Abstract
A large series of plasma cell dyscrasias (n=2207) was examined for translocations which deregulate the MAF genes, t(14;20)(q32;q12) and t(14;16)(q32;q23), and their disease behavior was compared to a group characterized by the t(4;14)(p16;q32) where CCND2 is also up-regulated. The t(14;20) showed low prevalence in myeloma (27/1830, 1.5%) and smoldering myeloma (1/148, <1%) with a higher incidence in MGUS (9/193, 5% P=0.005). Strong associations with del(13) (76%), non-hyperdiploidy (83%) and gain of 1q (58%) were seen but no association with an IgA M-protein or absence of bone disease was noted. All three translocations were associated with poor outcome in myeloma, but strikingly all t(14;20) MGUS/smoldering myeloma cases (n=10) had stable, low level disease. In contrast, the 10 t(14;16) and 25 t(4;14) MGUS/smoldering myeloma cases were associated with both evolving and non-evolving disease. None of the associated genetic abnormalities helped to predict for progression from MGUS or smoldering myeloma. (Clinicaltrials.gov identifier: ISRCTN 68454111; UKCRN ID 1176)
Keywords: plasma cell, myeloma, MGUS, chromosome abnormality, disease progression
Introduction
Approximately half of myeloma (MM) cases are characterized by translocations into the immunoglobulin heavy chain locus (IgH). Each translocation subgroup is associated with deregulation of a D group cyclin either directly, such as occurs with the t(11;14) (cyclin D1) and t(6;14) (cyclin D3) or indirectly, such as occurs with the t(4;14) or in the MAF translocation group.1 The MAF translocation group includes the t(14;16) and t(14;20), both of which are rare in myeloma, but are thought to be associated with poor prognosis.2 The mechanism of this poor outcome is thought to involve the consequences of MAF upregulation, which include upregulation of cyclin D2, effects on cell interaction and upregulation of apoptosis resistance.3 As upregulation of cyclin D2 is also seen in the t(4;14) group where poor prognosis is well established,4–8 it may be deregulation of this D group cyclin which is important in this respect. MM cases with t(4;14) show an excess of IgA M-protein type9 and have been reported to be less likely to present with bone disease (1) but it is not clear whether this also applies to the other cyclin D2 dysregulating translocations, t(14;16) and t(14;20) cases.
MGUS is a benign premylomatous condition lacking the clinical sequelae of myeloma, but with a rate of transformation to myeloma of approximately 1% per year.10 This relationship has led to the generation of disease models of myeloma based on the multistep progression of normal to MGUS through to myelomatous plasma cells.11 In these models, initial genetic hits result in an immortalized plasma cell clone and additional changes lead to its transformation to clinical myeloma. With the recent recognition that essentially all myeloma cases have a pre-existing asymptomatic phase,12–13 it becomes even more important to recognize which abnormalities affect the rate of progression.
The t(4;14) has been reported to be rare in MGUS and smoldering/asymptomatic MM (SMM), leading to the suggestion that it is associated with an aggressive disease process effectively bypassing this stage.9,14 However, there are reports of several cases of stable MGUS and SMM with t(4;14), which argues against this hypothesis.9,15–17 We report here the prevalence, genetic associations and outcome of patients with the t(14;20) and t(14;16) in a large series of MM, MGUS, and SMM cases and compare these to cases with a t(4;14). Particular emphasis has been placed on t(14;20) cases due to the almost complete absence of published information.
Design and methods
Patients
Bone marrow (BM) samples were received from UK hospitals with informed consent for cytogenetic testing. Adequate material was received from 2207 patients between January 2001 and November 2007. The diagnoses (made on standard criteria18 with central revue of values but not slides) were MM 1,830 (with 1,695 diagnostic samples), plasma cell leukemia (PCL) 10, SMM 149, MGUS 192, amyloidosis (AL) not meeting the criteria for MM 26. The age range was 23 to 93 (MM, median 65 with 21% ≥ 75, MGUS median 69, SMM median 68, AL median 58, PCL median 59) with 1,284 male and 923 female patients. MGUS cases showed a slight excess of females (98F:94M). MM patients were treated with a variety of UK standard therapies; 1,020 were in the MRC myeloma IX trial19 and the majority of younger patients received at least one autologous transplant.
