Abstract
Artemin, a member of the glial-derived neurotrophic factor family, promotes robust regeneration of sensory axons after dorsal root crush. We report here that several classes of sensory axons regenerate to topographically appropriate regions of the dorsal horn with artemin treatment. Projections of regenerated muscle and cutaneous myelinated sensory afferents are restricted to the correct spinal segments and to appropriate regions within spinal gray matter. Regenerated unmyelinated axons expressing calcitonin gene-related peptide project only to superficial laminae of the dorsal horn, where uninjured nociceptive afferents project normally. In contrast, intraventricular infusion of a soluble form of the Nogo receptor that blocks the action of several myelin-associated inhibitory proteins promotes relatively unrestricted regeneration of sensory axons throughout the dorsal white and gray matter of the spinal cord. These results demonstrate that cues capable of guiding regenerating axons to appropriate spinal targets persist in the adult mammalian cord, but only some methods of stimulating regeneration allow the use of these cues by growing axons.
Keywords: artemin, central nervous system regeneration, dorsal root, soluble Nogo receptor peptide, specificity
Growth of damaged axons in the adult spinal cord is inhibited by the presence of myelin-associated proteins, up-regulation of proteoglycan expression, and the absence of appropriate growth factors. Several agents that can partially overcome this inhibition permit some regeneration of spinal axons in contusion or transaction models of spinal cord injury (SCI) (1–4); however, the limited regeneration and difficulty in labeling specific subclasses of axons with known projection patterns within the spinal cord have impeded progress in determining whether these regenerated projections are specific.
The regeneration of sensory axons into the spinal cord after dorsal root (DR) crush provides a useful preparation for assessing the precision with which regenerating axons project back to appropriate target areas within the central nervous system (CNS). Regrowth of damaged sensory axons within the spinal cord can be visualized by injecting neurotracers into peripheral nerves. Identification of individual classes of axons can be achieved by making injections close to target tissues where nerve branches contain single classes of sensory afferents. Because only the DRs are damaged, the architecture of the spinal cord is left intact, allowing clear identification of the central projections of regenerated axons.
Without treatment, sensory axons regenerate only to the DR entry zone (DREZ), where they encounter inhibitory barriers within the CNS (5, 6). Application of two agents—a soluble Nogo receptor peptide (sNgR), which binds to Nogo receptor ligands and abrogates their inhibitory effect (7), and artemin (ART), a member of the glial-derived neurotrophic factor family—promote robust regeneration of sensory axons after DR crush (8, 9). The specificity of projections of specific classes of sensory axons to appropriate target areas within the spinal cord has not yet been investigated, however. In this report, we compare the topographic specificity of regenerated projections of sensory afferents promoted by sNgR and ART into the spinal cord. Our experiments demonstrate that treatment with ART, but not with sNgR, promotes topographically specific regeneration. These results indicate that cues capable of guiding regenerating axons to appropriate spinal target areas persist in the adult mammalian cord, but that only some methods of stimulating regeneration allow the use of these cues by growing axons.
Results
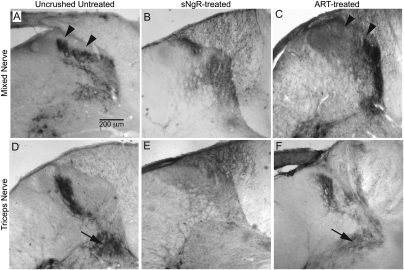
The normal projections of myelinated sensory afferents were assessed via injection of cholera toxin b (CTB) into peripheral nerves. CTB binds to GM1 gangliosides present on myelinated sensory and motor axons. Labeled sensory axons were seen to project throughout all but the most dorsal laminae of the dorsal horn. Some extended ventrally toward motor neurons, but none arborized within laminae I and IIo, where unmyelinated sensory afferents project (Fig. 1A). After cervical DR crush, sensory projections within the cord degenerated, and no projections were labeled by CTB in the dorsal horn, confirming the complete interruption of this pathway into the spinal cord (8, 9).
Fig. 1.
Regenerated myelinated sensory afferents project to topographically correct regions of the dorsal horn with ART treatment, but not with sNgR treatment. Central projections of sensory afferents were labeled with CTB injections on the uninjured side (A and D) and on the injured, regenerated side (B, C, E, and F). Experimental groups were untreated (A and D), sNgR treated (B and E), or ART treated (C and F). Injections were made into radial and/or median nerves (A–C) or the triceps muscle nerve (D–F). Arrowheads in A and C indicate laminae I and IIo. Arrows in D and F indicate the ventral projections of muscle afferents. Images in control panels (uncrushed axons) have been reversed horizontally in A and D, to facilitate comparison with projections of regenerated axons.
