Abstract
Aim
The primary aim of this investigation was to examine genotype and clinical phenotype differences in individuals with juvenile neuronal ceroid lipofuscinosis (JNCL) who were homozygous for a common disease-causing deletion or compound heterozygous. The secondary aim was to cross-validate the Child Behavior Checklist (CBCL) and the Unified Batten Disease Rating Scale (UBDRS), a disease-specific JNCL rating scale.
Method
Sixty individuals (28 males, 32 females; mean age 15y 1mo, SD 4y 9mo, range 5y 9mo–31y 1mo) with JNCL completed the UBDRS.
Results
No significant genotype and clinical phenotype differences were identified when comparing individuals homozygous for the deletion with a heterogeneous group of compound heterozygous individuals. There were significant correlations among related behaviour items and scales on the CBCL and UBDRS (Spearman’s rho ranging from 0.39 [p<0.05] to 0.72 [p<0.01]). Behaviour and physical function ratings were uncorrelated, supporting divergent validity of these two constructs in JNCL.
Interpretation
Previous reports of genotype and clinical phenotype differences were unsupported in this investigation, which did not find differences between individuals homozygous or heterozygous for the CLN3 deletion. The CBCL, an already validated measure of behaviour problems, appears valid for use in JNCL and cross-validates well with the UBDRS.
The neuronal ceroid lipofuscinoses are fatal, autosomal recessive, lysosomal storage diseases and are the most commonly occurring neurodegenerative diseases of childhood. There are several forms of neuronal ceroid lipofuscinosis (e.g. congenital, infantile, late infantile, and juvenile), which are genetically distinct but share common clinical features, including vision loss, seizures, cognitive and motor decline, premature death, and the accumulation of autofluorescent storage material in neurons and other cells.1 The age of onset of juvenile neuronal ceroid lipofuscinosis (JNCL) is between 4 and 8 years of age and is the most prevalent form of neuronal ceroid lipofuscinosis, with an estimated incidence of 1:12 500 worldwide.2 JNCL is caused by mutations in the CLN3 gene. The most frequently seen mutation is a 1.02kb deletion that removes exons 7 and 8 of this gene; 80 to 85% of affected children and young adults are homozygous for this deletion.3 Most of the remaining individuals with JNCL are compound heterozygotes for the common deletion and another mutation. The typical clinical course of JNCL involves vision loss between the ages of 5 and 7 years, followed by seizures, cognitive and behavioural disturbances, and motor decline, although there is variability in the temporal order of symptom onset and rate of disease progression. Adaptive skills and cognitive function are significantly negatively correlated with disease duration.4,5
Two Finnish studies report potentially slower disease progression among patients who are compound heterozygous for the common deletion. Järvelä et al.6 reported that individuals homozygous for the CLN3 deletion exhibited greater cognitive and motor impairments than compound heterozygotes, although the two groups had similar onset and progression of seizures and vision loss. A second study described 10 compound heterozygous individuals with either preserved cognitive abilities in adulthood (n=2) or milder seizures and/or cognitive and motor decline (n=8) compared with deletion homozygous individuals.7 However, these findings are by no means definitive. Järvelä et al. also observed a high degree of between-participant and intrafamilial variability in clinical presentation, especially among compound heterozygotes. Another investigation, which genetically re-evaluated patients diagnosed previously on the basis of clinicopathological findings, reported that, of eight compound heterozygous individuals, all but two (with delayed onset of dementia and motor decline in the fifth decade of life) exhibited a fairly typical disease course.8 Identification of genotype–phenotype patterns associated with CLN3 mutations may help advance our understanding of genomic and biochemical correlates or modifiers of the clinical disease.
