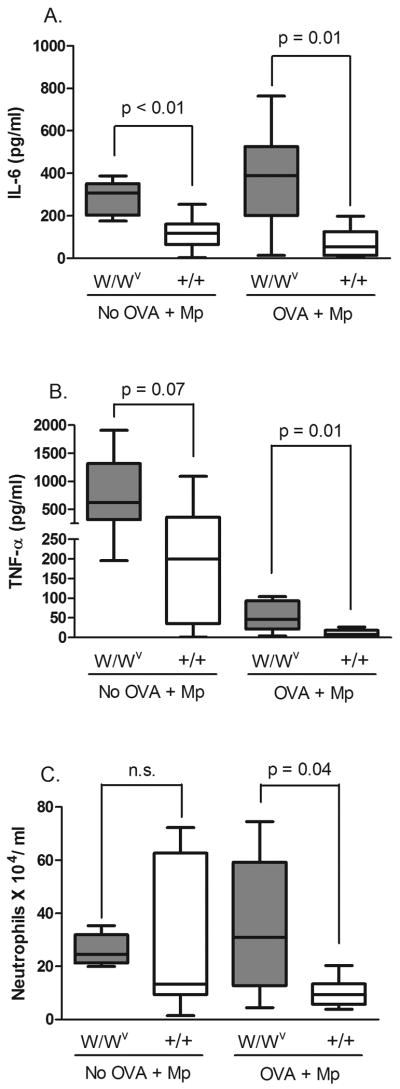
W/Wv mice exhibit a heightened cytokine response acutely after infection
The results from our adoptive transfer experiment indicated that mast cell-derived IL-6 was not necessary for Mp host defense in an allergic setting. To further show that mast cell-derived IL-6 was not necessary for lung Mp clearance, we examined cytokine levels in the BAL fluid of W/Wv mice. We assayed cytokine levels 7 days after infection; however, levels were too low to be detected in infected mice. Therefore, we determined IL-6, and also TNF-α levels, 24 hours after infection to gauge the acute response to Mp. We found levels of IL-6 and TNF-α were elevated in the BAL supernatant of infected W/Wv mice compared to littermates, regardless of OVA challenge (Fig. 4 A, B). Both IL-6 and TNF-α levels were increased in response to Mp as levels were significantly lower in non-infected mice (p < 0.05). We further examined the composition of white blood cells in the BAL fluid as we did not observed any differences in cell numbers 7 days after infection and wanted to verify the same 24 hours after infection. In the case of 24 hours after infection we found a higher number of neutrophils in allergic and infected W/Wv compared to littermate controls (Fig. 4 C), demonstrating neutrophil influx is not impaired in the lungs of W/Wv mice.
Fig. 4.
Quantification of cytokine levels and neutrophils in the BAL fluid of OVA-naïve and OVA-challenged W/Wv and wild type mice 24 hours after Mp infection. (A) IL-6 levels were increased in infected W/Wv mice compared to control littermates in both OVA-naïve and OVA-challenged mice. (B) TNF-α levels were increased in W/Wv mice compared to controls. (C) The total number of neutrophils was slightly increased in infected and OVA-challenged W/Wv mice compared to littermate controls. n = 6-8 mice per group. The data are presented in box and whisker plots with the 75th and 25th quartiles and the max and min indicated by box and whiskers respectively. The bar represents the median. P values were determined using the Mann-Whitney U test.
