Abstract
TPM1κ is an alternatively spliced isoform of the TPM1 gene whose specific role in cardiac development and disease is yet to be elucidated. Although mRNA studies have shown TPM1κ expression in axolotl heart and skeletal muscle, it has not been quantified. Also the presence of TPM1κ protein in axolotl heart and skeletal muscle has not been demonstrated. In this study, we quantified TPM1κ mRNA expression in axolotl heart and skeletal muscle. Using a newly developed TPM1κ specific antibody, we demonstrated the expression and incorporation of TPM1κ protein in myofibrils of axolotl heart and skeletal muscle. The results support the potential role of TPM1κ in myofibrillogenesis and sarcomeric function.
Keywords: Ambystoma mexicanum, Heart, Skeletal muscle, Growth & development, Reverse Transcriptase Polymerase Chain Reaction, Tropomyosin
INTRODUCTION
Tropomyosins (TM) are a family of highly conserved actin binding proteins which are expressed in all eukaryotic cells. TMs are found in muscle- skeletal, cardiac, and smooth, as well as non-muscle cells and are involved in regulating the function of actin filaments in these tissues. TMs play an important role in the control of calcium regulated thin filament function and striated muscle contraction.
There are four known TM genes (designated as TPM1, TPM2, TPM3, and TPM4) in vertebrates. They generate a multitude of tissue and developmental specific isoforms through the use of different promoters, alternative mRNA splicing, different 3′-end mRNA processing and possibly tissue specific translational control [Gunning et al., 2008; Lees-Miller and Helfman, 1991].
The TPM1 gene is the most versatile of the four genes, encoding at least 10 isoforms via alternative splicing in vertebrates. The TPM1 gene contains 15 exons, 5 of which are common to all isoforms. TM isoforms containing exon 1a are 284 amino acids long (high molecular weight, HMW) whereas TM isoforms containing exon 1b are 248 amino acids long (low molecular weight, LMW) [Pittenger et al., 1994]. Alternative splice sites are found internally at exons 2a/2b and 6a/6b and in the C-terminus at exons 9a, 9b, 9c and 9d.
In mammals, the predominant cardiac isoform is TPM1α. We described a novel tropomyosin isoform designated as TPM1κ in human [Denz et al., 2004], rat (unpublished data), chicken [Zajdel et al., 2003], and axolotl [Luque et al., 1997]. TPM1α and TPM1κ share 9 exons and differ at exon 2: TPM1κ having exon 2a and TPM1α exon 2b. The classic splicing pattern for the striated muscle TPM1α isoform is 1a, 2b, 3, 4, 5, 6b, 7, 8, 9a, b and TPM1κ is 1a, 2a, 3, 4, 5, 6b, 7, 8, 9 a, b. In humans [Denz et al., 2004] and chicken [Zajdel et al., 2003], TPM1κ expression is restricted to the heart. In axolotl, three sarcomeric tropomyosin isoforms (TPM1α, TPM1κ, TPM4α) are expressed in cardiac muscle and unlike what is known in other vertebrates, TPM1κ expression is seen in skeletal muscle also in addition to the heart [Spinner et al., 2002].
It is known that cardiac mutant axolotl hearts are deficient in tropomyosin and are unable to contract [Spinner et al., 2002; Humphrey, 1972] due to a lack of organized myofibrils [Lemanski, 1973, 1979]. However ectopic expression of TPM1α, TPM1κ or TPM4α in mutant hearts in culture leads to formation of organized myofibrils and induce contractility [Zajdel et al., 1998, 2002]. Knockdown of TPM1κ in vitro with isoform specific anti sense oligonucleotides has been shown to inhibit contractility and cause disruption of myofibrillar organization [Zajdel et al., 2005].
TPM1κ protein is also expressed and incorporated into organized myofibrils in human hearts. In humans higher TPM1κ protein expression is seen in dilated cardiomyopathy and heart failure. Transgenic mice over-expressing TPM1κ developed dilated cardiomyopathy and demonstrated decreased fractional shortening, systolic and diastolic dysfunction and decreased myofilament calcium sensitivity with no change in maximum developed tension [Rajan et al., 2010]. These findings underscore the important role of TPM1κ isoform in cardiac myofibrillogenesis. However, its specific role in cardiac development and disease is yet to be elucidated.
Although previous studies have demonstrated expression of TPM1κ mRNA in axolotl heart and skeletal muscle, it has never been quantified. Also the presence of TPM1κ protein in axolotl heart and skeletal muscle has not been demonstrated. In this study, for the first time we quantified TPM1κ mRNA expression and demonstrated the expression and incorporation of TPM1κ protein in axolotl heart and skeletal muscle to further support its potential role in myofibrillogenesis and sarcomeric function.
MATERIALS AND METHODS
Embryo care
Normal and cardiac mutant axolotl embryos were obtained from the Ambystoma Genetic Stock Center, at the University of Kentucky (Lexington, KY). Embryos were maintained in Holtfreter’s solution (3.46 g NaCl, 0.05 g KCl, 0.1 g CaCl2, 0.2 g NaHCO3, 0.2 g MgSO4, [pH 7.4] per liter of distilled H20) until desired stages of maturation was reached.