In this investigation, we compared the physical and neurobehavioral presentation of CLN3 deletion homozygotes with CLN3 compound heterozygotes. We also examined the external validity of the neurobehavioral symptom portion of a JNCL-specific rating system developed by our research group (Unified Batten Disease Rating Scale; UBDRS).9 We examined associations between the UBDRS behaviour examination and related items and higher-order scales from the Child Behavior Checklist (CBCL),10 an established and well-validated measure of child behaviour problems. We have previously reported the use of the CBCL as a standardized method for quantifying behavioural symptoms in JNCL.4 At that time we found that JNCL-affected children and young adults exhibited various behaviour problems in comparison with age- and sex-based typically developing children, including aggressiveness and clinically notable impairments in areas of social skills, attention, and thought problems. We did not find any significant sex differences in behaviour problems. The present study extended our work with the CBCL so that we could cross-validate it with the UBDRS – currently one of only two clinical assessment tools specifically designed for JNCL.9,11
METHOD
The UBDRS is a clinical rating scale developed to measure physical and other neurological symptom severity and symptom progression over time in individuals with JNCL. It has been developed for use with individuals with JNCL across the full range of age, disease duration, and disease severity. Full details of the UBDRS examination are described in an earlier paper by the University of Rochester Medical Center (URMC) Batten Study Group.9 Included in the UBDRS are eight behaviour assessment items that evaluate emotional and behavioural status, based on parental report (sad mood, apathy, anxiety, aggression to self, aggression to others, repetitive behaviours, hallucinations, and obsessive thoughts). Each of these problem items is rated separately for frequency (never, sometimes, frequent, almost always) and severity (none, mild, moderate, severe). Earlier work has established the interrater reliability of the UBDRS.9 The product of behaviour items frequency (i.e. frequency rating) and their severity (i.e. severity rating) was used to establish overall impairment within each symptom domain. Higher scores indicate greater problems; the possible range for each product is 0–9. To cross-validate the UBDRS, a subset of parents whose child had JNCL also rated their child’s behavioural and emotional function using the CBCL,10 an established, measure of child behaviour problems with age-based forms that are validated from the age of 1 year and 6 months through to adulthood. For our longitudinal studies, participants are evaluated on an annual basis. In the present cross-validation analyses, we examined the most recent UBDRS assessment up to 2008, concurrent with the CBCL. Finally, some participants were rated on the UBDRS by more than one examiner for concurrent interrater reliability studies; for these individuals, we utilized the most recent (up to 2008) median UBDRS scores across raters. As with our previous study of the CBCL, there are no sex differences in UBDRS-rated physical function (J Kwon, personal communication, December 2009). The UBDRS also contains questions to evaluate the (parent reported) frequency of seizures, estimated duration of postictal states, and the impact of seizures (e.g. seizure-related injuries, hospitalizations, medication adjustments). These are summarized by the examiner using the UBDRS Clinical Global Impression (CGI) seizure score (scores range from 1 [none] to 5 [severe]). We have previously reported that, in most individuals with JNCL, seizures are infrequent and well controlled.9
We carried out genetic analysis in all participants. Before 2005, DNA samples were obtained by blood collection; subsequently, we employed a non-invasive method by obtaining buccal epithelial cell samples. DNA was extracted and purified from the specimens using standard methods. CLN3 mutation analysis started with screening for the common CLN3 deletion using a method developed in our laboratory12 and approved by the New York State Department of Health. In participants who were not homozygous for the common deletion, we then sequenced the exons and promoter and at least 40bp of flanking intronic sequences. The UBDRS, CBCL, assessments and genetic samples were obtained at annual meetings of the Batten Disease Support & Research Association and at the Batten Disease Diagnostic & Clinical Research Center at our institution, using a research protocol approved by the University of Rochester’s Institutional Review Board. The parents of all participants provided written informed consent for their child’s participation. All statistical analyses were carried out using Statistical, version 6.1 for Windows (StatSoft, Inc., Tulsa, OK, USA). Analyses of median scores from the UBDRS were performed using the non-parametric Mann–Whitney U or Spearman’s rank order tests, as appropriate. The Student’s t-test for two independent samples (for comparisons by group: genotype) was used to analyse mean scores from the CBCL. To evaluate correlations between the median UBDRS scores and CBCL scores, we utilized the Spearman’s rank order test.
