Abstract
Background
Allogeneic hematopoietic stem cell transplantation is an effective treatment for patients with poor risk lymphoma, at least in part because of the graft-versus-lymphoma effect. Over the past decade, reduced intensity conditioning regimens have been shown to offer results similar to those of conventional high-dose conditioning regimens but with lower toxicity early after transplantation, especially in patients with chemosensitive disease at transplant.
Design and Methods
The aim of this study was to analyze the long-term outcome of patients with follicular lymphoma who received an HLA identical sibling allogeneic stem cell transplant with a reduced intensity conditioning regimen within prospective trials. The prospective multicenter studies considered included 37 patients with follicular lymphoma who underwent allogeneic stem cell transplantation between 1998 and 2007 with a fludarabine plus melphalan-based reduced intensity conditioning regimen.
Results
The median age of the patients was 50 years (range, 34–62 years) and the median follow-up was 52 months (range, 0.6 to 113 months). Most patients (77%) had stage III-IV at diagnosis, and patients had received a median of three lines of therapy before the reduced intensity conditioning allogeneic stem cell transplantation. At the time of transplantation, 14 patients were in complete remission, 16 in partial remission and 7 had refractory or progressive disease after salvage chemotherapy. The 4-year overall survival rates for patients in complete remission, partial remission, or with refractory or progressive disease were 71%, 48% and 29%, respectively (P=0.09), whereas the 4-year cumulative incidences of non-relapse mortality were 26% (95% CI, 11–61), 33% (95% CI, 16–68) and 71% (95% CI, 44–100), respectively. The incidence of relapse for the whole group was only 8% (95% CI, 2–23).
Conclusions
We conclude that this strategy of reduced intensity conditioning allogeneic stem cell transplantation may be associated with significant non-relapse mortality in heavily pre-treated patients with follicular lymphoma, but a remarkably low relapse rate. Long-term survival is likely in patients without progressive or refractory disease at the time of transplantation.
Keywords: follicular lymphoma, reduced intensity conditioning, HLA identical sibling donor, allogeneic stem cell transplantation, graft-versus-host disease
Introduction
Among patients with follicular lymphoma (FL), allogeneic hematopoietic stem cell transplantation (SCT) was initially used in those patients who relapsed after autologous SCT or who were not candidates for autologous SCT because of the extent of their disease or marrow involvement. The results of several retrospective, comparative studies between allogenic and autologous SCT suggested that allogenic SCT was associated with a very low relapse rate and might be a curative option for FL.1–4 The presumed advantage of allogeneic SCT was attributed in part to a graft-versus-lymphoma (GVL) effect. This presumption was supported by several studies, which showed very low rates of relapse.1–7 However, the benefit of allogeneic SCT was counteracted by a high non-relapse mortality (NRM) rate.1,4 With the aim of reducing NRM while retaining the GVL effect, reduced intensity conditioning (RIC) regimens were developed for allogeneic SCT.8,9
RIC extends the indication for allogeneic SCT to older and less medically fit patients. This strategy has shown comparable results to high-dose conditioning regimens for several hematologic diseases.10–12 A recent report from the CIBMTR registry also supports the use of RIC allogeneic SCT in patients with FL.9 However, the risk of organ toxicity and infections associated with graft-versus-host disease (GVHD) is still a major concern in this setting. Robinson et al. reported a disappointing 39% NRM and 29% progression-free survival at just 1 year for patients with FL after RIC allogeneic SCT.12 In line with such results, restrospective studies by Vigouroux et al. and Rezvani et al. also reported high NRM rates in patients with low-grade lymphoma following various RIC allogeneic SCT strategies.6,13
The anti-tumor effect of RIC allogeneic SCT is based at least partly on the GVL effect, which is usually associated with GVHD, especially chronic GVHD; the presence of a GVL effect in FL is suggested by the encouraging results reported by Khouri et al. for patients with chemosensitive FL conditioned with a fludarabine, cyclophosphamide and rituximab-based non-myeloablative protocol, without T-cell depletion in most cases: the estimated overall survival and progression-free survival rates of these patients were 85% and 83%, respectively, at a median follow-up of 60 months.14 The French group also reported a very low relapse rate (9%) after a long follow-up in patients with low-grade lymphoma who received various RIC allogeneic SCT protocols.6 In contrast, in a study by Morris et al.,15 the incidence of relapse among patients who received a RIC allogeneic SCT protocol which incorporated in vivo T-cell depletion with alemtuzumab, in combination with fludarabine and melphalan, was relatively high (44%).