RNA isolation from embryonic axolotl and chicken
Axolotl hearts were removed after heartbeat initiation at stage 35. The embryos were removed from their jelly coats and anesthetized using MS-222 (Tricaine methanesulfonate). Hearts were dissected out using watchmaker forceps under a dissecting microscope in Steinberg’s solution (3.4 g NaCl, 0.05 g KCl, 0.05 g CaCl2, 0.205 g MgSO4, 1.1 g HEPES [pH 7.4] per liter of distilled H20 and vacuum filtered). They were quickly frozen in microcentrifuge tubes submerged in absolute ethanol containing dry ice. Fertile chicken eggs (Leghorn) were incubated at 37°C for 10–15 days. Heart and skeletal muscle were dissected free and placed in liquid nitrogen. The frozen tissue was ground in a mortar pestle in the presence of liquid nitrogen. RNA was isolated from frozen tissue using the RiboPure kit (Ambion), following manufacturers’ protocol.
RT-PCR for gene expression in embryonic axolotl tissue
cDNA was made with total RNA from axolotl heart or skeletal muscle using AxTPM1 (−) gen. 5′-TTACATTGAAGTCATATCGTTGTTGAG-3′ primer that is common to both TPM1α and TPM1κ located at the exon 9 of the TPM1 gene. For real time RT-PCR, TPM1α and TPM1κ were amplified using isoform-specific primer pairs as described below. The positive primers were TPM1κ1 (+) 5′-TAGAGGAGGAGATTGTGCA-3′ from exon 2a and TPM1α1 (+) 5′-CTTGAGGACGAGCTAGTAGCCC-3′ from exon 2b. The common negative primer used to amplify both isoforms was from exon 3: TPM1α/κ (−) 5′-CAGACGCTCCTGAGCACG-3′. Glyceraldehyde 3- phosphate dehydrogenase (GAPDH) was used as a housekeeping gene to demonstrate consistent RNA input from the different tissues. It was amplified with the primer pair GAPDH 1(+) 5′-TGGCGTGTTCACCACAAT-3′ and GAPDH2 (−) 5 ′-AAAGCCATTCCAGTGAGT-3′ and identified after ethidium bromide staining.
QRT-PCR was carried out to quantify TPM1κ and TPM1α from embryonic axolotl hearts. First, optical density was taken of an axolotl TPM1κ and TPM1α TA clone using a spectrophotometer. The copy number per volume of clone in solution was determined using the equation, number of copies = (ng of plasmid DNA * 6.02×1023)/(bp length of plasmid * 1×109 * 650), which was simplified by Andrew Staroscik at the URI Genomics and Sequencing Center. A dilution series of the clone was done for 1×101 – 1×104 copies of template, which would be used to create a standard curve post-amplification. iQ® SYBR green supermix (BioRad) and a Bio-Rad iCycler thermocycler were used and melt curve was determined using manufacturer’s protocol.
The reaction mixture contained 12.5μl of the SYBR green supermix, 1μl of both positive and negative 10mM primer, 9.5μl DEPC-treated H20, 1μl of cDNA for the unknowns, or 1μl of DNA from the dilution series of axolotl TPM1κ and TPM1α TA clones for the standards, or 1μl of H20 for the primer control. To verify the specificity of the primer pair, PCR products were run on an agarose gel after real-time analysis.
Myofibrillar and skeletal muscle protein analyses
Protein was extracted from axolotl heart using cell extraction buffer (Invitrogen), supplemented with 1mM PMSF and complete protease inhibitors (Roche). Protein quantitation was done using the EZQ kit (Invitrogen) and a fluorescence-based microplate reader following the manufacturer’s protocol. LDS sample buffer and sample reducing agent (Invitrogen) were added to the protein extracts, and SDS-PAGE was carried out. Between 30–50μg of axolotl and chicken protein extract was loaded into Novex NuPAGE 4–12% Bis-Tris gels in MOPS running buffer with antioxidant added (all Invitrogen), and run for 1 hour at 200v in Xcell SureLock Mini-Cell apparatus (Invitrogen). The gels were transferred to nitrocellulose membranes using the same apparatus, under transfer buffer (Invitrogen) supplemented with methanol and antioxidant (Invitrogen). Ponceau reversible staining was done to test loading consistency and transfer efficiency. The blots were blocked in 5% dry milk powder (Carnation) in TBST (1x TBS, 0.05% Tween 20) overnight at 4°C. Primary antibody incubations were done for 2 hours at room temperature. Secondary antibody incubations were done for 1 hour at room temperature. Blots were washed in TBST after both primary and secondary antibody incubations. Chemiluminescence was accomplished by using ECL detection reagents (Amersham) and exposing the blot to x-ray film, following the manufacturer’s protocol. Antibodies were diluted in the 5% milk powder blocking solution. As a positive control, 75μg of total protein extract from a 20 week old human fetal heart (Biochain) was run alongside the axolotl heart extracts.
A 1:100 dilution of affinity purified rabbit anti-TPM1κ was used to detect this isoform of TPM1 gene. Myofibril extracts from transgenic mice over-expressing human TPM1κ in a cardiac-specific manner was used as positive control. Anti-rabbit HRP (Amersham) was diluted 1:5000 and used as the secondary antibody. A 1:50 dilution of the mouse monoclonal antibody CH1 (Sigma), which detects all sarcomeric tropomyosins, was used on the TPM1κ blot after stripping the first antibodies off (PBS with 2%SDS, 0.7% β-mercaptoethanol at 65°C for 10 minutes). ECL was done on the blot to verify that stripping reaction was successful, prior to staining with CH1. A 1:5000 dilution of anti-mouse HRP (Amersham) was used as the secondary antibody for CH1.
