Abstract
Background
A pharmacokinetically derived schedule of flavopiridol administered as a 30 min intravenous bolus followed by 4-hour continuous intravenous infusion (IVB/CIVI) is active in fludarabine-refractory chronic lymphocytic leukemia, but no studies examining the feasibility and maximum tolerated dose of this schedule have been reported in acute leukemia.
Design and Methods
We conducted a phase I dose escalation trial of single-agent flavopiridol in adults with relapsed/refractory acute leukemias, utilizing a modification of the intravenous bolus/continuous intravenous infusion approach, intensifying treatment for administration on days 1, 2, and 3 of 21-day cycles.
Results
Twenty-four adults with relapsed/refractory acute myeloid leukemia (n=19) or acute lymphoblastic leukemia (n=5) were enrolled. The median age was 62 years (range, 23–78). The maximum tolerated dose of flavopiridol was 40mg/m2 intravenous bolus plus 60mg/m2 continuous intravenous infusion (40/60). The dose limiting toxicity was secretory diarrhea. Life-threatening hyperacute tumor lysis syndrome requiring hemodialysis on day 1 was observed in one patient. Pharmacokinetics were dose-dependent with increased clearance observed at the two highest dose levels. Diarrhea occurrence and severity significantly correlated with flavopiridol concentrations at the end of the 4-hour infusion, volume of distribution, and elimination half-life. Modest anti-leukemic activity was observed, with most patients experiencing dramatic but transient reduction/clearance of circulating blasts lasting for 10–14 days. One refractory acute myeloid leukemia patient had short-lived complete remission with incomplete count recovery.
Conclusions
Flavopiridol as a single agent given by intravenous bolus/continuous intravenous infusion causes marked, immediate cytoreduction in relapsed/refractory acute leukemias, but objective clinical responses were uncommon. With this schedule, the dose is limited by secretory diarrhea (ClinicalTrials.gov Identifier: NCT00101231).
Keywords: flavopiridol, acute leukemia, relapsed, refractory, pharmacokinetics
Introduction
Flavopiridol is a novel anti-cancer agent that broadly targets cyclin dependent kinases (CDK).1–3 Although it is currently synthetically produced, its chemical structure is identical to a product obtained from Dysoxylum binectariferum, a plant indigenous to India.4 Its mechanisms of action remain incompletely defined but include targeting of cyclin dependent kinases including the CDK9/cyclin T complex (preventing activation of RNA polymerase II),5–8 downregulation of Mcl-1 and other antiapoptotic proteins,9–11 induction of mitochondrial permeability changes,12 and others. Initial in vitro studies suggested that a long infusion schedule of administration would be most effective clinically, but Sausville and colleagues demonstrated a marked in vivo dose response curve with bolus administration of flavopiridol in human leukemia cells, compared to 72-hour continuous exposure.13 In this in vivo human leukemia xenograft model system, flavopiridol was shown to be most effective when given on a repeated bolus dosing schedule of administration.13
Clinically, a variety of different schedules of administration have been explored with flavopiridol in solid and hematologic malignancies including 72-hour continuous infusion,14, 15 24-hour continuous infusion,16, 17 and 1-hour bolus.18 Reports with these different schedules all noted short-duration neutropenia, diarrhea, cytokine release syndrome,19 and fatigue. No significant clinical activity was observed in phase II testing with single agent flavopiridol using the 72-hour infusion.20–23 Modest activity was noted in chronic lymphocytic leukemia24 and mantle cell non-Hodgkin’s lymphoma25 with a 1-hour bolus at 50 mg/m2 daily for three days. Notably, based on pre-clinical studies demonstrating the ability of flavopiridol to recruit leukemic cells into a proliferative state, increasing sensitivity to cytotoxic chemotherapy,26 significant clinical activity was seen in refractory acute leukemias with flavopiridol given as a 1-hour bolus followed by high-dose cytarabine and mitoxantrone in timed-sequential fashion.27,28
Flavopiridol is highly protein bound when in human serum, compared to protein binding seen in fetal bovine serum. This difference helps to explain the previous lack of clinical activity of flavopiridol with the continuous infusion schedules that targeted plasma concentrations based on in vitro cytotoxicity IC50s determined with fetal bovine serum-supplemented media. Considering the issue of low levels of free flavopiridol when in human serum, together with pharmacokinetic data derived from a previous negative study of flavopiridol given as a 24-hour infusion in chronic lymphocytic leukemia,17 a novel schedule of administration was designed to achieve and maintain target plasma levels predicted to be active in chronic lymphocytic leukemia from pre-clinical studies performed in human serum: 30-minute intravenous bolus (IVB) followed by 4-hour intravenous infusion (IVB/CIVI). This schedule, given for four of six weeks, is highly active in fludarabine refractory, genetically high-risk chronic lymphocytic leukemia.29, 30
We hypothesized that a similar schedule, intensified to administer the drug on three consecutive days given the experience from the human leukemia xenograft model system, would be active in relapsed/refractory acute leukemia. We designed a phase I dose escalation study to establish the maximum tolerated dose (MTD) and describe toxicities associated with single agent flavopiridol using the “hybrid” IVB/CIVI schedule of administration in this population.
Design and Methods
Eligibility criteria and study design
This study enrolled patients (≥18 years) with relapsed/refractory non-M3 acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL), between April 2005 and August 2007. Patients were required to have total bilirubin less than or equal to 2 x upper limit normal (ULN), creatinine less than or equal to 2.0 mg/dL, ALT/AST less than or equal to 5 x ULN, left ventricular ejection fraction at least 40%, and Eastern Cooperative Oncology Group performance status under or equal to 2. Active infection was permitted if controlled. Informed written consent approved by The Ohio State University Human Studies Committee was obtained on all patients prior to study entry.