The published studies on RIC allogeneic SCT in FL are mostly retrospective and often include several different conditioning regimens or have a short follow-up. The purpose of the current study was to determine the long-term outcome of patients with advanced FL who were entered in consecutive prospective RIC allogeneic SCT trials carried out in Spain; in contrast to many previous studies, a homogeneous conditioning regimen was used in these trials, and the GVHD prophylaxis, donor type and stem cell source were also homogeneous.
Design and Methods
Patients
The current study includes 37 patients diagnosed with FL grade I to III who were included in two prospective protocols, which were run in parallel in nine Spanish stem cell transplantation centers from October 1999 to August 2007. Twenty-five (68%) of the 37 patients were included in the prospective trial registered as MINIALO-99-00/270, in which the dose of melphalan used was 140 mg/m2, while the remaining 12 (32%) patients were included in the GELTAMO-99-0151 study in which the dose of melphalan was 80 mg/m2. Patients with relapsed or refractory FL who were not candidates for autologous transplantation and who had a suitable HLA identical related donor were eligible for inclusion in the study. Patients were required to have an ECOG performance status score of 2 or lower, serum bilirubin lower than 2 mg/dL, serum creatinine lower than 1.6 mg/dL, no symptomatic cardiac or pulmonary disease, and no active infection. All participants gave written informed consent to participation in the studies, which were approved by the national and local ethics committees. All data records were obtained from the Grupo Español de Linfomas/Transplante de Médula Osea (GELTAMO) database, and were completed by each center. All donors were HLA-identical siblings and the source of stem cells was granulocyte colony-stimulating factor-mobilized peripheral blood stem cells in all cases.
Conditioning and graft-versus-host disease prophylaxis
The conditioning regimen used has been described in detail elsewhere.16 Briefly, fludarabine (125–150 mg/m2) was combined with melphalan (80–140 mg/m2). GVHD prophylaxis consisted of cyclosporine A from day -7 plus methotrexate (10 mg/m2), administered on days +1, +3, and +6 and, in five cases, also on day +11. All patients received standard antimicrobial prophylaxis during the early post-transplant period.
Hematopoietic recovery, graft-versus-host disease assessment and chimerism analyses
Neutrophil and platelet engraftment were defined as the first of three consecutive days with an absolute neutrophil count of greater than 0.5×109/L and an untransfused platelet count of greater than 20×109/L, respectively.
The diagnosis of acute GVHD was based on the classical clinical presentation with confirmatory pathological findings in all patients. Acute and chronic GVHD were assessed and graded according to published criteria.17,18
Chimerism was evaluated between days 21 and 28 after transplantation and every 15 days thereafter until complete T-cell donor chimerism was achieved. The chimerism analyses were done in separated T cells and granulocytes from peripheral blood in most patients (n=28). Chimerism was determined using polymerase chain reaction analysis of informative minisatellite loci, as previously described.19 Complete donor chimerism was defined as the presence of at least 95% donor DNA in the sample analyzed.
Clinical evaluation and response assessment
Patients underwent computed tomography of the chest, abdomen and pelvis, and bone marrow aspiration and biopsies with immunohistochemical and flow cytometric immunophenotyping analysis. Disease stage was evaluated using the Ann Arbor criteria. Patients were evaluated for disease stage and response at 3, 6, and 12 months after transplantation and every 6 months thereafter. Responses were evaluated according to standard criteria for patients with lymphoma, as described by Cheson et al.20 A complete response was defined as the complete disappearance of all detectable clinical, pathological (i.e. bone marrow) and radiographic evidence of disease, and all disease-related symptoms as well as the normalization of all biochemical abnormalities. A partial response was defined as a decrease of at least 50% of all measurable lesions. Stable disease was defined as no response or a less than 50% decrease in measurable lesions. Progressive disease was defined as at least a 50% increase of any measurable lesion or appearance of any new lesion during or after therapy.
Statistical analysis
The primary end-point of this study was to analyze the long-term overall survival after RIC allogeneic SCT. Secondary end-points were to analyze the regimen-related toxicity, disease-free survival, relapse rate, and NRM, as well as to verify the impact of possible prognostic factors for these outcomes. The incidences of acute and chronic GVHD, NRM and relapse were calculated using cumulative incidence estimates, taking into account competing risks.21 The probability of overall survival was estimated from the time of transplantation using Kaplan-Meier curves22 and compared using the Tarone-ware and log rank tests. Median times were compared by Wilcoxon’s rank sum test. Risk factors for NRM were estimated using a univariate Cox regression model; multivariate analyses were not done because of the small number of patients. All tests of significance were two-sided, with P levels of 0.05 or less being considered statistically significant. All statistical analyses were performed using SPSS version 15.0 (SPSS, Chicago, IL, USA), with the exception of the cumulative incidence analyses, which were carried out with NCSS 2004 (Number Cruncher Statistical System, Kaysville, UT, USA).
