Abstract
As chronic myeloid leukemia is rare in children, most data on imatinib mesylate therapy is derived from adult studies. We retrospectively evaluated pediatric (<14 years) patients with Ph+ chronic myeloid leukemia treated with imatinib mesylate, from January 2003 through June 2008. Of the 12 chronic myeloid leukemia patients (2% of all leukemias) 11 were in chronic phase while one had myeloid blast crisis. Six subsequently underwent stem cell transplantation. Five patients had grade 3–4 arthralgia requiring therapy alteration. None achieved complete molecular remission (MR) with imatinib mesylate alone. In contrast 3/6 patients post stem cell transplantation have undetectable BCR-ABL. Three patients relapsed to chronic phase (1 imatinib mesylate; 2 stem cell transplantation). Relapse free survival is 65.6% at four years and all are alive. Imatinib mesylate is effective therapy for children with chronic myeloid leukemia. However, cure probably requires stem cell transplantation. Acute toxicity of imatinib mesylate is tolerable, but long-term effects on growing children are unknown. Pediatric patients with chronic myeloid leukemia should undergo stem cell transplantation when appropriate related donors are available.
Keywords: chronic myeloid leukemia, BCR-ABL, adult studies, stem cell transplantation
Introduction
The treatment of chronic myeloid leukemia (CML) has been revolutionized by the introduction of molecularly directed therapy that specifically targets and inhibits the tyrosine kinase (TK) activity of the BCR-ABL protein. Imatinib mesylate (IM) is extremely effective in suppressing Ph+ cells resulting in significant responses in CML. Imatinib mesylate has resulted in not only achieving complete hematologic remissions (CHR), but also results in complete cytogenetic responses (CCR) and molecular remission.1–4 It is, however, still unclear if imatinib mesylate achieves cure and hematopoietic stem cell transplantation (SCT) remains the only modality with documented curative results, achieving 50–80% long-term survivals for patients in chronic phase, and progressively lower results for patients transplanted beyond first chronic phase.5,6
While most data regarding the use of imatinib mesylate for chronic myeloid leukemia comes from studies in adult patients, this treatment strategy has now been expanded to include children with Ph+ chronic myeloid leukemia.7,8 However, little information is available regarding response, outcome and toxicity of imatinib mesylate in children. At our institution, we have used this adult-based strategy for children keeping stem cell transplantation as the ultimate treatment of choice when a suitable donor source is available.
Design and Methods
At our institution, 598 pediatric patients (<14 years old) with leukemia were treated from January 2003 through June 2008. Following institutional ethical review and approval, medical records of all patients diagnosed with chronic myeloid leukemia were retrospectively reviewed and data were collected.
Details of the diagnostic process and molecular analysis methodology for detection and quantification of the BCR/ABL gene rearrangement are available in the Online Supplementary Appendix.
Mainly descriptive statistics were generated and relapse free survival (RFS) for the patients was calculated using the Kaplan-Meier method. Relapse was considered when patients reverted to at least cytogenetic positivity after having achieved major molecular responses (MMR; > 3 log reduction in BCR-ABL transcript).
Results and Discussion
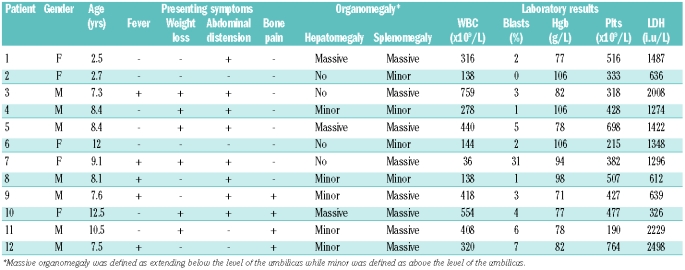
Twelve patients were diagnosed with chronic myeloid leukemia, constituting 2% of all pediatric leukemias seen at our institution during that time. Clinical characteristics are shown in Table 1.
Table 1.
Patients’ characteristics at presentation.