RESULTS
A total of 60 children and young adults with genetically confirmed JNCL participated in this study (28 males, 32 females); their mean age at the time of evaluation was 15 years 1 month (SD 4y 8mo; range 5y 8mo–31y 1mo). Of these, 44 (73.3%) were common deletion homozygotes, 15 were compound heterozygotes (mutations reported in Table I), and one participant was homozygous for the R334H mutation. The mean age at onset of first symptom was retrospectively determined by parent interview and was 5 years 4 months (SD 1y 7mo; range 1y–8y 7mo). Mean disease duration (age at most recent assessment minus age at first symptom onset) was 9 years 8 months (SD 4y 6mo; range 2mo–24y 8mo). The number of participants whose most recent UBDRS assessment was carried out in a given year was as follows: 2002, n=4; 2003, n=6; 2004, n=2; 2005, n=3; 2006, n=4; 2007, n=9; and 2008, n=32. Participants’ age at the time of evaluation and disease duration were significantly correlated (r=0.94; p<0.001). Seizures were generally rated as mild on the CGI seizure score (median and modal score=3, or ‘mild’ seizure rating).
Table 1.
Frequency of specific CLN3 mutations in the sample (n=60)
| Mutations | No. |
|---|---|
| del_del | 44 |
| del_IVS7+1G>A | 2 |
| del _944insA | 1 |
| del _R334H | 2 |
| del _D416G | 1 |
| del _W35X | 1 |
| del _ivs11+56GtoA | 2 |
| del _L384P | 1 |
| del _c.240delG | 1 |
| del _V330F | 1 |
| del _c.424delG | 3 |
| R334H_R334H | 1 |
del_del, homozygous for 1.02kb common deletion; R334H_R334H, homozygous for R334H point mutation; all others are compound heterozygotes with one copy of the 1.02kb common deletion.
Genotype–phenotype relationships
Because of several earlier reports of an attenuated clinical phenotype among individuals with JNCL who are compound heterozygotes, we examined genotype–phenotype clinical behaviour and relationships in our larger sample of 60 participants. For these analyses, the individual who was homozygous for a point mutation (R334H_R334H) was excluded, resulting in a total of 59 participants (n=44 deletion homozygotes; n=15 compound heterozygotes). All patients who were compound heterozygotes, regardless of CLN3 mutation, were grouped together for the purpose of these analyses.
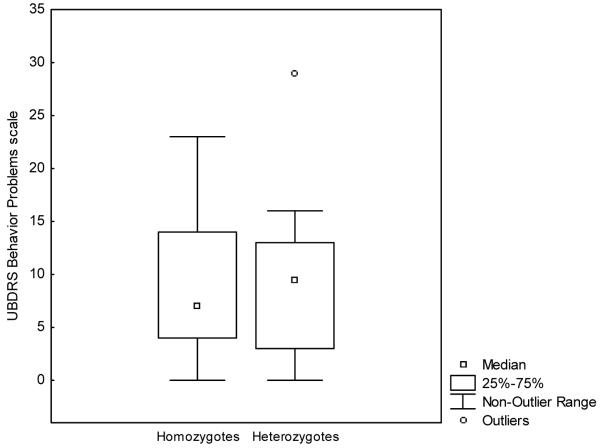
Box plots of the median UBDRS behaviour problems score by genotype do not show differences in the expression of symptoms between homozygotes and heterozygotes (Fig. 1). One extreme outlier is visible in the box plot for heterozygotes; this outlier has an identical genotype to another participant who is not an outlier. We also performed a series of Mann–Whitney U-tests to examine differences in UBDRS behaviour scores by genotype. There was no significant difference between common deletion homozygotes and a heterogeneous group of compound heterozygotes on any UBDRS behavioural item. Similarly, there were no significant differences in CBCL scores between the groups in the subset of patients who were also rated on this measure (n=35; 23 deletion homozygotes; 12 compound heterozygotes). These data are reported in Table IIa and b. Additionally, the median UBDRS physical severity scores by genotype (homozygotes vs compound heterozygotes) were not significantly different from one another (Mann–Whitney U-test=262, Z=−1.18, p=0.24). Finally, we performed a series of Brown and Forsythe homogeneity of variance tests to determine whether there were significant differences in the variability of median scores between the two genotype groups (homozygotes and compound heterozygotes). These tests were performed for individual severity and frequency ratings across eight UBDRS items (sad, apathy, anxiety, aggressive to self, aggressive to others, repetitive behaviours, obsessions, and hallucinations). Among these 16 tests, there was only one significant result: for ‘sad’ frequency (p=0.02). Thus, the variation in median scores on any of these ratings did not appear to be systematically different between the two genotype groups.