Results
Patients’ characteristics
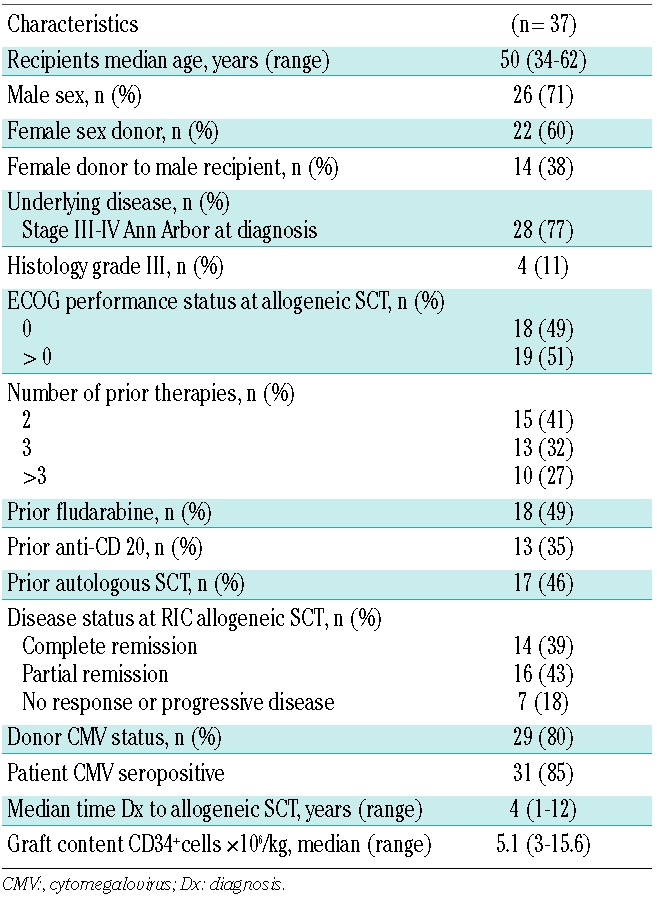
The patients’ characteristics are summarized in Table 1. We first compared the patients’ characteristics, toxicity profile and outcomes according to the melphalan dose, and found no differences in conditioning-related toxicity, engraftment kinetics, occurrence and timing of GVHD, or any post-transplant outcomes (data not shown). There were 26 (71%) male patients with a median age of 50 years (range, 34 to 62 years). The time from diagnosis to transplantation ranged from 1 to 12 years (median of 4 years). Most patients had been heavily pretreated (59% of patients had received three or more lines of chemotherapy) prior to the RIC allogeneic SCT. Thirty patients (82%) were transplanted with sensitive disease and 18% with refractory disease. Fourteen patients transplanted in complete remission had been heavily pretreated with a median of three lines (range, 2 to 5 lines) of systemic therapy, and 46% of the patients had previously undergone autologous SCT.
Table 1.
Patients’ characteristics before RIC allogeneic stem cell transplantation.
