Abstract
Double umbilical cord blood transplantation with a reduced intensity regimen is an effective strategy for adult patients without matched donors. However, the risk of second cancers is not yet established. Ninety-eight adults with hematologic malignancies received a double umbilical cord blood transplant. Seventy patients received the reduced intensity regimen of fludarabine 30 mg/m2/day × 6 days, melphalan 100 mg/m2/day × 1 day, and rabbit antithymocyte globulin 1.5 mg/kg/day × 4 days, and 28 patients received an ablative total body radiation containing conditioning regimen. Sixty-three patients received sirolimus-based graft versus host disease prophylaxis and 35 patients received non-sirolimus based graft versus host disease prophylaxis. Median age was 48 (range 19-67) years. Eighteen patients developed a second malignancy at a median of 134 days after transplant. Sixteen patients had lymphoma and two patients had myelodysplasia/myeloproliferative disorder. Sixteen of these second cancers (both MDS/MPD and fourteen of the lymphomas) were donor derived; the origin of the others was not determined. GVHD prophylaxis, HLA matching, primary disease, age, total nucleated cell dose, and CD34+ cell dose were not associated with a higher rate of second malignancy. Second myeloid malignancies of donor origin occur after double umbilical cord blood transplantation, suggesting that a search for donor origin should be performed in all patients with suspected relapse.
Introduction
Umbilical cord blood is an alternative stem cell source for patients without matched related or unrelated donors. A projected disease-free survival of approximately 30-60% has been observed in adults receiving umbilical cord blood-derived hematopoietic stem cell transplantation (HSCT) using either a single cord blood product (1-4) or two cord blood products. (5-7) However, most of these transplantations utilize donors that are mismatched at multiple HLA antigens. Moreover, the product includes only naïve T cells, and those are present in small numbers. Thus, there is an inherent risk of post transplantation lymphoproliferative disorders (PTLD). Stimulation of limiting numbers of stem and progenitor cells may increase the risk of myelodysplasia and AML. (8) Finally, mutations have been identified in post-partum umbilical cord blood that are associated with the later development of leukemia. (9)
Second malignancies may be of recipient or more rarely donor origin. (10-13) Recent analyses of the risk of second malignancy after allogeneic stem cell transplantation suggest an incidence of 6-12%. (14-15) However, the risk of solid tumors does not appear to plateau. A retrospective study of 18,000 patients reported to the Center for International Blood and Marrow Transplant Research (CIBMTR) indicates that the incidence of PTLD was 1%, with 82% of cases occurring in the first year after transplant. (14) Risk factors for developing PTLD included Epstein Barr Virus (EBV) infection, HLA mismatch, use of antithymocyte globulin (ATG), T cell depletion, and chronic Graft versus Host Disease (GVHD). The incidence of PTLD after reduced-intensity double cord blood transplantation ranges from 3% to as high as 21%, especially if ATG is used in the conditioning regimen. (16)
Second myeloid malignancies are rare after allogeneic transplantation using living donor marrow or PBSC. There have been individual case reports of secondary myeloid malignancies of donor origin after allogeneic stem cell transplant; the incidence is estimated at less than <0.25%. (17-23)
Since relapse of the primary disease may be difficult to distinguish from donor derived secondary leukemia unless cytogenetic or molecular techniques are used, the actual incidence of donor derived second malignancies may be underreported. In this study, we describe the incidence and outcomes of secondary hematologic malignancies in 98 patients who received a double umbilical cord blood HSCT.
Materials and Methods
Patients
Consecutive patients with hematologic malignancies undergoing double cord blood transplantation were retrospectively analyzed. Patients were eligible for reduced intensity double cord blood HSCT if they had no 5/6 or 6/6 HLA-A, B, DR allele level matched related donor or no 6/6 HLA A, B, DR allele level matched unrelated donor. Patients with acute leukemia were eligible if they were in first remission with high-risk cytogenetics or in second or subsequent remission. Patients with refractory leukemia were not eligible. Patients with chemotherapy sensitive relapsed lymphoma were eligible. In addition, patients with myelodysplasia, chronic myelogenous leukemia refractory to tyrosine kinase inhibitors, or chronic lymphocytic leukemia progressive after at least two regimens were also eligible.