FISH
Plasma cell purification and fluorescence in situ hybridization (FISH) studies using probes for 13q, IgH break-apart, t(11;14), t(4;14), t(14;16), MAFB break-apart, CCND3 break-apart, t(8;14), 17p deletion, enumeration of chromosomes 5, 9, 15 and 3, 7 and 22 was performed as described previously20;21 with the addition of BAC RP11307C12 for CKS1B at 1q21, and confirmation that split IgH and MAFB indicated t(14;20) by hybridization of a single color (red) MAFB probe along with the FGFR3/IgH probe, resulting in fusions in all cases. One hundred cells were scored for each probe and the European Myeloma Network FISH workshop recommendations used for cut-offs (fusion/break-apart probes 10%, numerical abnormalities 20%). Ploidy was primarily deduced from the 5/9/15 probe combination22 but all results were taken into account where only one of the 5/9/15 probes was gained.
Statistical analysis
Median follow-up was 31.7 months (range 4–290). Kaplan-Meier survival curves were calculated using MINITAB 14. Survival from diagnosis of MM was accepted for primary IgH translocations and ploidy regardless of the time of FISH testing as these are early changes. Analysis for deletions of 13q, 16q and 17p were only performed on patients studied at diagnosis. Incidences of genetic abnormalities and clinical associations were compared using Fisher’s exact test.
Results and Discussion
Prevalence
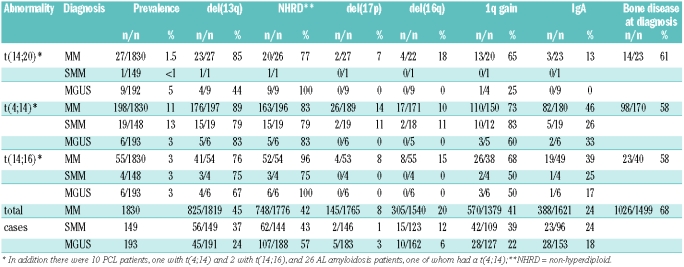
In this series, overall, the MAF translocations have a prevalence of approximately 5%, making them a clinically significant subgroup of patients (Table 1). The t(14;20) is rare in MM or SMM with a prevalence of 1.5 and <1% respectively, but was unexpectedly higher (5%) in MGUS (P=0.005). This finding is consistent with a single report in a smaller number of cases.23 Both the MM and MGUS cases showed the translocation in at least 70% of cells. The t(14;16) showed a prevalence of 3% (n=67) with a consistent distribution in each of the major disease subgroups. The prevalence of t(4;14) in MM was 11%, which is at the lower end of the range described (11–20%).5–8 Nevertheless this appears to be an accurate reflection of the incidence of t(4;14) in UK MM patients. We have shown that the rate of IgH translocations is inversely proportional to age20 and when only patients under the age of 66 are considered the incidence of t(4;14) in MM rose to 13%. In keeping with previous reports9;14 the t(4;14) was significantly less frequent in MGUS than in MM or SMM (P=0.0002 and P=0.001 respectively).
Table 1.
Prevalence of t(14;20), t(4;14) and t(14;16) in different plasma cell dyscrasias and associations with other factors.
Association with other factors
The t(14;20) is similar in its genetic associations to the t(14;16) and t(4;14). Of 37 patients with t(14;20), 28 (76%) also had a del(13q) (P<0.0001 cf total del(13) cases). There was a strong association with a non-hyperdiploid (NHRD) karyotype (30/36, P<0.0001 cf total NHRD cases). Fewer cases could be tested for 1q. There was a strong association between 1q and all three translocations in MM but t(14;20) MGUS cases did not show an excess of 1q gain, although the difference from t(4;14) or t(14;16) cases was not significant.
The t(14;16), like the t(4;14) (Ref. #9 and Table 1; P<10−7), has a higher prevalence in IgA myeloma (19/49 cf 388/1621 in total MM cases, P=0.02). No association with IgA isotype was seen with t(14;20) (only 3/23 cases IgA, 13%). Interestingly, none of the translocations showed an IgA excess in MGUS/SMM. The incidence of bone disease at diagnosis (Table 1) was significantly lower in t(4;14) and t(14;16) cases (both 58%) than for MM overall (68%, P=0.006 and P=0.05 respectively). Although the trend was also lower for t(14;20) at 61% (14/23) this was not significantly different from the overall incidence (P=0.19) which may be due to the small numbers.