With intraventricular administration of sNgR, peripheral nerve injections with CTB demonstrated extensive regeneration of myelinated axons after DR crush. Regenerated axons projected aberrantly within the dorsal white matter and throughout the dorsal laminae of the gray matter, with no deficit of projections to laminae I and IIo, as is seen in normal rats (Fig. 1B; see also fig. 3C and E in ref. 8). These heavy projections into the most dorsal laminae suggest that at least some regenerating axons were projecting inappropriately. In contrast, systemic ART treatments promoted robust regeneration of CTB-labeled afferents throughout the dorsal horn except within the upper laminae, thereby restoring the normal pattern of projections (Fig. 1C) (9).
Robust regeneration of myelinated afferents was seen with both sNgR and ART, albeit in different patterns in the spinal cord. With ART treatment, myelinated afferents projected to the correct spinal laminae, avoiding the most superficial laminae that are normally occupied by unmyelinated axons. This suggests that regeneration promoted by ART might be topographically specific. Injection of CTB into peripheral nerves labeled a mixed population of sensory afferents in the spinal cord, however, making it impossible to identify the projection pattern of a specific class of axons.
To examine the specificity of regenerated projections, we used more specific labeling techniques to isolate three classes of sensory afferents: myelinated muscle axons, myelinated cutaneous axons, and unmyelinated nociceptive axons. The projection pattern of myelinated muscle afferents was assessed via injection of CTB into the triceps muscle nerves. In uninjured animals, muscle afferents were seen to project into laminae III–VII of the spinal cord and extend ventrally, where they innervate motor neurons (Fig. 1D). The projections of myelinated cutaneous sensory afferents were identified via small intradermal injections of CTB into the C6 dermatome. Normally, these afferents arborize only within the C6 spinal segment and are restricted to laminae III and IV in the lateral portion of the dorsal horn (Fig. 2A). Unmyelinated nociceptive sensory afferents were identified using antibodies against calcitonin gene–related peptide (CGRP). CGRP labeling is normally restricted to laminae I and IIo (Fig. 3A) (10), and is reduced by 85% in the C6 spinal segment following the crush of five cervical roots and by 100% after crush of seven cervical roots (8).
Fig. 2.
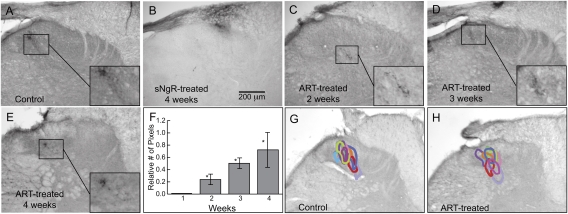
ART promotes topographically specific regeneration of myelinated cutaneous afferents in the dorsal horn. (A–E) Central projections of cutaneous sensory afferents visualized by small intradermal injections of CTB into the C6 dermatome. (A) Projections in a control cord. (B) Regenerated projections in an sNgR-treated cord after 4 wk. (C–E) Regenerated projections in ART-treated cords after 2 wk (C), 3 wk (D), and 4 wk (E). (F) Time course of appearance of synaptic puncta from regenerated cutaneous sensory axons in ART-treated cords. Asterisks denote significant differences from those of 4-wk untreated cords or after 1 wk of ART treatment (P < 0.001). (G and H) Artemin promotes regeneration of cutaneous sensory afferents to topographically correct regions of the dorsal horn. The areas in the dorsal horn occupied by CTB-labeled puncta are shown in different colors for nine ART-treated animals. Colors are matched for each animal on the control and regenerated sides. See Materials and Methods for details of the analysis. Images in control panels (uncrushed axons) have been reversed horizontally in A and G to facilitate comparison with projections of regenerated axons.
Fig. 3.