Quantification of TPM1κ protein in axolotl heart, skeletal muscle
For quantification of axolotl myofibrillar TPM1κ using actin normalization, blots containing the myofibrillar fraction were stripped and reacted with anti- actin monoclonal antibody, clone 5C5 (Sigma) at a dilution of 1:5000 followed by a similar dilution of anti- mouse HRP (Amersham) as the secondary antibody. The blots were developed as described before. The intensity of the bands was quantified with ImageQuant 5.1 software. Actin levels were used to normalize TM values in the myofibrillar fractions.
Immunostaining of embryonic axolotl hearts, skeletal muscle and paraffin embedded tissue sections of human heart
Embryonic axolotl hearts were first partially fixed and permeabilized in 1mM DTSP/DMSO in Steinberg’s solution for 15 minutes. Soluble proteins were removed in 0.5%NP-40 in Steinberg’s for 10 minutes. To stop this reaction, two 10 minute washes in 0.1M glycine in Steinberg’s were done. The tissues were blocked overnight at 4°C in 2%BSA. Primary antibody incubation was carried out overnight at 4°C. Affinity purified rabbit polyclonal antibody against human TPM1κ was used at a 1:50 dilution. Monoclonal sacrcomeric myosin antibody, MF20 (Developmental Hybridoma Bank, University of Iowa) was used at a 1:50 dilution. Mouse monoclonal antibody, CH1 which detects all sarcomeric tropomyosins CH1 was used at 1:30 dilution. After primary antibody incubation, tissues were washed in 0.1M BSA for 15 minutes. The tissues were then incubated in the appropriate secondary antibody at a 1:100 dilution for 1 hour at 37°C. FITC conjugated anti-rabbit and rhodamine red x conjugated anti-mouse (Jackson Immunological; West Grove, PA) were used as the secondary antibodies for polyclonal TPM1κ, and either CH1 or MF20, respectively. The tissues were then fixed in 2% paraformaldehyde for 1 hour. Another two 15 minute washes in glycine were followed by a 20 minute wash in Steinberg solution. The tissues were mounted on slides in Flurogard antifade reagent (Bio-Rad) with nail polish spacers. Cover slips were then placed and sealed with nail polish. Whole mount tissues were viewed using a BioRad MRC-1024 confocal laser microscope attached to a Nikon Eclipse E600 microscope. 60X and 100X objectives were used, and optical sections were taken for each experimental condition. Adobe Photoshop software was used to create figures from single section images.
Paraffin embedded tissue sections of human hearts obtained at autopsy were stained with affinity purified polyclonal rabbit anti TPM1κ specific antibody. Sections of left ventricular posterior area were used. Immunostaining with anti-rabbit LSAB system- HRP was used according to the manufacturer’s protocol (DakoCytomation). Antigen retrieval was performed in target retrieval solution (DakoCytomation) for 15–20 min at 95°C. TPM1κ antibody was used at a dilution of 1:200 and incubated at 37°C for 1 hr. For control staining, preimmune serum was used instead of the primary antibody. Sections were counterstained with hematoxylin (Vector Labs) and images obtained using 20 X objective.
RESULTS AND DISCUSSION
Defining the role of TM in cardiac development and function has been problematic due to the lack of suitable isoform specific antibodies to assess the complex and diverse TM isoforms expressed in heart. TPM1α, the predominant cardiac isoform shares 9 exons with TPM1κ and differs only at exon 2: TPM 1κ having exon 2a and TPM 1α exon 2b. Exon 2a is also present in some smooth muscle isoforms; however those isoforms differ with TPM1κ in exon 9, where TPM1κ shares homology with TPM1α.
To distinguish the TPM1κ protein from other TM isoforms, we used an isoform specific antibody with an epitope that resides in exon 2a sequence which is encoded within TPM1κ but not in TPM1α protein. A conserved 16-mer from exon 2a region of the human TPM1κ peptide was used to develop a rabbit polyclonal antibody against TPM1κ (Table 1) which was affinity purified (Sigma- Genosys). The specificity of TPM1κ antibody was confirmed using western blot analysis with bacterially expressed recombinant TPM1α and TPM1κ proteins used as controls. The TPM1κ specific antibody which was raised against the human TPM1κ peptide could detect the TPM1κ protein in heart and skeletal muscle, but did not react to the recombinant TPM1α protein. Differential mobility of the two isoforms was noted possibly due to amino acid sequence differences between them [Rajan et al., 2010].
Table 1.
Alignment of exon 2a from human TPM1κ with the homologous regions of chicken and axolotl. The underlined portion is the peptide sequence of antigen used to develop the rabbit polyclonal antibody against human TPM1κ. Corresponding areas of exon 2b from human and axolotl TPM1α are also shown to highlight the differences between exons 2a and 2b.