Discussion
The key finding of our study is that mast cells aid Mp clearance in a mouse model of allergic inflammation and we are the first to report that mast cells aid host defense under allergic conditions. In vivo, mast cells have been shown to provide a protective advantage against a number of infectious agents including Mycobacterium tuberculosis [12], enterobacteria [10], and the natural murine pathogen Mycoplasma pulmonis [13]. With particular relevance to our studies, M. pulmonis is the mouse homolog to Mp and mast cell deficient mice were shown to have a higher burden of M. pulmonis in the early stages of infection. We would like to note M. pulmonis is more pathogenic than Mp in mice, and that while Xu et. al. found bacterial burden was initially lower in wild type mice compared to mast cell deficient mice, bacterial burden increased in wild type mice over the course of 28 days to levels similar to those in the mast cell deficient strain [13]. In contrast we have found Mp levels are highest in mice 24 hours after infection and decline thereafter with no detectable levels after two weeks, thereby suggesting increased bacterial load in allergic W/Wv mice is strictly related to impaired bacterial clearance mechanisms.
The mechanism of mast cell-mediated lung Mp clearance has not been demonstrated; however, previous findings have demonstrated a central role for mast cell-derived TNF-α in defense against peritoneal infections [9, 10], and we have shown IL-6 is necessary for lung Mp clearance in non-allergic mice [16]. In addition, both IL-6-/- mice and mast cell deficient mice were demonstrated to be more susceptible to lung infection with Klebsiella pneumoniae and administration of exogenous IL-6 to mast cell deficient mice was demonstrated improve survival [31]. We choose to focus our studies on the role of mast cell-derived IL-6 in lung Mp defense due to the aftermentioned studies. In our model, we hypothesized mast cells aid Mp clearance in an allergic environment by maintaining production of host defense cytokines, namely IL-6. We demonstrated after IL-4 pretreatment in BMCMCs, Mp infection resulted in slightly diminished TNF-α production and had no impact on IL-6 production. Our in vitro data suggested under allergic conditions mast cells maintain production of IL-6 in response to Mp infection; however, in vivo we demonstrated by adoptive transfer that IL-6-/- mast cells reduced lung Mp burden in W/Wv mice to levels comparable to +/+ mice and to W/Wv mice that had received wild type mast cells. Furthermore, we found W/Wv mice have significantly higher levels of IL-6 in the BAL fluid after infection, further indicating that mast cell-derived IL-6 is not essential for host defense. In our model, the increase in IL-6 levels in infected W/Wv mice is perhaps not surprising considering the ubiquitous nature of IL-6 and we propose increased cytokine production was due to increased Mp burden. We observed that compared to littermates, allergic and non-allergic W/Wv mice had a significantly higher burden of Mp (p < 0.05) 24 hours after infection. Other cell types, in particular epithelial cells and dendritic cells, have been demonstrated to release IL-6 in response to Mp [16, 32], which could account for the elevated levels of cytokines in W/Wv mice. Alternatively, a deficiency in secreted mast cell mediators could explain the increased cytokine levels in our study as mast cells have recently been recognized to function in an immunomodulatory context [33]. In one report, deletion of the mast cell cysteine protease dipeptidly peptidase I (DPPI) resulted in increased levels of IL-6 with concomitant protection against bacterial peritonitis, although not bacterial burden [34]. We would like to note in our study the results show that reconstitution using bone marrow-derived cultured mast cells is sufficient to improve lung Mp clearance in W/Wv mice, suggesting that although these cells have an immature phenotype in vitro [35-37] they are still able to carry out clearance functions in vivo.