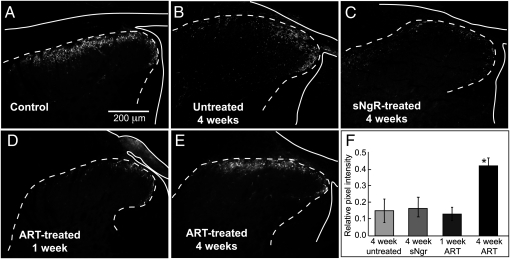
Expression of CGRP recovers after DR crush treated with ART, but not with sNgR. The outline of the cord and DR is indicated by a solid white line; the dotted line indicates the border between gray and white matter. (A) The normal projection of CGRP+ afferents was limited to upper laminae of the dorsal horn. (B and C) Four weeks after crush of five cervical DRs, CGRP expression fell to ~15% of normal in untreated cords (B) or with sNgR treatment (C). (D and E) Little recovery of CGRP expression was seen after 1 wk with ART treatment (D), but after 4 wk, expression recovered to ~40% of normal and was restricted to superficial laminae (E). (F) Quantitative assessment of recovery of CGRP expression in superficial laminae of untreated and sNgR or ART-treated cords with DR crush compared with uncrushed controls. The asterisk denotes significant difference from all other values (P < 0.001). Images in control panel (uncrushed axons) has been reversed horizontally in A, to facilitate comparison with projections of regenerated axons.
In sNgR-treated rats, the projection of muscle sensory axons was limited principally to upper laminae of the dorsal horn; relatively few labeled muscle afferents projected ventrally (Fig. 1E). However, with ART treatment, regenerated muscle afferents projected ventrally into the topographically appropriate regions of the cord (Fig. 1F). A quantitative assessment of the depth of these projections indicated that with ART treatment, regenerated muscle afferents projected nearly as deeply as the undamaged muscle afferents on the control side (91 ± 1% of normal). In contrast, with sNgR treatment, regenerated axons projected only one-third as deeply (34 ± 4% of normal; P < 0.001). This suggests that treatment with ART, but not with sNgR, promotes the regeneration of muscle afferents to the correct laminae of the cord.
The specificity of cutaneous axon regeneration also depends on how regeneration is promoted. With sNgR treatment, myelinated cutaneous axons projected diffusely within the dorsal white matter, with few projections into gray matter. Those regenerated cutaneous axons that did project into the dorsal horn did not terminate in the appropriate mediolateral position (Fig. 2B; see also fig. 3G in ref. 8); however, regeneration of cutaneous afferents in ART-treated rats was appropriately restricted within the dorsal horn (Fig. 2 C–E). The areas occupied by CTB-labeled puncta (representing synaptic terminals) on the regenerated and uncrushed sides were located at similar mediolateral and dorsoventral positions within the cord (Fig. 2 G and H; composite results from nine animals). Regenerated cutaneous axons also reestablished the appropriate projections rostrocaudally. The majority of sections in segments C5–C7 with labeled projections on the control side also had labeling on the denervated side, and none of the sections without labeling on the control side (i.e., outside the rostrocaudal extent of normal projections) had labeling on the denervated side. The extent of regeneration, as assessed by pixel density within the region of CTB-labeled puncta, increased gradually over the 4-wk period (Fig. 2F). Interestingly, the small amount of regeneration at 2 wk was already restricted appropriately (Fig. 2C), suggesting that these axons establish correct projections from the outset.
We evaluated regeneration of a restricted population of unmyelinated nociceptive sensory axons using antibodies against calcitonin gene–related peptide (CGRP). After 4 wk, expression of CGRP was the same with or without sNgR treatment (Fig. 3 B, C, and F). The absence of new CGRP expression implies that sNgR does not promote significant regeneration of unmyelinated axons, as has been reported previously (8). Because unmyelinated axons lack NgR expression, they might be less responsive to a blockade of myelin-associated inhibition than myelinated axons (11). In contrast, CGRP expression recovers following ART treatment, consistent with expression of the specific ART receptor, GFRα3, on unmyelinated neurons (9, 12, 13), suggesting that these afferents are regenerating (Fig. 3E). CGRP expression within the dorsal horn increased from 15% to 42% of control, similar to results obtained in an earlier study (9) (Fig. 3 E and F). Nearly all of the CGRP expression in the dorsal horn was limited to laminae I and IIo, as in normal cords (97 ± 4%) (Fig. 3E). Thus, ART treatment promotes regeneration of unmyelinated CGRP+ afferents to their appropriate target areas in the spinal cord.
Regeneration of sensory axons following DR crush also has been reported using several other neurotrophic factors, including nerve growth factor (NGF), neurotrophin 3 (NT3), and glial-derived neurotrophic factor (GDNF) (14–17); however, in all of these cases, the regeneration did not appear to be topographically specific (Discussion). A possible reason for the absence of specificity is that these factors were applied directly to the spinal cord either by viral expression within the cord or by intrathecal infusion, suggesting that the method of application is critical for topographically specific regeneration. In our experiments, ART was supplied systemically and thus might not have had access to the spinal cord.