Conditioning Regimen and Graft versus Host Disease Prophylaxis
All reduced intensity patients received a conditioning regimen of fludarabine 30mg/m2/day on Days −8 through −3 (total dose 180 mg/m2), melphalan 100 mg/m2/day on Day −2, and rabbit antithymocyte globulin (Genzyme, Cambridge MA) 1.5 mg/kg/day on Days −7,-5,-3,-1 (total dose 6.0 mg/kg). Patients under age 45 with no comorbid conditions were eligible to receive a myeloablative conditioning regimen which consisted of fludarabine 25 mg/m2/day on Days -6,-5,-4 (total dose 75 mg/m2), cyclophosphamide 1800 mg/m2/day Days -6 and -5 (total dose 3600mg/m2), and total body radiation 14 Gy in 7 fractions Days -3,-2,-1,0. Four patients also received ATG in addition to the above regimen. Sixty-three patients received a sirolimus based GVHD prophylaxis with sirolimus (target trough serum concentration 3-12 ng/ml) and tacrolimus (target trough serum concentration 5-10 ng/ml) for GVHD prophylaxis. (24) GVHD prophylaxis was tapered after Day +100, if there was no evidence of acute GVHD. Thirty-five patients received GVHD prophylaxis with either tacrolimus as above or cyclosporine (CYA) continuous intravenous infusion beginning on Day −3 and mycophenolate mofetil (MMF) 15 mg/kg twice daily starting on Day 0, with tapering of MMF after Day +60 and CYA after Day+100, if there was not evidence of acute GVHD. (6) Eleven patients received parathyroid hormone at 100 mcg a day for 28 days after transplantation on a concomitant trial. The protocols were reviewed and approved by the Institutional Review Board of the Dana Farber/Harvard Cancer Center. Written informed consent was obtained from all patients.
Cord Unit Selection
Cord blood units were obtained from national and international registries. Cord blood units had to meet a minimum cell dose requirement of >1.5 × 107 NC/kg pre freeze for each individual unit and 3.7 × 107 NC/kg pre freeze for both units combined. Cord blood units had to be at least a 4/6 A, B, DR allele level match with the patient and with each other. HLA C typing was performed on the cord units but was not used in the match strategy. Cord units were washed with dextran and albumin and were infused sequentially 1 to 6 hours apart. (25)
Second Malignancies
All patients are followed for survival, relapse, and second cancers. When possible, donor origin of the second cancer was assessed by routine cytogenetics or chimerism studies. Chimerism assays of peripheral blood buffy coat isolates were performed by short tandem repeat (STR) analysis using a multiplex kit with primers for 10 different loci (Profiler Plus, Applied Biosystems, Foster City, CA), as previously described. (26) Patients were monitored for EBV every other week by DNA analysis, and rituximab was instituted for sustained levels greater than 1000.
Statistical analysis
The time and each outcome event were measured from Day 0 of cord blood infusion. Overall survival was measured until the date of death or was censored at the last follow-up for patients who were still alive. Disease-free survival was measured until the earlier date of relapse or death, while it was censored at the last follow-up for patients who were alive and free of relapse. Overall and disease-free survivals were estimated by the Kaplan-Meier method. Treatment-related mortality was based on the cumulative incidence estimated in the presence of relapse as a competing risk. (27) The absolute risk of second cancer was based on the cumulative incidence estimated by considering death as a competing risk using Gray's method. Patients who had not developed a second malignancy or died were censored at their last follow-up. To adjust for confounding, the associations between patient or cord unit characteristics and second cancer incidence were analyzed in a multivariate model. In particular, competing risks regression using the Fine and Gray method (28) was used to assess the joint effects of age, primary disease, conditioning regimen, GVHD prophylaxis, HLA matching, nucleated cell dose and CD34+ dose. The relative risk of developing a second cancer was based on the hazard ratio estimate, with the 95% confidence interval (95% CI) calculated by taking exponents of the 95% CI for the regression coefficient. SAS 9.1 was used to perform the general computations, while the cmprsk package in R version 2.5.1 was used in the analysis of competing risks. All p-values are based on two-sided hypothesis tests. Due to the modest sample size available for a multivariate model with several covariates, p-values <0.200 were considered to be suggestive of a trend within a hypothesis-generating framework.
Results
Patient Characteristics
Patient characteristics are outlined in Table 1. The median age of the 98 patients was 48 years (range 19-67 years). The majority of patients had acute leukemia (40 patients) or relapsed non-Hodgkin lymphoma (19 patients). Eighty percent of patients were Caucasian. Twenty percent of leukemia patients were considered standard risk and 80% high risk. The median follow-up was 29 months (range 6-69 months) with 43 patients still alive. Twenty-nine percent of patients received an ablative regimen. Patients with myelodysplasia, chronic myelogeneous leukemia and aplastic anemia were more common in the myeloablative group (56% vs 21%) whereas patients with lymphoma were more often treated with the reduced intensity regimen (28% vs 3%).
Table 1.