Initially, the protocol required discontinuation of hydroxyurea 24 hours prior to the first dose of flavopiridol; however, due to tumor lysis occurring in one patient with high white blood cell count, the protocol was amended to allow hydroxyurea until the evening before flavopiridol was administered (but not within eight hours) for patients with highly proliferative disease. No other therapies were allowed within 30 days. Flavopiridol was given with the “hybrid” regimen of a 30 minute intravenous bolus (IVB) followed by a 4-hour continuous intravenous infusion (CIVI), daily for 3 days. A second cycle of treatment was permitted, based on a 21-day cycle, depending on cytoreduction. Dosing began at 20mg/m2 IVB and 30mg/m2 CIVI (20/30) and dose was escalated by approximately 25% increments following a classic 3+3 phase I design schema to determine the maximum tolerated dose of the schedule. After the dose limiting toxicity (DLT) was identified, additional patients were treated at the recommended phase II dose.
Adverse events were graded according to the National Cancer Institute (NCI) Common Toxicity Criteria for Adverse Events (CTCAE), version 3.0. Clinical responses were defined according to NCI published criteria as complete response (CR), complete remission with incomplete count recovery (CRi), or partial response (PR).31
Definition of dose limiting toxicity
Grade 3 to 4 non-hematologic toxicity attributable to the flavopiridol, except alopecia, fatigue, fever, deep vein thrombosis (DVT) at the site of the central line, or toxicities directly related to tumor lysis syndrome were defined as dose limiting toxicity. For hematologic toxicity, dose limiting toxicity was defined as failure to recover counts by day 42 [neutrophil (≥1,000/μL) and platelets (≥20×109/L without transfusion for seven additional days)] in patients with less than 5% blasts in the bone marrow. For patients with 5% blasts or under, failure to recover neutrophil and/or platelet count was not considered dose limiting toxicity. Dose limiting toxicity also included Grade 2 non-reversible non-hematologic toxicity except alopecia, fatigue, and fever according to the NCI CTCAE version 3.0 that was attributable to flavopiridol treatment. Dose limiting toxicity was defined with the first cycle of treatment.
Tumor lysis was not a dose limiting toxicity on this protocol, as this was an expected toxicity based on the chronic lymphocytic leukemia experience with flavopiridol given on this schedule of administration. In the event of severe tumor lysis syndrome, subsequent doses of flavopiridol were held until the patient recovered from the tumor lysis. During the study, a provision for re-treatment on days 4 and 6 (rather than days 2 and 3) was implemented for patients with severe tumor lysis.
Pharmacokinetic analysis
Plasma concentrations of flavopiridol and of flavopiridol-glucuronide metabolites (flavo-G) were measured on days 1–3 of the first cycle using a validated LC-MS/MS method as previously described.30,32 Flavo-G concentrations were determined with the use of a flavo-G standard and comparison of flavopiridol concentrations before and after sample treatment with β-glucuronidase as previously described.30,33 Sodium heparinized blood was obtained during the first dose of administration at the following time points: prior to dosing (t=0), and at 0.5, 1, 3, 4.5, 6, and 8 hours of treatment on day 1; prior to dosing, 0.5, and 4.5 hours on day 2; prior to dosing, and at 0.5, 4.5, 6, 8, and 24 hours of treatment on day 3. Calculated parameters were obtained using standard non-compartmental methods with WinNonlin version 3.0 (Pharsight, Mountain View, CA).
Statistical analysis
Descriptive statistics to include means, standard deviations, and frequencies were computed for pharmacokinetic variables. Student’s t-tests or analysis of variance (ANOVA) were used for pharmacokinetic comparisons with clinical outcomes.
Results
Patients’ characteristics and treatment groups
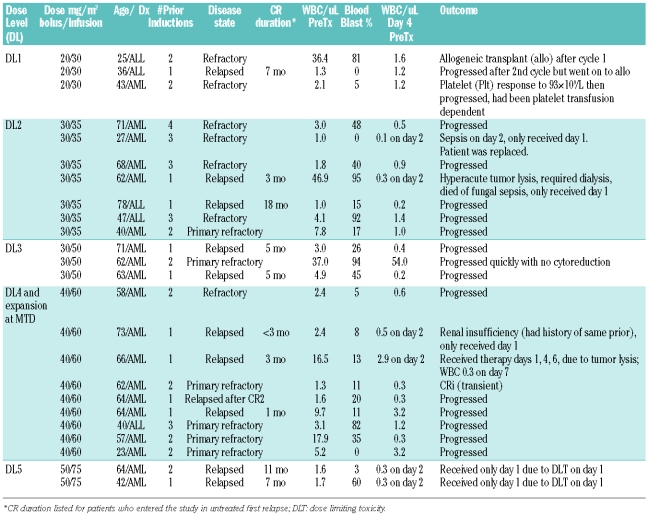
Twenty-four adults were treated on this phase I study; 19 with acute myeloid leukemia and 5 with acute lymphoblastic leukemia. The median age of patients was 62 years (range, 23–78). The median number of prior induction therapies was 2 (range 1–4). All patients had either relapsed or refractory acute leukemia. Eleven patients had relapsed disease, all with prior complete remission duration of less than one year except for a 76-year old acute lymphoblastic leukemia patient who relapsed beyond one year but who was still receiving intensification therapy at the time of relapse. Thirteen patients were refractory to the most recent prior therapy, including 6 patients with primary refactory disease who each entered the study after failure of at least 2 conventional regimens. Two patients entered the study with relapsed acute myeloid leukemia following prior donor stem cell transplantation (in CR1 and CR2, respectively). Five patients had secondary acute myeloid leukemia. Twenty patients had abnormal karyotype, 10 with adverse risk by Cancer and Leukemia Group B criteria.34,35 Additional patient data is shown in Table 1.