We further examined the composition of neutrophils in the BAL fluid of allergic and infected W/Wv mice to determine if the mechanism of mast cell-mediated Mp clearance in the lungs was dependent on neutrophil influx. It has previously been demonstrated that W/Wv mice are more susceptible to bacterial infection in the peritoneal cavity due to a deficiency in TNF-α and subsequently impaired neutrophil influx [10]. However, in response to lung infection with M. pulmonis, neutrophils were demonstrated to be a primary source of histamine in mast cell deficient Wsh mice (an alternative to the W/Wv mouse), suggesting neutrophils compensate for the lack of mast cells in the lung [38]. Furthermore, we observed an increased number of neutrophils in the BAL fluid of W/Wv mice. It has further been reported that W/Wv mice have low blood neutrophil levels versus Wsh mice [39]; however, we confirmed allergic Wsh mice respond similarly to Mp infection as W/Wv mice (unpublished data). We concluded the increased Mp burden in the lungs of W/Wv mice was not likely due to a deficiency in neutrophil recruitment, although we cannot rule out impaired neutrophil function in the presence of airway inflammation. Our evidence suggests that the mechanism of mast cell-mediated host defense in the lungs is different from that in the peritoneal cavity. As mast cells have different phenotypes in different tissues [40, 41], it is logical that the mechanism of mast cell-mediated bacterial clearance would vary in different organs. Additional mast cell-derived mediators such as the leukotrienes LTB4 and LTC4, cathelicidins, and properdin, a positive regulator of complement activation, have been demonstrated to play a protective role in bacterial infection [42-44] and will serve as candidate molecules for our future investigations. A further possibility that we are also considering is the kinetics of TNF-α release. Since mast cells are unique in their ability to store TNF-α in preformed granules, the rate of release could aid immediate defense against bacterial infections and we are currently examining this angle using adoptive transfer of TNF-α-/- in mast cell deficient mice.
Based on published literature we might have expected mast cells to exacerbate Mp burden in an allergic host through induction of airway inflammation [20-22], but the key finding of our study is that under allergic conditions mast cells do not potentiate Mp burden but rather aid host defense. As a caveat to our findings, the induction of allergic inflammation was not mast cell-dependent in our model and hence mast cells may not have had the same response that would normally be observed in a mast cell-driven model of allergy. In our model we used the combination of aluminum hydroxide (alum) and OVA to sensitize mice, which Williams and Galli have previously shown induces airway hyperreactivity and inflammation through a mast cell-independent mechanism [19]. Furthermore, recent work from Kool et. al. demonstrated that the use of alum enhances adaptive and cellular immunity through a dendritic cell-driven process [45], implying the induction of allergic inflammation in this model relies less heavily on cells of the innate immune system (i.e. mast cells). As our aim was to determine the impact of mast cells on Mp burden under allergic conditions, and not the role of mast cells in the development of allergic inflammation, use of the alum-OVA model was valid in our studies. Nonetheless, as cytokine release from mast cells was shown to be enhanced in the presence of both IgE-allergen and TLR agonists [23, 24], the impact of mast cell activation on Mp burden may be different in a model where allergic inflammation is driven primarily by mast cells.
The role of mast cells in host defense in an allergic milieu has previously not been reported in the literature. Here we demonstrate that mast cells aid host defense even in an allergic context although not through an IL-6 dependent mechanism. Since airway Mp has been demonstrated in both acute asthma exacerbations as well as in chronic asthma, and the number of mast cells is increased in asthma, this emphasizes the necessity of understanding how mast cells function in host defense in an allergic milieu not only in an acute allergic model but more importantly in a model of chronic allergic inflammation.