| 39 | 77 | |
|---|---|---|
| Hum TPM1κ | LEEDIAAKEKLLRVSEDERDRVLEELHKAEDSLLAAEEA | |
| Axolotl TPM1κ | ...E.VQL..Q..I.........D....S.E...T.D.K | |
| Chicken TPM1κ | ..D..VQL..Q...T..S..Q....L..S....LS...N | |
| Human TPM1α | ..DELVSLQ.KLKGT...L.KYS.A.KD.QEK.EL..KK | |
| Axolotl TPM1α | ..DELV.LQ.K.KGT...L.KYS.S.KD.QEK.EL.DKK | |
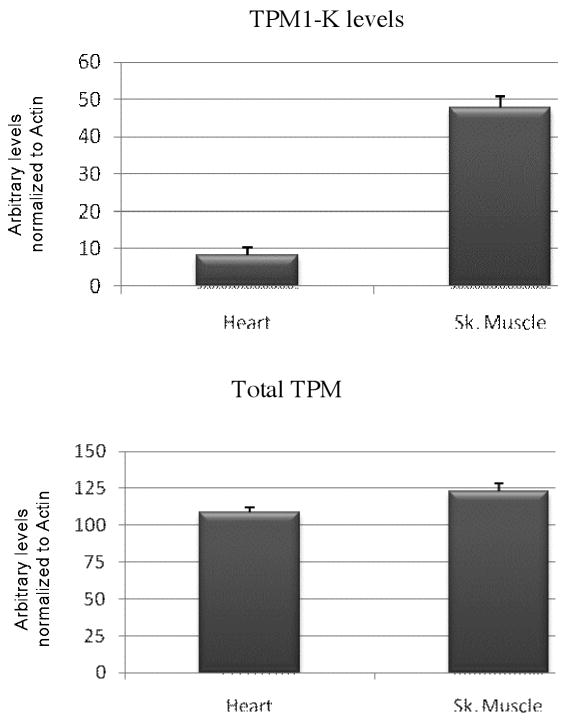
Western blots (WB) using this antibody demonstrated that TPM1κ protein was expressed in both adult axolotl skeletal muscle and heart. Higher expression of TPM1κ was observed in skeletal muscle (Figure 1a: Panel A lanes 3 and 4 in two different concentrations) than heart (Figure 1a: Panel B lanes 1 and 2 in two different concentrations). Myofibril extracts from transgenic mice heart over-expressing human TPM1κ in a cardiac-specific manner was used as positive control. The TPM1κ protein levels were normalised to actin and quantified (Figure 2). The results show that TPM1κ expression in axolotl heart is significantly lower compared to the total sarcomeric TPM protein expression as determined by staining with CH1 monoclonal antibody. CH1 antibody recognises both TPM1α and TPM1κ. The epitope recognised by CH1 is at exon 9a which is common to all sarcomeric TPMs. TPM1κ protein expression was not seen in either chicken heart or skeletal muscle (Figure 3). There are three amino acid changes between the human peptide sequence used to develop the antibody and the axolotl TPM1κ sequence. However, chicken TPM1κ sequence differs from the human peptide sequence at five amino acids. The difference in amino acid sequences may explain why we have been unable to detect chicken TPM1κ protein expression with this antibody. WB of axolotl heart with TPM1κ antibody (Fig 2, lanes 1 and 2) showed a lighter band of higher MW above the dark band. This could be due to non specific binding of antibody with protein present in axolotl heart or the antibody binding to another isoform of TPM1 which is yet to be confirmed. mRNA of an isoform of TPM1 gene containing both exons 2a and 2 b, coding for 326 amino acids, has been sequenced from humans (Genbank acc no: EAW77624.1). However it is not known if this mRNA sequence is translated to protein.
Figure 1.
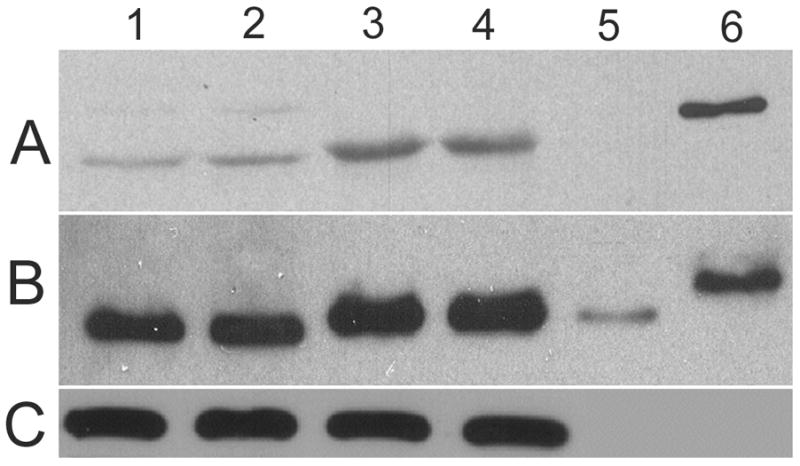
Western blot (WB) of adult axolotl extracted myofibrils and skeletal muscle with TPM1κ specific antibody.
Panel A: TPM1κ antibody. The band size of TPM1κ is 37kDa
Panel B: CH1 antibody that recognizes both TPM1α and TPM1κ
Panel C: Anti α sarcomeric actin antibody
Lanes 1 and 2. Axolotl heart myofibrils
Lanes 3 and 4. Axolotl skeletal muscle myofibrils
Lane 5. Purified recombinant TPM1α protein
Lane 6. Purified recombinant TPM1κ protein
Figure 2.
Quantification of total TPM and TPM1κ levels, based on estimation of western blot intensity bands in axolotl heart and skeletal muscle. Actin levels were used to normalize TM values in the myofibrillar fractions.
Figure 3.