Figure 1.
Box plots of the median United Batten Disease Rating Scale (UBDRS) behaviour problems score by genotype group.
Table II.
|
a Genotype comparisons for Unified Batten Disease Rating Scale behavioural items | |||||
|---|---|---|---|---|---|
| Median scores |
Mann- Whitney U-test |
Z- score |
P value |
||
| Homozygote s (n=44) |
Heterozygote s (n=15) |
||||
| Sad | 2.0 | 0.0 | 234.5 | −1.23 | 0.22 |
| Apathy | 0.0 | 0.0 | 261.0 | 0.62 | 0.53 |
| Anxiety | 2.0 | 0.0 | 270.0 | −0.57 | 0.57 |
| Aggression to Others |
0.0 | 2.0 | 209.0 | 1.71 | 0.09 |
| Aggression to Self | 0.0 | 0.0 | 272.5 | 0.53 | 0.60 |
| Repetitive behaviours |
1.0 | 2.0 | 274.5 | −0.49 | 0.62 |
| Hallucinations | 0.0 | 0.0 | 251.5 | −0.42 | 0.97 |
| Obsessions | 2.0 | 2.0 | 294.0 | −0.13 | 0.90 |
|
b: Genotype comparisons for Child Behavior Checklist scales | ||||
|---|---|---|---|---|
| Mean scores |
t | p value | ||
| Homozygotes (n=23) |
Heterozygotes (n=12) |
|||
| Anxious/depressed | 3.43 | 4.00 | 0.40 | 0.69 |
| Withdrawn/depressed | 3.52 | 4.00 | 0.40 | 0.69 |
| Aggressive behaviour | 8.43 | 13.75 | 1.70 | 0.10 |
Validation of UBDRS behaviour assessment
Of the 60 participants who had completed at least one UBDRS examination, 35 (20 males, 15 females) also completed the CBCL – an independent assessment of behavioural and emotional function – on at least one occasion. The mean age of these 35 children and young adults at the time of evaluation was 13 years 11 months (SD=5y; range 4y 9mo–31y 1mo). The mean age at first symptom onset was 5 years (SD=1y 6mo; range 9mo–8y 6mo). The mean disease duration (age at most recent assessment minus age to first symptom onset) was 8 years 11 months (SD 4y 9mo; range 10mo–24y 7mo). Seizure severity (CGI) was not significantly correlated with internalizing (Spearman’s rho 0.12), externalizing (Spearman’s rho 0.13, not significant) or total behaviour problems on the UBDRS (Spearman’s rho 0.33, not significant).
We studied the most recent examination within the same year for both UBDRS and CBCL, between the years 2002 and 2008. The CBCL consists of over 100 behaviour problem items, which are empirically organized into lower-order factors reflecting problem areas (e.g. depression, anxiety, aggression). Lower-order factors are then organized into higher-level factors reflecting more general areas of difficulty (e.g. overall mood symptoms). Table IIIa–c shows the Spearman’s rank order correlations among CBCL problem scales anxious/depressed, withdrawn/depressed, aggressive behaviour) and conceptually similar items on the UBDRS behaviour assessment. We then combined three items from the UBDRS (anxiety + apathy + sad mood) to create an overall mood problems scale, and similarly combined aggression to self and aggression to others to create a total aggression scale. These were examined in relation to conceptually similar CBCL higher-order scales: internalizing problems (reflecting overall mood and anxiety) and externalizing problems (reflecting overall aggression and non-compliance/defiance), shown in Table IVa–c. In general, there were significant positive correlations among conceptually similar UBDRS and CBCL items and scales, although the UBDRS Anxiety scale did not correlate significantly with CBCL scales. There were also modest but significant correlations between CBCL anxious/depressed problems and UBDRS aggression scales (aggression to others, aggression to self). The aggression to self UBDRS scale may have a meaningful relationship to items on the CBCL anxious/depressed scale, which includes reference to self-injurious and suicidal behaviours. Table V shows the intercorrelations among UBDRS items, showing some overlap among internalizing and externalizing symptoms.