Table 1.
Patient data, dose level, and response.
Dose escalation
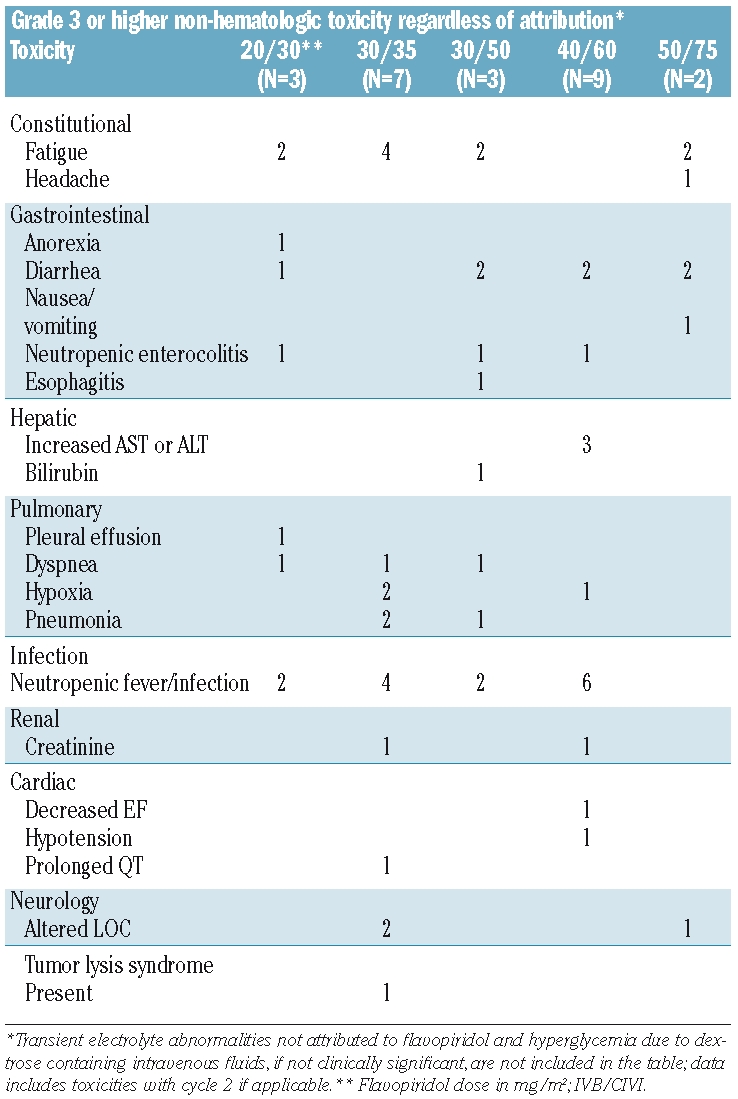
Dose was escalated from 20/30 up to 50/75. Three patients were treated at dose level 1 (DL1, 20/30). Seven patients were treated at DL2 (30/35). The cohort was expanded to 6 patients due to the occurrence of hyperacute tumor lysis in one patient, then one patient was replaced due to failure to complete the treatment (sepsis after day 1 of treatment). Three patients were treated at DL3 (30/50). Two patients had grade 3 diarrhea at this dose level (Table 2), but alternative causes of the diarrhea were present at the time of the event, and the toxicity did not recur on days 2 or 3 of treatment in either case. Nine patients were treated at DL3 (40/60) following initial expansion due to toxicity and subsequent treatment of additional patients in a maximum tolerated dose expansion. One patient at this dose level had grade 3 renal insufficiency, another had transient drug-related grade 3 elevations in AST/ALT that resolved within 72 hours and was not clinically significant. Two patients at this dose level had grade 3 diarrhea. Two patients were treated at DL5 (50/75); both had dose limiting diarrhea.
Table 2.
Toxicities.
Toxicities
The treatment approach was intensive, with universal pancytopenia, and toxicities were common as expected in this poor risk cohort of patients. A summary of grade 3 or higher non-hematologic toxicities regardless of attribution is listed in Table 2. The dose limiting toxicity was diarrhea, occurring on the first day of administration in both patients at DL5. Grade 2 diarrhea was common, occurring in 7 patients. Diarrhea attributed to flavopiridol had a typical pattern of onset within hours after initiation of treatment with cessation early in the evening of day 1. Interestingly, most patients self-reported a marked reduction in side effects including diarrhea on days 2 and 3 of administration as compared to day 1, though this does not appear to be reflected objectively in toxicity grading. Mucositis was infrequent, with serious mucositis occurring in only one patient (herpes simplex related in this case).
One patient at DL4 experienced transient grade 3 elevations in AST/ALT attributed to flavopiridol that were reversible (72 hours) and not clinically significant. Two others at that dose level had grade 3 AST/ALT, but in these cases the elevations were not felt to be drug related, including one patient with rising ALT/AST occurring together with a rapid rise in white blood cell count due to treatment failure three weeks after treatment. One patient experienced grade 3 hyperbilirubinemia due to progressive hepatosplenomegaly related to leukemic organ infiltration; pre-treatment bilirubin was 1.9mg/dL. One patient with refractory acute myeloid leukemia experienced hyperacute tumor lysis syndrome on DL2 (30/35); chemical tumor lysis with rise in lactate dehydrogenase at the time of falling white blood cell count was common across dose levels. Infection was a frequent and expected toxicity in this population of relapsed/refractory acute myeloid leukemia patients, with febrile neutropenia or infection occurring in 14 patients. Pulmonary toxicities described in Table 2 were of infectious etiology. One patient with a past history of drug-induced renal failure developed grade 3 creatinine after one dose of flavopiridol; he had the lowest creatinine clearance on the study pre-treatment.
