Abstract
Many animals learn to follow habitual routes between important locations, but how they encode their routes is still largely unknown. Desert ants traveling between their nest and a food site develop stable, visually guided routes that can wind through desert scrub without the use of trail pheromones. Their route memories are sufficiently robust that if a nest-bound ant is caught at the end of its route and replaced somewhere earlier along it, the ant will recapitulate the route from the release site. Insects appear to use panoramas to recognize when they are on a familiar route. I examine here the cues then used for their guidance. Several mechanisms are known for straight segments of a route; but how does an ant encode a curved route along which both the views it sees, and the directions it takes, are constantly changing? The results here suggest that when an ant travels past a landmark on a familiar route, it uses the gradually changing direction of the landmark to trigger a set of associated learned heading directions. A route through a complex 2D environment could thus be encoded and followed economically if it is divided into panorama-defined segments, with each segment controlled by such a 1D mapping. The solution proposed for the ants would be simple to implement in an autonomous robot.
Keywords: insect, navigation, algorithm, encoding
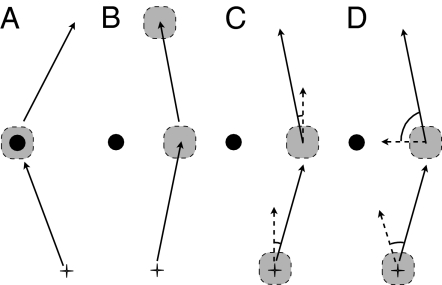
For many animals, the ability to find their way efficiently between important locations is a vital part of foraging. A wide range of animals increase the reliability of their navigation, and reduce their travel costs, by learning to follow habitual routes (1, 2). For humans, as we have all experienced, a route that has become habitual can be followed with relatively little thought. Guidance along the route appears to be largely controlled by processes involving the basal ganglia (3). Travel off of a route, or before a route has become habitual, generally requires more thought. Guidance then involves the hippocampus (4). Insects such as honey bees or desert ants also have a variety of navigational mechanisms, some of which provide guidance along habitual routes (2). Desert ants, many of which do not lay trail pheromones, can develop stable, visually guided routes between their nest and a food site (5–7). The ants use memories of both en route landmarks (5, 8) as well as features viewed from the final goal (9–11). These memories are sufficiently robust that if a nest-bound ant is caught at the end of its route and replaced somewhere earlier along it, the ant will recapitulate the route from the release site (6–8). We are beginning to understand how ants encode straight segments of simple routes (12, 13); however, how they encode routes that wind through more complex environments is still largely unknown. This paper evaluates three possible classes of guidance mechanism (Fig. 1) and suggests what information is encoded.
Fig. 1.
Three classes of landmark-based guidance. Landmark schematized as filled circle, recognized location as broken circle, and an individual's path as arrow. (A) Beaconing toward a recognized feature. (B) Image matching toward a remembered location that is encoded as a snapshot of surrounding features. (C and D) Familiar view of surrounding features acts as signpost, triggering remembered heading direction. (C) Heading direction encoded as compass direction. (D) Heading direction encoded as the position of the landmark on the retina.
Remembering a route requires both recognizing the locations along the route and activating guidance commands appropriate to those locations. A desert ant or honey bee will use a distinctive panorama to identify a familiar location such as its nest or a food-site (14, 15) or a habitual route (7, 16). They are thought to learn visual “snapshots” at specific locations that are composed of elements of their retinal image (9–11) when facing a particular direction (17). Such elements may include the apparent size (18) or the retino-topic positions of the edges (19) of landscape features viewed from a location. Skyline silhouettes, which tend to provide the most prominent edges in a desert ant world, seem particularly important and can be recognized in the absence of, or with conflicting, compass cues (15, 20, 21). The results in this paper suggest that, to determine its fine-grain position along a segment of a habitual route, an ant uses fewer cues than it does either to determine whether it is on the route or to determine its position when off of a route.
