Abstract
Equatorial Africa has among the highest incidences of Kaposi’s sarcoma (KS) in the world, thus earning the name “KS Belt.” This was the case even prior to the HIV . To date, there is no clear evidence that HHV-8 seroprevalence is higher in this region, but interpretation of the available literature is tempered by differences in serologic assays used across studies. We examined representatively sampled ambulatory adults in Uganda, which is in the “KS Belt”, and in Zimbabwe and South Africa which are outside the Belt, for HHV-8 antibodies. All serologic assays were uniformly performed in the same reference laboratory by the same personnel. In the base-case serologic algorithm, seropositivity was defined by reactivity in an immunofluorescence assay or in two enzyme immunoassays. A total of 2375 participants were examined. In Uganda, HHV-8 seroprevalence was high early in adulthood (35.5% by age 21) without significant change thereafter. In contrast, HHV-8 seroprevalence early in adulthood was lower in Zimbabwe and South Africa (13.7% and 10.8%, respectively), but increased with age. After age adjustment, Ugandans had 3.24-fold greater odds of being HHV-8-infected than South Africans (p<0.001) and 2.22-fold greater odds than Zimbabweans (p<0.001). Inferences were unchanged using all other serologic algorithms evaluated. In conclusion, HHV-8 infection is substantially more common in Uganda than in Zimbabwe and South Africa. These findings help explain the high KS incidence in the “KS Belt” and underscore the importance of a uniform approach to HHV-8 antibody testing.
Even prior to the HIV epidemic, the incidence of Kaposi’s sarcoma (KS) in equatorial Africa was among the highest in the world. In portions of Uganda, Tanzania, and what is now known as the Democratic Republic of Congo, the lifetime incidence of KS approached 16 per 1000,1 thus earning the region the name “KS Belt” (Figure 1). Although KS case reporting is imperfect in Africa, it is clear that non-HIV-related KS incidence was about 3 to 10 times higher in this region as compared to countries further north and south on the continent.1 While several theories have been offered (e.g., regional volcanic salts2), none has been proven to explain this substantial difference in KS incidence over such a relatively small geographic area.
Figure 1.
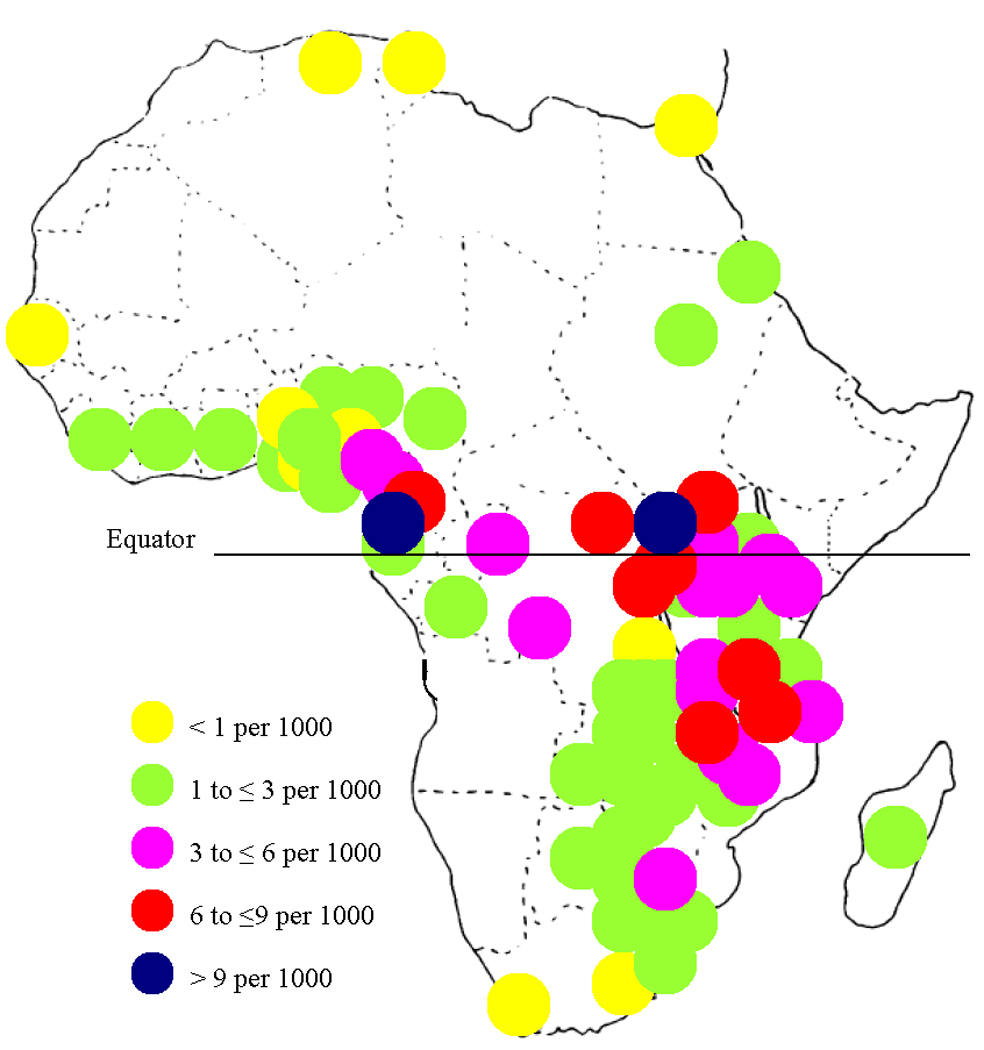
Cumulative incidence from birth to age 64 years of endemic (non-HIV-related) Kaposi’ sarcoma among men in Africa. Estimates are from Cook-Mozaffari et al. (1).