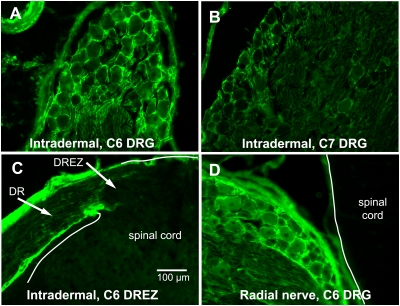
To investigate the extent to which ART is transported after systemic application, we assessed the distribution of ART immunohistochemically after two methods of delivery. Intradermal injections into a single dermatome, like those used to label cutaneous afferent projects, labeled the DR ganglia (DRG) of the corresponding segment most heavily (Fig. 4A). Minimal ART immunoreactivity (IR) was present in the immediately adjacent DRGs (Fig. 4B), and no label was present in DRGs more than one segment away. ART-IR also was present in the C6 DR, but stopped abruptly at the DREZ (Fig. 4C). At 16 h after the direct injection of ART into the radial nerve, ART-IR was present in the DRGs of the cervical spinal cord in a pattern consistent with the entry of sensory afferents from the radial nerve into the spinal cord (Fig. 4D). With both types of injection, no ART-IR was visible within the spinal cord proximal to the DREZ (Fig. 4 C and D). After intradermal or direct nerve injection, labeling was most prominent around both large and small sensory neurons (Fig. 4 A and D), suggesting binding either to surface receptors on the neurons or to the surrounding extracellular matrix. These results strongly favor the idea that systemically administered ART promotes regeneration through its action on sensory neurons within the DRG rather than acting within the spinal cord.
Fig. 4.
DRG accumulate ART delivered by peripheral application. The distribution of ART was assessed immunohistochemically. (A and B) Intradermal injections of ART into the upper forelimb within the C6 dermatome resulted in ART immunoreactivity around many sensory neurons in the C6 DRG (A), but only weak staining in adjacent ganglia (B). (C) Intradermal ART injections into the C6 dermatome also labeled connective tissue in the C6 DR, but the label stopped at the DREZ. (D) Direct injection of ART into the radial nerve also resulted in ART immunoreactivity surrounding most sensory neurons, but with no immunoreactivity within the white matter of the spinal cord.
Discussion
Although various treatments have been shown to promote axonal regeneration in the spinal cord, relatively little information is available on the precision with which regenerating axons reestablish projections to their original target areas. The DR crush model is useful for such studies, because specific classes of sensory fibers can be labeled in peripheral nerves, allowing an anatomical determination of their spinal projections. Using this model, we found that two treatments that promote robust anatomical and functional regeneration differed dramatically in terms of the specificity of this regeneration. Different classes of sensory axons projected diffusely through the dorsal columns and dorsal horn when regeneration was promoted by the soluble Nogo receptor peptide, sNgR. In contrast, a 2-wk regimen of systemic artemin treatment led to the reestablishment of projections of three different classes of sensory axons to their appropriate topographic locations within the cord.
One successful approach to stimulating regeneration of axons in the CNS has been to block the inhibition to growth caused by oligodendrocytes. Many previous studies have used a contusion model of SCI, blocking the inhibition caused by CNS myelin with antibodies or peptides directed against Nogo ligands or the Nogo receptor NgR1. (See ref. 18 for a discussion of these studies.) Several groups have reported significant improvements in spinal cord function through blockage of this inhibition, but determining the degree to which normal spinal circuitry is reestablished has proven difficult. We found that interfering with Nogo receptor signaling with sNgR also promoted robust regeneration of myelinated sensory axons into the cord, with restoration of synaptic function and behavioral recovery of the affected limb (8).
In the present study, we assessed the degree to which regeneration promoted by sNgR is topographically specific. Although myelinated axons supplying both muscle and skin regenerated through the inhibitory barrier of the DREZ, both classes of axons projected throughout the dorsal white matter and superficial laminae of the dorsal horn. This projection pattern is distinctly different from normal and also from the undamaged afferents on the contralateral side of the cord, where these afferents project only within laminae III–VI of the dorsal horn. This indicates that reduction of myelin inhibition with sNgR treatment allows robust regeneration but with an inappropriate anatomical distribution, suggesting that some inhibition might be required to direct growth to the appropriate regions of the spinal cord.