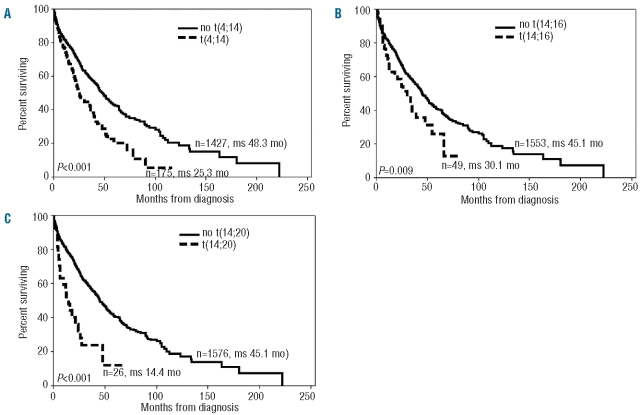
The survival curves for t(4;14), t(14;16) and t(14;20) are shown in Figure 1 A–C, which make it clear that all three translocations are associated with a poor prognosis in MM. The t(14;20) patients had a short median survival of only 14.4 months.
Figure 1.
Kaplan-Meier survival curves for patients with (A) t(4;14), (B) t(14;16), (C) t(14;20).
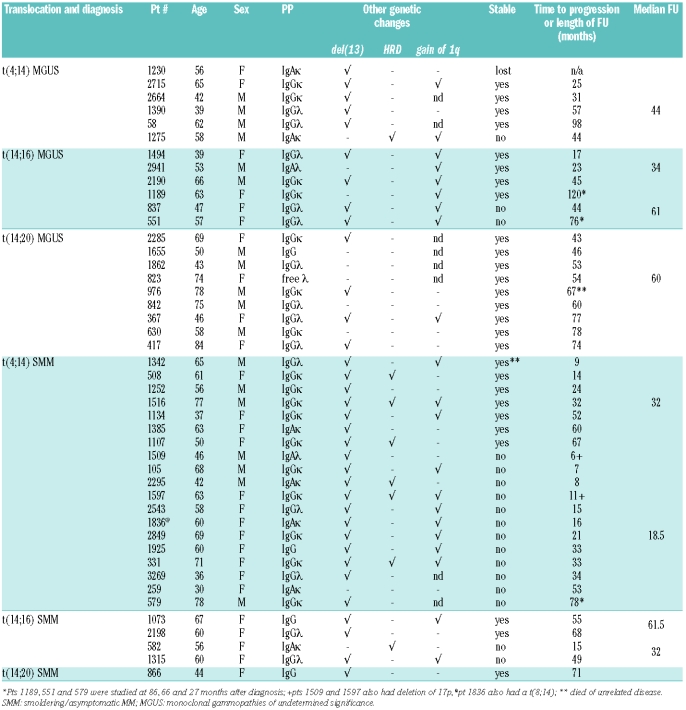
In contrast to myeloma patients, the t(14;20) MGUS/SMM patients appear to do particularly well. All are alive with stable paraprotein levels and no evidence of progression from 43–78 months after diagnosis. This observation does not hold for t(4;14) and t(14;16) MGUS patients, who constitute a less uniform group (Table 2) with one of 5 t(4;14) and 2 of 6 t(14;16) MGUS cases having progressed (follow-up range 17–120 months from diagnosis). Not surprisingly, SMM cases show a higher progression rate, with 12/19 t(4;14) and 2/4 t(14;16) cases progressing. There appeared to be two patterns of progression with 7 patients showing steadily increasing M-protein and requiring treatment by less than 1.5 years from presentation, and the remainder having a longer indolent period followed by a sudden rise in M-protein or onset of other symptoms of end organ damage, thus conforming to both the evolving and non-evolving patterns suggested by Rosinol et al.24 The range of time to progression of the latter group was 33 to 78 months. Overall only 11 of 27 t(4;14) and t(14;16) MGUS and SMM patients with follow-up of at least three years required treatment.
Table 2.
Genetic abnormalities, patient’s characteristics and disease course in smoldering/asymptomatic MM and monoclonal gammopathies of undetermined significance patients with t(4;14), t(14;16) or t(14;20)
Conclusions
These results provide important information about the impact of these three translocations on the etiology and outcome of myeloma. While they are associated with short survival in MM the translocations alone cannot be responsible for this clinical behavior and additional events must be required. Cases characterized by these translocations, particularly the t(14;20), can be stable as either MGUS or SMM for years before progression occurs. All three translocations are strongly associated with deletion of 13q, NHRD and gain of 1q, but none of these additional markers are sufficient to distinguish the clinical behavior of t(4;14), t(14;16) or t(14;20) cases.