With the discovery that human herpesvirus 8 (HHV-8, also known as Kaposi’s sarcoma-associated herpesvirus) is the viral etiologic agent of KS,3–8 it was naturally hypothesized that regional differences in HHV-8 seroprevalence in Africa would explain differences in KS incidence, similar to how high HHV-8 seroprevalence in homosexual men in the U.S.9–11 and Europe12–14 was quickly determined to explain their high KS incidence.15 However, despite many studies of HHV-8 seroprevalence in Africa, clear regional differences have not been established, resulting in the current view by many that the prevalence of HHV-8 is uniform throughout Africa.16–18 However, a closer inspection of work to date reveals that estimates of HHV-8 seroprevalence from different African regions are difficult to compare. In addition to demographic differences in the subjects tested, differences in serologic testing importantly often preclude comparisons of studies across regions. This is because there is no commercially standardized HHV-8 antibody assay, a variety of different antigens are being targeted and serologic platforms being used across studies, and there is proven assay discordance between assays.19
To address whether underlying regional differences in HHV-8 seroprevalence may in part explain differences in KS incidence, we compared HHV-8 seroprevalence in three countries with different pre-AIDS epidemic incidences of KS—Uganda, with high KS incidence, versus Zimbabwe and South Africa with lower KS incidence. Importantly, to preclude differences in serologic techniques, we tested all samples with assays for HHV-8 antibodies performed in the same laboratory.
Methods
Participants
Participants were sampled in three cross-sectional studies of primarily young adults in Uganda, Zimbabwe, and South Africa. In Uganda, subjects were consecutive blood donors, age 17 years or older, at the Nakasero Blood Bank in 2000 to 2001 who resided in Kampala and surrounding periurban and rural areas. Blood donation was voluntary and without monetary incentive. All blood donors in this analysis had been screened and found by the blood bank to be seronegative for HIV, hepatitis B virus, and Treponema pallidum infections. A portion of these subjects have been previously described 20. In Zimbabwe, as part of a more intensive study of HHV-8 seroepidemiology and virologic shedding, consecutive women age 17 years or older seeking family planning services at four clinics (Harare Hospital, Spilhaus Clinic, Chitungwiza Clinic, and Epworth Clinic) in Harare and surrounding periurban areas were recruited from 2001 to 2004. Participants were selected based on their HIV infection status in a 2:1 infected to uninfected ratio. In South Africa, as part of a larger study of horizontal transmission of HHV-8 to children, primary female caregivers of young children were selected from a community-based household sample conducted in 2003 in Cato Manor, an urban settlement in Durban, and, KwaXimba, a rural area outside Durban. All subjects provided their informed consent.
Measurements
Demographic characteristics
Age and gender were collected by questionnaire at all three sites.
Laboratory testing
All blood samples were tested for antibodies to KSHV with two enzyme immunoassays (EIAs) and one indirect immunofluorescence assay (IFA), each previously described and performed by the same personnel at the Centers for Disease Control and Prevention (CDC). The two EIAs target antibodies to open reading frame (ORF) K8.1 and ORF 65 respectively, using synthetic peptides as antigen substrates.21, 22The IFA uses KSHV-infected BCBL-1 cells as antigen substrate, in which KSHV is induced to its replicative phase23; specimens were evaluated at a dilution of 1:80. In the base-case algorithm, specimens reactive in any two tests, or the IFA alone were classified as KSHV-antibody positive. Specimens that were non-reactive in all tests or reactive in only one EIA were categorized as KSHV-antibody negative; all other patterns were considered equivocal and excluded from analysis. This serologic algorithm has an estimated specificity of 97.5%, as evidenced by testing blood donors,24 and sensitivity of 96.3% based on testing patients with KS.25 HIV antibody testing performed in Uganda used an EIA (MUREX HIV-1.2.O; Murex Biotech, Kent, United Kingdom), in Zimbabwe used two EIAs (Uni-Gold, Trinity Biotech, Bray, Ireland; and Determine HIV-1/2, Abbot Diagnostics, Abbott Park, IL) followed by a Western Blot for positive confirmation, and in South African samples used two EIAs (Vironostika HIV-1 Microelisa, bioMérieux, Durham, N.C.; and Genetic Systems rLAV EIA, Bio-Rad, Redmond, WA).
Statistical Analysis
The primary analysis compared HHV-8 seroprevalence, determined by the base-case serologic algorithm, in Uganda, Zimbabwe, and South Africa. Multivariable logistic regression was performed to adjust for confounding and to assess for potential effect modification by age. As sensitivity analyses, we varied the serologic definition of HHV-8 seropositivity to include various permutations of single assays evaluated individually or in combination. All analyses were carried out using Stata version 9.1 (College Station, TX).