Toxicity
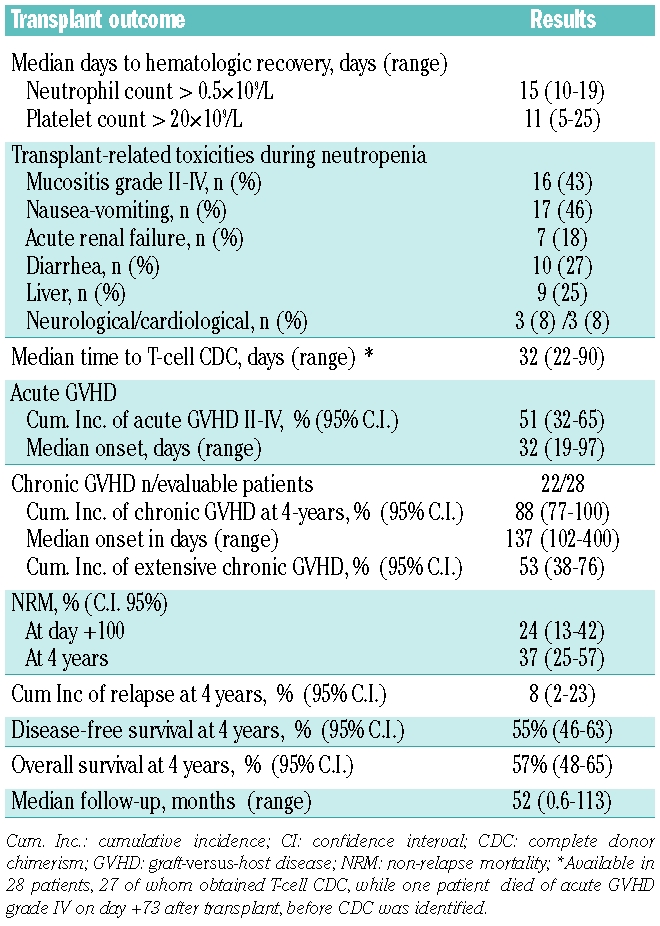
As shown in Table 2, the main conditioning-related toxicity was gastro-intestinal. Seventeen (46%) patients suffered nausea or vomiting, but only six had grade 2 emesis and one had grade 3. Mucositis grades II-IV was observed in 16 (43%) of the 37 patients, and diarrhea in 10 (27%) cases, which was grade 3 in only one case. Overall, only six (16%) patients had grade III to IV toxicity. Only one patient died from toxicity directly attributed to the conditioning regimen (death on day +18 from a severe sinusoidal obstruction syndrome).
Table 2.
Toxicity, hematopoietic recovery and transplant outcome.
Hematopoietic recovery and chimerism analyses
The median times of neutrophil and platelet recovery as well as other post-transplant outcomes are shown in Table 2. Information on peripheral blood leukocyte subset chimerism was available for 28 patients. Twenty-seven (96%) of the 28 patients studied achieved complete donor T-cell chimerism at a median time of 32 days (range, 23–90 days) after transplantation.
Graft-versus-host disease
The details on the characteristics and incidence of acute and chronic GVHD are shown in Table 2. Acute GVHD grades I-IV was diagnosed in 19 (53%) of 36 evaluable patients with a median onset at 32 days (range, 19–97 days). Five patients had grade IV acute GVHD, three had grade III, nine had grade II and two developed grade I acute GVHD. Steroid-refractory acute GVHD occurred in six (31%) of the 19 patients. Nine patients were not assessable for chronic GVHD, since they died before day +120. Twenty-two of 28 (78%) evaluable patients developed chronic GVHD, and 12 (42%) of them had extensive chronic GVHD.
Non-relapse mortality
With a median follow-up for all 37 patients of 52 months, 15 (41%) patients had died, 13 due to transplant-related complications at a median time after allogeneic SCT of 94 days (range, 18–1567 days) and two due to disease progression. The most common causes of NRM were GVHD and infections (four patients died from GVHD with infection, three from GVHD without infection, four from infection without active GVHD and two from other causes). Univariate analysis for NRM identified refractory or progressive FL before transplant as the only risk factor for NRM (univariate HR 3; 95% CI 1.01–8.8; P=0.04). Other variables such as age, sex, time from diagnosis to transplant, stage at diagnosis, prior anti-CD20 therapy, prior fludarabine, prior autologous SCT, number of treatment lines before transplantation, ECOG performance status and donor sex mismatch did not show any impact on NRM.
Disease response
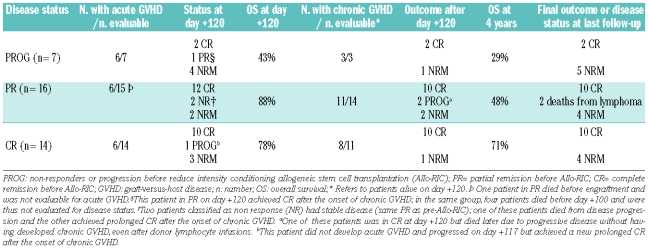
Table 3 summarizes the response to transplant and other outcomes. Twenty-eight patients were evaluable for disease status at 3–4 months after RIC allogeneic SCT, while nine patients were not evaluable because of early death (NRM before day +120). Fifteen of 23 patients (63%) with active disease (partial response, refractory disease or progression of FL) at the time of transplantation showed a response to the transplant (14 achieved a complete remission and one a partial remission). As shown in Table 3, three of seven patients transplanted with refractory or progressive disease were evaluable and all three responded (two complete remissions and one partial remission) after developing acute followed by chronic GVHD. At last follow-up both patients who had a complete response remain in remission with extensive chronic GVHD, while the patient with a partial remission died due to a non-relapse-related cause.