Regeneration of sensory axons after DR crush also has been reported using various neurotrophic factors, including nerve growth factor (NGF), neurotrophin 3 (NT3), and glial-derived neurotrophic factor (GDNF). Expression of NGF or fibroblast growth factor by viral transfection of cells in the dorsal horn was found to promote massive regeneration of unmyelinated CGRP+ axons, restoring nociceptive sensation in the affected limb (19). These projections are not limited to the most dorsal laminae of the gray matter, however; rather, they occupy a large fraction of the entire dorsal horn. Intrathecal infusions of NT3 or GDNF promote the regeneration of large-diameter sensory afferents through the DREZ and into the dorsal horn gray matter, restoring functional synaptic transmission with spinal neurons and leading to significant behavioral improvement (15). However, these regenerated afferents occupy an aberrant position in lamina IIo and grow along the pial surface, abnormal locations for this class of sensory axon (16, 17).
Similar to the effects of intrathecal GDNF or NT3 treatment, systemic injections of ART promote regeneration of sensory axons through the DREZ and into the gray matter of the spinal cord despite the presence of myelin (9). This finding might be explained by a dynamic inhibitory influence of myelin. Intact myelin and myelin debris produced by damaged axons have different inhibitory properties (18). Inhibition mediated by myelin produced by oligodendrocytes increases during the first week after DR crush injury as myelin degenerates (5). Myelin debris might expose an increased number of epitopes that bind receptors mediating growth cone collapse. Indeed, in the absence of DR injury, transplanted primary afferent neurons from mice into rat DRG grow past the DREZ and into the spinal cord within an environment of intact myelin. Growth is inhibited when DRs are crushed at the time of transplantation (5). Ramer et al. (16) postulated that with the support of certain growth factors, such as NT3, regenerating sensory axons might enter the spinal cord before inhibition mediated by myelin debris is increased. Our data support this hypothesis; 2 wk after injury, regenerated axons were found in the gray matter in more ventral locations with ART treatment than with sNgR treatment. Thus, rapid growth promoted by growth factors such as NT3, GDNF, and ART might promote growth of sensory axons through the DREZ before inhibitory mechanisms are up-regulated.
Unlike the effects of intrathecal NGF or NT3 treatment, however, systemic administration of ART after DR crush promotes regeneration of both myelinated and unmyelinated sensory neurons. This positive result is confounded by the expression patterns of GFRα3 in neuronal subpopulations in the DRG. Based on immunohistochemical staining, GFRα3 is expressed largely on unmyelinated neurons. Variable expression on myelinated neurons, ranging from 0 to 14% of the total number of myelinated neurons in the DRG, has been reported (9, 12, 13). Behavioral recovery attributed to regeneration of unmyelinated axons is more robust than that by myelinated axons, consistent with a larger fraction of unmyelinated neurons expressing GFRα3 (9). Nonetheless, substantial regeneration of myelinated DRG neurons is observed with administration of ART. It is possible that these neurons express a low level of GFRα3 that cannot be detected with standard immunohistochemical techniques.
In contrast to the results with other treatments that promote regeneration, we report here that regeneration of sensory axons with systemic ART is topographically specific. We characterized the projection pattern of regenerated myelinated muscle and cutaneous afferents and found that these projections are localized to the appropriate regions of the spinal cord, avoiding areas that are normally occupied by unmyelinated nociceptive afferents. Furthermore, regenerated CGRP-expressing nociceptive axons project to the dorsal laminae of the dorsal horn, in a distribution similar to that of undamaged nociceptive axons. Because the effects of myelin proteins are unlikely to be interrupted with ART treatment, we hypothesize that myelin proteins might contribute to the guidance of growing axons toward appropriate regions of the gray matter. Data from other experiments are consistent with this hypothesis. For example, the rostrocaudal projections of regenerating axons with NT3 and GDNF treatment avoid the white matter, instead projecting rostrocaudally in the gray matter, where the influence of these proteins is less significant (15, 16). Thus, the presence of intact myelin at early time points after DR injury might act in concert with other guidance cues in the cord to allow a short window of opportunity for axons to regenerate before inhibition is increased by myelin debris and a glial scar develops.