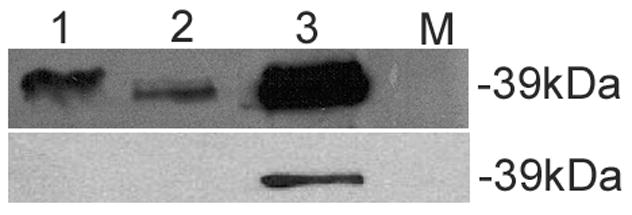
Western blot of chicken myofibrils and skeletal muscle with TPM1κ specific antibody.
Top panel: WB with CH1 antibody that recognizes both TPM1α and TPM1κ
Bottom panel: WB with TPM1κ antibody. The band size of TPM1κ is 37kDa
Lane 1: Chicken heart
Lane 2: Chicken skeletal muscle
Lane 3: Heart from transgenic mice over- expressing TPM1κ
Lane 4: Molecular marker
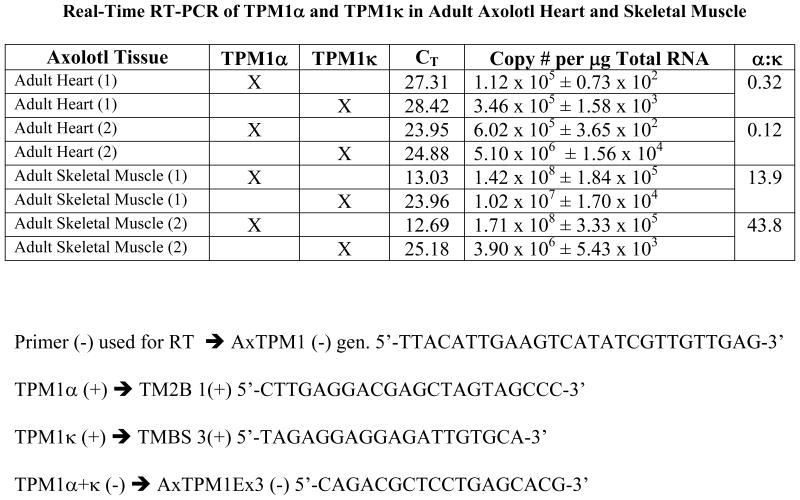
Previous studies have demonstrated TPM1κ transcript expression in axolotl heart and skeletal muscle [Zajdel et al., 1998]. We studied the quantitative expression of mRNA using QRT-PCR. Real-time RT-PCR of TPM1α and TPM1κ in adult axolotl showed markedly elevated copy numbers of both the isoforms in skeletal muscle compared to the heart (Figure 4). However expression patterns of the isoforms were different in both tissues. TPM1κ mRNA was expressed at a higher level in the axolotl heart compared to TPM1α. In contrast TPM1α mRNA expression was higher in skeletal muscle compared to TPM1κ. However TPM1κ protein expression signals were significantly lower in both skeletal muscle and heart. This is possibly due to the three amino acid changes between the human peptide sequence used for antibody synthesis and the axolotl TPM1κ sequence causing suboptimal antigen- antibody interaction. Also, TPM1κ may have lower translational efficiency compared to TPM1α.
Figure 4.
Real-time RT-PCR data of TPM1α and TPM1κ expression in adult axolotl heart and skeletal muscle. Primer pairs used for the reaction are as follows:
Negative primer:
TPM1α/κ (−) 5′-CAGACGCTCCTGAGCACG-3′
Positive primers:
TPM1α1 (+) 5′-CTTGAGGACGAGCTAGTAGCCC-3′
TPM1κ1 (+) 5′-TAGAGGAGGAGATTGTGCA-3′
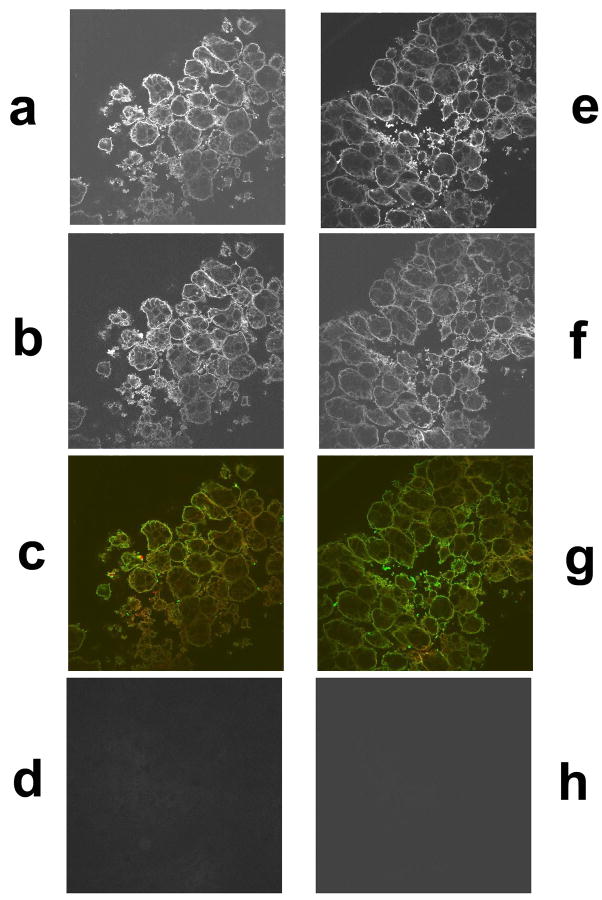
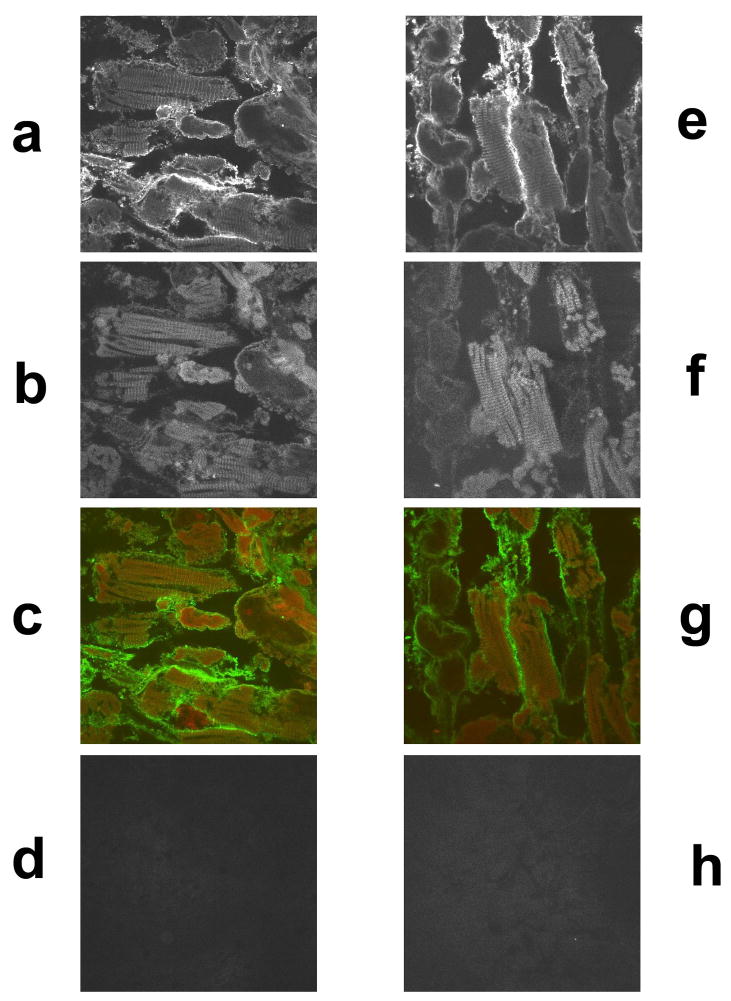
Using confocal laser scanning microscopy, immunostained tissues were examined to study the expression of TPM1κ protein and its localization in embryonic axolotl heart and skeletal muscle. CH1, a monoclonal antibody that detects all sarcomeric tropomyosin isoforms and MF20, a monoclonal antibody against myosin heavy chain were used for localization studies. Double labeled immunofluorescence experiments with TPM1κ and MF20 in axolotl heart (Figure 5a–d) showed expression and incorporation of TPM1κ presumably into the thin filaments. MF20 recognizes sarcomeric myosin, a component of thick filament in both skeletal muscle and cardiac muscle. Hence we used the same antibody for localization