Abstract
Small molecule inhibitors of PI3K for oncology mainly target the class I PI3Ks, comprising the p110α, β, γ, and δ isoforms, of which only p110α is mutated in cancer. To assess the roles of class I PI3K isoforms in cell proliferation and survival, we generated immortalized mouse leukocyte and fibroblast models in which class I PI3Ks were inactivated by genetic and pharmacological approaches. In IL3-dependent hemopoietic progenitor cells (which express all four class I PI3K isoforms), genetic inactivation of either p110α or p110δ did not affect cell proliferation or survival or sensitize to p110β or p110γ inactivation. Upon compound inactivation of p110α and p110δ, which removed >90% of p85-associated PI3K activity, remarkably, cells continued to proliferate effectively, with p110β assuming an essential role in signaling and cell survival. Furthermore, under these conditions of diminished class I PI3K activity, input from the ERK pathway became important for cell survival. Similar observations were made in mouse embryonic fibroblasts (which mainly express p110α and p110β) in which p110α or p110β could sustain cell proliferation as a single isoform. Taken together, these data demonstrate that a small fraction of total class I PI3K activity is sufficient to sustain cell survival and proliferation. Persistent inhibition of selected PI3K isoforms can allow the remaining isoform(s) to couple to upstream signaling pathways in which they are not normally engaged. Such functional redundancy of class IA PI3K isoforms upon sustained PI3K inhibition has implications for the development and use of PI3K inhibitors in cancer.
Keywords: apoptosis, cancer therapeutics, inhibitors, combination therapy, MAP kinase
The class I subset of PI3K comprises the p110 catalytic subunits, which have been further subdivided into class IA and IB, depending on whether they occur in complex with a p85- (in the case of p110α, p110β, and p110δ) or p87/p101-type regulatory subunit (in the case of p110γ). Unlike p87/p101, which have no homology to other proteins, the p85 subunits have SH2 domains, which can link the class IA PI3Ks to tyrosine kinase signaling pathways. In mammals, the four class I PI3K isoforms are present in all cell types, with p110δ and p110γ highly enriched in leukocytes (1). Among the p110s, the most prominent role in cancer has thus far been attributed to p110α, encoded by the PIK3CA gene, found to be amplified and mutated in a wide range of solid tumors (2, 3). Consistent with their roles in signaling downstream of tyrosine kinases and Ras, class IA PI3Ks are currently being pursued as therapeutic targets in oncology (4, 5). However, the relative importance of p110β in tyrosine kinase signaling is not entirely clear, as this isoform has recently been shown to mainly signal downstream of G protein-coupled receptors (GPCRs) (6). A role for the GPCR-coupled p110γ in cancer is less clear, but activity against this isoform is often represented in small PI3K molecule inhibitors under development or in trials. Moreover, overexpression of non-p110α class I PI3K isoforms in cell-based models can confer characteristics of cell transformation (7).
A better understanding of the role of the individual class I PI3K isoforms in cell survival and proliferation is important for the development of therapeutics targeting the PI3K pathway, especially to determine whether inhibition of multiple PI3K isoforms is essential to block cell proliferation and survival. In this study, we addressed this question by derivation of cell lines from mice with homozygous inactivation of p110α (8, 9), p110δ (10), or their intercrosses, in combination with the use of small molecule inhibitors against p110β (11) or p110γ (12). We initially focused our studies on hemopoietic cells, given that these cells express all four class I PI3K isoforms, allowing us to test the contribution of each p110 isoform to cell proliferation and survival. This cell model also allowed us to test the role of p110δ in the context of p110α and p110β. Indeed, it has now become apparent that many nonhematological cancers, unlike their normal tissue counterparts, can express high levels of p110δ (13–15), increasing the relevance of this model to the study of solid tumors. We also studied mouse embryonic fibroblasts (MEFs), which mainly express p110α and p110β, with low or undetectable levels of p110δ and p110γ (6). We have used these cells to assess the effect of inhibiting class I PI3K isoforms, alone or in combination, on cell proliferation and survival, and on sensitivity to treatment with various stressors. Our data show that cells can proliferate and survive with very low levels of class I PI3K activity. Cells only showed a reduction in proliferation, with associated cell death in the case of hemopoietic cells, upon full inactivation of class IA PI3K activity. This also resulted in sensitization of cells to selected signal transduction inhibitors, including those targeting the MEK/ERK pathway. However, inactivation of class IA PI3Ks did not sensitize hemopoietic cells or MEFs to genotoxic stress. The implications of these findings for the ongoing development of PI3K inhibitors and their use in cancer therapy are discussed.
Results
Expression of a Single Active Class IA PI3K species Can Sustain Cell Survival and Proliferation in Hemopoietic Cells.
Homozygous p110α knock-in (KI) mice (8) die around day 10 of embryonic development, partially due to defective developmental angiogenesis (9). Embryos die before the stage of liver development, precluding isolation of hemopoietic cells from this organ. We therefore used the yolk sac, an extraembryonic tissue and a site of primitive hemopoiesis in the mouse (16), to derive hemopoietic progenitor cells (HPCs) from E10.5 embryos. HPCs, which express all four class I PI3K isoforms, were derived from homozygous p110α KI (8) or p110δ KI mice (10) or from their intercrosses (further referred to as p110α/δ KI). In this KI approach, the endogenous p110 alleles are inactivated by the introduction of a germline KI mutation in the kinase domain of the p110 isoforms. In these mice and cells derived thereof, the mutated p110 protein is expressed in equivalent amounts as in WT mice but in an inactive form. Such genetic intervention better reflects kinase inhibition by small molecule inhibitors than gene deletion approaches (17).