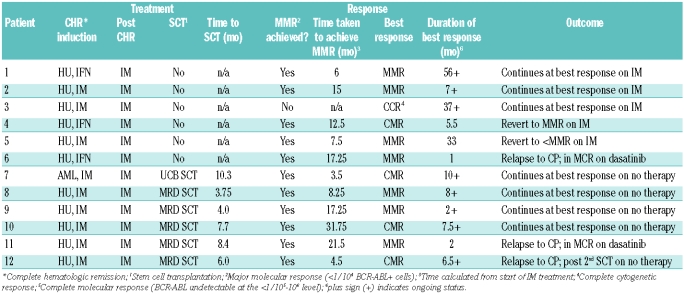
Treatment and patient outcome are shown in Table 2. All patients in chronic phase started therapy with hydroxyurea (HU); in 8 patients imatinib mesylate was added within one week, following cytogenetic confirmation. HU was continued for a median of 21 days until white blood cell count reduction to around 10×109/L. The 3 other patients in chronic phase were treated with HU and α-interferon (IFα) for 3.75–8 months and started on imatinib mesylate when it became available in our institution. The starting dose of imatinib mesylate was 300 mg/m2/day, rounded to the nearest 100 mg. The patient in CML-BP received induction chemotherapy according to our acute myeloid leukemia protocol only (idarubicin 12 mg/m2 IV x 3 days, cytarabine 100 mg/m2/day IV CI × 7 days and thioguanine 60 mg/m2 PO × 7 days). She achieved a bone marrow response but continued to have splenomegaly. Imatinib mesylate was started with the second induction cycle. All patients continued on therapy with imatinib mesylate alone. The relapse free survival for all 12 patients is 65.6% at four years and all are currently alive.
Table 2.
Treatment provided and outcome for the patients with chronic myeloid leukemia.
All patients achieved a complete hematologic response within the first three months, except patient #4 who was treated with HU/IFα for four months before being switched to imatinib mesylate. Cytogenetic assessment of response was tested in 9 patients; 7 achieved complete cytogenetic response by 12 months of diagnosis. One patient was in complete cytogenetic response at 13 months while one other did not have routine cytogenetic testing until 24 months into therapy.
Five of the 6 non-transplant patients have achieved major molecular response at a median of 7.5 months (range 3–17.25 months). One patient (#3) remains in complete cytogenetic response at 35 months on imatinib mesylate, probably related to suboptimal compliance. Patient #6 relapsed to CML-CP at 4.13 years since diagnosis, following a brief major molecular response at 17.25 months on imatinib mesylate. This patient is currently receiving dasatinib and has achieved a major cytogenetic response (MCR; BCR-ABL 5% by FISH).
Six patients underwent allogeneic stem cell transplantation at a median of 6.8 months (range 3.75–10.3 months) from diagnosis. Time to transplant was dependant on identification of an appropriate donor and completion of pre-transplant workup. The five patients diagnosed in chronic phase were transplanted from matched sibling donors (MSD), while the one patient diagnosed in blast phase underwent one class II antigen mismatched unrelated umbilical cord blood (UCB) transplantation. Conditioning regimen for the MSD stem cell transplantation was with cyclophosphamide (CY) and busulfan, while the conditioning for the UCB stem cell transplantation was with CY, busulfan, etoposide and anti-thymocyte globulin (ATG). The bone marrow recipients averaged 3.75×106/kg (range 0.92–6.16×106/kg) CD34+ cells, while the UCB transplant patient received 0.17×106/kg CD34+ cells. The median time to neutrophil and platelet engraftments was 21.5 days (mean 20.5; range 15–30 days) and 31.5 days (mean 33.8; range 27–70 days), respectively. All stem cell transplantation patients were in at least a complete hematologic response at the time of transplantation and only one had achieved a complete molecular response (CMR; BCR-ABL not detectable at <1/105–106 level).
Three of the five patients tested post stem cell transplantation achieved complete molecular response at a mean duration of 15.3 months (range 2–27 months), while the remaining patients (#8 and 9) have achieved major molecular response at five and ten months post stem cell transplantation. Patient #12, who was transplanted in complete molecular response, relapsed to CML-CP 22 months post stem cell transplantation. He underwent a second, CY/TBI conditioned, stem cell transplantation from the same matched sibling donor after imatinib mesylate therapy for 14 months. The second patient who relapsed post stem cell transplantation (#11) had achieved a transient major molecular response at 13 months and eventually relapsed to CML-CP 39 months post stem cell transplantation. He is currently on dasatinib therapy having achieved a complete molecular response.