The question arises as to why sensory regeneration promoted by systemic treatment with ART is more specific than that promoted by other neurotrophic factors. One possibility is that the other factors have been tested either by infusion into the cerebrospinal fluid (15–17) or by viral infection directly in the spinal cord (19, 20). Direct neurotropic effects of these neurotrophic factors within the spinal cord may overpower molecular cues that could otherwise guide growing axons back to their original target areas in the cord. In contrast, we found that systemic application of ART, whether injected intradermally, subcutaneously, or into peripheral nerves, results in increased ART levels within the DRG, but not within the spinal cord itself. ART contains a heparin-binding site that could possibly mediate its binding to the extracellular matrix within the DRG. Heparin binding is a requirement for the optimal activation of GFRα3 (21). Thus, stable positioning of ART near these receptors might maximize interactions, especially on the larger neurons that do not express high levels of GFRα3. Therefore, systemic ART would be capable of up-regulating growth programs directly in sensory neurons within the DRG without interfering with guidance cues in the cord. If this hypothesis were correct, it could motivate a change in strategies for promoting regeneration in the CNS. Rather than attempting to overcome the inhibitory environment within the area of axon regeneration, effective treatments should be developed to stimulate growth programs in neuronal cell bodies. Recent experiments demonstrating robust axon growth via stimulation of the mTOR pathway in retinal ganglion cells and of Arg1 in sensory neurons suggest that these approaches can be successful (22, 23), although the specificity of the regeneration that they promote remains unknown.
To the best of our knowledge, no other agent or program of treatment has been shown to promote topographically specific regeneration of axons in the adult mammalian CNS after DR injury. Our findings suggest that molecular cues capable of directing the growth of regenerating sensory axons to their targets are present in the adult mammalian spinal cord. Treatment with artemin may thus enable functional restoration of specific sensory input to the spinal cord after brachial plexus injury. A more general implication of our findings is that other guidance cues within the gray matter also may be available to guide the regeneration of other classes of spinal axons, such as those damaged in contusion injuries. Although artemin is unlikely to promote regeneration of these axons, which do not express known artemin receptors, nevertheless it might be possible to develop generalized strategies for promoting specific regeneration of these axons while keeping intact the inhibitory influences in the spinal cord that may guide appropriate targeting of regenerated axons.
Materials and Methods
Dorsal Root Crush Surgery.
All research procedures were approved by the Institutional Animal Care and Use Committee at Tufts University School of Medicine and conformed to National Institutes of Health guidelines. Surgery was performed aseptically using isoflurane anesthesia. Unilateral C5-T1 DR crush was performed on male Sprague–Dawley rats (200–250 g; Charles River Labs), as described previously (8). In brief, rats were anesthetized with 2% isoflurane (vol/vol), and unilateral hemilaminectomy was performed under sterile conditions. The spinal cord and about 1 mm of the C5–T1 DRs, which innervate the forelimb and parts of the shoulder and flank, were exposed. Each DR was crushed three times for 10 s per crush, midway between the DRG and the cord, using sharpened no. 7 Dumostar forceps. On completion of the operation, the muscles were sutured in layers, and the skin was closed with metal clips or sutures. Continuous s.c. delivery of rat artemin or saline vehicle over a 2-wk course was achieved via an osmotic mini-pump (Alzet). A total of 2 mg of recombinant artemin was administered. Preparation and purification of artemin was as described by Wang et al. (9). Rat sNgR was prepared and administered intraventricularly as described by Harvey et al. (8).
Neuroanatomy.
For transganglionic tracing of the central projections of sensory axons, the radial and/or median nerves were exposed under sterile conditions, and 1–2 μL of a 2–5% (wt/vol) solution of CTB (Sigma-Aldrich) was injected using a Hamilton syringe 4–7 d before the rats were killed. The rats were perfused intracardially with 4% paraformaldehyde, and the cervical spinal cord with DRGs intact was removed. Tissue was cryoprotected in 30% sucrose in PBS. Sections cut on a cryostat were processed using a polyclonal anti-CTB antibody (List Biologicals; 1:80,000), followed by biotinylated anti-goat secondary antibodies (Vector Laboratories; 1:200) and avidin-biotin conjugates (ABC Kit; Vector Laboratories). Antibodies were visualized using a diaminobenzidine reaction (DAB Kit; Vector Laboratories).
Unmyelinated sensory axons were identified using a polyclonal antibody against CGRP and a fluorescent secondary antibody (Alexa Fluor 568; see ref. 8 for details). Intradermal injections of CTB were used to reveal the spinal projections of small groups of myelinated cutaneous afferents innervating a patch of skin in the C6 dermatome. Cutaneous afferents labeled in this manner are localized exclusively within the C6 segment of the spinal cord in a restricted region of laminae III and IV in the dorsal horn (24). Myelinated sensory axons supplying the triceps muscle were labeled by injecting 1–2 μL of CTB directly into the triceps muscle nerve, as described above.