Clinical responses
There was one objective response seen on the study. A patient with relapsed acute myeloid leukemia treated at DL3 (30/50) experienced a transient complete remission without platelet recovery (CRi). This response lasted only one month. Overall, marked cytoreduction of white blood cell count was frequent, with 20/24 patients (83%) experiencing at least 50% reduction (Table 1, assessed on the day after last dose of treatment). Unfortunately, reappearance of circulating blasts around day 14 of cycle 1 was typical. Two patients received a second course of therapy due to reduction in marrow blasts and/or perceived clinical benefit, but both progressed after cycle 2. Only one patient had bone marrow hypoplasia following cycle 1. Two patients went on to allogeneic transplantation following completion of protocol therapy.
Pharmacokinetics
Plasma samples were collected prior to dosing and at various times up to 72 hours after start of initial bolus infusion during the first course of treatment; flavopiridol and flavo-G concentrations were measured via LC-MS/MS. Data from 23 patients (a total of 60 concentration-time profiles) were available for analysis. Plots of concentration versus time data for both flavopiridol and flavo-G are shown in Figure 1.
Figure 1.
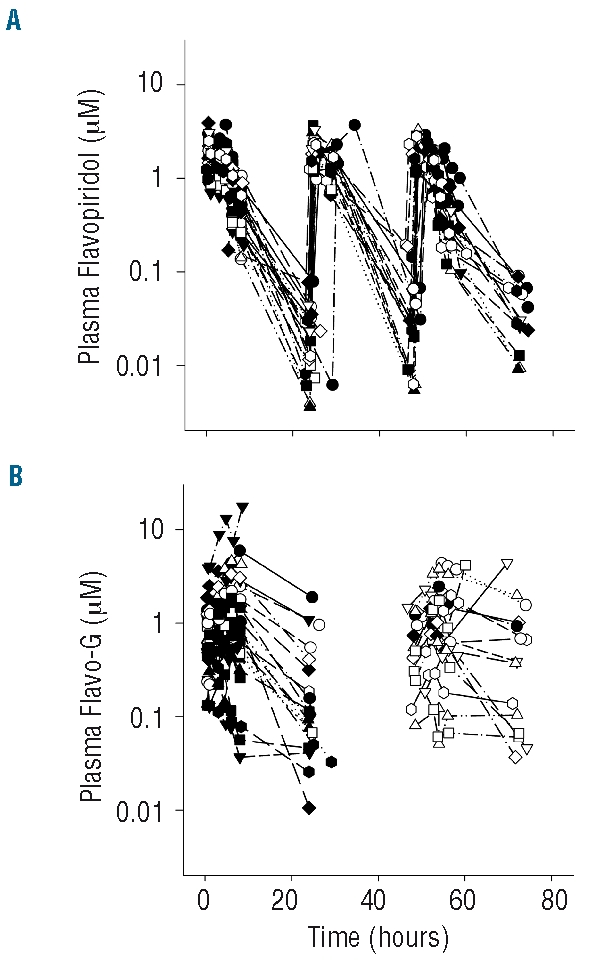
Flavopiridol pharmacokinetics. Plasma flavopiridol (A) and flavopiridol glucuronide (flavo-G, B) concentration-time profiles through 80 hours for cycle 1 in 23 of 24 patients with evaluable PK profiles (PK data from one patient extends through 150 hours due to dosing delays resulting from toxicity). Flavopiridol glucuronide concentrations were not determined for day 2.
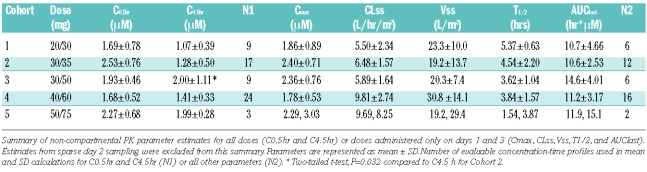
Concentration-time data was used to generate steady-state pharmacokinetic parameters via non-compartmental analysis for both flavopiridol and flavo-G. A summary of these parameters is presented in Table 3. Five doses, ranging from 50 to 125 mg/m2, were administered in this study. Mean plasma flavopiridol concentrations at the end of the 0.5 and 4 hour infusions (C0.5hr and C4.5hr, respectively) were similar to the previously reported chronic lymphocytic leukemia study with this “hybrid” schedule administered weekly, where a significant increase in C4.5hr was observed between the 30/30 and 30/50 dose groups.29,30 A statistically significant increase in C4.5hr was observed between these 2 dose groups in the current study (Cohorts 1 and 3, two-tailed t-test, P=0.03). However, C0.5hr and C4.5hr did not consistently increase with increasing doses between cohorts in the overall study among the 5 dose groups.
Table 3.
Flavopiridol pharmacokinetic parameters.
Inter-day pharmacokinetic parameter comparisons for days 1 and 3 were made with all individuals treated for three consecutive days (N=17). Flavopiridol accumulation was not evident from area under curve (AUC) in this analysis, as no significant increase in AUC was observed from day 1 (11.91±3.86 μM x h) to day 3 (12.169±3.10 μM x h, P=0.35, paired t-test, N=17). Higher trough concentrations were observed on day 3 (47±27 nM) compared to day 1 (28±23 nM, P=0.007, paired t-test) for the 4 dose groups evaluated (note: neither of the 2 patients given the highest 50/75 mg/m2 dose received day 2 or 3 treatment). Overall there was a significant difference in CL among the 5 dose groups (P=0.001, ANOVA). Mean CLss across the 50, 65 and 80 mg/m2 groups was 6.36±1.89 L/hr/m29, 30, while the mean CLss for the combined 100 and 125 mg/m2 groups was significantly higher at 9.72±2.60 L/hr/m2 (P=0.00004, Student’s t-test). Neither Vss or Cmax were significantly affected by dose (P>0.05, ANOVA).