Once an individual has determined where it is along a route, it can recall the guidance commands appropriate to that location. Three classes of mechanisms have been proposed to explain the guidance that is triggered by landscape memories. One mechanism that insects can use is beaconing. An individual recognizes a feature as matching a snapshot memory and then heads toward the center of the feature (22). A route produced in this way would zigzag from landmark to landmark (Fig. 1A). A second way that snapshot memories could be used is known as retinal image matching (9). For this mechanism, a view along a route would trigger the snapshot memory that had been acquired at a nearby location, and the individual would move until its view matched the retinal positions and sizes of the features in the remembered snapshot (23). A route would then be encoded as a set of snapshot-defined locations, each of which would act as an attractor from nearby positions (Fig. 1B). Third, snapshots could act as signposts that indicate habitual directions of movement from the identified locations (24). The remembered heading directions could be encoded as compass directions (Fig. 1C) or in terms of the position of landscape features on the retina (13, 15, 25) (Fig. 1D). A route could then be composed of a sequence of such directional memories (25).
This paper first investigates what class of navigational mechanism best predicts how the desert ant Cataglyphis fortis uses a single large landmark for guidance along a curved route. The analysis suggests that ants encode the route as an association between a set of location-specific visual cues and remembered heading directions. It suggests too that the visual cue an ant uses to identify its fine-grain position along the route is simply the direction in which a landmark is viewed. Finally, it is suggested how this mechanism of route guidance may be integrated with the rest of the ant's navigational toolbox.
Results
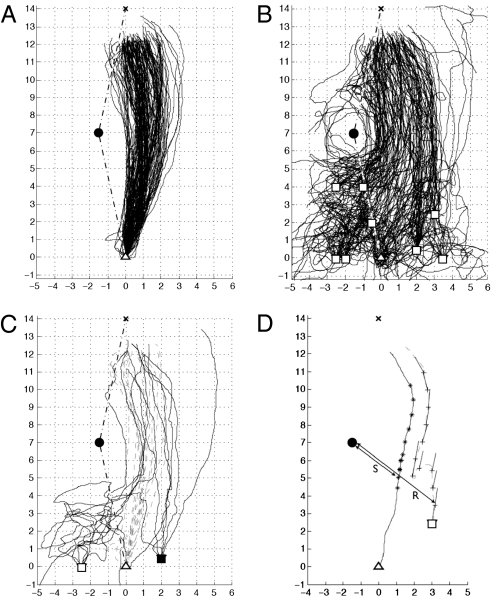
The study site chosen was particularly flat and featureless, ensuring that an artificial landmark was the only prominent feature in some directions for hundreds of meters (Fig. S1). This landmark, a 0.7-m-high and 0.4-m-wide black cylinder, lay 1.5 m to the side of the direct route between the nest and a feeder 14 m to the North. The ants’ trajectories curved gently around the landmark (Fig. 2A). The directions of final approach to, and departure from, the feeder appeared to correspond approximately to the prevailing direction of wind (26), but this was not systematically studied.
Fig. 2.
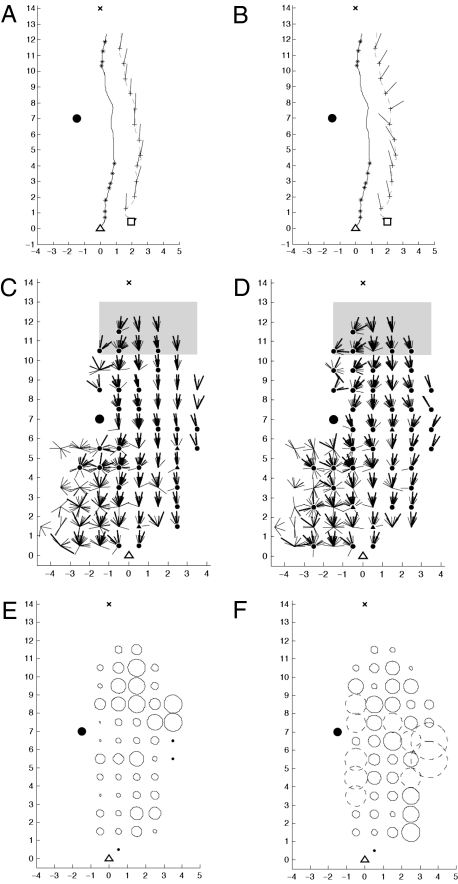
Sample and recapitulation trajectories. Feeder (▵) at (0,0) nest (X) at (0,14) and the cylinder-landmark (•) is at (−1.5,7). One unit corresponds to 1 m. Dot-dashed lines indicate limits of “familiar” sector within which the homeward trajectories lie during training. (A) Sample homeward trajectories from the feeder. (B) Recapitulation trajectories. Each ant was collected near the nest at the end of the sample trajectory and displaced to one of eight release sites (□). (C) Initial trajectories (dashed) and recapitulation trajectories (solid) of the six focal ants. (D) An example comparison between a paired sample (solid) and recapitulation (dashed) homeward trajectories by an ant. Probe points (+) every 1 m along the recapitulation trajectory and the matched reference points (*) on the sample trajectory. R and S indicate the distances from the landmark to the recapitulation and sample trajectories, respectively, at an example probe point and matched reference point. Line segments from probe points show the heading directions taken from their matched reference points.