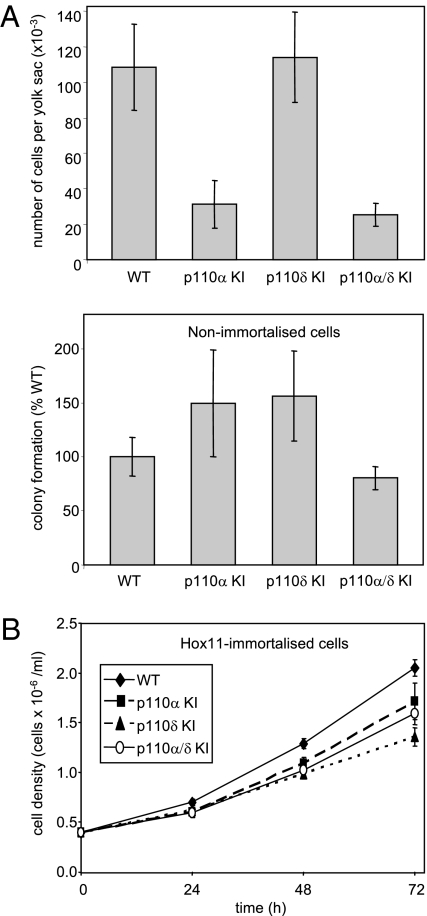
p110α/δ KI embryos were obtained at a frequency somewhat below the predicted Mendelian ratio (20% versus expected 25%) and were often severely malformed. When the condition of the embryo allowed isolation of the yolk sac, this tissue (like p110α KI embryos) contained substantially fewer cells than WT embryos (Fig. 1A Upper).
Fig. 1.
HPC survival and proliferation upon genetic inactivation of p110α, p110δ, or both. (A) Primary cells were isolated from yolk sacs of E10.5 embryos of the designated genotypes and counted (Upper), followed by measurement of colony formation capacity in methylcellulose-based semisolid media (Lower) (equal numbers of cells were plated per condition). Pooled data from cells isolated from three embryo litters per each genotype (i.e., nine independent colony assays) are shown. (B) Immortalized Hox11-expressing HPCs of the indicated genotypes were seeded at the same density and cultured in the presence of IL3, followed by cell counting at the indicated time points. Pooled data from three independent experiments performed in triplicate are shown.
Primary HPCs from all genotypes very effectively produced colonies upon plating in cytokine/growth factor-supplemented methylcellulose media, albeit with some variable efficiency (Fig. 1A Lower).
We next transduced these primary HPCs with a retrovirus encoding Hox11 (18), a protooncogene originally identified in pediatric T cell acute lymphoblastic leukemia. In vitro transduction of HPCs with Hox11 is known to give rise to immortal but still factor-dependent cell lines (19). Immortalized IL3-dependent HPC pools with single or combined inactivation of p110α and p110δ could be obtained and found to proliferate well in liquid cultures, albeit at a slightly reduced rate compared to WT cells, but with no apparent differences between the three genotypes (Fig. 1B).
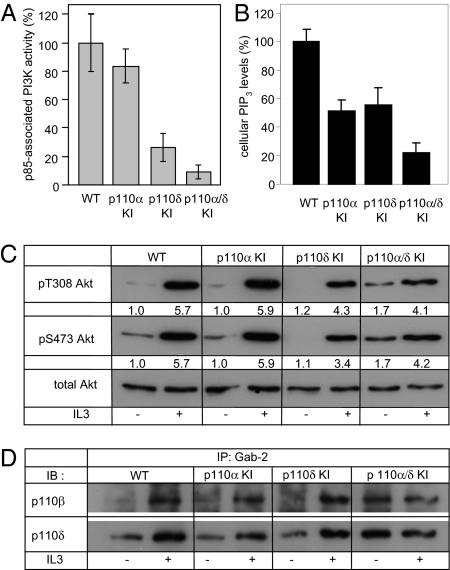
Lipid kinase activity assays on p85 immunoprecipitates from each of these HPC lines indicated that p110δ contributed the largest fraction (approximately 70%) of the total class IA PI3K activity in WT cells (Fig. 2A), with p110α accounting for approximately 20% of PI3K activity. These observations are in line with p110δ being the predominant PI3K isoform in leukocytes (20, 21). p110α/δ KI cells had about 10% of p85-associated PI3K activity remaining, presumably conferred by p110β. In vitro kinase assays on immunoprecipitated PI3K do not necessarily reflect the impact of individual PI3K isoform inactivation on the cellular levels of their in vivo product phosphatidylinositol(3,4,5,)trisphosphate (PIP3) (22). We therefore measured cellular PIP3 levels in HPCs with inactivation of the various PI3K isoforms using a time-resolved FRET assay (23). As shown in Fig. 2B, inactivation of p110α or p110δ resulted in a similar reduction in the PIP3 levels in IL3-stimulated HPCs. This implies that p110α contributes higher (and p110δ lower) amounts of PIP3 in vivo than indicated by the p85-associated PI3K activity levels. Combined inactivation of p110α and p110δ further reduced the PIP3 levels to approximately 20% of those in WT cells, a value roughly in line with the p85-associated activity in these cells. Taken together, these data show that HPCs, either primary or immortalized, can survive and proliferate in the absence of the class IA catalytic isoforms p110α and p110δ and maintain sufficient levels of PIP3 for survival and proliferation in the face of only 10% of total class IA PI3K activity present.
Fig. 2.