Results
A total of 2375 participants were examined, 482 in South Africa, 539 in Zimbabwe, and 1354 in Uganda. Overall, 42 (1.8%) of individuals were classified as equivocal in HHV-8 antibody testing and not considered in the primary analysis, leaving 471 participants in South Africa, 532 in Zimbabwe, and 1330 in Uganda. Of those in the primary analysis, the median age was 28 years (interquartile range [IQR] 21 to 37) in South Africa, 29 years (IQR 25 to 33) in Zimbabwe, and 22 years (IQR 19 to 28) in Uganda. In Uganda, 21.4% of participants were women, and by design, all participants in South Africa and Zimbabwe were women. Prevalence of HIV infection among South African participants was 38.2%, while, by design, 63.7% of Zimbabwe participants and no Uganda participants were HIV-infected.
Crude HHV-8 seroprevalence was 37.3% in Uganda, 20.3% in Zimbabwe, and 17.6% in South Africa. This equates to, unadjusted for age, Ugandans having 2.33-fold greater odds (95% confidence interval [CI] 1.83 to 2.95, p < 0.001) of being HHV-8-infected than Zimbabweans and a 2.77-fold greater odds (95% CI 2.13 to 3.60, p < 0.001) than South Africans. The high seroprevalence of HHV-8 infection in Uganda was apparent early in adulthood, with 35.5% of participants infected before age 21 years (Table 1). The higher seroprevalence in Uganda did not substantially change throughout adulthood, only rising to 43.4% in those 41 years or older (p = 0.30 for age trend). In contrast, HHV-8 seroprevalence was substantially lower amongst the youngest participants in Zimbabwe and South Africa (13.7% and 10.8%, respectively, in those 25 years and younger), but then increased significantly with age in both Zimbabwe (p = 0.023) and South Africa (p < 0.001). Gender differences were evaluable only in Uganda, where infection was more common among men (38.6%) than among women (32.0%, p = 0.085). In both South Africa and Zimbabwe, HHV-8 infection appeared to be unrelated to HIV infection status (p = 0.44 and p = 0.40, respectively).
Table 1.
Factors associated with HHV-8 infection in South Africa, Zimbabwe, and Uganda.
| No. tested |
No. (%) HHV-8 antibody-positive |
Adjusted odds ratio (95% CI)* |
p value | |
|---|---|---|---|---|
| South Africa | ||||
| Age, years | 1.04 (1.02 – 1.06)† | <0.001 | ||
| 14 – 20 | 106 | 13 (12.3) | ||
| 21 – 25 | 89 | 8 (9.0) | ||
| 26 – 30 | 81 | 15 (18.5) | ||
| 31 – 35 | 62 | 11 (17.7) | ||
| 36 – 40 | 43 | 9 (20.9) | ||
| > 40 | 90 | 27 (30.0) | ||
| HIV infection status | ||||
| Uninfected | 291 | 53 (18.2) | reference | |
| Infected | 180 | 30 (16.7) | 1.23 (0.73 – 2.09) | 0.44 |
| Zimbabwe | ||||
| Age | 1.04 (1.004 – 1.08)† | 0.026 | ||
| 14 – 20 | 20 | 1 (5.0) | ||
| 21 – 25 | 133 | 20 (15.0) | ||
| 26 – 30 | 172 | 37 (21.5) | ||
| 31 – 35 | 116 | 28 (24.1) | ||
| 36 – 40 | 52 | 12 (23.1) | ||
| > 40 | 39 | 10 (25.6) | ||
| HIV infection status | ||||
| Uninfected | 193 | 40 (20.7) | reference | |
| Infected | 339 | 68 (20.1) | 0.82 (0.51– 1.30) | 0.40 |
| Uganda | ||||
| Age | 1.01 (0.99 – 1.02)† | 0.27 | ||
| 14 – 20 | 513 | 182 (35.5) | ||
| 21 – 25 | 367 | 132 (38.7) | ||
| 26 – 30 | 195 | 73 (37.4) | ||
| 31 – 35 | 118 | 41 (34.8) | ||
| 36 – 40 | 54 | 21 (38.9) | ||
| > 40 | 83 | 36 (43.4) | ||
| Sex | ||||
| Female | 284 | 91 (32.0) | reference | |
| Male | 1046 | 404 (38.6) | 1.29 (0.97 – 1.72) | 0.081 |
Adjusted for age and other factor, either HIV infection (South Africa and Zimbabwe) or gender (Uganda)
Assessed as a continuous variable, per one year increase in age
To formally compare HHV-8 seroprevalence across countries, we restricted the analysis to only the 1287 women—all of the participants from South Africa and Zimbabwe and the 284 women from Uganda—given the slightly higher seroprevalence among men in Uganda. In a multivariable logistic regression analysis adjusting for age, there was no strong evidence for a difference in HHV-8 seroprevalence in Zimbabwe versus South Africa (odds ratio [OR] = 1.32, 95% CI 0.95 to 1.83; p = 0.095). In contrast, Ugandans had a 3.24-fold greater odds (95% CI 2.19 to 4.81; p < 0.001) of being HHV-8-infected than South Africans and a 2.22-fold greater odds (95% CI 1.49 to 3.30; p < 0.001) than Zimbabweans. Because there was no significant difference between South Africa and Zimbabwe, we combined participants from these two countries and found that Ugandans had a 2.72-fold greater odds (95% CI 1.96 to 3.79; p < 0.001) of being HHV-8-infected than the combination of South Africans and Zimbabweans (Table 2). When we further restricted the analysis to just those participants who were HIV-uninfected, the inferences were unchanged. Among HIV-uninfected individuals, Ugandans had a 3.47-fold greater odds (95% CI 2.14 to 5.62; p < 0.001) of being HHV-8-infected than South Africans and a 2.06-fold greater odds (95% CI 1.27 to 3.33; p = 0.004) than Zimbabweans. Finally, we used the multivariable model to determine whether the relationship between age and HHV-8 infection differed by country, as suggested by the within-in country analyses above. We found no evidence that the effect of age on HHV-8 seroprevalence differed in Zimbabwe vs. South Africa (p = 0.89 for interaction term). While we did find suggestive evidence that the effect of age differed in Uganda versus the combined population of South Africa and Zimbabwe, we cannot exclude that this is chance occurrence (p = 0.13 for interaction term).