Pharmacokinetics and toxicity
The dose limiting and primary toxicity in this study was secretory diarrhea. Although some patients experienced less severe diarrhea on day 3 compared to day 1 and subjectively the treatment was much better tolerated after day 1, the overall average diarrhea grade level did not change from day 1 to 3 for the full set of patients. Pre-treatment albumin level did not appear to affect pharmacokinetics/toxicity in this study. Pharmacokinetic parameters were evaluated for correlations with the occurrence and severity of diarrhea during course 1 of treatment. Mean pharmacokinetic parameters were calculated for each patient (means of days 1 and 3 for AUClast, CLss, Vss Cmax, T1/2, and means of days 1, 2 and 3 for C0.5hr and C4.5hr) and compared to the maximum diarrhea grading during the first course of treatment (days 1 through 3). Significant relationships were observed between diarrhea grade and C4.5hrs, T1/2 and AUClast (P<0.05, ANOVA) Vss trends downward as diarrhea grade increased, but the differences among the grades were not significant. Neither CL nor C0.5hrs correlated with diarrhea grade.
Previous studies have evaluated the relationship of diarrhea to the rate and extent of flavopiridol glucuronidation.30,36 To further evaluate this relationship, we quantified flavo-G levels, calculated flavo-G/flavopiridol AUC ratios, and compared among diarrhea grades, but we found no apparent relationship between this ratio and diarrhea.
Discussion
This study showed that single agent flavopiridol has early cytoreductive activity in acute leukemias, but only one objective response was seen (CRi) in this cohort of relapsed/refractory adult acute leukemia patients. The maximum tolerated dose was 40mg/m2 IV bolus over 30 min followed by 60mg/m2 IV over four hours, given on days 1, 2, 3. The dose limiting toxicity was secretory diarrhea, though other toxicities common to treatment of relapsed/refractory acute leukemias were frequent. Hyperacute tumor lysis syndrome was observed in one patient with refractory acute myeloid leukemia.
Limited pharmacokinetic evaluations have been reported for this “hybrid” dosing schedule, and no data is available in acute leukemia. The study in chronic lymphocytic leukemia previously reported by our group evaluated only 2 dose levels, 60 mg/m2 (30/30, IVB/CIVI) and 80 mg/m2 (40/40 and 30/50). Dose escalation in the chronic lymphocytic leukemia study was halted due to tumor lysis; the data from this study suggested possible non-linearity over this limited dose range. Non-linearity has been reported by Rudek and colleagues at doses greater than 50 mg/m2/d on a 72-hour infusion schedule.37 The validity of this observation is underscored by the large number of doses evaluated (13 doses, range 4–122.5 mg/m2). The increasing CL observed in our study is consistent with that reported by Rudek and colleagues. Their proposed explanations included a potential interaction with cholestyramine and/or upregulation of uridine glucuronosyltransferase (UGT) activity. Loperamide, a Pgp and cytochrome P-450 substrate, but not cholestyramine, was used to treat diarrhea in our study. Drug-drug interactions would not be expected with loperamide and flavopiridol, which is eliminated primarily by glucuronidation and biliary excretion of both parent and glucuronide metabolites.38–41 Additionally, our flavo-G data do not support the latter hypothesis, as we saw no indication of upregulation of UGT activity between days 1 and 3 (data not shown). Measureable increases in flavopiridol trough levels were observed in this study, although AUCs did not significantly change between days 1 and 3. Accumulation was not reported in previous studies with daily × 5 or daily × 3 1-hour infusion schedules.24, 41–43 The increasing trough levels are expected to be clinically insignificant given the relatively low trough concentrations (less than 100 nM troughs).
Secretory diarrhea was the dose limiting toxicity in this study. Significant correlations were identified between diarrhea severity and pharmacokinetic parameters, C4.5hr, AUClast and T1/2. While all clinical studies with flavopiridol have reported diarrhea as a frequent and potentially severe toxicity, no reports indicate strong correlations with flavopiridol pharmacokinetics. Innocenti and colleagues observed an inverse relationship between diarrhea occurrence and the ratio of flavopiridol glucuronide metabolite to flavopiridol,36 although our group failed to identify such a relationship in chronic lymphocytic leukemia.30 The observations in this current study with the “hybrid” dosing schedule in acute leukemias suggest severe diarrhea is tied most closely to flavopiridol end-of-infusion concentrations (C4.5hr).
We intensified the promising weekly “hybrid” (IVB/CIVI) schedule of flavopiridol administered successfully to chronic lymphocytic leukemia patients29 to give treatment on three consecutive days to patients with relapsed or refractory acute leukemias. This change was based on the knowledge that: 1) acute leukemia has a high proliferative rate that is less amenable to intermittent dosing than chronic lymphocytic leukemia; 2) drug-related neutropenia is of lesser concern in acute leukemia as standard chemotherapy treatment often produces cytopenias for 3–4 weeks; and 3) available clinical pharmacokinetics suggested little or no accumulation of drug would occur during the 3-day induction. The IVB/CIVI regimen given in this trial allowed administration of slightly higher total doses than previous schedules. Marked cytoreductive activity of flavopiridol as a single agent in acute myeloid leukemia was previously observed with a 1-hour bolus schedule of administration, in a study of flavopiridol followed by high-dose cytarabine and mitoxantrone as timed sequential therapy.28 In that phase II study, there was significant clinical activity noted including complete remissions (CRs). The CR rate of 75% (12/15) in previously untreated poor-risk patients was higher than expected, compared to previously published variations on the timed sequential therapy theme in the same patient population with a CR rate of 39–44%.28,44,45 Likewise, the regimen was active in first relapse (even if previous remission was of short duration), with 75% complete remission (18/24). Not unexpectedly, complete remission was uncommon in patients with primary refractory or multiply relapsed acute myeloid leukemia (CR rate 9%, 2/23).28 Though difficult to compare across phase I/II studies, in terms of early cytoreduction the IVB/CIVI schedule of single agent flavopiridol appears to be more active than the 1-hour bolus (as a single agent), as 83% of patients with the IVB/CIVI schedule had at least 50% reduction in white blood cell count by day 4 of treatment in the current study compared to 44% with the 1-hour bolus in the timed sequential therapy study noted above (before chemotherapy given).