Table IIIa-c.
Intercorrelations among similar Unified Batten Disease Rating Scale (UBDRS) and Child Behaviour Checklist (CBCL) symptoms
| (a) Frequency of sad mood, anxiety, and aggression (Spearman’s rho) | |||||
|---|---|---|---|---|---|
| UBD RS sad mood |
UBDRS apathy |
UBDRS anxiety |
UBDRS aggressio n to others |
UBDRS: aggression to self (frequency)Aggress ion to self |
|
| CBCL anxious/depressed problems scale |
0.49b | 0.34a | 0.26 | 0.39a | 0.41a |
| CBCL withdrawn/depresse d problems scale |
0.38a | 0.47b | −0.01 | 0.34a | 0.31 |
| CBCL aggressive behaviour scale |
0.34a | 0.27 | 0.20 | 0.68b | 0.57b |
| (b) Severity of sad mood, anxiety, and aggression (Spearman’s rho) | |||
|---|---|---|---|
| UBDRS sad mood |
UBDRS apathy |
UBDRS anxiety |
|
| CBCL anxious/depressed problems scale | 0.42a | 0.36a | 0.28 |
| CBCL withdrawn/depressed problems scale | 0.34a | 0.45a | −0.03 |
| CBCL aggressive behaviour scale | 0.29 | 0.28 | 0.18 |
| (c) Product of frequency and severity ratings for sad mood, anxiety, and aggression (Spearman’s rho) | |||||
|---|---|---|---|---|---|
| UBDRS sad mood |
UBDRS apathy |
UBDRS anxiety |
UBDRS aggression to others |
UBDRS aggression Aggression to self |
|
| CBCL anxious/depressed problems scale | 0.46a | 0.35a | 0.28 | 0.40a | 0.42a |
| CBCL withdrawn/depressed problems scale | 0.37a | 0.46b | −0.01 | 0.36a | 0.31 |
| CBCL aggressive behaviour scale | 0.32 | 0.28 | 0.19 | 0.69b | 0.57b |
p<0.05
p<0.01.
Table IVa-c.
Intercorrelations among similar Unified Batten Disease Rating Scale (UBDRS) and Child Behaviour Checklist (CBCL) higher-order scales
| (a) Frequency of mood, aggression, and total problems (Spearman’s rho) | |||
|---|---|---|---|
| UBDRS mood |
UBDRS aggression |
UBDRS total problems |
|
| UBDRS physical subscale |
0.14 | −0.23 | 0.01 |
| CBCL internalizing problems |
0.41a | 0.46b | 0.53b |
| CBCL externalizing problems |
0.31 | 0.72b | 0.64b |
| CBCL total problems | 0.39a | 0.65b | 0.72b |
| (b) Severity of mood, aggression, and total problems (Spearman’s rho) | |||
|---|---|---|---|
| UBDRS mood |
UBDRS aggression |
UBDRS total problems |
|
| UBDRS physical subscale |
0.11 | −0.24 | 0.05 |
| CBCL internalizing problems |
0.41a | 0.46b | 0.47b |
| CBCL externalizing problems |
0.30 | 0.73b | 0.59b |
| CBCL total problems | 0.36a | 0.65b | 0.64b |
| (c) Product of frequency and severity ratings for mood, aggression, and total problems (Spearman’s rho) | |||
|---|---|---|---|
| UBDRS mood |
UBDRS aggression |
UBDRS total problems |
|
| UBDRS physical subscale |
0.11 | −0.24 | 0.01 |
| CBCL internalizing problems | 0.41a | 0.47b | 0.52b |
| CBCL externalizing problems | 0.34a | 0.72b | 0.54b |
| CBCL total problems | 0.39a | 0.65b | 0.58b |
p<0.05
p<0.01.
UBDRS mood: UBDRS sad + apathy + anxiety. UBDRS total aggression: UBDRS aggression to self + aggression to others. UBDRS total: UBDRS total behaviour problems (UBDRS mood + UBDRS aggression + UBDRS hallucinations + UBDRS obsessions + UBDRS repetitive behaviours).
Table V.