Table 2.
Comparison of HHV-8 seroprevalence in women in South Africa, Zimbabwe, and Uganda using different antibody interpretation algorithms.
| Age-Adjusted Odds Ratios (95% CI) | ||||
|---|---|---|---|---|
| Algorithm | Zimbabwe vs. South Africa |
Uganda vs. South Africa |
Uganda vs Zimbabwe |
Uganda vs. Pooling of South Africa and Zimbabwe |
| Base-case* | 1.32 (0.95 – 1.83) | 3.24 (2.19 – 4.81) | 2.22 (1.49 – 3.30) | 2.72 (1.96 – 3.79) |
| Es defined reactive† | 1.25 (0.91 – 1.71) | 2.98 (2.04 – 4.36) | 2.15 (1.46 – 3.17) | 2.60 (1.88 – 3.59) |
| Es defined non-reactive‡ | 1.34 (0.97 – 1.85) | 3.25 (2.19 – 4.81) | 2.20 (1.48 – 3.25) | 2.71 (1.95 – 3.76) |
| IFA alone§ | 1.22 (0.88 – 1.68) | 2.93 (1.98 – 4.33) | 2.13 (1.43 – 3.18) | 2.60 (1.87 – 3.63) |
| K8.1 EIA alone§ | 0.92 (0.65 – 1.31) | 2.43 (1.61 – 3.68) | 2.58 (1.65 – 4.03) | 2.55 (1.78 – 3.67) |
| ORF65 EIA alone§ | 1.23 (0.76 – 1.99) | 5.44 (3.16 – 9.37) | 3.43 (2.02 – 5.80) | 4.76 (3.07 – 7.37) |
| Both EIA reactive‖ | 1.07 (0.62 – 1.85) | 3.44 (1.82 – 6.50) | 2.42 (1.27 – 4.60) | 3.30 (1.94 – 5.61) |
| One EIA reactive¶ | 0.99 (0.71 – 1.38) | 3.24 (2.20 – 4.79) | 3.20 (2.11 – 4.84) | 3.26 (2.33 – 4.55) |
| All three assays reactive** | 1.09 (0.63 – 1.89) | 2.79 (1.46 – 5.33) | 1.92 (1.00 – 3.70) | 2.64 (1.53 – 4.55) |
| Any assay reactive†† | 1.19 (0.88 – 1.60) | 3.13 (2.19 – 4.48) | 2.63 (1.81 – 3.82) | 2.82 (2.08 – 3.84) |
Seropositivity defined as either reactive on IFA or on both EIA assays; equivocal results discarded
As for base-case, with equivocal results coded as reactive
As for base-case, with equivocal results coded as non-reactive
Seropositivity defined as reactive on the assay listed, without regard to other assays; equivocal results discarded in case of IFA
Seropositivity defined as reactive on both the K8.1 EIA and the ORF65 EIA
Seropositivity defined as reactive on either the K8.1 EIA or the ORF65 EIA
Seropositivity defined as reactive on IFA, K8.1 EIA, and ORF65 EIA; equivocal results discarded
Seropositivity defined as reactive on either IFA, K8.1 EIA, or ORF65 EIA; equivocal results discarded
To determine if our finding of a significant difference in HHV-8 seroprevalence in Uganda versus either Zimbabwe or South Africa was dependent on the base-case algorithm we used to determine seropositivity, we also evaluated a number of other algorithms by which to interpret our antibody assays. We found that the principal inferences—no difference between South Africa and Zimbabwe but substantially higher seroprevalence in Uganda than in either Zimbabwe or South Africa—were unchanged regardless of the algorithm used (Table 2). In the most strict algorithm, where reactivity in all three antibody assays is required to determine overall seropositivity, the unadjusted country-specific estimates of HHV-8 seroprevalence were predictably the lowest (South Africa: 6.8%; Zimbabwe: 5.6%; Uganda: 8.2%), but nonetheless after age-adjustment, Ugandans again had a 2.64-fold greater odds of being HHV-8-infected than the South African and Zimbabweans. The least strict algorithm, where reactivity in any one of the three assays was deemed seropositive, produced the highest country-specific prevalence estimates (South Africa: 23.1%; Zimbabwe: 24.7%; Uganda: 47.1%), with Ugandans again having significantly greater seroprevalence. We also evaluated algorithms where we considered all the equivocal results as either reactive or non-reactive, and again the principal findings were unchanged.