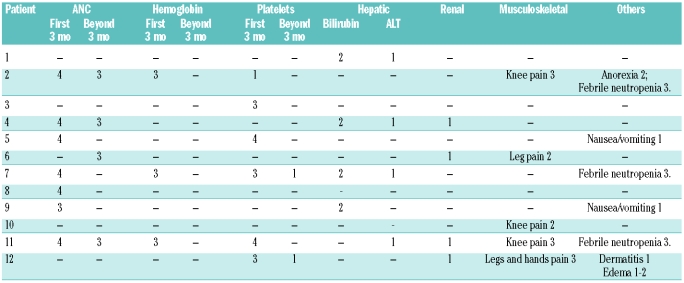
Imatinib was reasonably well tolerated. Hematologic toxicity was more frequent and severe during the first three months of therapy (Table 3). Non-hematologic toxicities were generally mild and transient, except for joint pains. Five patients developed significantly severe lower extremity and joint pain, resulting in major limitation of movement and altered normal function. Imatinib mesylate dosage had to be reduced in all patients and discontinued in 3. While imatinib mesylate could be restarted at lower doses and gradually increased to full dose in most patients, it was not tolerated by one patient (#11). Only one of these patients had mild to moderate lower extremity edema associated with the joint pain. Cardiac dysfunction has not been observed in any of our patients to date as determined by routine echocardiographic evaluations.
Table 3.
Toxicity encountered during treatment with imatinib mesylate. Toxicity is graded according to the NCI common toxicity criteria.
Chronic myeloid leukemia is a rare diagnosis in children. This is reflected in the paucity of data relating to pediatric patients with chronic myeloid leukemia in the international literature. While pediatric studies are being conducted, treatment of children with chronic myeloid leukemia is primarily based on the experience derived from adult studies.
Imatinib mesylate is considered the primary first line treatment for chronic myeloid leukemia in adult patients.5,9 Phase I/II studies of imatinib mesylate in children confirmed its safety and efficacy,7,10 and although no phase III clinical trials in children have been reported, the benefit seen in adults provided sufficient evidence to warrant use in children. However, the therapeutic aim in children differs from that in adults; in children a definitive cure is sought while in adults the aim is to delay progression. Evident data exist confirming that imatinib mesylate may not be curative for most patients with chronic myeloid leukemia even after achieving undetectable BCR-ABL levels. Quiescent leukemia stem cells are believed resistant to imatinib mesylate and repopulate the bone marrow if imatinib myelate is discontinued.11 Current recommendation for adult patients on imatinib mesylate as first-line therapy, without stem cell transplantation, is to continue the treatment indefinitely.5 Whether this should also apply to the pediatric patient remains to be seen. Although most of our patients on imatinib mesylate achieved molecular responses, all still have detectable BCR-ABL. This would confirm the supposition that imatinib mesylate, at least over the relatively short-term duration used in our patients, is not curative. In contrast, at least half of the transplanted patients have achieved undetectable BCR-ABL levels and actual cures can be expected for them.
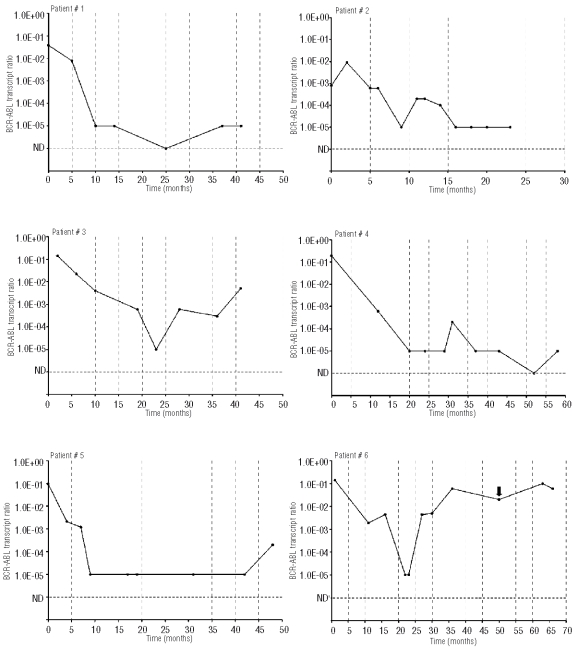
Figure 1.
BCR-ABL transcript ratio depicted on a logarithmic scale against time for all the patients. Figure shows the graphs for patients ns 1–6 who did not undergo SCT and have continued therapy on TK inhibitor therapy alone. BCR-ABL values ——●——; time of relapse depicted by the black arrow.