Patient Characteristics
| Median Age (range) | 48 years (19-67) | |
|---|---|---|
| Male Sex | 57% | |
| Malignancy | ||
| AML | 33 (34%) | |
| Non-Hodgkin lymphoma | 19 (19%) | |
| Hodgkin lymphoma | 8 (8%) | |
| Aplastic Anemia | 7 (7%) | |
| MDS | 12 (12%) | |
| CLL/PLL | 6 (6%) | |
| ALL | 6 (6%) | |
| CML | 4 (4%) | |
| Other | 3 (3%) | |
| Transplant Risk | ||
| Standard | 18 (20%) | |
| High Risk | 70 (80%) | |
| Conditioning Regimen | ||
| Reduced Intensity | 70 (71%) | |
| Myeloablative | 28 (29%) | |
| GVHD Prophylaxis | ||
| Sirolimus-based | 63 (64%) | |
| Non-sirolimus Based | 35 (36%) | |
Standard Risk: AML/ALL CR 1, MDS RA/RARS, CML CP1
High Risk: All others, excluding aplastic anemia and lymphoma
Cord Unit Characteristics
Cord unit characteristics are outlined in Table 2. The median nucleated cell (NC) dose infused for the combined two cord blood units was 4.4 × 107NC/kg (range 1.3-8.5). The median CD34+ dose infused for the combined two cord blood units was 2.4 × 105 CD34+ cells/kg (range 0.3-11.5). Seventy-six percent of patients received two 4/6 HLA A, B, DR allele level matched cords. Fourteen percent received one 4/6 and one 5/6 matched cord unit. The remaining 8% received two 5/6 matched cord units. No patients received a 6/6 matched cord unit.
Table 2.
Cord Blood Characteristics
Second Malignancies
Eighteen patients (18%) developed second malignancies, corresponding to an actuarial rate of 19% at 26 months after transplantation. The median time to second cancers was 134 days (range 39 days to 26 months). Thirteen of these patients died, all from second malignancy. Table 3 outlines the patients with second malignancies. Eleven patients in this study received parathyroid hormone after transplantation; one of these patients developed a second cancer. No solid tumors were observed in this population with a median follow-up after transplantation of 29 months.
Table 3.
Second Malignancies
| Primary Disease | Type of Second Cancer | Derivation | Outcomes | |
|---|---|---|---|---|
| AML | MDS/MPD | Donor | Fatal | |
| Hodgkin lymphoma | PTLD | Donor | Fatal | |
| Non-Hodgkin lymphoma (NHL) | MDS/MPD | Donor | Fatal | |
| Hodgkin lymphoma | PTLD | Unknown | Fatal | |
| CLL | PTLD | Donor | Fatal | |
| MDS | PTLD | Donor | Fatal | |
| AML | PTLD | Donor | Fatal | |
| MDS | PTLD | Donor | Alive, in CR | |
| NHL | PTLD | Donor | Fatal | |
| Hodgkin lymphoma | PTLD | Donor | Fatal | |
| AML | PTLD | Donor | Fatal | |
| AML | PTLD | Donor | Alive, in CR | |
| NHL | PTLD | Donor | Fatal | |
| NHL | PTLD | Donor | Fatal | |
| MDS | PTLD | Donor | Fatal | |
| Aplastic Anemia | PTLD | Donor | Alive, in CR | |
| Aplastic Anemia | PTLD | Donor | Alive, in CR | |
| CML | PTLD | Unknown | Alive, in CR | |
Epstein Barr Virus Lymphoproliferative Disorders
Sixteen patients had EBV-PTLD; 14 patients had donor derived disease as measured by chimerism assay, and the origin of the disease in the other two patients was not tested. In one patient, the bone marrow showed chimerism from one cord blood unit and the lung tissue with the lymphoproliferative disorder showed chimerism from the other cord blood unit. Post transplantation lymphoma developed at a median of 117 days after transplantation (range 39 to 368 days). The primary diseases were Hodgkin lymphoma (3), non-Hodgkin lymphoma (3), chronic lymphocytic leukemia (1), chronic myelogeneous leukemia (1), aplastic anemia (2), myelodysplasia (3), and acute myelogeneous leukemia (3). The median maximum EBV viral load was 9500 copies/ml (range 240-5.4 million). Thirteen patients had widespread organ involvement at the time of diagnosis, and two patients were treated for a rising EBV viral load. Twelve patients were treated with rituximab; three patients received rituximab and CHOP (cyclophosphamide, adriamycin, vincristine, and prednisone) therapy. Eight patients died with fulminant disease within 2 weeks of the diagnosis. Three patients also received EBV-specific cytotoxic T lymphocytes after failure of rituximab, and one of these patients is alive in a sustained remission. EBV PTLD resulted in the deaths of 11 patients.
Overall, five patients are alive and in remission, a median of 12 months from the diagnosis of PTLD. These surviving patients developed PTLD at a median of 5 months post transplant (range 4-12 months), similar to the general cohort of PTLD patients. The median EBV viral load in these 5 patients was 7000 (range 603-340,000). All five patients received rituximab, one patient received R-CHOP and one patient, as above, also received EBV-specific cytotoxic T lymphocytes.