Discussion
The “KS Belt” in equatorial Africa has long been investigated to determine the reasons why KS is so common in the region. Prior work addressing whether underlying HHV-8 infection is more common in this region as compared to other parts of Africa has produced inconsistent results and has often been interpreted as the entire continent being monolithically HHV-8-infected.18 We believe that the inability of prior studies to establish regional differences in HHV-8 seroprevalence in Africa is partially explained by differences in the antibody assays used by these studies. Using the same assay, performed in the same laboratory by the same personnel, we here show the odds of HHV-8-seropositivity to be as much as three-fold higher in the “KS Belt” (Uganda) than in areas of Africa outside from the Belt (Zimbabwe and South Africa).
Although the available literature on HHV-8 seroprevalence in Africa nominally gives the impression of no substantial differences between regions, a close dissection of studies that, in part, use identical, or very similar, antibody assays substantiates our conclusions. Specifically, two studies of adults from South Africa26, 27 and one from Uganda28 each include an IFA targeting antibodies to the latency-associated nuclear antigen (LANA) of HHV-8. When comparing only serologic results from the LANA-specific IFA, HHV-8 seroprevalence in Uganda was found to be 48%28 compared to 14%26 and 16%27 in South Africa. These estimates are very similar to our findings. Furthermore, separate reports from Tanzania (within the “KS Belt”) and Nigeria (outside the Belt), both using the same K8.1-based EIA, found HHV-8 seroprevalence to be substantially higher in Tanzania.29, 30 In the only other report we are aware of that used the same antibody assay in two different populations representing within and outside the “KS Belt”, there was higher HHV-8 seroprevalence in the Democratic Republic of Congo compared to Botswana when using an ORF65 EIA, but not when using the LANA IFA or K8.1 EIA.31 The very high seroprevalence of HHV-8 found in this report in Botswana (>75% by K8.1 EIA or LANA IFA) has not been replicated in other parts of the country and may possibly reflect the isolated nature of the population studied.
Not only is the overall level of HHV-8-seropositivity higher in Uganda than in Zimbabwe and South Africa but the patterns of HHV-8 infection for these three countries also appear different. In Uganda, high levels of infection are already apparent by early adulthood, with only minimal increases thereafter, suggesting scant primary infection during adulthood. In South Africa and Zimbabwe, in contrast, prevalence was low in the youngest adults, and increased steadily throughout adulthood, suggesting ongoing transmission. This finding that most of the HHV-8 transmission in Uganda has occurred prior to adulthood while most transmission in South Africa occurs after adulthood is supported by recent direct work in children, which found that HHV-8 seroprevalence among Ugandan children was much higher than among South African children.32
In addition to geographic differences in HHV-8 seroprevalence, we found evidence of differences by gender in the one region where this was evaluable. In Uganda, men had a trend towards higher seroprevalence than women, which is consistent with some,29 but not all,28 prior reports from this region. This is notable because endemic KS, the form of KS that existed in Africa prior to the spread of HIV, was about 10 times more common among males than females.1 However, the magnitude of the difference we found in HHV-8 seroprevalence between men and women, if true, would explain only a small portion of the difference observed for KS. We did not find any difference in HHV-8 seroprevalence by HIV infection status in Zimbabwe or South Africa, which is compatible with some reports,26, 28, 33–37but not others.30, 38–41 This inconsistency in the relationship between HIV and HHV-8 in Africa across studies has defied explanation to date.
A potential limitation of this study is that the participants were not sampled in identical ways in the three countries. In particular, the Ugandan sample is from blood donors who were pre-screened for three sexually transmitted diseases. If HHV-8 is sexually transmitted in Africa among adults, as been suggested in some reports,30, 42, 43 then our analysis underestimates the true prevalence of HHV-8 infection among Ugandans and underestimates the difference between Uganda and Zimbabwe and South Africa. Similarly, it could be argued that the inclusion in South Africa of only women who were caregivers to a young child might have selected for those were naturally exposed to more infections. However, if this were the case, then our sample overestimates the true prevalence of HHV-8 in South Africa and our analysis would again underestimate the difference between Uganda and South Africa. A more significant limitation is that we could only formally compare HHV-8 seroprevalence across countries in women, and it is not known if these same results will hold true for men.
In conclusion, the use of a standardized approach to antibody testing, some of the strongest evidence that HHV-8 seroprevalence is substantially higher in the “KS Belt” than regions south of this Belt. We hypothesize that this difference in underlying HHV-8 seroprevalence in part explains the higher rate of endemic KS within the Belt. The question remains as to why these regional differences in HHV-8 seroprevalence exist. Possible explanations include differences in viral strain infectiousness, host susceptibility to infection, environmental factors, and behavioral practices. That HHV-8 infection, unlike many other herpesvirus infections, does not appear to be ubiquitous in any of the African regions studied should provide opportunities to study HHV-8 transmission dynamics that may ultimately be exploitable for the development of effective prevention strategies.
Acknowledgments
Financial Support: Supported by the National Institutes of Health (U01 CA078124, R01 CA119903, P30 MH062246, D43 TW000003, R03 TW006054, P01 DE007946, K01 HD052020 and P30 AI027763), the AIDS Malignancy Consortium (U01 CA071375), the University of California Universitywide AIDS Research Program (CC99-SF-001), the South Africa National Research Foundation (Thuthuka Programme Grant 2054349), and the AIDS Care Research in Africa Program.
Footnotes
Potential conflicts of interest. All authors: no conflicts.
Presented In Part: Oral Presentation at the Seventh International Workshop on Kaposi’s Sarcoma Associated Herpesvirus and Related Agents, Santa Cruz, CA, 2004.