studies in skeletal muscle (Figure 6a–d and figure 7b). TPM1κ co- localized with known thin filament component TPM1α as detected by the CH1 antibody in skeletal muscle. TPM1κ localized in between two thick filaments as stained by MF20, presumably to the thin filament area of contracted skeletal muscle sarcomere. Negative staining controls consisting of identical stages of axolotl heart and skeletal muscle without primary antibody, with only rhodamine red conjugated anti-mouse and FITC conjugated anti-rabbit antibodies showed no detectable signals when visualized under identical settings. Peripheral staining of TPM1κ was seen in both axolotl heart and skeletal muscle. While we cannot exclude the possibility of localization of TPM1κ along the peripheries of cell, it is more likely an artifact. The stains were performed on whole hearts and skeletal muscles, which might lead to incomplete penetration of antibodies into multiple layers of cardiomyocytes.
Figure 5.
Confocal images of immunohistochemical staining of stage 42 embryonic axolotl hearts with TPM1κ antibody, CH1, a monoclonal antibody that detects all sarcomeric tropomyosin isoforms and MF20, a monoclonal sacrcomeric myosin antibody. (60X magnification)
a. TPM1κ staining of heart double-labeled with TPM1κ and MF20
b. MF20 staining of heart double-labeled with TPM1κ and MF20
c. Co-localization of TPM1κ (green) and MF20 (red)
d. Secondary antibody control for rhodamine red conjugated anti-mouse
e. TPM1κ staining of heart double-labeled with TPM1κ and CH1
f. CH1 staining of heart double-labeled with TPM1κ and CH1
g. Co-localization of TPM1κ (green) and CH1 (red)
h. Secondary antibody control of FITC conjugated anti-rabbit.
Figure 6.
Confocal images of immunohistochemical staining of embryonic axolotl skeletal muscle with TPM1κ antibody, CH1, a monoclonal antibody that detects all sarcomeric tropomyosin isoforms and MF20, a monoclonal sacrcomeric myosin antibody. (60X magnification)
a. TPM1κ staining of skeletal muscle double-labeled with TPM1κ and MF20
b. MF20 staining of skeletal muscle double-labeled with TPM1κ and MF20
c. Co-localization of TPM1κ (green) and MF20 (red)
d. Secondary antibody control for rhodamine red conjugated anti-mouse
e. TPM1κ staining of skeletal muscle double-labeled with TPM1κ and
f. CH1 staining of skeletal muscle double-labeled with TPM1κ and CH1
g. Co-localization of TPM1κ (green) and CH1 (red)
h. Secondary antibody control of FITC conjugated anti-rabbit
Figure 7.