PI3K activity and signaling in immortalized HPCs upon genetic inactivation of p110α, p110δ, or both. (A) Lipid kinase activity was assayed in anti-p85 immunoprecipitates from HPC lysates. Data from one experiment performed in quadruplicate are shown. (B) Cellular PIP3 levels in IL3-stimulated HPCs. Pooled data from three independent experiments performed in duplicate are shown. The average value of p110α/δ KI HPCs treated with 1 μM TGX-221 for 16 h was subtracted as background signal. (C and D) HPCs, deprived of serum and IL3 for 3 h, were stimulated with 20 ng/mL IL3 for 5 min at 37 °C, followed by cell lysis and immunoblotting of 50 μg of total protein with the indicated antibodies to Akt (C) or by immunoprecipitation of 1.5 mg of total protein using an antibody to Gab2, followed by immunoblotting using antibodies to the indicated p110 isoforms (D). In C, relative (fold over vehicle-treated WT cells) signal intensities, indicated under each blot, were quantified by densitometry.
p110β Can Substitute for p110α/p110δ in Cell Survival and Proliferation Signaling in Hemopoietic Cells.
We next investigated signaling in cells lacking functional p110α, p110δ or both. As shown in Fig. 2C, IL3-induced phosphorylation of Akt was unaffected in p110α KI cells and only modestly reduced in p110δ and p110α/δ KI cells. Following IL3 receptor stimulation, the adaptor protein Gab2 is known to bind the bulk of PI3K activity (24). In p110α- or p110δ-null cells, p110 binding to Gab2 was only observed upon IL3 stimulation (Fig. 2D) (note that p110α could not be detected in this complex, probably due to its expression levels being below the detection limit of the assay). In p110α/δ-deficient cells, p110β and p110δ tended to associate with Gab2, even in quiescent conditions of no IL3 stimulation (Fig. 2D). This was observed in two independently-derived p110α/δ KI HPC pools (Fig. S1 Upper). The underlying mechanism for this constitutive association of PI3K subunits with Gab2 is not clear at the moment, but is unlikely to be the result of full constitutive activation of the IL3 signaling pathway, given that basal phosphorylation of ERK under IL3-free conditions was not observed in these cells (Fig. S1B).
Inhibition of p110β Blocks Proliferation and Induces Apoptosis in Hemopoietic Cells Only upon Coinactivation of p110α and p110δ.
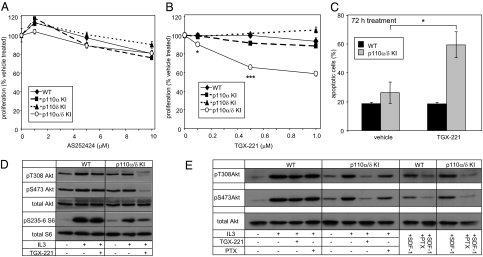
We next tested the sensitivity of HPCs to treatment with small molecule inhibitors with selectivity for p110β (TGX-221) (11) or p110γ (AS252424) (12). Treatment with even a high dose of AS252424 had little effect on proliferation of cells of any genotype (Fig. 3A), excluding a potential involvement of p110γ in cell survival and proliferation in this cell model. Inhibition of p110β only affected the proliferation of p110α/δ-deficient cells (Fig. 3B), with prolonged (72-h) treatment inducing apoptosis only in these cells (Fig. 3C).
Fig. 3.
p110β can support survival and proliferation of p110α/δ KI cells in a largely Gβγ-independent fashion. (A and B) Dose-dependent effect of p110γ inhibitor AS252424 (A) or p110β inhibitor TGX-221 (B) on HPC proliferation assessed by MTS assay after treatment for 24 h. Pooled data from two and three independent experiments, respectively, performed in triplicate, are shown. (C) Apoptosis induced by 72-h treatment with TGX-221 (1 μM). Pooled data from four independent experiments are shown. (D) WT and p110α/δ KI HPCs cultured in the presence of 500 nM TGX-221 or vehicle for 24 h were stimulated with 20 ng/mL IL3 for 5 min at 37 °C, followed by lysis and immunoblot analysis of Akt and S6 phosphorylation using the indicated antibodies. (E) WT and p110α/δ KI HPCs cultured in serum-free RPMI medium in the presence or absence of 100 ng/mL PTX for 3 h were treated with 100 nM TGX-221 or vehicle for 30 min, followed by stimulation with 20 ng/mL IL3 for 5 min at 37 °C. Akt was analyzed by immunoblotting using the indicated antibodies. Pertussis toxin-treated WT and p110α/δ KI cells were stimulated for 5 min with the GPCR ligand SDF-1 (250 ng/mL) as a positive control for the effectiveness of PTX treatment.
In line with the observations above, inhibition of p110β by TGX-221 only affected PI3K pathway signaling in p110α/δ-null cells, abrogating IL3-induced Akt phosphorylation and substantially decreasing phosphorylation of the ribosomal S6 protein (Fig. 3D). Treatment with the Gi protein inhibitor pertussis toxin (PTX) only modestly affected IL3-induced phosphorylation of Akt in these cells (Fig. 3E), suggesting that p110β signaling in p110α/δ-deficient HPCs predominantly operates through tyrosine kinase signaling pathways, rather than through GPCR signaling (6).
We also found that proliferation was unaffected in p110α/δ KI cells, TGX-221–treated p110α KI and TGX-221–treated p110δ KI cells (in which, respectively, only p110β, p110δ, or p110α remain as a single active class IA PI3K isoform), suggesting that any class IA PI3K isoform is sufficient to sustain cell survival and proliferation.
PI3K Inactivation in Hemopoietic Cells Results in Enhanced Sensitivity of Proliferation to Blockade of mTOR or MEK.