References
- 1.Cook-Mozaffari P, Newton R, Beral V, Burkitt DP. The geographical distribution of Kaposi's sarcoma and of lymphomas in Africa before the AIDS epidemic. Br J Cancer. 1998;78:1521–1528. doi: 10.1038/bjc.1998.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ziegler JL. Endemic Kaposi's sarcoma in Africa and local volcanic soils. Lancet. 1993;342:1348–1351. doi: 10.1016/0140-6736(93)92252-o. [DOI] [PubMed] [Google Scholar]
- 3.Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 4.Whitby D, Howard MR, Tenant-Flowers M, Brink NS, Copas A, Boshoff C, Hatzioannou T, Suggett FE, Aldam DM, Denton AS, et al. Detection of Kaposi sarcoma associated herpesvirus in peripheral blood of HIV-infected individuals and progression to Kaposi's sarcoma. Lancet. 1995;346:799–802. doi: 10.1016/s0140-6736(95)91619-9. [DOI] [PubMed] [Google Scholar]
- 5.Gao SJ, Kingsley L, Hoover DR, Spira TJ, Rinaldo CR, Saah A, Phair J, Detels R, Parry P, Chang Y, Moore PS. Seroconversion to antibodies against Kaposi's sarcoma-associated herpesvirus-related latent nuclear antigens before the development of Kaposi's sarcoma. N Engl J Med. 1996;335:233–241. doi: 10.1056/NEJM199607253350403. [DOI] [PubMed] [Google Scholar]
- 6.Martin JN, Ganem DE, Osmond DH, Page-Shafer KA, Macrae D, Kedes DH. Sexual transmission and the natural history of human herpesvirus 8 infection. N Engl J Med. 1998;338:948–954. doi: 10.1056/NEJM199804023381403. [DOI] [PubMed] [Google Scholar]
- 7.Renwick N, Halaby T, Weverling GJ, Dukers NH, Simpson GR, Coutinho RA, Lange JM, Schulz TF, Goudsmit J. Seroconversion for human herpesvirus 8 during HIV infection is highly predictive of Kaposi's sarcoma. Aids. 1998;12:2481–2488. doi: 10.1097/00002030-199818000-00018. [DOI] [PubMed] [Google Scholar]
- 8.O'Brien TR, Kedes D, Ganem D, Macrae DR, Rosenberg PS, Molden J, Goedert JJ. Evidence for concurrent epidemics of human herpesvirus 8 and human immunodeficiency virus type 1 in US homosexual men: rates, risk factors, and relationship to Kaposi's sarcoma. J Infect Dis. 1999;180:1010–1017. doi: 10.1086/315039. [DOI] [PubMed] [Google Scholar]
- 9.Martin JN, Ganem DE, Osmond DH, Page-Shafer K, Macrae D, Kedes DH. Sexual transmission and the natural history of human herpesvirus 8 infection. N Engl J Med. 1998;338:948–954. doi: 10.1056/NEJM199804023381403. [DOI] [PubMed] [Google Scholar]
- 10.Kedes DH, Operskalski E, Busch M, Kohn R, Flood J, Ganem D. The seroepidemiology of human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus): distribution of infection in KS risk groups and evidence for sexual transmission. Nat Med. 1996;2:918–924. doi: 10.1038/nm0896-918. [DOI] [PubMed] [Google Scholar]
- 11.Engels EA, Atkinson JO, Graubard BI, McQuillan GM, Gamache C, Mbisa G, Cohn S, Whitby D, Goedert JJ. Risk factors for human herpesvirus 8 infection among adults in the United States and evidence for sexual transmission. J Infect Dis. 2007;196:199–207. doi: 10.1086/518791. [DOI] [PubMed] [Google Scholar]
- 12.Dukers NH, Renwick N, Prins M, Geskus RB, Schulz TF, Weverling GJ, Coutinho RA, Goudsmit J. Risk factors for human herpesvirus 8 seropositivity and seroconversion in a cohort of homosexual men. Am J Epidemiol. 2000;151:213–224. doi: 10.1093/oxfordjournals.aje.a010195. [DOI] [PubMed] [Google Scholar]
- 13.Regamey N, Cathomas G, Schwager M, Wernli M, Harr T, Erb P. High human herpesvirus 8 seroprevalence in the homosexual population in Switzerland. J Clin Microbiol. 1998;36:1784–1786. doi: 10.1128/jcm.36.6.1784-1786.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melbye M, Cook PM, Hjalgrim H, Begtrup K, Simpson GR, Biggar RJ, Ebbesen P, Schulz TF. Risk factors for Kaposi's-sarcoma-associated herpesvirus (KSHV/HHV-8) seropositivity in a cohort of homosexual men, 1981–1996. Int J Cancer. 1998;77:543–548. doi: 10.1002/(sici)1097-0215(19980812)77:4<543::aid-ijc12>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 15.Beral V, Peterman TA, Berkelman RL, Jaffe HW. Kaposi's sarcoma among persons with AIDS: a sexually transmitted infection? Lancet. 1990;335:123–128. doi: 10.1016/0140-6736(90)90001-l. [DOI] [PubMed] [Google Scholar]