Acknowledgments
Funding: This work was funded by NIH P01HL0703907
Abbreviations
- BAL
Bronchoalveolar lavage
- BMCMCs
Bone marrow-derived cultured mast cells
- Mp
Mycoplasma pneumoniae
- OVA
Ovalbumin
References
- 1.Biscardi S, Lorrot M, Marc E, Moulin F, Boutonnat-Faucher B, Heilbronner C, Iniguez JL, Chaussain M, Nicand E, Raymond J, Gendrel D. Mycoplasma pneumoniae and asthma in children. Clin Infect Dis. 2004;38:1341–1346. doi: 10.1086/392498. [DOI] [PubMed] [Google Scholar]
- 2.Cosentini R, Tarsia P, Canetta C, Graziadei G, Brambilla AM, Aliberti S, Pappalettera M, Tantardini F, Blasi F. Severe asthma exacerbation: role of acute Chlamydophila pneumoniae and Mycoplasma pneumoniae infection. Respir Res. 2008;9:48. doi: 10.1186/1465-9921-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hassan J, Irwin F, Dooley S, Connell J. Mycoplasma pneumoniae infection in a pediatric population: analysis of soluble immune markers as risk factors for asthma. Hum Immunol. 2008;69:851–855. doi: 10.1016/j.humimm.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Lieberman D, Printz S, Ben-Yaakov M, Lazarovich Z, Ohana B, Friedman MG, Dvoskin B, Leinonen M, Boldur I. Atypical pathogen infection in adults with acute exacerbation of bronchial asthma. Am J Respir Crit Care Med. 2003;167:406–410. doi: 10.1164/rccm.200209-996OC. [DOI] [PubMed] [Google Scholar]
- 5.Seggev JS, Lis I, Siman-Tov R, Gutman R, Abu-Samara H, Schey G, Naot Y. Mycoplasma pneumoniae is a frequent cause of exacerbation of bronchial asthma in adults. Ann Allergy. 1986;57:263–265. [PubMed] [Google Scholar]
- 6.Martin RJ, Kraft M, Chu HW, Berns EA, Cassell GH. A link between chronic asthma and chronic infection. J Allergy Clin Immunol. 2001;107:595–601. doi: 10.1067/mai.2001.113563. [DOI] [PubMed] [Google Scholar]
- 7.Brown JM, Wilson TM, Metcalfe DD. The mast cell and allergic diseases: role in pathogenesis and implications for therapy. Clin Exp Allergy. 2008;38:4–18. doi: 10.1111/j.1365-2222.2007.02886.x. [DOI] [PubMed] [Google Scholar]
- 8.Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454:445–454. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Echtenacher B, Mannel DN, Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381:75–77. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- 10.Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 11.Siebenhaar F, Syska W, Weller K, Magerl M, Zuberbier T, Metz M, Maurer M. Control of Pseudomonas aeruginosa skin infections in mice is mast cell-dependent. Am J Pathol. 2007;170:1910–1916. doi: 10.2353/ajpath.2007.060770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlos D, de Souza Junior DA, de Paula L, Jamur MC, Oliver C, Ramos SG, Silva CL, Faccioli LH. Mast cells modulate pulmonary acute inflammation and host defense in a murine model of tuberculosis. J Infect Dis. 2007;196:1361–1368. doi: 10.1086/521830. [DOI] [PubMed] [Google Scholar]
- 13.Xu X, Zhang D, Lyubynska N, Wolters PJ, Killeen NP, Baluk P, McDonald DM, Hawgood S, Caughey GH. Mast cells protect mice from Mycoplasma pneumonia. Am J Respir Crit Care Med. 2006;173:219–225. doi: 10.1164/rccm.200507-1034OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Supajatura V, Ushio H, Nakao A, Okumura K, Ra C, Ogawa H. Protective roles of mast cells against enterobacterial infection are mediated by Toll-like receptor 4. J Immunol. 2001;167:2250–2256. doi: 10.4049/jimmunol.167.4.2250. [DOI] [PubMed] [Google Scholar]