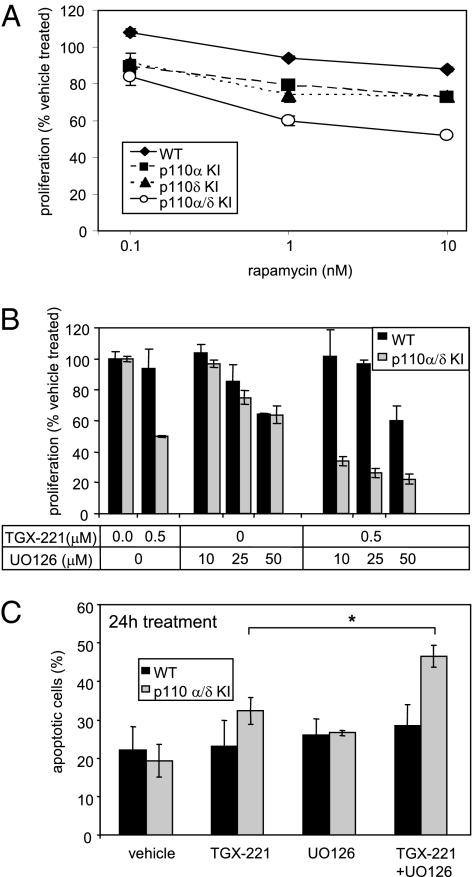
We next investigated whether inhibition of PI3K affects sensitivity to blockade of signaling pathways that are partially controlled by PI3K (such as mTOR) or that operate in parallel with the PI3K pathway (such as the MEK/ERK pathway). Inhibition of proliferation by rapamycin was enhanced in cells with one or more inactive PI3K isoforms (Fig. 4A), but this treatment did not lead to apoptosis, even in p110α/δ KI HPCs treated with TGX-221 (Fig. S2). The MEK inhibitor UO126 also had an additive effect on inhibition of proliferation of p110α/δ-deficient HPCs by TGX-221 (Fig. 4B and Fig. S3) but, unlike rapamycin, also enhanced and accelerated the apoptosis induced by TGX-221, with cell death now clearly evident by 24 h (Fig. 4C) (note that treatment with either TGX-221 or UO126, as single agents, over 24 h had a minimal impact on apoptosis in these cells). This highlights the importance of combined pan-class IA PI3K and ERK inhibition to achieve a rapid apoptotic response.
Fig. 4.
mTOR and ERK pathway inhibition augments the effect of class IA PI3K inhibition on HPC survival and proliferation. (A and B) Dose-dependent effect of rapamycin (A) or TGX-221 combined with the MEK inhibitor UO126 (B) on HPC proliferation, assessed by MTS assay after 48 h. Representative experiments performed in triplicate are shown (values = mean ± SD). (C) Apoptosis in WT and p110α/δ KI HPCs upon incubation in the presence of TGX-221 (1 μM) with or without UO126 (10 μM). Data pooled from three independent experiments are shown.
Either p110α or p110β Is Sufficient for Proliferation of MEFs.
We next tested the impact of PI3K isoform inactivation in MEFs, which mainly express p110α and p110β, and low or undetectable p110δ and p110γ (6). PI3K isoform inactivation in these cells was achieved by genetic (p110α) or pharmacological (for p110β only, as no p110α-selective inhibitors are available) approaches.
To inactivate p110α, we immortalized MEF (by p53 knock-down) from mice homozygous for a conditional p110α allele (p110αflox/flox mice). Efficient deletion of the floxed p110α allele was achieved by the introduction of tamoxifen-inducible Cre recombinase. Indeed, no p110α protein expression (Fig. S4A) or activity (Fig. S4B) could be detected in total lysates or p110α immunoprecipitates, respectively, from p110α-deficient MEFs (further referred to as p110αDEL/DEL MEFs).
Basal proliferation of p110αDEL/DEL MEFs was essentially unaffected, despite the fact that the remaining p85-associated PI3K activity in these cells was as low as 7% of control (p110αflox/flox) cells (Fig. S4C).
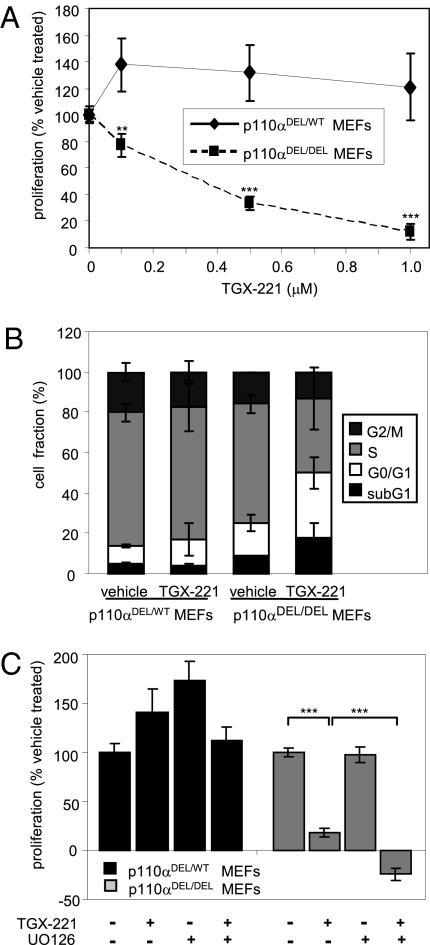
Similar to HPCs, inhibition of p110β by TGX-221 blocked proliferation of p110αDEL/DEL MEFs in a dose-dependent manner (Fig. 5A). In these cells, p110β inhibition resulted in a G0/G1 cell cycle arrest rather than in induction of apoptosis (Fig. 5B). Similar to HPCs, the MEK inhibitor UO126 enhanced the negative impact of TGX-221 on proliferation of p110αDEL/DEL MEFs (Fig. 5C).