- 16.Sissolak G, Mayaud P. AIDS-related Kaposi's sarcoma: epidemiological, diagnostic, treatment and control aspects in sub-Saharan Africa. Trop Med Int Health. 2005;10:981–992. doi: 10.1111/j.1365-3156.2005.01491.x. [DOI] [PubMed] [Google Scholar]
- 17.Ablashi DV, Chatlynne LG, Whitman JE, Jr, Cesarman E. Spectrum of Kaposi's sarcoma-associated herpesvirus, or human herpesvirus 8, diseases. Clin Microbiol Rev. 2002;15:439–464. doi: 10.1128/CMR.15.3.439-464.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dedicoat M, Newton R. Review of the distribution of Kaposi's sarcoma-associated herpesvirus (KSHV) in Africa in relation to the incidence of Kaposi's sarcoma. Br J Cancer. 2003;88:1–3. doi: 10.1038/sj.bjc.6600745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enbom M, Sheldon J, Lennette E, Schulz T, Ablashi DV, Neipel F, Biberfeld P, Carlberg H, Ljungman P, Nilsson A, Soderstrom T, Wadstrom J, et al. Antibodies to human herpesvirus 8 latent and lytic antigens in blood donors and potential high-risk groups in Sweden: variable frequencies found in a multicenter serological study. J Med Virol. 2000;62:498–504. [PubMed] [Google Scholar]
- 20.Hladik W, Dollard SC, Mermin J, Fowlkes AL, Downing R, Amin MM, Banage F, Nzaro E, Kataaha P, Dondero TJ, Pellett PE, Lackritz EM. Transmission of human herpesvirus 8 by blood transfusion. N Engl J Med. 2006;355:1331–1338. doi: 10.1056/NEJMoa055009. [DOI] [PubMed] [Google Scholar]
- 21.Pau CP, Lam LL, Spira TJ, Black JB, Stewart JA, Pellett PE, Respess RA. Mapping and serodiagnostic application of a dominant epitope within the human herpesvirus 8 ORF 65-encoded protein. J Clin Microbiol. 1998;36:1574–1577. doi: 10.1128/jcm.36.6.1574-1577.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spira TJ, Lam L, Dollard SC, Meng YX, Pau CP, Black JB, Burns D, Cooper B, Hamid M, Huong J, Kite-Powell K, Pellett PE. Comparison of serologic assays and PCR for diagnosis of human herpesvirus 8 infection. J Clin Microbiol. 2000;38:2174–2180. doi: 10.1128/jcm.38.6.2174-2180.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dollard SC, Nelson KE, Ness PM, Stambolis V, Kuehnert MJ, Pellett PE, Cannon MJ. Possible transmission of human herpesvirus-8 by blood transfusion in a historical United States cohort. Transfusion. 2005;45:500–503. doi: 10.1111/j.0041-1132.2005.04334.x. [DOI] [PubMed] [Google Scholar]
- 24.Pellett PE, Wright DJ, Engels EA, Ablashi DV, Dollard SC, Forghani B, Glynn SA, Goedert JJ, Jenkins FJ, Lee TH, Neipel F, Todd DS, et al. Multicenter comparison of serologic assays and estimation of human herpesvirus 8 seroprevalence among US blood donors. Transfusion. 2003;43:1260–1268. doi: 10.1046/j.1537-2995.2003.00490.x. [DOI] [PubMed] [Google Scholar]
- 25.Cannon MJ, Operskalski EA, Mosley JW, Radford K, Dollard SC. Lack of evidence for human herpesvirus-8 transmission via blood transfusion in a historical US cohort. Journal of Infectious Diseases. 2009;199:1592–1598. doi: 10.1086/598859. [DOI] [PubMed] [Google Scholar]
- 26.Dedicoat M, Newton R, Alkharsah KR, Sheldon J, Szabados I, Ndlovu B, Page T, Casabonne D, Gilks CF, Cassol SA, Whitby D, Schulz TF. Mother-to-child transmission of human herpesvirus-8 in South Africa. J Infect Dis. 2004;190:1068–1075. doi: 10.1086/423326. [DOI] [PubMed] [Google Scholar]
- 27.Bourboulia D, Whitby D, Boshoff C, Newton R, Beral V, Carrara H, Lane A, Sitas F. Serologic evidence for mother-to-child transmission of Kaposi sarcoma-associated herpesvirus infection. JAMA. 1998;280:31–32. doi: 10.1001/jama.280.1.31-a. [DOI] [PubMed] [Google Scholar]
- 28.Newton R, Ziegler J, Bourboulia D, Casabonne D, Beral V, Mbidde E, Carpenter L, Reeves G, Parkin DM, Wabinga H, Mbulaiteye S, Jaffe H, et al. The sero-epidemiology of Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) in adults with cancer in Uganda. Int J Cancer. 2003;103:226–232. doi: 10.1002/ijc.10817. [DOI] [PubMed] [Google Scholar]
- 29.Mbulaiteye SM, Pfeiffer RM, Whitby D, Brubaker GR, Shao J, Biggar RJ. Human herpesvirus 8 infection within families in rural Tanzania. J Infect Dis. 2003;187:1780–1785. doi: 10.1086/374973. [DOI] [PubMed] [Google Scholar]
- 30.Eltom MA, Mbulaiteye SM, Dada AJ, Whitby D, Biggar RJ. Transmission of human herpesvirus 8 by sexual activity among adults in Lagos, Nigeria. Aids. 2002;16:2473–2478. doi: 10.1097/00002030-200212060-00014. [DOI] [PubMed] [Google Scholar]