The limited objective response rate observed here dampens enthusiasm for further work with flavopiridol as a single agent in acute leukemia. However, the observation of early rapid cytoreduction in acute leukemia is encouraging for further work with this drug in combination with other agents for patients eligible to receive intensive therapy. Indeed, studies with flavopiridol on this “hybrid” schedule of administration in combination with cytotoxic chemotherapy in acute leukemia are already being conducted by other investigator groups. Combination of flavopiridol with novel compounds that target anti-apoptotic pathways should also be pursued.
Acknowledgments
the authors thank all of the patients who participated in this trial as well as the dedicated nurses and nurse practitioners who cared for them in the James Cancer Hospital inpatient/outpatient Leukemia units and the Clinical Treatment Unit.
Footnotes
Funding: this work was supported by: NIH/NCI K23CA120708 (WB), NCI U01 CA 76576 (MRG), D. Warren Brown Foundation (JCB), and Specialized Center of Research, Leukemia and Lymphoma Society.
Authorship and Disclosures
WB was the principal investigator and takes primary responsibility for the paper together with MAP. WB, BR, RBK, SMD, GM and JCB recruited and/or treated the patients. MAP, DMR, WN, KAA, JMK, LL and PJ performed the pharmacokinetic work for this study. DML, AJ, JCB and MRG developed the novel dosing schedule employed. CK and LJS coordinated data and the trial operation.
The authors reported no potential conflicts of interest.
References
- 1.Carlson BA, Dubay MM, Sausville EA, Brizuela L, Worland PJ. Flavopiridol induces G1 arrest with inhibition of cyclin-dependent kinase (CDK) 2 and CDK4 in human breast carcinoma cells. Cancer Res. 1996;56(13):2973–8. [PubMed] [Google Scholar]
- 2.Losiewicz MD, Carlson BA, Kaur G, Sausville EA, Worland PJ. Potent inhibition of CDC2 kinase activity by the flavonoid L86-8275. Biochem Biophys Res Commun. 1994;201(2):589–95. doi: 10.1006/bbrc.1994.1742. [DOI] [PubMed] [Google Scholar]
- 3.Kaur G, Stetler-Stevenson M, Sebers S, Worland P, Sedlacek H, Myers C, et al. Growth inhibition with reversible cell cycle arrest of carcinoma cells by flavone L86-8275. J Natl Cancer Inst. 1992;84(22):1736–40. doi: 10.1093/jnci/84.22.1736. [DOI] [PubMed] [Google Scholar]
- 4.Naik RG, Kattige SL, Bhat SV, et al. An anti-inflamatory cum immunomodulatory. Tetrahedron. 1988;44:2081–6. [Google Scholar]
- 5.Chao SH, Price DH. Flavopiridol inactivates P-TEFb and blocks most RNA polymerase II transcription in vivo. J Biol Chem. 2001;276(34):31793–9. doi: 10.1074/jbc.M102306200. [DOI] [PubMed] [Google Scholar]
- 6.Chao SH, Fujinaga K, Marion JE, Taube R, Sausville EA, Senderowicz AM, et al. Flavopiridol inhibits P-TEFb and blocks HIV-1 replication. J Biol Chem. 2000;275 (37):28345–8. doi: 10.1074/jbc.C000446200. [DOI] [PubMed] [Google Scholar]
- 7.de Azevedo WF, Jr, Canduri F, da Silveira NJ. Structural basis for inhibition of cyclin-dependent kinase 9 by flavopiridol. Biochem Biophys Res Commun. 2002;293 (1):566–71. doi: 10.1016/S0006-291X(02)00266-8. [DOI] [PubMed] [Google Scholar]
- 8.Lam LT, Pickeral OK, Peng AC, Rosenwald A, Hurt EM, Giltnane JM, et al. Genomic-scale measurement of mRNA turnover and the mechanisms of action of the anti-cancer drug flavopiridol. Genome Biol. 2001;2(10):RESEARCH0041. doi: 10.1186/gb-2001-2-10-research0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gojo I, Zhang B, Fenton RG. The cyclin-dependent kinase inhibitor flavopiridol induces apoptosis in multiple myeloma cells through transcriptional repression and down-regulation of Mcl-1. Clin Cancer Res. 2002;8(11):3527–38. [PubMed] [Google Scholar]
- 10.Kitada S, Zapata JM, Andreeff M, Reed JC. Protein kinase inhibitors flavopiridol and 7-hydroxy-staurosporine down-regulate anti-apoptosis proteins in B-cell chronic lymphocytic leukemia. Blood. 2000;96(2):393–7. [PubMed] [Google Scholar]
- 11.Wittmann S, Bali P, Donapaty S, Nimmanapalli R, Guo F, Yamaguchi H, et al. Flavopiridol down-regulates antiapoptotic proteins and sensitizes human breast cancer cells to epothilone B-induced apoptosis. Cancer Res. 2003;63(1):93–9. [PubMed] [Google Scholar]
- 12.Hussain SR, Lucas DM, Johnson AJ, Lin TS, Bakaletz AP, Dang VX, et al. Flavopiridol causes early mitochondrial damage in chronic lymphocytic leukemia cells with impaired oxygen consumption and mobilization of intracellular calcium. Blood. 2008;111(6):3190–9. doi: 10.1182/blood-2007-10-115733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arguello F, Alexander M, Sterry JA, Tudor G, Smith EM, Kalavar NT, et al. Flavopiridol induces apoptosis of normal lymphoid cells, causes immunosuppression, and has potent antitumor activity In vivo against human leukemia and lymphoma xenografts. Blood. 1998;91(7):2482–90. [PubMed] [Google Scholar]