Table 3.
Response to transplant and other outcomes according to disease status at transplantation and the development of graft-versus-host disease.
Among the patients transplanted in partial remission, 14 were evaluable for response, and 12 achieved a complete remission by 3 months post-transplant while two had stable disease. One patient with stable disease reached a complete remission at day +206, upon developing chronic GVHD. The other patient with stable disease showed progressive disease 166 days after transplantation, without having developed chronic GVHD, and died. One patient who was in complete remission on day +120 relapsed on day +122; the patient did not develop GVHD despite donor lymphocyte infusions and died from FL.
Finally, among the 14 patients who were in complete remission at the time of allogeneic SCT, ten (71%) remain in complete remission, three died early after the transplant and one patient relapsed on day +117, but after rapid tapering of the cyclosporine A dose, he developed chronic GVHD and a new complete remission was obtained, which is ongoing 102 months after the allogeneic transplant.
Relapse, overall survival and disease-free survival
Relapse or progression occurred in only three patients at a median time post-transplant of 122 days (range, 117–166 days), and two of these patients died from disease progression, while one obtained a prolonged complete remission, as described above. The median overall survival for the whole group was 85 months (range, 16–112 months) at last follow-up, and the probability of overall survival at 4 years was 54%. According to the disease status at transplantation, the overall survival was 29%, 48% and 71% for patients with refractory or progressive disease, partial remission and complete remission at transplantation, respectively (P=0.09) (Figure 1). The 4-year probability of disease-free survival for the whole group was 57%, with a median follow-up in surviving patients of 87 months (range, 16–113 months). The disease-free survival for patients with refractory or progressive disease, partial remission and complete remission at transplantation was 29%, 48% and 64%, respectively (P=0.2).
Figure 1.
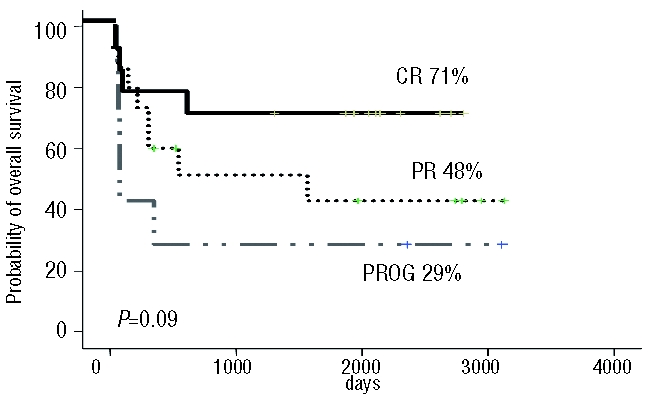
Overall survival according to the status of the FL at the time of transplantation. PROG; patients with no response or progressive disease prior to RIC allogeneic SCT; PR: patients in partial remission prior to the transplant; CR: patients in complete remission prior to the transplant.
Discussion
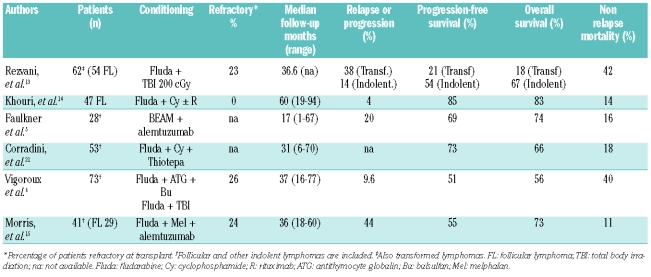
This prospective multicenter study has a long follow-up and reports on the efficacy of a homogeneous RIC regimen in patients with advanced FL undergoing allogeneic SCT, showing 4-year overall and disease-free survival rates above 50%. The results of larger, previously reported series, which were mostly analyzed retrospectively, are summarized in Table 4. Although direct comparisons of the results of the studies are quite difficult to interpret given the important differences in study populations and follow-up periods, in line with previous reports, the relapse rate in our study was very low (8%), and a GVL effect was apparent, with some durable responses seen only after the onset of chronic GVHD. This finding is, however, in contrast with the higher relapse rate (44%) reported by Morris et al. in a study in which a similar regimen was used but with the addition of alemtuzumab in order to decrease GVHD,14 thus highlighting the importance of a GVL effect in this indolent malignancy, which is usually linked to the appearance of acute and/or chronic GVHD. Nevertheless, and as reported by Vigouroux et al.,6 the day +100 and 4-year NRM were high. Progressive or refractory disease at the time of transplantation was associated with a significantly higher NRM, emphasizing that selection of patients is crucial for current RIC allogeneic SCT strategies to be successful. Recent studies by Khouri et al.14 and Corradini et al.23 showed similar relapse rates with better overall survival due to lower NRM. The differences between the results are probably due to the fact that our cohort of patients, unlike those of the other studies, included patients with progressive/chemorefractory disease. In fact, irrespective of the type of non-Hodgkin’s lymphoma, patients with refractory disease fare very poorly.4,24,25 Other relevant differences between the studies were the high number of patients in our cohort in whom a prior autologous SCT had failed (46%), and the large proportion of patients who did not