Fig. 5.
Simultaneous inactivation of p110α and p110β in MEFs results in G0/G1cell cycle arrest. (A) Dose-dependent effect of the p110β inhibitor TGX-221 on proliferation of p110α-deficient (p110αDEL/DEL) MEF assessed by MTS assay after treatment for 48 h. Heterozygous p110αDEL/WT MEFs (i.e., tamoxifen-treated p110αflox/WT MEFs) were used as a control. Pooled data from three independent experiments are shown. (B) Cell cycle distribution of p110α-deficient MEFs assessed by FACS analysis of BrdU incorporation following 48-h treatment with 1 μM TGX-221. Pooled data from three independent experiments are shown. (C) Effect of combined treatment with TGX-221 (1 μM) and MEK UO126 (10 μM) on MEF proliferation, assessed by MTS assay after 48 h. Pooled data from three independent experiments are shown.
Conversely, inhibition of p110β with TGX-221 did not diminish proliferation of p110αDEL/WT MEFs (Fig. 5A), indicating that p110α can support proliferation as a single class I PI3K isoform in these cells.
Sensitivity of PI3K-Deficient Cells to Cellular Stress.
Pan-PI3K inhibitors such as LY294002 have previously been shown to enhance sensitivity to DNA-damaging agents (25–27). However, LY294002 also inhibits protein kinase members of the PI3K family that are induced in response to DNA damage, including ATM, ATR, and DNA-PK (28–30). Therefore, it is important to determine the extent to which LY294002-induced sensitization to genotoxic agents may be attributable specifically to PI3K inhibition. TGX-221-treated p110αDEL/DEL MEFs were not sensitized to doxorubicin-induced apoptosis (Fig. S5A). Also in HPCs, there was no correlation between inactivation of selected PI3K isoforms and sensitivity to doxorubicin or etoposide, including full inactivation of class IA PI3Ks (i.e., by treatment of p110α/δ-deficient cells with TGX-221) (Fig. S5C). Furthermore, various cellular stresses such as the autophagy inhibitor chloroquine, serum deprivation, and H2O2 had a similar impact on all HPC pools, irrespective of genotype (Fig. S6). These data suggest that class IA PI3K inhibition per se does not sensitize cells to the stressors tested.
Discussion
An important consideration in targeting the PI3K pathway for therapy is whether to interfere with one or more of the isoforms of PI3K, especially in cancer (31). Current drugs developed for use in oncology mainly inhibit class I PI3Ks. In this study, we used two cell models with different class I PI3K isoform expression profiles to address the relative contribution of these PI3K isoforms to cell signaling, proliferation, and survival. Class I PI3K isoforms were inactivated either in isolation or in combination, using genetic or pharmacological approaches.
The ubiquitously expressed p110α has been considered the main PI3K isoform supporting cell survival and proliferation. However, the fact that embryos can progress until the 10th day of development upon full inactivation of p110α (8, 9, 32) implies that cells can survive and proliferate in the absence of functional p110α, in line with the observation that MEFs without functional p110α survive and proliferate (33). Likewise, genetic or pharmacological inactivation of p110β does not seem to have a major impact on cell proliferation and survival (6, 34, 35). In this study, we show that immortalized HPCs can tolerate combined loss-of-function of p110α and p110δ, which together account for 90% of the p85-associated PI3K activity in these cells. In other words, only about 10% of the cellular class IA PI3K activity appears to be sufficient to sustain survival and proliferation in these cells. In the case of MEFs, approximately 7% of total p85-associated PI3K activity still allows these cells to effectively proliferate. A recent study presented evidence for a role of both p110α and p110β in cell cycle progression (36), and it is conceivable that both PI3K isoforms might be necessary for optimal cell proliferation. Nevertheless, the present data demonstrate that, in principle, any class IA PI3K isoform can sustain cell proliferation and survival.
An important conclusion of the present study is that p110β as a single class I PI3K can sustain proliferation in both hemopoietic cells and MEFs. The mode of regulation of p110β has recently been the subject of intense investigation, not least because a role for this isoform in the development of certain cancers has started to emerge (34, 35, 37), especially in tumors lacking functional PTEN (38, 39). Despite the fact that p110β exists as a dimer with p85 and can become recruited to phosphotyrosine complexes, recent studies indicate that its activity is mainly regulated by GPCRs rather than by tyrosine kinase pathways (6, 34, 35). Here, we have shown that in the absence of catalytically active p110α and p110δ, p110β can productively engage with Gab2 signaling complexes in hemopoietic cells. Upon chemical inhibition of p110β in these cells, and thus complete inactivation of class IA PI3K activity, cell proliferation is inhibited and apoptosis is induced. Our data suggest that almost complete class IA inhibition is required to lead to cell death of immortalized HPCs. Such full inhibition of PI3K activity might be difficult to achieve with ATP-competitive inhibitors. This could partially explain why PI3K inhibition by small molecule inhibitors in vitro and in vivo does not appear to result in apoptosis but rather leads to cytostasis, due to a G0/G1 arrest (40–42). In other words, very potent pan-class IA PI3K inhibitors and full target inhibition might be required if apoptosis is the desired outcome. However, it is still possible that MEFs might be more reflective of most cell types in the context of sensitivity to PI3K inhibition, in that these cells show inhibition of proliferation rather than apoptosis upon inactivation of the bulk of class IA PI3K activity.