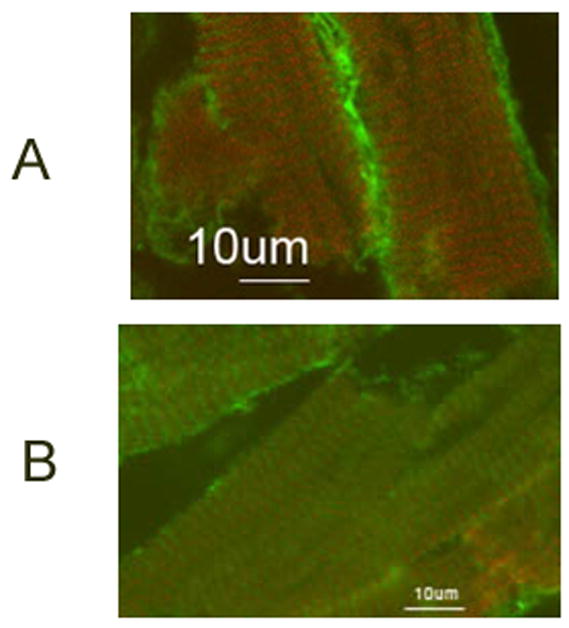
Higher magnification (100X) of confocal images of immunohistochemical staining of embryonic axolotl skeletal muscle with TPM1κ antibody, CH1, a monoclonal antibody that detects all sarcomeric tropomyosin isoforms and MF20, a monoclonal sacrcomeric myosin antibody.
a. Co-localization of TPM1κ (green) and CH1 (red)
b. Co-localization of TPM1κ (green) and MF20 (red)
We also stained paraffin embedded tissue sections of human heart obtained at autopsy studies with TPM1κ antibody. Expression and incorporation of TPM1κ into the myofibrillar bundles of the hypertrophied ventricular section was confirmed. It was uniformly distributed in all the bundles of the section (Figure 8).
Figure 8.
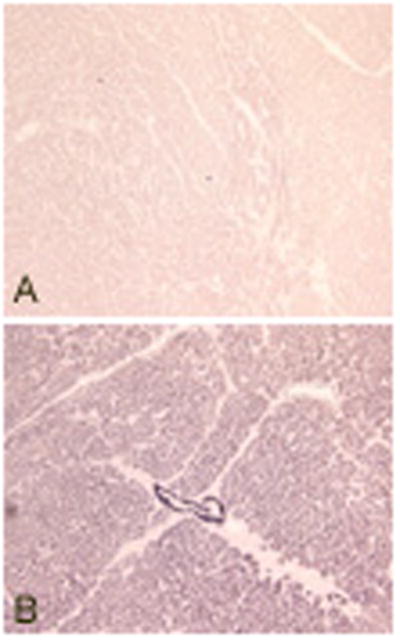
Paraffin embedded tissue sections of human heart stained with TPM1κ antibody.
Panel A: Negative control with no TPM1κ antibody
Panel B: Stained with TPM1κ antibody
Our previous studies have shown that TPM1κ is essential for myofibril organization. Inhibiting the expression of TPM1κ using an exon 2a specific anti-sense oligonucleotide led to disruption of myofibril organization, decreased cardiac contractility and lack of tropomyosin staining in normal axolotl heart [Zajdel et al., 2005]. Mexican axolotl has a cardiac mutant that has reduced expression of tropomyosin in the ventricle. Ectopic expression of TPM1κ protein promoted the formation of organized myofibrils and thus contractility of heart [Zajdel et al., 2002]. TPM1κ is also known to be expressed in human hearts. Transgenic mice over expressing TPM1κ in hearts show a dilated phenotype, as detected by echocardiogram. They also exhibit a significant decrease in rates of contraction and relaxation and decreased myofilament calcium sensitivity with no change in maximum developed tension. It is to be noted that these abnormalities do not lead to morphological changes as determined by histological analyses [Rajan et al., 2007, 2010]. These findings support the essential role of TPM1κ in myofibrillogenesis.
In this study we quantified TPM1κ mRNA expression in axolotl heart and skeletal muscle using qRT- PCR. Using a newly developed TPM1κ specific antibody, we demonstrated expression of TPM1κ protein in axolotl heart and skeletal muscle to further support its role in myofibrillogenesis and sarcomeric function. However the specific role TPM1κ in cardiac and sarcomeric myofibril formation and the role of interactions between various isoforms of TPM in this process remain to be elucidated. Further studies on TPM1κ expression at various transitory stages of myofibrillogenesis: premyofibrils to nascent myofibrils to mature myofibrils [Wang et al., 2007] and on knock-in and knock-out animal models will answer these questions.
Acknowledgments
Grant sponsors: American Heart Association; 02 550rrN to DKD, Children’s Miracle Network at Syracuse, NY; award # 8784 & 8821 to DKD, NHLBI grant; HL-71952 to DFW.
Axolotl embryos were provided by Ambystoma genetic stock center, University of Kentucky. Lexington, KY.