Thus, stem cell transplantation remains possibly the only curative option for patients with chronic myeloid leukemia. However, the 5-year survival is significantly better for pediatric patients in CP1 following matched sibling donor stem cell transplantation than with alternative donors.8 Based on such data, our own practice has been to offer matched sibling donor stem cell transplantation to patients with chronic myeloid leukemia in CP1 and to continue imatinib mesylate for the remaining patients until availability of a suitable donor or further data is generated that would allow changes in practice. A recent survey of pediatric oncologists and transplant specialists also highlights the non-uniformity in chronic myeloid leukemia treatment for pediatric patients.12 However, 63% of the respondents continue to recommend matched sibling donor stem cell transplantation in first chronic phase after using imatinib mesylate upfront.
As has been reported previously,13 and is evident from our data, reduction of BCR-ABL post stem cell transplantation to undetectable levels requires several months. The three patients who achieved complete molecular response post stem cell transplantation required a mean of 15.5 months; likely a result of graft versus leukemia effect. Patient n. 9 demonstrated this clearly as a decline in engraftment corresponded with a rise in BCR-ABL levels, with subsequent reduction as the donor engraftment approached 100%. (Online Supplementary Figure S1). Quantitative monitoring of the BCR-ABL transcript post stem cell transplantation, therefore, becomes critically important in defining reduction in the leukemic clone and facilitating early detection of failures.
Imatinib mesylate was generally well tolerated. While it does directly cause some myelosuppression, hematologic toxicities seen during initial therapy are likely related to reduction in the leukemic clone without marrow repopulation by normal stem cells. As the proportion of imatinib mesylate sensitive cells decreases, cytopenias also become less frequent. Lack of significant hematologic toxicities in patients with solid tumors treated with imatinib mesylate vouches for this mechanism.14,15 Non-hematologic toxicity, however, was quite different when compared to adults. Fluid retention and edema, commonly encountered in adult patients, was only noted in one of our patients. Among adult patients, 15–20% may suffer from mild joint pains.16 Arthralgia was probably the most debilitating toxicity in our patients, forcing therapy alterations. Interestingly, this toxicity was mostly transient and after dose reduction and symptom resolution most patients could tolerate full dose. Only minor hepatic dysfunction was noted and none of the patients suffered cardiac abnormalities.
The long-term effects of imatinib mesylate in children are unknown. Multiple metabolic effects of imatinib mesylate, including glucose, lipid and bone metabolism, have been reported.17 While in adult patients these may have some beneficial impact, their consequences on the growing child remain unknown. Extended study in children is required to determine whether prolonged tyrosine kinase inhibition results in alterations in growth and development.
Until such time as more long-term data are available, our strategy seems to be valid. All patients with Ph+ chronic myeloid leukemia should be treated with imatinib mesylate. Those with a suitably matched family donor should undergo stem cell transplantation. Patients without a family donor may continue on imatinib mesylate keeping in mind that this may likely not achieve curative results and at present the duration of therapy is not determined.
Acknowledgments
the authors would like to thank Khawar Siddiqui and Saada Mansour for their help in data management and technical support.
Footnotes
The Online version of this article has a Supplementary Appendix.
Authorship and Disclosures
AFB designed the study, analyzed the results and wrote the manuscript. AA-S collected the data and co-wrote the paper. MA, MA-M, AA-S, AA-A and HE-S assisted in study design and data analysis, and reviewed the manuscript.
The authors declare no competing financial interests.