- 15.Edelson BT, Stricker TP, Li Z, Dickeson SK, Shepherd VL, Santoro SA, Zutter MM. Novel collectin/C1q receptor mediates mast cell activation and innate immunity. Blood. 2006;107:143–150. doi: 10.1182/blood-2005-06-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Q, Martin RJ, Lafasto S, Efaw BJ, Rino JG, Harbeck RJ, Chu HW. Toll-like receptor 2 down-regulation in established mouse allergic lungs contributes to decreased mycoplasma clearance. Am J Respir Crit Care Med. 2008;177:720–729. doi: 10.1164/rccm.200709-1387OC. [DOI] [PubMed] [Google Scholar]
- 17.Hoek KL, Cassell GH, Duffy LB, Atkinson TP. Mycoplasma pneumoniae-induced activation and cytokine production in rodent mast cells. J Allergy Clin Immunol. 2002;109:470–476. doi: 10.1067/mai.2002.121951. [DOI] [PubMed] [Google Scholar]
- 18.Beisswenger C, Kandler K, Hess C, Garn H, Felgentreff K, Wegmann M, Renz H, Vogelmeier C, Bals R. Allergic airway inflammation inhibits pulmonary antibacterial host defense. J Immunol. 2006;177:1833–1837. doi: 10.4049/jimmunol.177.3.1833. [DOI] [PubMed] [Google Scholar]
- 19.Williams CM, Galli SJ. Mast cells can amplify airway reactivity and features of chronic inflammation in an asthma model in mice. J Exp Med. 2000;192:455–462. doi: 10.1084/jem.192.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakae S, Ho LH, Yu M, Monteforte R, Iikura M, Suto H, Galli SJ. Mast cell-derived TNF contributes to airway hyperreactivity, inflammation, and TH2 cytokine production in an asthma model in mice. J Allergy Clin Immunol. 2007;120:48–55. doi: 10.1016/j.jaci.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 21.Reuter S, Heinz A, Sieren M, Wiewrodt R, Gelfand EW, Stassen M, Buhl R, Taube C. Mast cell-derived tumour necrosis factor is essential for allergic airway disease. Eur Respir J. 2008;31:773–782. doi: 10.1183/09031936.00058907. [DOI] [PubMed] [Google Scholar]
- 22.Nigo YI, Yamashita M, Hirahara K, Shinnakasu R, Inami M, Kimura M, Hasegawa A, Kohno Y, Nakayama T. Regulation of allergic airway inflammation through Toll-like receptor 4-mediated modification of mast cell function. Proc Natl Acad Sci U S A. 2006;103:2286–2291. doi: 10.1073/pnas.0510685103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fehrenbach K, Port F, Grochowy G, Kalis C, Bessler W, Galanos C, Krystal G, Freudenberg M, Huber M. Stimulation of mast cells via FcvarepsilonR1 and TLR2: the type of ligand determines the outcome. Mol Immunol. 2007;44:2087–2094. doi: 10.1016/j.molimm.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 24.Qiao H, Andrade MV, Lisboa FA, Morgan K, Beaven MA. FcepsilonR1 and toll-like receptors mediate synergistic signals to markedly augment production of inflammatory cytokines in murine mast cells. Blood. 2006;107:610–618. doi: 10.1182/blood-2005-06-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, Corry DB. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 27.Ryan JJ, DeSimone S, Klisch G, Shelburne C, McReynolds LJ, Han K, Kovacs R, Mirmonsef P, Huff TF. IL-4 inhibits mouse mast cell Fc epsilonRI expression through a STAT6-dependent mechanism. J Immunol. 1998;161:6915–6923. [PubMed] [Google Scholar]
- 28.Wolters PJ, Mallen-St Clair J, Lewis CC, Villalta SA, Baluk P, Erle DJ, Caughey GH. Tissue-selective mast cell reconstitution and differential lung gene expression in mast cell-deficient Kit(W-sh)/Kit(W-sh) sash mice. Clin Exp Allergy. 2005;35:82–88. doi: 10.1111/j.1365-2222.2005.02136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanzola MB, Robbie-Ryan M, Gutekunst CA, Brown MA. Mast cells exert effects outside the central nervous system to influence experimental allergic encephalomyelitis disease course. J Immunol. 2003;171:4385–4391. doi: 10.4049/jimmunol.171.8.4385. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki K, Nakajima H, Watanabe N, Kagami S, Suto A, Saito Y, Saito T, Iwamoto I. Role of common cytokine receptor gamma chain (gamma(c))- and Jak3-dependent signaling in the proliferation and survival of murine mast cells. Blood. 2000;96:2172–2180. [PubMed] [Google Scholar]