Intercorrelations among UBDRS symptoms (Spearman’s rho)
| Apath y |
Anxiet y |
Aggression to others |
Aggressio n to Self |
Repet . |
Halluc . |
Obsess . |
|
|---|---|---|---|---|---|---|---|
| Sad | 0.30a | 0.41b | 0.37b | 0.37b | 0.11 | 0.19 | 0.42b |
| Apathy | 0.15 | 0.05 | 0.22 | −0.06 | 0.09 | 0.15 | |
| Anxiety | 0.13 | 0.26 | 0.38b | 0.16 | 0.54b | ||
| Aggression to others |
0.26a | 0.12 | 0.05 | 0.36b | |||
| Aggression to self |
0.06 | −0.06 | 0.31a | ||||
| Repet. | 0.20 | 0.33a | |||||
| Halluc. | 0.18 |
UBDRS scores reflect product of frequency × severity ratings.
p<0.05
p<0.01.
Repet., Repetitive Behaviors; Halluc., Hallucinations; Obsess., Obsessions.
We were also interested in determining whether or not behavioural/emotional and physical symptoms in JNCL were independent of one another, as divergence between these constructs has been reported in other neurodegenerative conditions such as Huntington disease.13 The UBDRS behavioural subscale total score and UBDRS physical scale total score were not significantly correlated (Spearman’s rho 0.02, not significant). In addition, the CBCL total problems score (all behaviour problems endorsed on the entire measure) was not significantly correlated with the UBDRS physical scale total score (Spearman’s rho 0.05, not significant).
DISCUSSION
Our primary aim was to search for associations of genotype with behavioural function in a group of children and young adults with genetically confirmed JNCL. At least 40 disease-causing mutations in CLN3 have been identified (CLN3 mutation database: http://www.ucl.ac.uk/ncl/cln3.shtml); however, the function of the protein encoded by this gene is unknown. We did not find significant associations between genotype and clinical behavioural phenotype in this cross-sectional examination of the data. Our compound heterozygous participants included individuals with a diverse set of genotypes, some of which have been individually reported to be associated with alternative clinical progression. However, we did not find consistent genotype–phenotype differences using our objective rating scale data. The collective grouping of these compound heterozygotes may partly explain why the findings of the present study are different from those of a previous Finnish investigation that reported phenotypic variability among heterozygotes. However, the difference may also reflect the smaller sample size of the previous investigation and the different methodology for rating motor neurological function.14 Another possibility is that variable progression in individual cases is due to other modifier genes or mutations in CLN3 that are not yet understood. Identification of genotype–phenotype patterns associated with either CLN3 mutations or modifier genes may help further our understanding of genomic and biochemical correlates or modifiers of clinical disease and provide a focus for targeted interventions with respect to both anticipation and management of symptoms, and improved models for future clinical trials.
We also recognize that the use of an ordinal scale can affect the interpretation of clinical ratings of symptom frequency and severity. More work is needed to refine our understanding of what type of numerical score would best describe the clinical state of individuals with JNCL, but to our knowledge no better scoring system yet exists for describing disease and disease progression in this rare disease. The UBDRS was modelled after two other clinical rating scales, also using ordinal rankings, that are criterion standard assessment tools for clinical studies of particular diseases: UHDRS for Huntington disease13 and UPDRS for Parkinson disease15.
Our second goal was to examine the validity of the behaviour assessment portion of the UBDRS for evaluation of JNCL. Previous investigations by the URMC Batten Study Group established good reliability of the UBDRS,9 and also established the use of the CBCL for quantifying behavioural problems in this disease.4 Our present study extends and integrates the work of these earlier projects. There was good convergence of the UBDRS behavioural scale and the CBCL – an externally valid, omnibus measure of child behaviour problems. Further, there was evidence of divergence or lack of association between the UBDRS physical scale and the CBCL. In addition, we demonstrated that, within the UBDRS itself, the behavioural and physical scales appear to measure independent constructs. The significant correlations among related items and scales from the UBDRS behavioural scale and the CBCL, and divergence between mood and behavioural symptoms (on both UBDRS and CBCL) and physical manifestations, help support the validity of the UBDRS behavioural construct. Our group has also previously reported that cognitive and physical symptoms are not closely related, particularly in earlier disease stages,5 and this also seems to be the case when considering behavioural and physical symptoms. The finding that physical and psychiatric features are uncorrelated is consistent with findings in Huntington disease, another neurodegenerative disease, but one with onset primarily in adulthood.13,16 A future step of our research will be to examine whether there is differing progression of the longitudinal course of physical versus behavioural symptoms.