- 14.Thomas JP, Tutsch KD, Cleary JF, Bailey HH, Arzoomanian R, Alberti D, et al. Phase I clinical and pharmacokinetic trial of the cyclin-dependent kinase inhibitor flavopiridol. Cancer Chemother Pharmacol. 2002;50(6):465–72. doi: 10.1007/s00280-002-0527-2. [DOI] [PubMed] [Google Scholar]
- 15.Senderowicz AM, Headlee D, Stinson SF, Lush RM, Kalil N, Villalba L, et al. Phase I trial of continuous infusion flavopiridol, a novel cyclin-dependent kinase inhibitor, in patients with refractory neoplasms. J Clin Oncol. 1998;16(9):2986–99. doi: 10.1200/JCO.1998.16.9.2986. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz GK, O'Reilly E, Ilson D, Saltz L, Sharma S, Tong W, et al. Phase I study of the cyclin-dependent kinase inhibitor flavopiridol in combination with paclitaxel in patients with advanced solid tumors. J Clin Oncol . 2002;20(8):2157–70. doi: 10.1200/JCO.2002.08.080. [DOI] [PubMed] [Google Scholar]
- 17.Flinn IW, Byrd JC, Bartlett N, Kipps T, Gribben J, Thomas D, et al. Flavopiridol administered as a 24-hour continuous infusion in chronic lymphocytic leukemia lacks clinical activity. Leuk Res. 2005;29(11):1253–7. doi: 10.1016/j.leukres.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Tan AR, Headlee D, Messmann R, Sausville EA, Arbuck SG, Murgo AJ, et al. Phase I clinical and pharmacokinetic study of flavopiridol administered as a daily 1-hour infusion in patients with advanced neoplasms. J Clin Oncol. 2002;20(19):4074–82. doi: 10.1200/JCO.2002.01.043. [DOI] [PubMed] [Google Scholar]
- 19.Messmann RA, Ullmann CD, Lahusen T, Kalehua A, Wasfy J, Melillo G, et al. Flavopiridol-related proinflammatory syndrome is associated with induction of inter-leukin-6. Clin Cancer Res. 2003;9(2):562–70. [PubMed] [Google Scholar]
- 20.Aklilu M, Kindler HL, Donehower RC, Mani S, Vokes EE. Phase II study of flavopiridol in patients with advanced colorectal cancer. Ann Oncol. 2003;14(8):1270–3. doi: 10.1093/annonc/mdg343. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz GK, Ilson D, Saltz L, O'Reilly E, Tong W, Maslak P, et al. Phase II study of the cyclin-dependent kinase inhibitor flavopiridol administered to patients with advanced gastric carcinoma. J Clin Oncol. 2001;19(7):1985–92. doi: 10.1200/JCO.2001.19.7.1985. [DOI] [PubMed] [Google Scholar]
- 22.Stadler WM, Vogelzang NJ, Amato R, Sosman J, Taber D, Liebowitz D, et al. Flavopiridol, a novel cyclin-dependent kinase inhibitor, in metastatic renal cancer: a University of Chicago Phase II Consortium study. J Clin Oncol. 2000;18(2):371–5. doi: 10.1200/JCO.2000.18.2.371. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro GI, Supko JG, Patterson A, Lynch C, Lucca J, Zacarola PF, et al. A phase II trial of the cyclin-dependent kinase inhibitor flavopiridol in patients with previously untreated stage IV non-small cell lung cancer. Clin Cancer Res. 2001;7(6):1590–9. [PubMed] [Google Scholar]
- 24.Byrd JC, Peterson BL, Gabrilove J, Odenike OM, Grever MR, Rai K, et al. Treatment of relapsed chronic lymphocytic leukemia by 72-hour continuous infusion or 1-hour bolus infusion of flavopiridol: results from Cancer and Leukemia Group B study 19805. Clin Cancer Res. 2005;11(11):4176–81. doi: 10.1158/1078-0432.CCR-04-2276. [DOI] [PubMed] [Google Scholar]
- 25.Kouroukis CT, Belch A, Crump M, Eisenhauer E, Gascoyne RD, Meyer R, et al. Flavopiridol in untreated or relapsed mantle-cell lymphoma: results of a phase II study of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2003;21(9):1740–5. doi: 10.1200/JCO.2003.09.057. [DOI] [PubMed] [Google Scholar]
- 26.Karp JE, Ross DD, Yang W, Tidwell ML, Wei Y, Greer J, et al. Timed sequential ther-apy of acute leukemia with flavopiridol: in vitro model for a phase I clinical trial. Clin Cancer Res. 2003;9(1):307–15. [PubMed] [Google Scholar]
- 27.Karp JE, Passaniti A, Gojo I, Kaufmann S, Bible K, Garimella TS, et al. Phase I and Pharmacokinetic Study of Flavopiridol followed by 1-{beta}-D-Arabinofuranosyl-cytosine and Mitoxantrone in Relapsed and Refractory Adult Acute Leukemias. Clin Cancer Res. 2005;11(23):8403–12. doi: 10.1158/1078-0432.CCR-05-1201. [DOI] [PubMed] [Google Scholar]
- 28.Karp JE, Smith BD, Levis MJ, Gore SD, Greer J, Hattenburg C, et al. Sequential flavopiridol, cytosine arabinoside, and mitoxantrone: a phase II trial in adults with poor-risk acute myelogenous leukemia. Clin Cancer Res. 2007;13(15 Pt 1):4467–73. doi: 10.1158/1078-0432.CCR-07-0381. [DOI] [PubMed] [Google Scholar]