Acknowledgments
we thank Leukaemia Research for funding this work, Catherine Stacey-Richardson and the referring clinicians and their research nurses who have provided clinical details, and Faith Davies, Sue Bell and Walter Gregory for various aspects of the Myeloma IX trial.
Footnotes
Funding: this work was funded by Leukaemia Research.
Authorship and Disclosures
FMR was the principal investigator and takes primary responsibility for the paper. LC, GPD, RKMP, DMS, and MTD performed the laboratory work for this study. AJS participated in the statistical analysis, FMR, NCPC, CJH and GJM co-ordinated the research. All authors contributed to the final version of the paper.
The authors reported no potential conflicts of interest.
References
- 1.Bergsagel PL, Kuehl WM. Molecular pathogenesis and a consequent classification of multiple myeloma. J Clin Oncol. 2005;23(26):6333–8. doi: 10.1200/JCO.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 2.Fonseca R, Blood E, Rue M, Harrington D, Oken MM, Kyle RA, et al. Clinical and biologic implications of recurrent genomic aberrations in myeloma. Blood. 2003;101(11):4569–75. doi: 10.1182/blood-2002-10-3017. [DOI] [PubMed] [Google Scholar]
- 3.Hurt EM, Wiestner A, Rosenwald A, Shaffer AL, Campo E, Grogan T, et al. Overexpression of c-maf is a frequent oncogenic event in multiple myeloma that promotes proliferation and pathological interactions with bone marrow stroma. Cancer Cell. 2004;5(2):191–9. doi: 10.1016/s1535-6108(04)00019-4. [DOI] [PubMed] [Google Scholar]
- 4.Avet-Loiseau H, Attal M, Moreau P, Charbonnel C, Garban F, Hulin C, et al. Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myelome. Blood. 2007;109(8):3489–95. doi: 10.1182/blood-2006-08-040410. [DOI] [PubMed] [Google Scholar]
- 5.Cremer FW, Bila J, Buck I, Kartal M, Hose D, Ittrich C, et al. Delineation of distinct subgroups of multiple myeloma and a model for clonal evolution based on inter-phase cytogenetics. Genes Chromosomes Cancer. 2005;44(2):194–203. doi: 10.1002/gcc.20231. [DOI] [PubMed] [Google Scholar]
- 6.Gutierrez NC, Castellanos MV, Martin ML, Mateos MV, Hernandez JM, Fernandez M, et al. Prognostic and biological implications of genetic abnormalities in multiple myeloma undergoing autologous stem cell transplantation: t(4;14) is the most relevant adverse prognostic factor, whereas RB deletion as a unique abnormality is not associated with adverse prognosis. Leukemia. 2007;21(1):143–50. doi: 10.1038/sj.leu.2404413. [DOI] [PubMed] [Google Scholar]
- 7.Keats JJ, Reiman T, Maxwell CA, Taylor BJ, Larratt LM, Mant MJ, et al. In multiple myeloma, t(4;14)(p16;q32) is an adverse prognostic factor irrespective of FGFR3 expression. Blood. 2003;101(4):1520–9. doi: 10.1182/blood-2002-06-1675. [DOI] [PubMed] [Google Scholar]
- 8.Gertz MA, Lacy MQ, Dispenzieri A, Greipp PR, Litzow MR, Henderson KJ, et al. Clinical implications of t(11;14)(q13;q32), t(4;14)(p16.3;q32), and –17p13 in myeloma patients treated with high-dose therapy. Blood. 2005;106(8):2837–40. doi: 10.1182/blood-2005-04-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avet-Loiseau H, Facon T, Grosbois B, Magrangeas F, Rapp MJ, Harousseau JL, et al. Oncogenesis of multiple myeloma: 14q32 and 13q chromosomal abnormalities are not randomly distributed, but correlate with natural history, immunological features, and clinical presentation. Blood. 2002;99(6):2185–91. doi: 10.1182/blood.v99.6.2185. [DOI] [PubMed] [Google Scholar]
- 10.Kyle RA, Therneau TM, Rajkumar SV, Offord JR, Larson DR, Plevak MF, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346(8):564–9. doi: 10.1056/NEJMoa01133202. [DOI] [PubMed] [Google Scholar]