Patients were monitored every other week until Day +180 for the development of EBV viremia. After Day +180, patients were monitored as clinically indicated. An additional 12 patients developed an EBV viremia, but did not progress to a lymphoproliferative disorder. The median EBV viral load for these patients was 500 copies/ml with a range of 200 to 3300 copies/ml. The EBV viremia occurred at a median of 89 days after transplantation (range 22-1460 days). Two of these patients received rituximab, as part of treatment for relapsed disease.
Second Cancer with Myelodysplasia/Myeloproliferative Disorder
Two patients developed myelodysplasia or myeloproliferative disorders, and both patients died of their second cancers. The actuarial rate of donor derived myeloid disease was 3%. Both patients received reduced intensity transplantations with the conditioning regimen of fludarabine, melphalan, and rabbit ATG. One occurred in a male patient transplanted for non-Hodgkin lymphoma at 21 months after double cord blood transplantation. He had been slow to engraft and was receiving filgrastim. He developed myelodysplasia with bone marrow showing 11% myeloblasts consistent with refractory anemia with excess blasts, and 100% XX cytogenetics. He was treated with 5 azacytidine without improvement. A second patient (female) was transplanted for AML, and developed a confirmed donor derived secondary MDS/MPD at 26 months after HSCT. Bone marrow revealed a hypercellular marrow with left shifted myeloid cells, erythroid dysplasia, and 100% XY cytogenetics. She was treated with steroids and splenectomy without improvement.
Both cases of donor derived second myeloid malignancies were reported to the cord blood banks, which provided the cord blood units. In one case, follow up revealed that both cord blood donors were healthy. No feedback was given from the cord blood banks in the second instance.
Predictors for Second Malignancies
Age, primary disease, conditioning regimen, GVHD prophylaxis, HLA matching, nucleated cell dose and CD34+ dose were studied in a multivariate analysis to determine independent predictors of second cancers (Table 4). Reduced intensity conditioning was associated with a trend towards increased incidence of second cancers compared to myeloablative regimens, 24% versus 7%, but the effect is confounded with the effect of ATG. ATG was used in all patients receiving a reduced intensity regimen, compared to only 4 (14%) patients receiving a myeloablative regimen. A reduced intensity regimen with ATG was associated with a trend towards an increased risk of developing a second cancer, but the effect was not significant. (p=0.23). Patients with primary Hodgkin lymphoma had a 38% incidence of second cancers that was higher than the 18% rate associated with other diseases, but this effect was also not significant (p=0.06). Older age, GVHD prophylaxis, HLA matching, total nucleated cell dose and CD34+ cell dose had no association with an increased incidence of second cancers. Both patients with donor derived MDS/MPD had received ATG but risk factors for MDS/MPD could not be determined due to the low patient numbers.
Table 4.
Predictors for Second Cancers
| Patient or Cord Unit Characteristic | Risk Groups | Cumulative Incidence | Hazard Ratio (95% CI) | p-value |
|---|---|---|---|---|
| Age | > 50 years | 26% | 1.8 (0.8-4.8) |
0.270 |
| ≤ 50 years | 14% | |||
| Primary disease | Hodgkin lymphoma | 38% | 2.8 (0.6-4.8) |
0.064 |
| Other malignancies | 18% | |||
| Conditioning Regimen | Reduced Intensity | 24% | 2.5 (0.6-10.6) |
0.230 |
| Myeloablative | 7% | |||
| GVHD prophylaxis | Sirolimus Based | 20% | 1.1 (0.5-2.9) |
0.800 |
| Other Agents | 18% | |||
| HLA matching | 4/6,5/6 or 5/6, 5/6 | 30% | 0.9 (0.3-2.6) |
0.350 |
| 4/6, 4/6 | 16% | |||