The small but significant correlation between aggression and mood symptoms was unexpected. Children and young adults with emotional distress may also have more generalized behavioural problems, as was suggested in this study by significant correlations between total problems scores (on UBDRS and CBCL) and both mood problems and aggressive/externalizing problems (as assessed by either the UBDRS or CBCL). There may also be overlapping features of these symptom clusters (internalizing or mood problems) and aggression, such as an irritable or labile mood that can be accompanied by aggressive outbursts. As noted previously, the ‘aggressive to self’ UBDRS item may overlap with features of the CBCL depressive scales, which contain reference to self-injurious behaviours. Finally, normative data from the CBCL10 also show modest but significant correlations between internalizing (i.e. mood problems) and externalizing (e.g. aggression, defiance) problems. The management of mood and behaviour symptoms in the setting of dementia is challenging at best, and there are no established guidelines for treatment of these dementia-related problems in paediatric groups. Prospective clinical trials of behavioural and pharmacological interventions are needed to establish best practices for improving affected individuals’ symptoms and quality of life. We have also previously acknowledged the challenges of evaluating the relative impacts of seizures, cognitive impairment, and behavioural symptoms in this disease.4 We view each of these symptoms as manifestations of the underlying neurodegenerative disease process, and recognize that further work is needed to establish a disease model clarifying the relationships among these symptoms.
In studying any rare disease, small sample sizes may limit the extent of analyses, but are an unavoidable limitation. Nonetheless, our studies of JNCL involve the largest known group of individuals with this disease. In our longitudinal studies, we will further compile an extensive body of data on within-participant progression of physical and behavioural symptoms (UBDRS, CBCL), other aspects of the disease (seizures, vision loss, and dementia), symptom management, and other relevant medical history and patient and family demographics. In doing so, we will be able to better describe the clinical phenotype and natural history of JNCL within individual participants and subgroups (e.g. by genotype, sex). The relatively smaller number of compound heterozygotes and their wide variety of mutations is also a potential limiting factor in understanding genotype–phenotype relationships. Although the overall percentage of compound heterozygotes as a function of the total sample is not predicted to increase, we expect that continuing enrolment will increase the absolute number as well as the range of compound heterozygotes available for study. Finally, in our sample, all participants were clinically symptomatic at the time of assessment. Consequently, little is known about early and perhaps subtle genotype–phenotype differences that may signal or accompany clinical onset of disease. A future goal is to better understand clinical phenotype–genotype associations across the full range of disease severity and disease duration, including children and young adults with genetically confirmed CLN3 but who are presymptomatic or in very early stages of phenoconversion.
What this paper adds
Previous reports of genotype and clinical phenotype differences were unsupported in this investigation
It furthers our understanding of neurobehavioral symptoms in JNCL.
The validity of a disease-specific rating scale for neurobehavioral symptoms in JNCL is established.
Neurobehavioral and physical symptoms in JNCL are differentiated.
ACKNOWLEDGEMENTS
This research was supported in part by the Batten Disease Support and Research Association and by NIH grant 5K23 NS058756-01 from the National Institute of Neurological Disorders and Stroke.
The authors thank the families of affected children and young adults for their participation in this study, and the Batten Disease Support & Research Association for assistance with study recruitment.