References
- Denz CR, Narshi A, Zajdel RW, Dube DK. Expression of novel cardiac-specific tropomyosin isoform in humans. Biochem Biophys Res Commun. 2004;320(4):1291–1297. doi: 10.1016/j.bbrc.2004.06.084. [DOI] [PubMed] [Google Scholar]
- Gunning P, O’neill G, Hardeman E. Tropomyosin-based regulation of the actin cytoskeleton in time and space. Physiol Rev. 2008;88:1–35. doi: 10.1152/physrev.00001.2007. [DOI] [PubMed] [Google Scholar]
- Humphrey RR. Genetic and experimental studies on a mutant gene (c) determining absence of heart action in embryos of the Mexican axolotl (Ambystoma mexicanum) Dev Biol. 1972;27(3):365–375. doi: 10.1016/0012-1606(72)90175-3. [DOI] [PubMed] [Google Scholar]
- Lees-Miller JP, Helfman DM. The molecular basis for tropomyosin isoform diversity. Bioessays. 1991;13:429–437. doi: 10.1002/bies.950130902. [DOI] [PubMed] [Google Scholar]
- Lemanski LF. Morphology of developing heart in cardiac lethal mutant Mexican axolotls, Ambystoma mexicanum. Dev Biol. 1973;33(2):312–333. doi: 10.1016/0012-1606(73)90140-1. [DOI] [PubMed] [Google Scholar]
- Lemanski LF. Role of tropomyosin in actin filament formation in embryonic salamander heart cells. J Cell Biol. 1979;82(1):227–238. doi: 10.1083/jcb.82.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luque EA, Spinner BJ, Dube S, Dube DK, Lemanski LF. Differential expression of a novel isoform of alpha-tropomyosin in cardiac and skeletal muscle of the Mexican axolotl (Ambystoma mexicanum) Gene. 1997;185(2):175–180. doi: 10.1016/s0378-1119(96)00606-3. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Kazzaz JA, Helfman DM. Functional properties of non-muscle tropomyosin isoforms. Curr Opin Cell Biol. 1994;6:96–104. doi: 10.1016/0955-0674(94)90122-8. [DOI] [PubMed] [Google Scholar]
- Rajan S, Jagatheesan G, D’Souza KM, Bulcao CF, Akhter SA, Boivin GP, Dube DK, Petrashevskaya N, Liggett SB, Karam CN, Solaro RJ, Wieczorek DF. Expression analysis and functional significance of a novel cardiac specific human tropomyosin in normal and cardiomyopathy patients [abstract] Circulation. 2007;116(2):143. [Google Scholar]
- Rajan S, Jagatheesan G, Karam CN, Alves ML, Bodi I, Schwartz A, Bulcao CF, D’Souza KM, Akhter SA, Boivin GP, Dube DK, Petrashevskaya N, Herr AB, Hullin R, Liggett SB, Wolska BM, Solaro RJ, Wieczorek DF. Molecular and functional characterization of a novel cardiac specific human tropomyosin isoform. Circulation. 2010 Jan 11; doi: 10.1161/CIRCULATIONAHA.109.889725. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinner BJ, Zajdel RW, McLean MD, Denz CR, Dube S, Mehta M, Choudhury A, Nakatsugawa M, Dobbins N, Lemanski LF, Dube DK. Characterization of a TM-4 type tropomyosin that is essential for myofibrillogenesis and contractile activity in embryonic hearts of the Mexican axolotl. J Cell Biochem. 2002;85(4):747–761. doi: 10.1002/jcb.10178. [DOI] [PubMed] [Google Scholar]
- Wang J, Sanger JM, Kang S, Thurston H, Abbott LZ, Dube DK, Sanger JW. Ectopic expression and dynamics of TPM1alpha and TPM1kappa in myofibrils of avian myotubes. Cell Motil Cytoskeleton. 2007;64(10):767–76. doi: 10.1002/cm.20221. [DOI] [PubMed] [Google Scholar]
- Zajdel RW, Denz CR, Lee S, Dube S, Ehler E, Perriard E, Perriard JC, Dube DK. Identification, characterization, and expression of a novel alpha-tropomyosin isoform in cardiac tissues in developing chicken. J Cell Biochem. 2003;89(3):427–439. doi: 10.1002/jcb.10504. [DOI] [PubMed] [Google Scholar]
- Zajdel RW, Denz CR, Narshi A, Dube S, Dube DK. Anti-sense-mediated inhibition of expression of the novel striated tropomyosin isoform TPM1kappa disrupts myofibril organization in embryonic axolotl hearts. J Cell Biochem. 2005;95(4):840–848. doi: 10.1002/jcb.20456. [DOI] [PubMed] [Google Scholar]
- Zajdel RW, McLean MD, Lemanski SL, Muthuchamy M, Wieczorek DF, Lemanski LF, Dube DK. Ectopic expression of tropomyosin promotes myofibrillogenesis in mutant axolotl hearts. Dev Dyn. 1998;213(4):412–420. doi: 10.1002/(SICI)1097-0177(199812)213:4<412::AID-AJA6>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Zajdel RW, Sanger JM, Denz CR, Lee S, Dube S, Poiesz BJ, Sanger JW, Dube DK. A novel striated tropomyosin incorporated into organized myofibrils of cardiomyocytes in cell and organ culture. FEBS Lett. 2002;520(1–3):35–39. doi: 10.1016/s0014-5793(02)02756-4. [DOI] [PubMed] [Google Scholar]