- 31.Engels EA, Sinclair MD, Biggar RJ, Whitby D, Ebbesen P, Goedert JJ, Gastwirth JL. Latent class analysis of human herpesvirus 8 assay performance and infection prevalence in sub-saharan Africa and Malta. Int J Cancer. 2000;88:1003–1008. doi: 10.1002/1097-0215(20001215)88:6<1003::aid-ijc26>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 32.Butler LM, Dorsey G, Hladik W, Rosenthal PJ, Brander C, Neilands TB, Mbisa G, Whitby D, Kiepiela P, Mosam A, Mzolo S, Dollard SC, et al. Kaposi sarcoma-associated herpesvirus (KSHV) seroprevalence in population-based samples of African children: evidence for at least 2 patterns of KSHV transmission. J Infect Dis. 2009;200:430–438. doi: 10.1086/600103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baeten JM, Chohan BH, Lavreys L, Rakwar JP, Ashley R, Richardson BA, Mandaliya K, Bwayo JJ, Kreiss JK. Correlates of human herpesvirus 8 seropositivity among heterosexual men in Kenya. Aids. 2002;16:2073–2078. doi: 10.1097/00002030-200210180-00013. [DOI] [PubMed] [Google Scholar]
- 34.Olsen SJ, Chang Y, Moore PS, Biggar RJ, Melbye M. Increasing Kaposi's sarcoma-associated herpesvirus seroprevalence with age in a highly Kaposi's sarcoma endemic region, Zambia in 1985. Aids. 1998;12:1921–1925. doi: 10.1097/00002030-199814000-00024. [DOI] [PubMed] [Google Scholar]
- 35.Avelleira JC, Lupi O, Caterino-de-Araujo A, Santos-Fortuna Ede L. Seroprevalence of HHV-8 infection in the pediatric population of two university hospitals in Rio de Janeiro, Brazil. Int J Dermatol. 2006;45:381–383. doi: 10.1111/j.1365-4632.2006.02523.x. [DOI] [PubMed] [Google Scholar]
- 36.Malope BI, Pfeiffer RM, Mbisa G, Stein L, Ratshikhopha EM, O'Connell DL, Sitas F, MacPhail P, Whitby D. Transmission of Kaposi sarcoma-associated herpesvirus between mothers and children in a South African population. J Acquir Immune Defic Syndr. 2007;44:351–355. doi: 10.1097/QAI.0b013e31802f12ea. [DOI] [PubMed] [Google Scholar]
- 37.Minhas V, Crabtree KL, Chao A, M'Soka TJ, Kankasa C, Bulterys M, Mitchell CD, Wood C. Early Childhood Infection by Human Herpesvirus 8 in Zambia and the Role of Human Immunodeficiency Virus Type 1 Coinfection in a Highly Endemic Area. Am J Epidemiol. 2008 doi: 10.1093/aje/kwn125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brayfield BP, Phiri S, Kankasa C, Muyanga J, Mantina H, Kwenda G, West JT, Bhat G, Marx DB, Klaskala W, Mitchell CD, Wood C. Postnatal human herpesvirus 8 and human immunodeficiency virus type 1 infection in mothers and infants from Zambia. J Infect Dis. 2003;187:559–568. doi: 10.1086/367985. [DOI] [PubMed] [Google Scholar]
- 39.Hladik W, Dollard SC, Downing RG, Kataaha P, Pellett PE, Karon JM, Mermin J, Lackritz EM. Kaposi's sarcoma in Uganda: risk factors for human herpesvirus 8 infection among blood donors. J Acquir Immune Defic Syndr. 2003;33:206–210. doi: 10.1097/00126334-200306010-00015. [DOI] [PubMed] [Google Scholar]
- 40.Enbom M, Tolfvenstam T, Ghebrekidan H, Ruden U, Grandien M, Wahren B, Linde A. Seroprevalence of human herpes virus 8 in different Eritrean population groups. J Clin Virol. 1999;14:167–172. doi: 10.1016/s1386-6532(99)00061-x. [DOI] [PubMed] [Google Scholar]
- 41.Lyall EG, Patton GS, Sheldon J, Stainsby C, Mullen J, O'Shea S, Smith NA, De Ruiter A, McClure MO, Schulz TF. Evidence for horizontal and not vertical transmission of human herpesvirus 8 in children born to human immunodeficiency virus-infected mothers. Pediatr Infect Dis J. 1999;18:795–799. doi: 10.1097/00006454-199909000-00010. [DOI] [PubMed] [Google Scholar]
- 42.Klaskala W, Brayfield BP, Kankasa C, Bhat G, West JT, Mitchell CD, Wood C. Epidemiological characteristics of human herpesvirus-8 infection in a large population of antenatal women in Zambia. J Med Virol. 2005;75:93–100. doi: 10.1002/jmv.20242. [DOI] [PubMed] [Google Scholar]
- 43.Lavreys L, Chohan B, Ashley R, Richardson BA, Corey L, Mandaliya K, Ndinya-Achola JO, Kreiss JK. Human herpesvirus 8: seroprevalence and correlates in prostitutes in Mombasa, Kenya. J Infect Dis. 2003;187:359–363. doi: 10.1086/367703. [DOI] [PubMed] [Google Scholar]