| Nucleated cell dose infused | > 4.4 × 107/kg | 21% | 1.5 (0.6-3.9) |
0.350 |
| ≤ 4.4 × 107/kg | 17% | |||
| CD34+ cell dose infused | ≤ 2.4 × 105/kg | 21% | 1.5 (0.6-3.6) |
0.380 |
| > 2.4 × 105/kg | 15% |
Survival
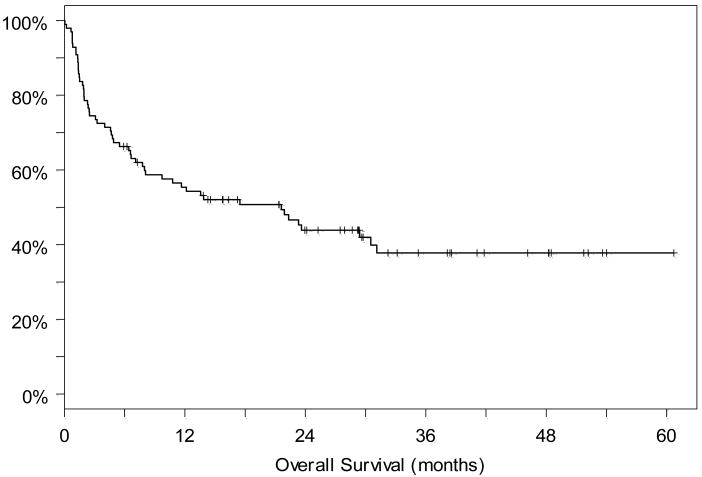
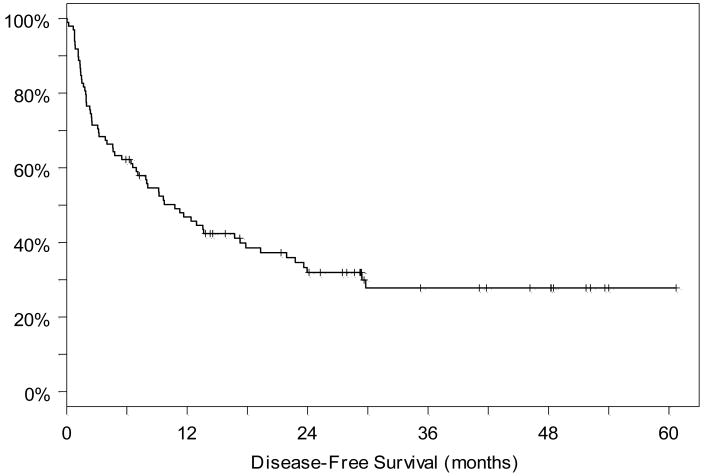
As reported previously, the reduced intensity CYA/MMF group had overall and disease-free survivals of 71% and 62% at one year, respectively. (6) Two year overall and disease-free survival was 57% and 57%. The reduced intensity group with either CYA/MMF or sirolimus/tacrolimus GVHD prophylaxis had overall and disease-free survivals of 63% and 51% respectively at one year and 48% and 33% at two years respectively. In the myeloablative group, overall and disease-free survivals were 35% and 35% at one and two years, respectively. For the entire cohort, 100 day transplant related mortality (TRM) was 26%, with infection the leading cause of death. The majority of deaths from second cancers occurred after 100 days and therefore these patients are not included in this 100-day TRM. With a median follow up of 29 months among the 43 patients still alive, the overall and disease-free survivals for the entire cohort were 55% and 47% at one year, and 44% and 32% at 2 years, respectively. (Figures 1 and 2) Twenty-four patients had relapse of their disease; in ten of these patients the relapse was documented by cytogenetics to be of recipient origin.
Figure 1.
Overall survival for the entire cohort from the time of transplantation.
Figure 2.
Disease-free survival for the entire cohort from the time of transplantation.
Discussion
Approximately 10,000 umbilical cord blood transplants have been performed worldwide, the majority for hematologic malignancies. (1-4) Recently, double umbilical cord blood transplantation has been employed in adult patients to shorten the engraftment times and reduce the risk of infections. (5-7, 29) While second malignancies have been reported after double cord blood transplantation, the magnitude of the risk is uncertain. We report a high incidence (19%) of second cancers, including a surprising 3% incidence of donor derived myeloid cancers.
As has been reported previously by the Minnesota group, we found a high incidence of PTLD in our double cord blood patients. (16) Our incidence of PTLD is higher than that reported in some double cord blood transplant series, but comparable to the incidence reported by the Minnesota group in patients receiving reduced intensity transplants with ATG. Brunstein and colleagues reported a 3% incidence of PTLD in patients who received a myeloablative conditioning regimen containing ATG (3% with ATG vs none with no ATG). However, the incidence of PTLD rose sharply when a reduced intensity preparative regimen was employed, to 21% with ATG vs 2% with no ATG. The increased incidence of PTLD after cord blood transplantation may be related to the absence of EBV specific memory T cells in cord blood grafts. (30) The increase with reduced intensity conditioning is not completely understood but may be related to the number of residual recipient B cells remaining after conditioning. (31) Although weekly or every other weekly EBV monitoring may help identify early viral reactivation, the PTLD after cord blood HSCT appears to be more aggressive than after solid organ transplantation, with rapid progression to multi organ involvement and lack of responsiveness to rituximab. (31,32) However, the risk of death in our series in patients who developed PTLD (69%) was similar to that seen in other stem cell transplantation series. For example, the German group reported 82% mortality in their stem cell transplant patients who developed PTLD. (33,34) PTLD after hematopoietic stem cell transplant of all sources has a poorer prognosis than PTLD after solid organ transplant.