- 11.Hallek M, Bergsagel PL, Anderson KC. Multiple myeloma: increasing evidence for a multistep transformation process. Blood. 1998;91(1):3–21. [PMC free article] [PubMed] [Google Scholar]
- 12.Landgren O, Kyle RA, Pfeiffer RM, Katzmann JA, Caporaso NE, Hayes RB, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009;113(22):5412–7. doi: 10.1182/blood-2008-12-194241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss BM, Abadie J, Verma P, Howard RS, Kuehl WM. A monoclonal gammopathy precedes multiple myeloma in most patients. Blood. 2009;113(22):5418–22. doi: 10.1182/blood-2008-12-195008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuehl WM, Bergsagel PL. Multiple myeloma: evolving genetic events and host inter-actions. Nat Rev Cancer. 2002;2(3):175–87. doi: 10.1038/nrc746. [DOI] [PubMed] [Google Scholar]
- 15.Malgeri U, Baldini L, Perfetti V, Fabris S, Vignarelli MC, Colombo G, et al. Detection of t(4;14)(p16.3;q32) chromosomal translocation in multiple myeloma by reverse transcription-polymerase chain reaction analysis of IGH-MMSET fusion transcripts. Cancer Res. 2000;60(15):4058–61. [PubMed] [Google Scholar]
- 16.Fonseca R, Bailey RJ, Ahmann GJ, Rajkumar SV, Hoyer JD, Lust JA, et al. Genomic abnormalities in monoclonal gammopathy of undetermined significance. Blood. 2002;100(4):1417–24. [PubMed] [Google Scholar]
- 17.Van Wier SA, Ahmann GJ, Henderson KJ, Greipp PR, Rajkumar SV, Larson D, et al. The t(4;14) is present in patients with early stage plasma cell proliferative disorders including MGUS and smoldering multiple myeloma (SMM) Blood. 106(11):1545. 1-11-2005. [Google Scholar]
- 18.Durie BG, Kyle RA, Belch A, Bensinger W, Blade J, Boccadoro M, et al. Myeloma management guidelines: a consensus report from the Scientific Advisors of the International Myeloma Foundation. Hematol J. 2004;5(3):285. [PubMed] [Google Scholar]
- 19.Morgan GJ, Davies FE, Gregory WM, Bell SE, Szubert AJ, Cocks K, et al. The addition of thalidomide to the induction treatment of newly presenting myeloma patients increases the CR rate which is likely to translate into improved PFS and OS. Blood. 2009;114(22):352. [Google Scholar]
- 20.Ross FM, Ibrahim AH, Vilain-Holmes A, Winfield MO, Chiecchio L, Protheroe RK, et al. Age has a profound effect on the incidence and significance of chromosome abnormalities in myeloma. Leukemia. 2005;19(9):1634–42. doi: 10.1038/sj.leu.2403857. [DOI] [PubMed] [Google Scholar]
- 21.Chiecchio L, Protheroe RK, Ibrahim AH, Cheung KL, Rudduck C, Dagrada GP, et al. Deletion of chromosome 13 detected by conventional cytogenetics is a critical prognostic factor in myeloma. Leukemia. 2006;20(9):1610–7. doi: 10.1038/sj.leu.2404304. [DOI] [PubMed] [Google Scholar]
- 22.Wuilleme S, Robillard N, Lode L, Magrangeas F, Beris H, Harousseau JL, et al. Ploidy, as detected by fluorescence in situ hybridization, defines different subgroups in multiple myeloma. Leukemia. 2005;19(2):275–8. doi: 10.1038/sj.leu.2403586. [DOI] [PubMed] [Google Scholar]
- 23.Chng WJ, Glebov O, Bergsagel PL, Kuehl WM. Genetic events in the pathogenesis of multiple myeloma. Best Pract Res Clin Haematol. 2007;20(4):571–96. doi: 10.1016/j.beha.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosinol L, Carrio A, Blade J, Queralt R, Aymerich M, Cibeira MT, et al. Comparative genomic hybridisation identifies two variants of smoldering multiple myeloma. Br J Haematol. 2005;130(5):729–32. doi: 10.1111/j.1365-2141.2005.05673.x. [DOI] [PubMed] [Google Scholar]