We acknowledge that the cell types used in this study might not be fully representative of cancer cells, which might be more addicted to the PI3K pathway, and therefore more sensitive to PI3K inhibition. However, our data demonstrate the principle that cells can be very resilient to PI3K pathway inactivation, something that might very well also occur in cancer cells, especially upon sustained PI3K inhibition. Our results are in line with our previous studies, which showed that immortalized cells, in contrast to primary cells, can often use multiple PI3K isoforms for the same function (43). Whether inhibition of p110α in cancers with an activating PIK3CA mutation will be sufficient as monotherapy remains to be determined. It is possible that the broader mutational landscape in which mutant p110α occurs may be a more critical factor. Also, p110α may be important at the initial stages of the oncogenesis but not once the cancer is established. In line with this possibility, a targeted p110α allele deficient for binding to Ras, or knockout of the p85 PI3K regulatory subunit, has been shown to protect mice from development of lung adenocarcinoma induced by mutated K-Ras (44, 45). However, established tumors in these models were found to be insensitive to inhibition of PI3K only, suggesting that PI3K is dispensable for tumor maintenance, at least in this lung cancer model (45).
Our results indicate that combining PI3K inhibition with some, but not all, therapeutic agents may improve efficacy. We found that concomitant treatment with the MEK inhibitor UO126 produced a stronger block in proliferation (in both HPCs and MEFs) and an accelerated apoptotic response (in HPCs) compared to pan-class IA PI3K inhibition only. These observations are in line with the recent demonstration of enhanced therapeutic efficacy of combined PI3K and MEK inhibition in a preclinical model of lung cancer (45). We also found that proliferation of HPCs with inactive PI3K isoforms, particularly those with combined p110α/δ inactivation, was more sensitive to treatment with the mTOR inhibitor rapamycin than WT cells. Full inactivation of class I PI3K, however, does not appear to render cells sensitive to any form of stress tested. HPCs or MEFs with inactive class IA PI3K did not show enhanced sensitivity to the genotoxic drugs doxorobucin and etoposide.
The PI3K pathway is a major transducer of growth factor signaling, which suppresses autophagy. In line with this, it has recently been shown that the pan-PI3K inhibitor PI-103 elicits an autophagic response in neuroblastoma cell lines (46) and that inhibition of Akt promotes autophagy and sensitizes PTEN-null tumors to lysosomotropic agents (47). We found that HPCs with inactive class I PI3Ks were not more sensitive to inhibition of autophagy, and thus do not appear to be evading apoptosis by resorting to autophagy (48).
Observations that implicate specific isoforms of PI3K in tumorigenesis [for example the selective mutation of p110α in cancer and the apparent selective role of p110β in some PTEN-deficient tumors (38, 39)] indicate that targeting of specific PI3K isoforms in cancer could have its merits. However, the data from the present study suggest that targeting of all class I PI3Ks will be essential to produce maximal inhibition of cell proliferation (probably in most cases) and to induce apoptosis (in some cases). Given that this might be difficult to achieve using ATP-competitive inhibitors, at least in a sustained manner for a long duration, it is most likely that combined blockade of additional signaling pathways, such as the MEK/ERK pathway, will be required to achieve a significant impact in cancer therapy.
Materials and Methods
Materials.
TGX-221, PI-103, LY294002, UO126, and rapamycin were from Calbiochem. AS252424 was kindly donated by Merck Serono. All other chemicals were from Sigma.
Mutant Mice.
p110α KI, p110αflox/flox, and p110δ KI mice used for isolation of HPCs or MEFs have been described before (8–10).
Proliferation and Apoptosis Assays.
Growth curves of HPCs were generated by counting cells using an automatic cell counter, CASY model TT (Innovatis). To test the effect of compounds on cell proliferation, HPCs (5 × 104 per well) or MEFs (2 × 103 per well) were cultured in 96-well plates, in the presence of the test compounds or the respective vehicle followed by MTS assay (Promega). Apoptosis was monitored by FACS analysis using a carboxyfluorescein multicaspase activity kit (Biomol) for HPCs or by APC annexin V (BD Pharmingen) for MEFs according to the manufacturers’ instructions.
In Vitro Lipid Kinase Assay and Cellular PIP3 Determination.
Lipid kinase activity was assayed in p85 immunoprecipitates using an equimolar mix of phosphatidylserine and phosphatidylinositol(4,5)bisphosphate as a substrate. Cellular PIP3 was measured in extracts from 3 to 4 × 107 HPCs per sample using a time-resolved FRET assay (23).
Statistical Analysis.
Values are presented as mean ± SEM. P values were calculated using unpaired two-tailed t test. P ≤ 0.05 was considered to be statistically significant (designated by a single asterisk; double asterisk, P ≤ 0.01; triple asterisk, P ≤ 0.001).
Supplementary Material
Acknowledgments
We thank Wayne Pearce and Khaled Ali for experimental help and Benoit Bilanges and Julie Guillermet-Guibert for critically reading the manuscript. This study was supported by the Leukemia Research Fund (Grant 06013), Cancer Research UK (CR-UK Programme C23338/A10200), and Queen Mary University of London. Personal support was from a Wellcome Trust VIP award to L.F. and a European Union Marie Curie Fellowship (PIEF-GA-2008-219945) to I.M.B. Part of this work was undertaken at University College London Hospitals/University College London, who received a proportion of funding from the National Institute for Health Research Biomedical Research Centres funding scheme of the UK Department of Health.
Footnotes
Conflict of interest statement: Bart Vanhaesebroeck is a consultant for Intellikine (San Diego, CA).
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0906461107/-/DCSupplemental.