have an ideal performance status (19/37 had ECOG performance status >0), a variable that has been associated with an increased risk of NRM.24 With minimal-intensity non-myeloablative conditioning, Rezvani et al.13 reported that patients with refractory disease at transplantation were five times more likely to relapse and two times more likely to experience NRM compared to patients with chemosensitive disease. Thus, to reduce the NRM associated with current RIC allogeneic SCT protocols it seems reasonable to try to select patients earlier in their disease course and use novel salvage strategies, such as new monoclonal antibodies or radio-immunotherapy, in order to obtain at least a partial remission before transplantation, as suggested by most authors.4,25,26
Table 4.
Reports of major series of patients with non-Hodgkin’s lymphoma undergoing RIC allogeneic stem cell transplantation.
As expected, the fludarabine-melphalan conditioning regimen was shown to have a direct anti-tumor response in many patients, since disease evaluation early after transplantation showed that 15/23 (63%) of patients with measurable disease obtained a response, which was a complete remission in most cases (Table 3). However, a GVL effect was also noted, since three patients with measurable disease both before and after allogeneic transplantation did not develop acute GVHD but showed a durable complete remission after developing chronic GVHD, and one patient who was in complete remission before transplantation had early disease progression without acute GVHD but achieved a complete remission after the onset of de novo chronic GVHD. In addition, the two patients who died from disease progression after day +120 had not developed GVHD, while none of the 22 patients who did develop chronic GVHD had progression of their FL, with 22 patients alive in complete remission after a long follow-up. The high incidence of acute (51%) and chronic (88%) GVHD is characteristic of most RIC allogeneic SCT protocols that do not include in vivo T-cell depletion with antithymocyte globulin or, especially, alemtuzumab. Although the rate of disease progression may be higher with in vivo T-cell depletion, donor lymphocyte infusions may lead to a high proportion of new complete remissions, and thus the current disease-free survival rate following T-cell depleted RIC allogeneic protocols may be significantly higher than the standard disease-free survival rate.5,14 Donor lymphocyte infusions may also be given to patients with low or decreasing mixed chimerism with the intention of achieving full donor chimerism, even when there is no disease progression. Of course, such infusions carry the risk of inducing GVHD, which may be severe. On the other hand T-cell-replete transplants are expected to lead to higher incidences of acute and chronic GVHD, which may lead to a higher NRM that may counterbalance the positive GVL effect, thus lowering the disease-free survival rate. The need for donor lymphocyte infusions in this setting is, of course, much less. Perhaps a preference for any of these strategies should depend partly on the risk of disease recurrence at the time of transplantation.
In summary, this prospective study reports the long-term follow-up of a cohort of patients with advanced and heavily pretreated FL who underwent RIC and an allogeneic SCT with a homogeneous transplant strategy, confirming that long-term disease-free survival was obtained in more than half of these patients, especially in those whose FL was not in frank progression at the time of transplantation; both the direct anti-tumor effect of the fludarabine-melphalan combination and a GVL effect are responsible for these prolonged remissions.
Acknowledgments
special thanks to Manuel Delgado for his contribution to maintenance of the GELTAMO. JLP is supported by grants from the Instituto de Salud Carlos III (CM06/00139, Ministerio de Sanidad, Spain).
Footnotes
Authorship and Disclosures
JLP, and RM: conception and design of the study, analysis and interpretation of data, drafting the article, revising it critically for important intellectual content and final approval of the version to be published. JG, JLDM, LV, RA, JFT, AS, CS and JSi: analysis and interpretation of data, revising the article critically for important intellectual content and final approval of the version to be published. AS, JdlS, and DC: conception and design, revising the article critically for important intellectual content and final approval of the version to be published.
The authors declare no competing interests.