In prior analyses in conventional stem cell transplantation, risk factors for PTLD include T cell depletion, HLA disparity, GVHD, and the use of ATG. (14,33,34) After the first year, however, the major risk factor identified is the presence of chronic GVHD. (14) In this cord blood cohort, no variables were found to be statistically significant, although reduced intensity conditioning with the use of ATG was associated with a trend to an increased risk of second malignancies. Intensive monitoring, lowering the dose of ATG, prompt use of EBV specific cytotoxic T cells, or adding rituximab to the transplantation regimen are strategies that might lower the incidence of PTLD. (35,36,37) For example, the Italian group employs a single dose of rituximab on Day +5 after cord blood transplantation. (38)
We did not find second solid tumors, although the follow-up in our series is too short at a median of 30 months after transplantation. Squamous cell cancers have also been reported with increased frequency in older patients with chronic GVHD, and some of these squamous cell cancers are of donor origin. (39-41)
The incidence of donor-derived MDS/MPD found here (3%) is higher than that reported in the literature after adult-donor bone marrow or peripheral blood stem cell transplantation (<0.25%). (17-23) Our incidence is higher than that reported in other cord blood transplant series; however, donor-derived myeloid malignancy may be underreported as donor derived AML would be difficult to distinguish from relapsed leukemia, in the absence of cytogenetic or chimerism data. (4,5,7) Donor derivation by chimerism or cytogenetics may not be routinely done after transplantation so the true incidence after cord blood or standard transplantation is unknown. The biology for the development of second cancers is unclear. Both patients in this series with donor derived MDS/MPD had received reduced intensity regimens containing ATG but the small numbers preclude determining any specific risk factors.
It is possible that donor cord blood may be more likely than adult blood to contain pre-leukemic cells and contribute to a first “hit” in the “two-hit” development of a second cancer. For example, the TEL-AML 1 translocation was detectable in 6 of 567 cord blood samples. (9) The investigators estimate that up to 5% of cord blood units may contain pre-leukemic clones. (42) The use of growth factors to drive a low number of viable hematopoietic stem cells may also foster pre leukemic mutations, as has been postulated to occur in patients with aplastic anemia. (8,43,44)
Comparison of the incidence of second myeloid malignancies seen after cord blood vs. adult-donor bone marrow or stem cell transplantation is difficult. In our institution, donor origin has not been routinely tested at the time of suspected relapse. We believe these findings, which have altered our own practices, would encourage other investigators to monitor donor origin for second cancers after all allogeneic, unrelated or cord blood transplantations.
Donor derived second myeloid malignancies present unique ethical issues, especially when the donor is an infant. A consistent system of testing for donor origin in all patients with suspected relapse would help distinguish relapsed disease from a donor derived second cancer. Should the donor and their parents be notified whenever a donor derived second malignancy is diagnosed? Should these donors have any special follow-up or monitoring? These issues will need to be addressed as we perform more cord blood transplants with modern technology that permits determination of donor origin.
Acknowledgments
Supported in part by National Heart, Lung and Blood Institute Grant U54HL081030 and PO1 CA142106
Footnotes
Financial Disclosure Statement: TS has received consulting fees from Genzyme.
YC is a Special Fellow in Clinical Research of the Leukemia Lymphoma Society
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Laughlin MJ, Eapen M, Rubinstein P, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl M Med. 2004;351:2255–2275. doi: 10.1056/NEJMoa041276. [DOI] [PubMed] [Google Scholar]
- 2.Rocha V, Labopin M, Sanz G, et al. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med. 2004;351:2276–2285. doi: 10.1056/NEJMoa041469. [DOI] [PubMed] [Google Scholar]
- 3.Eapen M, Rocha V, Scaradavou A, et al. Effect of stem cell source on transplant outcomes in adults with acute leukemia: a comparison of unrelated bone marrow (BM), peripheral blood (PB), and cord blood (CB) Blood. 2008;112:151a. abstract. [Google Scholar]