For ART transport experiments, ART was injected intradermally or into the radial nerve. For intradermal injections, 1–2 μL of ART (1 mg/mL) was injected into the skin in the C6 dermatome. For nerve injections, the radial nerve was exposed unilaterally, as described above, and injected with 1–2 μL of ART (1 mg/mL). The animals were perfused intracardially with 4% paraformaldehyde at 48 h (intradermal injections) or 16 h (radial nerve injections) after injections. DRG were removed and cryoprotected by overnight immersion in 30% sucrose. Then 10-μm cryostat sections were cut and processed with monoclonal anti-artemin antibodies (1:5,000; R&D Systems), followed by Alexa Fluor 488–conjugated anti-mouse antibodies (1:200; Molecular Probes).
Measurements of Extent and Specificity of Regeneration.
Sensory axons supplying muscle normally project through the dorsal horn toward targets in the intermediate zone and ventral horn of the spinal cord, whereas cutaneous sensory axon terminals remain confined to the dorsal horn. Thus, the depth of regenerated muscle sensory projections served as a measure of the precision with which these afferents reestablished their normal projections. The maximum depth of control (uncrushed) and regenerated muscle afferent projections was measured in each of 20 sections (10 sections from four sNgR-treated animals and 10 sections from four ART-treated animals) using Image J software. The depth of the regenerated connections in each section was then calculated as the ratio of regenerated depth to control depth.
The extent and anatomical precision of cutaneous axon regeneration was assessed by comparing the central projections of restricted groups of myelinated cutaneous afferents labeled bilaterally with intradermal CTB injections into the C6 dermatome (see above). Three transverse spinal cord sections were randomly selected from the C6 segment of each of nine rats, and regions of interest (ROI) representing the area containing CTB label were drawn on images of the crushed and uncrushed sides of each section. The number of labeled pixels within each ROI was then measured using Image J software (NIH). To assess the topographic specificity of regeneration, the three sections from each animal were digitally overlaid, and a second ROI was drawn to contain all labeled pixels. This ROI was copied to a composite image, using a different color for each animal (Fig. 2 G and H). The relative positions of the ROIs on the crushed and uncrushed sides provide a qualitative assessment of the precision with which regenerated cutaneous axons project back to their original locations in the spinal cord.
The extent and precision of regeneration of unmyelinated sensory axons was assessed by measuring CGRP immunofluorescence in images such as those shown in Fig. 3. Between three and five randomly selected sections from the C6 and C7 segments were chosen from each of 4 4-wk untreated rats, 3 1-wk ART-treated rats, 6 4-wk ART-treated rats, and 16 4-wk sNgR-treated rats. Pixel density was determined within laminae I and II and within the entire dorsal horn as reported previously (8). Values are reported as the relative pixel density in corresponding spinal segments from the lesion-containing and non–lesion-containing sides of each animal.
Acknowledgments
We thank Peter Cariani, Lukas Marzec, and Jason Rahal, who were instrumental in pilot experiments in this study. This work was supported by contracts from Biogen Idec, a gift from the Murray Winston Foundation, and grants from the Paralyzed Veterans Association Research Foundation (2457, to E.F.) and National Institutes of Health (NS064494, to E.F.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Bradbury EJ, et al. NT-3 promotes growth of lesioned adult rat sensory axons ascending in the dorsal columns of the spinal cord. Eur J Neurosci. 1999;11:3873–3883. doi: 10.1046/j.1460-9568.1999.00809.x. [DOI] [PubMed] [Google Scholar]
- 2.Bradbury EJ, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- 3.Li S, Strittmatter SM. Delayed systemic Nogo-66 receptor antagonist promotes recovery from spinal cord injury. J Neurosci. 2003;23:4219–4227. doi: 10.1523/JNEUROSCI.23-10-04219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schnell L, Schwab ME. Sprouting and regeneration of lesioned corticospinal tract fibres in the adult rat spinal cord. Eur J Neurosci. 1993;5:1156–1171. doi: 10.1111/j.1460-9568.1993.tb00970.x. [DOI] [PubMed] [Google Scholar]