References
- 1.Van Besien K, Sobocinski KA, Rowlings PA, Murphy SC, Armitage JO, Bishop MR, et al. Allogeneic bone marrow transplantation for low-grade lymphoma. Blood. 1998;92 (5):1832–6. [PubMed] [Google Scholar]
- 2.Peniket AJ, Ruiz de Elvira MC, Taghipour G, Cordonnier C, Gluckman E, de Witte T, et al. An EBMT registry matched study of allogeneic stem cell transplants for lymphoma: allogeneic transplantation is associated with a lower relapse rate but a higher procedure-related mortality rate than autologous transplantation. Bone Marrow Transplant. 2003;31(8):667–8. doi: 10.1038/sj.bmt.1703891. [DOI] [PubMed] [Google Scholar]
- 3.Verdonck LF. Allogeneic versus autologous bone marrow transplantation for refractory and recurrent low-grade non-Hodgkin’s lymphoma: updated results of the Utrecht experience. Leuk Lymphoma. 1999;34(1–2):129–36. doi: 10.3109/10428199909083388. [DOI] [PubMed] [Google Scholar]
- 4.Van Besien K, Loberiza FR, Bajorunaite R, Armitage JO, Bashey A, Burns LJ, et al. Comparison of autologous and allogeneic hematopoietic stem cell transplantation for follicular lymphoma. Blood. 2003;102(10):3521–9. doi: 10.1182/blood-2003-04-1205. [DOI] [PubMed] [Google Scholar]
- 5.Faulkner RD, Craddock C, Byrne JL, Mahendra P, Haynes AP, Prentice HG, et al. BEAM-alemtuzumab reduced-intensity allogeneic stem cell transplantation for lymphoproliferative diseases: GVHD, toxicity, and survival in 65 patients. Blood. 2004;103(2):428–34. doi: 10.1182/blood-2003-05-1406. [DOI] [PubMed] [Google Scholar]
- 6.Vigouroux S, Michallet M, Porcher R, Attal M, Ades L, Bernard M, et al. Long-term outcomes after reduced-intensity allogeneic stem cell transplantation for low-grade lymphoma: a survey by the French Society of bone marrow graft transplantation and cellular therapy (SFGM-TC) Haematologica. 2007;92(5):627–34. doi: 10.3324/haematol.10924. [DOI] [PubMed] [Google Scholar]
- 7.Mandigers C, Raemaekers JMM, Schattenberg A, Roovers EA, Bogman MJ, van der Maazen RW, et al. Allogeneic bone marrow transplantation with T-cell-depleted marrow grafts for patients with poor-risk relapsed low-grade non-Hodgkin’s lymphoma. Br J Haematol. 1998;100(1):198–206. doi: 10.1046/j.1365-2141.1998.00539.x. [DOI] [PubMed] [Google Scholar]
- 8.Khouri IF, Saliba RM, Giralt SA, Lee MS, Okoroji GJ, Hagemeister FB, et al. Nonablative allogeneic hematopoietic transplantation as adoptive immunotherapy for indolent lymphoma: Low incidence of toxicity, acute graft-versus-host disease, and treatment-related mortality. Blood. 2001;98(13):3595–9. doi: 10.1182/blood.v98.13.3595. [DOI] [PubMed] [Google Scholar]
- 9.Hari P, Carreras J, Zhang M-J, Gale RP, Bolwell BJ, Bredeson CN, et al. Allogeneic transplants in follicular lymphoma: higher risk of disease progression after reduced-intensity compared to myeloablative conditioning. Biol Blood Marrow Transplant. 2008;14(2):236–45. doi: 10.1016/j.bbmt.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diaconescu R, Flowers CR, Storer B, Sorror ML, Maris MB, Maloney DG, et al. Morbidity and mortality with nonmyeloablative compared with myeloablative conditioning before hematopoietic cell transplantation from HLA-matched related donors. Blood. 2004;104(5):1550–8. doi: 10.1182/blood-2004-03-0804. [DOI] [PubMed] [Google Scholar]
- 11.Robinson SP, Goldstone AH, Mackinnon S, Carella A, Russell N, de Elvira CR, et al. Chemoresistant or aggressive lymphoma predicts for a poor outcome following reduced-intensity allogeneic progenitor cell transplantation: an analysis from the Lymphoma Working Party of the European Group for Blood and Bone Marrow Transplantation. Blood. 2002;100(13):4310–6. doi: 10.1182/blood-2001-11-0107. [DOI] [PubMed] [Google Scholar]
- 12.Robinson SP, Mackinnon S, Goldstone AH, Slavin S, Carella AM, Russell NH, et al. Higher than expected transplant-related mortality and relapse following non-myeloablative stem cell transplantation for lymphoma adversely effects progression free survival. Blood. 2000;96 [Google Scholar]