- 4.Takahashi S, Ooi J, Tomoran A, et al. Comparative single institute analysis of cord blood transplantation from unrelated donors with bone marrow or peripheral blood stem-cell transplants from related donors in adult patients with hematologic malignancies after myeloablative conditioning regimen. Blood. 2007;109:1322–1330. doi: 10.1182/blood-2006-04-020172. [DOI] [PubMed] [Google Scholar]
- 5.Brunstein CG, Barker JN, Weisdorf DJ, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110:3064–70. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballen KK, Spitzer TR, Yeap BY, et al. Double unrelated reduced-intensity umbilical cord blood transplantation in adults. Biol Blood Marrow Transplant. 2007;13:82–89. doi: 10.1016/j.bbmt.2006.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105:1343–47. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 8.Socie G, Mary JY, Schrezenmeier H, et al. Granulocyte-stimulating factor and severe aplastic anemia: a survey by the European Group for Blood and Marrow Transplantation (EBMT) Blood. 2007;109:2794–6. doi: 10.1182/blood-2006-07-034272. [DOI] [PubMed] [Google Scholar]
- 9.Mori H, Colman SM, Xiao Z, et al. Chromosome translocations and covert leukemic clones are generated during normal fetal development. Proc Natl Acad Sci USA. 2002;99:8242–8247. doi: 10.1073/pnas.112218799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curtis RE, Rowlings PA, Deeg HJ, et al. Solid cancers after bone marrow transplantation. N Engl J Med. 1997;336:897–904. doi: 10.1056/NEJM199703273361301. [DOI] [PubMed] [Google Scholar]
- 11.Lowe T, Bhatia S, Somlo G, et al. Second malignancies after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:1121–34. doi: 10.1016/j.bbmt.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Rizzo JD, Curtis RE, Socie G. Solid cancers after allogeneic hematopoietic stem cell transplantation. Blood. 2009;113:1175–83. doi: 10.1182/blood-2008-05-158782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooley LD, Sears DA, Udden MM, et al. Donor cell leukemia: report of a case occurring 11 years after allogeneic bone marrow transplantation and review of the literature. Am J Hematol. 2000;63:46–53. doi: 10.1002/(sici)1096-8652(200001)63:1<46::aid-ajh11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 14.Curtis RE, Travis LB, Rowlings PA, et al. Risk of lymphoproliferative disorders after bone marrow transplantation: a multi-institutional study. Blood. 1999;94:2208–2216. [PubMed] [Google Scholar]
- 15.Baker KS, DeFor TE, Burns LJ, et al. New malignancies after blood or marrow stem cell transplantation in children and adults: incidence and risk factors. J Clin Oncol. 2003;21:1352–58. doi: 10.1200/JCO.2003.05.108. [DOI] [PubMed] [Google Scholar]
- 16.Brunstein CG, Weisdorf DJ, DeFor T, et al. Marked increased risk of Epstein-Barr virus-related complications with the addition of antithymocyte globulin to a nonmyeloablative conditioning prior to unrelated umbilical cord blood transplantation. Blood. 2006;108:2874–2880. doi: 10.1182/blood-2006-03-011791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flynn CM, Kaufman DS. Donor cell leukemia; insight into cancer stem cells and the stem cell niche. Blood. 2007;109:2688–2692. doi: 10.1182/blood-2006-07-021980. [DOI] [PubMed] [Google Scholar]
- 18.Brunstein CB, Hirsch BA, Hammerschmidt D, et al. Leukemia in donor cells after allogeneic stem cell transplant. Bone Marrow Transplant. 2002;29:999–1003. doi: 10.1038/sj.bmt.1703577. [DOI] [PubMed] [Google Scholar]
- 19.Cutting RJ, Welch E, Channer J, et al. Myelofibrosis as the initial presentation of donor-derived myelodysplastic syndrome/AML: failure of a lasting response to a second allogeneic transplant from the original donor. Bone Marrow Transplant. 2008;42:631–33. doi: 10.1038/bmt.2008.224. [DOI] [PubMed] [Google Scholar]
- 20.Fraser CJ, Hirsch BA, Dayton V, et al. First report of donor cell-derived acute leukemia as a complication of umbilical cord blood transplantation. Blood. 2005;106:4377–4380. doi: 10.1182/blood-2005-06-2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ando T, Yujiri T, Mitani N, et al. Donor cell-derived acute myeloid leukemia after unrelated umbilical cord blood transplantation. Leukemia. 2006;20:744–745. doi: 10.1038/sj.leu.2404121. [DOI] [PubMed] [Google Scholar]
- 22.Matsunaga T, Murase K, Yoshida M, et al. Donor cell derived acute myeloid leukemia after allogeneic cord blood transplantation in a patient with adult T-cell lymphoma. Am J Hematol. 2005;79:294–298. doi: 10.1002/ajh.20349. [DOI] [PubMed] [Google Scholar]
- 23.Konuma T, Ooi J, Takahashi S, et al. Donor cell-derived myelodysplastic syndrome after cord blood transplantation. Bone Marrow Transplant. 2009;43:429–31. doi: 10.1038/bmt.2008.344. [DOI] [PubMed] [Google Scholar]