References
- 1.Kok K, Geering B, Vanhaesebroeck B. Regulation of phosphoinositide 3-kinase expression in health and disease. Trends Biochem Sci. 2009;34:115–127. doi: 10.1016/j.tibs.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Shayesteh L, et al. PIK3CA is implicated as an oncogene in ovarian cancer. Nat Genet. 1999;21:99–102. doi: 10.1038/5042. [DOI] [PubMed] [Google Scholar]
- 3.Samuels Y, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 4.Vogt PK, Kang S, Elsliger MA, Gymnopoulos M. Cancer-specific mutations in phosphatidylinositol 3-kinase. Trends Biochem Sci. 2007;32:342–349. doi: 10.1016/j.tibs.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: Variations on a theme. Oncogene. 2008;27:5497–5510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guillermet-Guibert J, et al. The p110β isoform of phosphoinositide 3-kinase signals downstream of G protein-coupled receptors and is functionally redundant with p110γ. Proc Natl Acad Sci USA. 2008;105:8292–8297. doi: 10.1073/pnas.0707761105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang S, Denley A, Vanhaesebroeck B, Vogt PK. Oncogenic transformation induced by the p110beta, -gamma, and -delta isoforms of class I phosphoinositide 3-kinase. Proc Natl Acad Sci USA. 2006;103:1289–1294. doi: 10.1073/pnas.0510772103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foukas LC, et al. Critical role for the p110α phosphoinositide-3-OH kinase in growth and metabolic regulation. Nature. 2006;441:366–370. doi: 10.1038/nature04694. [DOI] [PubMed] [Google Scholar]
- 9.Graupera M, et al. Angiogenesis selectively requires the p110alpha isoform of PI3K to control endothelial cell migration. Nature. 2008;453:662–666. doi: 10.1038/nature06892. [DOI] [PubMed] [Google Scholar]
- 10.Okkenhaug K, et al. Impaired B and T cell antigen receptor signaling in p110δ PI 3-kinase mutant mice. Science. 2002;297:1031–1034. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- 11.Jackson SP, et al. PI 3-kinase p110beta: A new target for antithrombotic therapy. Nat Med. 2005;11:507–514. doi: 10.1038/nm1232. [DOI] [PubMed] [Google Scholar]
- 12.Pomel V, et al. Furan-2-ylmethylene thiazolidinediones as novel, potent, and selective inhibitors of phosphoinositide 3-kinase gamma. J Med Chem. 2006;49:3857–3871. doi: 10.1021/jm0601598. [DOI] [PubMed] [Google Scholar]
- 13.Arcaro A, et al. Two distinct phosphoinositide 3-kinases mediate polypeptide growth factor-stimulated PKB activation. EMBO J. 2002;21:5097–5108. doi: 10.1093/emboj/cdf512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sawyer C, et al. Regulation of breast cancer cell chemotaxis by the phosphoinositide 3-kinase p110delta. Cancer Res. 2003;63:1667–1675. [PubMed] [Google Scholar]
- 15.Boller D, et al. Targeting the phosphoinositide 3-kinase isoform p110delta impairs growth and survival in neuroblastoma cells. Clin Cancer Res. 2008;14:1172–1181. doi: 10.1158/1078-0432.CCR-07-0737. [DOI] [PubMed] [Google Scholar]
- 16.Dzierzak E, Speck NA. Of lineage and legacy: The development of mammalian hematopoietic stem cells. Nat Immunol. 2008;9:129–136. doi: 10.1038/ni1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vanhaesebroeck B, Ali K, Bilancio A, Geering B, Foukas LC. Signalling by PI3K isoforms: Insights from gene-targeted mice. Trends Biochem Sci. 2005;30:194–204. doi: 10.1016/j.tibs.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Yu W-M, Hawley TS, Hawley RG, Qu C-K. Immortalization of yolk sac-derived precursor cells. Blood. 2002;100:3828–3831. doi: 10.1182/blood-2002-03-0937. [DOI] [PubMed] [Google Scholar]
- 19.Hawley RG, et al. Transforming function of the HOX11/TCL3 homeobox gene. Cancer Res. 1997;57:337–345. [PubMed] [Google Scholar]
- 20.Vanhaesebroeck B, et al. P110delta, a novel phosphoinositide 3-kinase in leukocytes. Proc Natl Acad Sci USA. 1997;94:4330–4335. doi: 10.1073/pnas.94.9.4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geering B, Cutillas PR, Nock G, Gharbi SI, Vanhaesebroeck B. Class IA phosphoinositide 3-kinases are obligate p85-p110 heterodimers. Proc Natl Acad Sci USA. 2007;104:7809–7814. doi: 10.1073/pnas.0700373104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gold MR, Duronio V, Saxena SP, Schrader JW, Aebersold R. Multiple cytokines activate phosphatidylinositol 3-kinase in hemopoietic cells. Association of the enzyme with various tyrosine-phosphorylated proteins. J Biol Chem. 1994;269:5403–5412. [PubMed] [Google Scholar]
- 23.Gray A, Olsson H, Batty IH, Priganica L, Peter Downes C. Nonradioactive methods for the assay of phosphoinositide 3-kinases and phosphoinositide phosphatases and selective detection of signaling lipids in cell and tissue extracts. Anal Biochem. 2003;313:234–245. doi: 10.1016/s0003-2697(02)00607-3. [DOI] [PubMed] [Google Scholar]
- 24.Gu H, et al. New role for Shc in activation of the phosphatidylinositol 3-kinase/Akt pathway. Mol Cell Biol. 2000;20:7109–7120. doi: 10.1128/mcb.20.19.7109-7120.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Gorman DM, McKenna SL, McGahon AJ, Knox KA, Cotter TG. Sensitisation of HL60 human leukaemic cells to cytotoxic drug-induced apoptosis by inhibition of PI3-kinase survival signals. Leukemia. 2000;14:602–611. doi: 10.1038/sj.leu.2401726. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Y, et al. Distinctive regulation and function of PI 3K/Akt and MAPKs in doxorubicin-induced apoptosis of human lung adenocarcinoma cells. J Cell Biochem. 2004;91:621–632. doi: 10.1002/jcb.10751. [DOI] [PubMed] [Google Scholar]