- 29.Byrd JC, Lin TS, Dalton JT, Wu D, Phelps MA, Fischer B, et al. Flavopiridol administered using a pharmacologically derived schedule is associated with marked clinical efficacy in refractory, genetically high-risk chronic lymphocytic leukemia. Blood. 2007;109(2):399–404. doi: 10.1182/blood-2006-05-020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phelps MA, Lin TS, Johnson AJ, Hurh E, Rozewski DM, Farley KL, et al. Clinical response and pharmacokinetics from a phase 1 study of an active dosing schedule of flavopiridol in relapsed chronic lymphocytic leukemia. Blood. 2009;113(12):2637–45. doi: 10.1182/blood-2008-07-168583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–9. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 32.Phelps MA, Rozewski DM, Johnston JS, Farley KL, Albanese KA, Byrd JC, et al. Development and validation of a sensitive liquid chromatography/mass spectrometry method for quantitation of flavopiridol in plasma enables accurate estimation of pharmacokinetic parameters with a clinically active dosing schedule. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;868(1–2):110–5. doi: 10.1016/j.jchromb.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramirez J, Iyer L, Journault K, Belanger P, Innocenti F, Ratain MJ, et al. In vitro characterization of hepatic flavopiridol metabolism using human liver microsomes and recombinant UGT enzymes. Pharm Res. 2002;19(5):588–94. doi: 10.1023/a:1015341726183. [DOI] [PubMed] [Google Scholar]
- 34.Byrd JC, Mrozek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100(13):4325–36. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 35.Wetzler M, Dodge RK, Mrozek K, Carroll AJ, Tantravahi R, Block AW, et al. Prospective karyotype analysis in adult acute lymphoblastic leukemia: the cancer and leukemia Group B experience. Blood. 1999;93(11):3983–93. [PubMed] [Google Scholar]
- 36.Innocenti F, Stadler WM, Iyer L, Ramirez J, Vokes EE, Ratain MJ. Flavopiridol metabolism in cancer patients is associated with the occurrence of diarrhea. Clin Cancer Res. 2000;6(9):3400–5. [PubMed] [Google Scholar]
- 37.Rudek MA, Bauer KS, Jr, Lush RM, 3rd, Stinson SF, Senderowicz AM, Headlee DJ, et al. Clinical pharmacology of flavopiridol following a 72-hour continuous infusion. Ann Pharmacother. 2003;37(10):1369–74. doi: 10.1345/aph.1C404. [DOI] [PubMed] [Google Scholar]
- 38.Jager W, Zembsch B, Wolschann P, Pittenauer E, Senderowicz AM, Sausville EA, et al. Metabolism of the anticancer drug flavopiridol, a new inhibitor of cyclin dependent kinases, in rat liver. Life Sci. 1998;62(20):1861–73. doi: 10.1016/s0024-3205(98)00152-0. [DOI] [PubMed] [Google Scholar]
- 39.Jager W, Gehring E, Hagenauer B, Aust S, Senderowicz A, Thalhammer T. Biliary excretion of flavopiridol and its glucuronides in the isolated perfused rat liver: role of multidrug resistance protein 2 (Mrp2) Life Sci. 2003;73(22):2841–54. doi: 10.1016/s0024-3205(03)00699-4. [DOI] [PubMed] [Google Scholar]
- 40.Jager W, Gehring E, Hagenauer B, Aust S, Senderowicz A, Thalhammer T. The role of hepatic Mrp2 in the interaction of flavopiridol and bilirubin: impact on therapy. Int J Clin Pharmacol Ther. 2003;41(12):610–1. doi: 10.5414/cpp41610. [DOI] [PubMed] [Google Scholar]
- 41.Zhai S, Sausville EA, Senderowicz AM, Ando Y, Headlee D, Messmann RA, et al. Clinical pharmacology and pharmacogenetics of flavopiridol 1-h i.v. infusion in patients with refractory neoplasms. Anticancer Drugs. 2003;14(2):125–35. doi: 10.1097/00001813-200302000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Tan AR, Yang X, Berman A, Zhai S, Sparreboom A, Parr AL, et al. Phase I trial of the cyclin-dependent kinase inhibitor flavopiridol in combination with docetaxel in patients with metastatic breast cancer. Clin Cancer Res. 2004;10(15):5038–47. doi: 10.1158/1078-0432.CCR-04-0025. [DOI] [PubMed] [Google Scholar]
- 43.Burdette-Radoux S, Tozer RG, Lohmann RC, Quirt I, Ernst DS, Walsh W, et al. Phase II trial of flavopiridol, a cyclin dependent kinase inhibitor, in untreated metastatic malignant melanoma. Invest New Drugs. 2004;22(3):315–22. doi: 10.1023/B:DRUG.0000026258.02846.1c. [DOI] [PubMed] [Google Scholar]
- 44.Bolanos-Meade J, Karp JE, Guo C, Sarkodee-Adoo CB, Rapoport AP, Tidwell ML, et al. Timed sequential therapy of acute myelogenous leukemia in adults: a phase II study of retinoids in combination with the sequential administration of cytosine arabinoside, idarubicin and etoposide. Leuk Res. 2003;27(4):313–21. doi: 10.1016/s0145-2126(02)00177-7. [DOI] [PubMed] [Google Scholar]
- 45.Geller RB, Burke PJ, Karp JE, Humphrey RL, Braine HG, Tucker RW, et al. A two-step timed sequential treatment for acute myelocytic leukemia. Blood. 1989;74(5):1499–506. [PubMed] [Google Scholar]