- 5.McPhail LT, Plunet WT, Das P, Ramer MS. The astrocytic barrier to axonal regeneration at the dorsal root entry zone is induced by rhizotomy. Eur J Neurosci. 2005;21:267–270. doi: 10.1111/j.1460-9568.2004.03837.x. [DOI] [PubMed] [Google Scholar]
- 6.Ramon y Cajal S. Degeneration and Regeneration of the Nervous System. London: Oxford Univ Press; 1928. [Google Scholar]
- 7.MacDermid VE, et al. A soluble Nogo receptor differentially affects plasticity of spinally projecting axons. Eur J Neurosci. 2004;20:2567–2579. doi: 10.1111/j.1460-9568.2004.03715.x. [DOI] [PubMed] [Google Scholar]
- 8.Harvey PA, Lee DHS, Qian F, Weinreb PH, Frank E. Blockade of Nogo receptor ligands promotes functional regeneration of sensory axons after dorsal root crush. J Neurosci. 2009;29:6285–6295. doi: 10.1523/JNEUROSCI.5885-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang R, et al. Persistent restoration of sensory function by immediate or delayed systemic artemin after dorsal root injury. Nat Neurosci. 2008;11:488–496. doi: 10.1038/nn2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennett DL, Dmietrieva N, Priestley JV, Clary D, McMahon SB. trkA, CGRP and IB4 expression in retrogradely labelled cutaneous and visceral primary sensory neurones in the rat. Neurosci Lett. 1996;206:33–36. doi: 10.1016/0304-3940(96)12418-6. [DOI] [PubMed] [Google Scholar]
- 11.Josephson A, et al. Nogo-receptor gene activity: Cellular localization and developmental regulation of mRNA in mice and humans. J Comp Neurol. 2002;453:292–304. doi: 10.1002/cne.10408. [DOI] [PubMed] [Google Scholar]
- 12.Elitt CM, et al. Artemin overexpression in skin enhances expression of TRPV1 and TRPA1 in cutaneous sensory neurons and leads to behavioral sensitivity to heat and cold. J Neurosci. 2006;26:8578–8587. doi: 10.1523/JNEUROSCI.2185-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orozco OE, Walus L, Sah DW, Pepinsky RB, Sanicola M. GFRalpha3 is expressed predominantly in nociceptive sensory neurons. Eur J Neurosci. 2001;13:2177–2182. doi: 10.1046/j.0953-816x.2001.01596.x. [DOI] [PubMed] [Google Scholar]
- 14.Romero MI, et al. Extensive sprouting of sensory afferents and hyperalgesia induced by conditional expression of nerve growth factor in the adult spinal cord. J Neurosci. 2000;20:4435–4445. doi: 10.1523/JNEUROSCI.20-12-04435.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramer MS, Priestley JV, McMahon SB. Functional regeneration of sensory axons into the adult spinal cord. Nature. 2000;403:312–316. doi: 10.1038/35002084. [DOI] [PubMed] [Google Scholar]
- 16.Ramer MS, Duraisingam I, Priestley JV, McMahon SB. Two-tiered inhibition of axon regeneration at the dorsal root entry zone. J Neurosci. 2001;21:2651–2660. doi: 10.1523/JNEUROSCI.21-08-02651.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramer MS, et al. Neurotrophin-3–mediated regeneration and recovery of proprioception following dorsal rhizotomy. Mol Cell Neurosci. 2002;19:239–249. doi: 10.1006/mcne.2001.1067. [DOI] [PubMed] [Google Scholar]
- 18.Chivatakarn O, Kaneko S, He Z, Tessier-Lavigne M, Giger RJ. The Nogo-66 receptor NgR1 is required only for the acute growth cone-collapsing but not the chronic growth-inhibitory actions of myelin inhibitors. J Neurosci. 2007;27:7117–7124. doi: 10.1523/JNEUROSCI.1541-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romero MI, Rangappa N, Garry MG, Smith GM. Functional regeneration of chronically injured sensory afferents into adult spinal cord after neurotrophin gene therapy. J Neurosci. 2001;21:8408–8416. doi: 10.1523/JNEUROSCI.21-21-08408.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alto LT, et al. Chemotropic guidance facilitates axonal regeneration and synapse formation after spinal cord injury. Nat Neurosci. 2009;12:1106–1113. doi: 10.1038/nn.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silvian L, et al. Artemin crystal structure reveals insights into heparan sulfate binding. Biochemistry. 2006;45:6801–6812. doi: 10.1021/bi060035x. [DOI] [PubMed] [Google Scholar]
- 22.Park KK, et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma TC, et al. A large-scale chemical screen for regulators of the arginase 1 promoter identifies the soy isoflavone daidzeinas, a clinically approved small molecule that can promote neuronal protection or regeneration via a cAMP-independent pathway. J Neurosci. 2010;30:739–748. doi: 10.1523/JNEUROSCI.5266-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cafferty WB, et al. Chondroitinase ABC-mediated plasticity of spinal sensory function. J Neurosci. 2008;28:11998–12009. doi: 10.1523/JNEUROSCI.3877-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]