References
- 1.Druker BJ, Lydon LB. Lessons learned from an Abl tyrosine kinase inhibitor for chronic myelogenous leukemia. J Clin Invest. 2000;105(1):3–7. doi: 10.1172/JCI9083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. 5-year follow up of patients receiving Imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408–17. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic phase chronic myeloid leukemia. N Engl J Med. 2003;348(11):994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 4.Hochhaus A, Druker BJ, Sawyer C, Guilhot F, Schiffer CA, Cortes J, et al. Favorable long-term follow-up results over 6 years for response, survival and safety with imatinib mesylate therapy in chronic-phase chronic myeloid leukemia after failure with interferon-α treatment. Blood. 2008;111(3):1039–43. doi: 10.1182/blood-2007-07-103523. [DOI] [PubMed] [Google Scholar]
- 5.Goldman JM. How I treat chronic myeloid leukemia in the imatinib era. Blood. 2007;110(8):2828–37. doi: 10.1182/blood-2007-04-038943. [DOI] [PubMed] [Google Scholar]
- 6.Maziarz RT, Mauro MJ. Transplantation for chronic myelogenous leukemia: yes, no, maybe so…An Oregon experience. Bone Marrow Transplant. 2003;32(5):459–69. doi: 10.1038/sj.bmt.1704163. [DOI] [PubMed] [Google Scholar]
- 7.Champagne MA, Capdeville R, Krailo M, Qu W, Peng B, Rosamilia M, et al. Imatinib mesylate (STI571) for treatment of children with Philadephia chromosome-positive leukemia: results from a Children’s Oncology Group Phase I study. Blood. 2004;104(9):2655–60. doi: 10.1182/blood-2003-09-3032. [DOI] [PubMed] [Google Scholar]
- 8.Suttorp M. Innovative approaches of targeted therapy for CML of childhood in combination with paediatric haematopoietic SCT. Bone Marrow Transplant. 2008;42:S40–6. doi: 10.1038/bmt.2008.282. [DOI] [PubMed] [Google Scholar]
- 9.Hehlmann R, Berger U, Pfirrmann M, Heimpel H, Hocchaus A, Hasford J, et al. Drug treatment is superior to allografting as first-line therapy in chronic myeloid leukemia. Blood. 2007;109(11):4686–92. doi: 10.1182/blood-2006-11-055186. [DOI] [PubMed] [Google Scholar]
- 10.Millot F, Guilhot J, Nelken B, Leblanc T, De Bont ES, Bekassy AN, et al. Imatinib mesylate is effective in children with chronic myelogenous leukemia in late chronic and advanced phase and in relapse after stem cell transplantation. Leukemia. 2006;20(2):187–92. doi: 10.1038/sj.leu.2404051. [DOI] [PubMed] [Google Scholar]
- 11.Michor F, Hughes TP, Iwasa Y, Branford S, Shah NP, Sawyers CL, Nowak MA. Dynamics of chronic myeloid leukaemia. Nature. 2005;435(7046):1267–70. doi: 10.1038/nature03669. [DOI] [PubMed] [Google Scholar]
- 12.Burke MJ, Willert J, Desai S, Kadota R. The treatment of pediatric Philadelphia positive (Ph+) leukemias in the Imatinib era. Pediatr Blood Cancer. 2009;53(6):992–5. doi: 10.1002/pbc.22172. [DOI] [PubMed] [Google Scholar]
- 13.Otazu IB, Tavares RCB, Hassan R, Zalcberg I, Tabak DG, Seuanez HN. Estimations of BCR-ABL/ABL transcripts by quantitative PCR in chronic myeloid leukaemia after allogeneic bone marrow transplantation and donor lymphocyte infusion. Leuk Res. 2002;26(2):129–41. doi: 10.1016/s0145-2126(01)00109-6. [DOI] [PubMed] [Google Scholar]
- 14.Wen PY, Yung WKA, Lamborn KR, Dahia PL, Wang Y, Peng B, et al. Phase I/II study of imatinib mesylate for recurrent malignant gliomas. North American Brain Tumor Consortium Study 99–08. 2006;12(16):4899–907. doi: 10.1158/1078-0432.CCR-06-0773. [DOI] [PubMed] [Google Scholar]
- 15.van Oosterom AT, Judson IR, Verweij J, Stroobants S, Dumez H, di Paola ED, et al. Update of phase I study of imatinib (STI571) in advanced soft tissue sarcomas and gastrointestinal stromal tumors: a report of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer. 2002;38 (Suppl 5):S83–7. doi: 10.1016/s0959-8049(02)80608-6. [DOI] [PubMed] [Google Scholar]
- 16.Larson RA, Druker BJ, Guilhot F, O’Brien SG, Riviere GL, Krahnke T, et al. Imatinib pharmacokinetics and its correlation with response and safety in chronic-phase chronic myeloid leukemia: a subanalysis of the IRIS study. Blood. 2008;111(8):4022–8. doi: 10.1182/blood-2007-10-116475. [DOI] [PubMed] [Google Scholar]
- 17.Breccia M, Alimena G. The metabolic consequences of imatinib mesylate: Changes on glucose, lypidic and bone metabolism. Leuk Res. 2009;33(7):871–5. doi: 10.1016/j.leukres.2009.01.040. [DOI] [PubMed] [Google Scholar]