- 13.Rezvani AR, Storer B, Maris M, Sorror ML, Agura E, Maziarz RT, et al. Nonmyeloablative allogeneic hematopoietic cell tansplantation in relapsed, refractory, and transformed indolent non-Hodgkin’s jymphoma. J Clin Oncol. 2008;26(2):211–7. doi: 10.1200/JCO.2007.11.5477. [DOI] [PubMed] [Google Scholar]
- 14.Khouri IF, McLaughlin P, Saliba RM, Hosing C, Korbling M, Lee MS, et al. Eight-year experience with allogeneic stem cell transplantation for relapsed follicular lymphoma after nonmyeloablative conditioning with fludarabine, cyclophosphamide, and rituximab. Blood. 2008;111(12):5530–6. doi: 10.1182/blood-2008-01-136242. Erratum in: Blood. 2009;113(7):1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris E, Thomson K, Craddock C, Mahendra P, Milligan D, Cook G, et al. Outcomes after alemtuzumab-containing reduced-intensity allogeneic transplantation regimen for relapsed and refractory non-Hodgkin lymphoma. Blood. 2004;104 (13):3865–71. doi: 10.1182/blood-2004-03-1105. [DOI] [PubMed] [Google Scholar]
- 16.Martino R, Caballero MD, Canals C, Simón JA, Solano C, Urbano-Ispízua A, et al. ALLOPBSCT Subcommittee of the Spanish Group for Haematopoietic Transplantation (GETH); Group GEL-TAMO. Allogeneic peripheral blood stem cell transplantation with reduced-intensity conditioning: results of a prospective multicentre study. Br J haematol. 2001;115(3):653–9. doi: 10.1046/j.1365-2141.2001.03153.x. [DOI] [PubMed] [Google Scholar]
- 17.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J. Meeting report. Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15(6):825–8. [PubMed] [Google Scholar]
- 18.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SL, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-hostdisease:1. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–55. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Valcarcel D, Martino R, Caballero D, Mateos MV, Pérez-Simón JA, Canals C, et al. Chimerism analysis following allogeneic peripheral blood stem cell transplantation with reducedintensity conditioning. Bone Marrow Transplant. 2003;31(5):387–92. doi: 10.1038/sj.bmt.1703846. [DOI] [PubMed] [Google Scholar]
- 20.Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an International Workshop to standardize response criteria for non-Hodgkin’s lymphomas. J Clin Oncol. 1999;17(4):1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 21.Klein JP, Rizzo JD, Zhang MJ, Keiding N. Statistical methods for the analysis and presentation of the results of bone marrow transplants. Part I: unadjusted analysis. Bone Marrow Transplantat. 2001;28(10):909–15. doi: 10.1038/sj.bmt.1703260. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958:457–81. [Google Scholar]
- 23.Corradini P, Dodero A, Zallio F, Caracciolo D, Casini M, Bregni M, et al. Graft-versus-lymphoma effect in relapsed PTCL NHL after reduced-intensity conditioning followed by allogeneic transplantation of hematopoietic cells. J Clin Oncol. 2004;22 (11):2172–6. doi: 10.1200/JCO.2004.12.050. [DOI] [PubMed] [Google Scholar]
- 24.Sorror M. Impacts of pretransplant comorbidities on allogeneic hematopoietic cell transplantation (HCT) outcomes. Biol Blood Marrow Transplant. 2009;15(1 Suppl):149–53. doi: 10.1016/j.bbmt.2008.12.498. [DOI] [PubMed] [Google Scholar]
- 25.Bastion Y, Brice P, Haioun C, Sonet A, Salles G, Marolleau JP, et al. Intensive therapy with peripheral blood progenitor cell transplantation in 60 patients with poor-prognosis follicular lymphoma. Blood. 1995;86(8):3257–62. [PubMed] [Google Scholar]
- 26.Voso MT, Martin S, Hohaus S, Abdallah A, Schlenk RF, Ho AD, et al. Prognostic factors for the clinical outcome of patients with follicular lymphoma following high-dose therapy and peripheral blood stem cell transplantation (PBSCT) Bone Marrow Transplant. 2000;25(9):957–64. doi: 10.1038/sj.bmt.1702336. [DOI] [PubMed] [Google Scholar]