- 31.Sutherland RE, Olsen JS, McKinstry A, Villalta SA, Wolters PJ. Mast cell IL-6 improves survival from Klebsiella pneumonia and sepsis by enhancing neutrophil killing. J Immunol. 2008;181:5598–5605. doi: 10.4049/jimmunol.181.8.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang J, Hooper WC, Phillips DJ, Talkington DF. Regulation of proinflammatory cytokines in human lung epithelial cells infected with Mycoplasma pneumoniae. Infect Immun. 2002;70:3649–3655. doi: 10.1128/IAI.70.7.3649-3655.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol. 2008;8:478–486. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mallen-St Clair J, Pham CT, Villalta SA, Caughey GH, Wolters PJ. Mast cell dipeptidyl peptidase I mediates survival from sepsis. J Clin Invest. 2004;113:628–634. doi: 10.1172/JCI19062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galli SJ, Dvorak AM, Dvorak HF. Basophils and mast cells: morphologic insights into their biology, secretory patterns, and function. Prog Allergy. 1984;34:1–141. [PubMed] [Google Scholar]
- 36.Galli SJ, Dvorak AM, Marcum JA, Ishizaka T, Nabel G, Der Simonian H, Pyne K, Goldin JM, Rosenberg RD, Cantor H, Dvorak HF. Mast cell clones: a model for the analysis of cellular maturation. J Cell Biol. 1982;95:435–444. doi: 10.1083/jcb.95.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nabel G, Galli SJ, Dvorak AM, Dvorak HF, Cantor H. Inducer T lymphocytes synthesize a factor that stimulates proliferation of cloned mast cells. Nature. 1981;291:332–334. doi: 10.1038/291332a0. [DOI] [PubMed] [Google Scholar]
- 38.Xu X, Zhang D, Zhang H, Wolters PJ, Killeen NP, Sullivan BM, Locksley RM, Lowell CA, Caughey GH. Neutrophil histamine contributes to inflammation in mycoplasma pneumonia. J Exp Med. 2006;203:2907–2917. doi: 10.1084/jem.20061232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou JS, Xing W, Friend DS, Austen KF, Katz HR. Mast cell deficiency in Kit(W-sh) mice does not impair antibody-mediated arthritis. J Exp Med. 2007;204:2797–2802. doi: 10.1084/jem.20071391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kitamura Y. Heterogeneity of mast cells and phenotypic change between subpopulations. Annu Rev Immunol. 1989;7:59–76. doi: 10.1146/annurev.iy.07.040189.000423. [DOI] [PubMed] [Google Scholar]
- 41.Welle M. Development, significance, and heterogeneity of mast cells with particular regard to the mast cell-specific proteases chymase and tryptase. J Leukoc Biol. 1997;61:233–245. doi: 10.1002/jlb.61.3.233. [DOI] [PubMed] [Google Scholar]
- 42.Malaviya R, Abraham SN. Role of mast cell leukotrienes in neutrophil recruitment and bacterial clearance in infectious peritonitis. J Leukoc Biol. 2000;67:841–846. doi: 10.1002/jlb.67.6.841. [DOI] [PubMed] [Google Scholar]
- 43.Di Nardo A, Vitiello A, Gallo RL. Cutting edge: mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J Immunol. 2003;170:2274–2278. doi: 10.4049/jimmunol.170.5.2274. [DOI] [PubMed] [Google Scholar]
- 44.Stover CM, Luckett JC, Echtenacher B, Dupont A, Figgitt SE, Brown J, Mannel DN, Schwaeble WJ. Properdin plays a protective role in polymicrobial septic peritonitis. J Immunol. 2008;180:3313–3318. doi: 10.4049/jimmunol.180.5.3313. [DOI] [PubMed] [Google Scholar]
- 45.Kool M, Soullie T, van Nimwegen M, Willart MA, Muskens F, Jung S, Hoogsteden HC, Hammad H, Lambrecht BN. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008;205:869–882. doi: 10.1084/jem.20071087. [DOI] [PMC free article] [PubMed] [Google Scholar]