LIST OF ABBREVIATIONS
- CBCL
Child Behavior Checklist
- JNCL
Juvenile neuronal ceroid lipofuscinosis
- UBDRS
Unified Batten Disease Rating Scale
REFERENCES
- 1.Boustany R-M. Batten disease or neuronal ceroid lipofuscinosis. In: Moser HW, editor. Handbook of Clinical Neurology. Elsevier Science; Amsterdam: 1996. pp. 671–700. [Google Scholar]
- 2.Santavuori P, Lauronen L, Kirveskari E, Åberg L, Sainio K, Autti T. Neuronal ceroid lipofuscinoses in childhood. Neurol Sci. 2000;21(Suppl 3):S35–41. doi: 10.1007/s100720070038. [DOI] [PubMed] [Google Scholar]
- 3.Phillips SN, Benedict JW, Weimer JM, Pearce DA. CLN3, the protein associated with batten disease: structure, function and localization. J Neurosci Res. 2005;79:573–83. doi: 10.1002/jnr.20367. [DOI] [PubMed] [Google Scholar]
- 4.Adams H, de Blieck EA, Mink JW, Marshall FJ, Kwon J, Dure L, et al. Standardized assessment of behavior and adaptive living skills in juvenile neuronal ceroid lipofuscinosis. Dev Med Child Neurol. 2006;48:259–64. doi: 10.1017/S0012162206000570. [DOI] [PubMed] [Google Scholar]
- 5.Adams H, Kwon J, Marshall FJ, de Blieck EA, Pearce DA, Mink JW. Neuropsychological symptoms of Juvenile-onset Batten disease: experiences from two studies. J Child Neurol. 2007;22:621–627. doi: 10.1177/0883073807302603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Järvelä I, Autti T, Lamminranta S, Åberg L, Raininko R, Santavuori P. Clinical and magnetic resonance imaging findings in Batten disease: analysis of the major mutation (1.02-kb deletion) Ann Neurol. 1997;42:799–802. doi: 10.1002/ana.410420517. [DOI] [PubMed] [Google Scholar]
- 7.Lauronen L, Munroe PB, Järvelä I, Autti T, Mitchison HM, O’Rawe AM, et al. Delayed classic and protracted phenotypes of compound heterozygous juvenile neuronal ceroid lipofuscinosis. Neurology. 1999;52:360–5. doi: 10.1212/wnl.52.2.360. [DOI] [PubMed] [Google Scholar]
- 8.Wisniewski KE, Zhong N, Kaczmarski W, Kaczmarski A, Sklower-Brooks S, Brown WT. Studies of atypical JNCL suggest overlapping with other NCL forms. Pediatr Neurol. 1998;18:36–40. doi: 10.1016/s0887-8994(97)00188-4. [DOI] [PubMed] [Google Scholar]
- 9.Marshall FJ, de Blieck EA, Mink JW, Dure L, Adams H, Messing S, et al. A clinical rating scale for Batten disease: reliable and relevant for clinical trials. Neurology. 2005;65:275–9. doi: 10.1212/01.wnl.0000169019.41332.8a. [DOI] [PubMed] [Google Scholar]
- 10.Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms & Profiles. University of Vermont, Research Center for Children, Youth, and Families; Burlington, VT: 2001. [Google Scholar]
- 11.Kohlschütter A, Laabs R, Albani M. Juvenile neuronal ceroid lipofuscinosis (JNCL): quantitative description of its clinical variability. Acta Paediatr. 1988;77:867–72. doi: 10.1111/j.1651-2227.1988.tb10770.x. [DOI] [PubMed] [Google Scholar]
- 12.Rothberg PG, Ramirez-Montealegre D, Frazier SD, Pearce DA. Homogeneous polymerase chain reaction nucleobase quenching assay to detect the 1-kbp deletion in cln3 that causes Batten disease. J Mol Diagn. 2004;6:260–3. doi: 10.1016/S1525-1578(10)60519-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huntington Disease Study Group. Huntington Study Group Unified Huntington’s Disease Rating Scale: reliability and consistency. Mov Disord. 1996;11:136–42. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]
- 14.Jarvela I, Autti T, Lamminranta S, Aberg L, Raininko R, Santavuori P. Clinical and magnetic resonance imaging findings in Batten disease: analysis of the major mutation (1.02-kb deletion) Ann Neurol. 1997;42:799–802. doi: 10.1002/ana.410420517. [DOI] [PubMed] [Google Scholar]
- 15.Fahn S, Elton RL, Members of the UPDRS Development Committee . Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent Developments in Parkinson’s Disease. Macmillan Health Care Information; Floram Park: 1987. pp. 153–64. [Google Scholar]
- 16.Huntington Study Group Unified Huntington’s Disease Rating Scale: reliability and consistency. Mov Disord. 1996;11:136–42. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]