- 27.Wang YA, et al. Enhanced anti-cancer effect of a phosphatidylinositol-3 kinase inhibitor and doxorubicin on human breast epithelial cell lines with different p53 and oestrogen receptor status. Int J Cancer. 2008;123:1536–1544. doi: 10.1002/ijc.23671. [DOI] [PubMed] [Google Scholar]
- 28.Goodarzi AA, Lees-Miller SP. Biochemical characterization of the ataxia-telangiectasia mutated (ATM) protein from human cells. DNA Repair (Amst) 2004;3:753–767. doi: 10.1016/j.dnarep.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 29.Hall-Jackson CA, Cross DA, Morrice N, Smythe C. ATR is a caffeine-sensitive, DNA-activated protein kinase with a substrate specificity distinct from DNA-PK. Oncogene. 1999;18:6707–6713. doi: 10.1038/sj.onc.1203077. [DOI] [PubMed] [Google Scholar]
- 30.Izzard RA, Jackson SP, Smith GC. Competitive and noncompetitive inhibition of the DNA-dependent protein kinase. Cancer Res. 1999;59:2581–2586. [PubMed] [Google Scholar]
- 31.Jia S, Roberts TM, Zhao JJ. Should individual PI3 kinase isoforms be targeted in cancer? Curr Opin Cell Biol. 2009;21:199–208. doi: 10.1016/j.ceb.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bi L, Okabe I, Bernard DJ, Wynshaw-Boris A, Nussbaum RL. Proliferative defect and embryonic lethality in mice homozygous for a deletion in the p110α subunit of phosphoinositide 3-kinase. J Biol Chem. 1999;274:10963–10968. doi: 10.1074/jbc.274.16.10963. [DOI] [PubMed] [Google Scholar]
- 33.Zhao JJ, et al. The p110alpha isoform of PI3K is essential for proper growth factor signaling and oncogenic transformation. Proc Natl Acad Sci USA. 2006;103:16296–16300. doi: 10.1073/pnas.0607899103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jia S, et al. Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature. 2008;454:776–779. doi: 10.1038/nature07091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ciraolo E, et al. Phosphoinositide 3-kinase p110beta activity: Key role in metabolism and mammary gland cancer but not development. Sci Signal. 2008;1:ra3. doi: 10.1126/scisignal.1161577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marqués M, et al. Phosphoinositide 3-kinases p110alpha and p110beta regulate cell cycle entry, exhibiting distinct activation kinetics in G1 phase. Mol Cell Biol. 2008;28:2803–2814. doi: 10.1128/MCB.01786-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu Q, et al. Phosphoinositide 3-OH kinase p85alpha and p110beta are essential for androgen receptor transactivation and tumor progression in prostate cancers. Oncogene. 2008;27:4569–4579. doi: 10.1038/onc.2008.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Torbett NE, et al. A chemical screen in diverse breast cancer cell lines reveals genetic enhancers and suppressors of sensitivity to PI3K isoform-selective inhibition. Biochem J. 2008;415:97–110. doi: 10.1042/BJ20080639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wee S, et al. PTEN-deficient cancers depend on PIK3CB. Proc Natl Acad Sci USA. 2008;105:13057–13062. doi: 10.1073/pnas.0802655105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raynaud FI, et al. Pharmacologic characterization of a potent inhibitor of class I phosphatidylinositide 3-kinases. Cancer Res. 2007;67:5840–5850. doi: 10.1158/0008-5472.CAN-06-4615. [DOI] [PubMed] [Google Scholar]
- 41.Dan S, Yoshimi H, Okamura M, Mukai Y, Yamori T. Inhibition of PI3K by ZSTK474 suppressed tumor growth not via apoptosis but G0/G1 arrest. Biochem Biophys Res Commun. 2009;379:104–109. doi: 10.1016/j.bbrc.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 42.Fan QW, et al. A dual phosphoinositide-3-kinase alpha/mTOR inhibitor cooperates with blockade of epidermal growth factor receptor in PTEN-mutant glioma. Cancer Res. 2007;67:7960–7965. doi: 10.1158/0008-5472.CAN-07-2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Papakonstanti EA, et al. Distinct roles of class IA PI3K isoforms in primary and immortalised macrophages. J Cell Sci. 2008;121:4124–4133. doi: 10.1242/jcs.032763. [DOI] [PubMed] [Google Scholar]
- 44.Gupta S, et al. Binding of ras to phosphoinositide 3-kinase p110alpha is required for ras-driven tumorigenesis in mice. Cell. 2007;129:957–968. doi: 10.1016/j.cell.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 45.Engelman JA, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–1356. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guillard S, et al. Molecular pharmacology of phosphatidylinositol 3-kinase inhibition in human glioma. Cell Cycle. 2009;8:443–453. doi: 10.4161/cc.8.3.7643. [DOI] [PubMed] [Google Scholar]
- 47.Degtyarev M, et al. Akt inhibition promotes autophagy and sensitizes PTEN-null tumors to lysosomotropic agents. J Cell Biol. 2008;183:101–116. doi: 10.1083/jcb.200801099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lum JJ, et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005;120:237–248. doi: 10.1016/j.cell.2004.11.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.