- 24.Cutler C, Mitrovich R, Kao G, et al. Double umbilical cord blood transplantation with reduced intensity conditioning and sirolimus-based GVHD prophylaxis. Blood. 2007;118:2006a. doi: 10.1038/bmt.2010.192. abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubinstein P, Dobrila L, Rosenfield RE, et al. Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proc Natl Acad Sci USA. 1995;92:10119–10122. doi: 10.1073/pnas.92.22.10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haspel RL, Kao G, Yeap BY, et al. Preinfusion variables predict the predominant unit in the setting of reduced-intensity double cord blood transplantation. Bone Marrow Transplant. 2008;41:523–9. doi: 10.1038/sj.bmt.1705933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gray R. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–54. [Google Scholar]
- 28.Fine JP, Gray RJ. A proportional hazard model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 29.Barker JN, Weisdorf DJ, DeFor TE, et al. Rapid and complete donor chimerism in adult recipients of unrelated donor umbilical cord blood transplantation after reduced intensity conditioning. Blood. 2003;102:1915–19. doi: 10.1182/blood-2002-11-3337. [DOI] [PubMed] [Google Scholar]
- 30.Clave E, Agbalika F, Bajzik V, et al. Epstein-Barr virus (EBV) reactivation in allogeneic stem-cell transplantation: relationship between viral load, EBV-specific T-cell reconstitution and rituximab therapy. Transplantation. 2004;77:76–84. doi: 10.1097/01.TP.0000093997.83754.2B. [DOI] [PubMed] [Google Scholar]
- 31.Wagner HJ, Cheng YC, Huls H, et al. Prompt versus preemptive intervention for EBV lymphoproliferative disease. Blood. 2004;103:3979–81. doi: 10.1182/blood-2003-12-4287. [DOI] [PubMed] [Google Scholar]
- 32.Knight JS, Tsodikov A, Cibrik DM, et al. Lymphoma after solid organ transplantation: risk, response to therapy, and survival at a transplantation center. J Clin Oncol. 2009;27:3354–3362. doi: 10.1200/JCO.2008.20.0857. [DOI] [PubMed] [Google Scholar]
- 33.Hou HA, Yao M, Tang JL, et al. Poor outcome in post transplant lymphoproliferative disorder with pulmonary involvement after allogeneic hematopoietic SCT: 13 years experience in a single institute. Bone Marrow Transplant. 2009;43:315–21. doi: 10.1038/bmt.2008.325. [DOI] [PubMed] [Google Scholar]
- 34.Ocheni S, Kroeger N, Zabelina T, et al. EBV reactivation and post transplant lymphoproliferative disorders following allogeneic SCT. Bone Marrow Transplant. 2008;42:181–6. doi: 10.1038/bmt.2008.150. [DOI] [PubMed] [Google Scholar]
- 35.Hale D, Waldmann H. Risk of developing Epstein-Barr virus-related lymphoproliferative disorders after T-cell depleted marrow transplants: CAMPATH users. Blood. 1998;91:3079–83. [PubMed] [Google Scholar]
- 36.Gustafsson A, Levitsky V, Zou JZ, et al. Epstein-Barr Virus (EBV) load in bone marrow transplant recipients at risk to develop posttransplant lymphoproliferative disease: prophylactic infusion of EBV- specific cytotoxic T cells. Blood. 2000;95:807–14. [PubMed] [Google Scholar]
- 37.Heslop HE. How I treat EBV lymphoproliferation. Blood. 2009;114:4002–4008. doi: 10.1182/blood-2009-07-143545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bacigalupo A, Locatelli F, Lanino E, et al. Fludarabine, cyclophosphamide, and anti-thymocyte globulin for alternative donor transplants in acquired severe aplastic anemia: a report from the EBMT-SAA Working Party. Bone Marrow Transplant. 2005;36:947–50. doi: 10.1038/sj.bmt.1705165. [DOI] [PubMed] [Google Scholar]
- 39.Gallagher G, Forest DL. Second solid cancers after allogeneic hematopoietic stem cell transplantation. Cancer. 2007;109:84–92. doi: 10.1002/cncr.22375. [DOI] [PubMed] [Google Scholar]
- 40.Curtis RE, Metayer C, Rizzo JR, et al. Impact of chronic GVHD therapy on the development of squamous-cell cancers after hematopoietic stem-cell transplantation: an international case-control study. Blood. 2005;105:3802–11. doi: 10.1182/blood-2004-09-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janin A, Murata H, Leboeuf C, et al. Donor-derived oral squamous cell carcinoma after allogeneic bone marrow transplantation. Blood. 2009;113:1834–1840. doi: 10.1182/blood-2008-07-171702. [DOI] [PubMed] [Google Scholar]
- 42.Greaves MF. Cord blood donor cell leukemia in recipients. Leukemia. 2006;20:1633–34. doi: 10.1038/sj.leu.2404293. [DOI] [PubMed] [Google Scholar]
- 43.Armand P, Antin JH. Allogeneic stem cell transplantation for aplastic anemia. Biol Blood Marrow Transplant. 2007;13:505–516. doi: 10.1016/j.bbmt.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 44.Ditschkowski M, Haferlach C, Schulte C, et al. Occurrence of AML in cells of donor origin after treatment of CML in relapse with imatinib and donor stem cell boost 16 years after the original allogeneic BMT. Bone Marrow Transplant. 2009;44:265–66. doi: 10.1038/bmt.2009.8. [DOI] [PubMed] [Google Scholar]