After the landmark and feeder had been in place for 10 days, 139 pairs of trajectories were collected from individual ants to compare their homeward navigational responses at different positions with respect to the landmark. Each tested ant was given a crumb of biscuit while it was at the feeder, upon which it would then immediately run back to the nest. The “sample” homeward trajectory that it took back from the feeder was recorded (Fig. 2A). Although this trajectory could be guided both by cues from an ant's path integration (27, 28) and by route memories, in fact route memories tend to prevail for an experienced ant (2, 6, 7) (Fig. S2). At the end of its sample trajectory, within 1 m of the nest, the ant was caught and displaced to one of eight release sites (Fig. 2B, squares) from where its “recapitulation” homeward trajectory was recorded. Because the ant had already run the 14 m home, landscape features but not path integration could guide the recapitulation trajectories toward the nest (12, 29).
The sample and recapitulation trajectories from an ant form a pair of trajectories that, together, can be used to test the various models of navigation. When both of the trajectories in a pair were recorded from the feeder, the recapitulation trajectory and sample trajectory largely coincided (Figs. S3 and S4). A similar experiment using a test field shows that the ants can encode such curved trajectories using the landmark (Fig. S3). The following analysis investigates what landmark-based navigational mechanism could produce both the sample trajectories and the observed recapitulation trajectories from the various release sites.
Where Are Route Guidance Mechanisms Elicited?
The homeward trajectories from the feeder define a sector, centered on the landmark, within which the ants view the landmark in an accustomed direction (Fig. 2A). Within this “familiar” sector, the recapitulation trajectories were generally directed homewards, showing that the ants are using visual memories of the landscape to reach the nest (Fig. 2B). Where the recapitulation trajectories have not yet entered the familiar sector, they are generally disordered and exhibit frequent turns indicative of search behavior (Fig. 2C). Where trajectories later leave the sector, they also show a switch in behavior, turning to keep the landmark in the direction as viewed from the nest. Thus, even though the landmark itself looked the same from all of the directions, the habitual route behavior was activated only where the landmark lay in the accustomed directions.
Recapitulation trajectories were less likely to reach the nest if they started in the unfamiliar sector (48/61 successful vs. 72/78 in the familiar sector). Examining only recapitulation trajectories that started at the same distance from the landmark as the feeder, the 37 trajectories from release points outside the familiar sector, at (−2.5,0) and (−2,0), took longer to travel 3 m from their release points than did the 32 trajectories starting in the familiar sector at (2,0) (P < 0.05, Wilcoxon rank-sum) (Fig. S5). This pattern was confirmed by six focal ants that had immediately well-directed recapitulation trajectories in the familiar sector (Fig. 2C, filled square). The ants were marked, released, and then tested again 3 h later outside the familiar sector (Fig. 2C, open square). Their recapitulation trajectories then started with a lengthy search and became well directed only when inside the familiar sector. From the current data, it is not possible to say whether the landmark must be in an accustomed compass direction or whether it must appear within the accustomed part of the landscape panorama.
Determining the Guidance Mechanism.
Snapshots acquired at the nest can often guide the final approach to the nest (9–11) and may well allow direct homing from a large surrounding area (30). In the present case, for the last few meters of the approach, the recapitulation trajectories converge toward the nest. This convergence suggests that, for the final approach, the ants may be using the constellation of more distant features for some kind of image matching. However, snapshots near the nest cannot generally account for guidance along all sections of habitual routes (5–7). What are the memories that are activated earlier along the route as the ant approaches and passes the landmark?
Both sample and recapitulation trajectories curve around the landmark, indicating that the ants in the present case were not using the landmark as a beacon (Fig. 1A). This curve also suggests that ants were not using a single learned heading direction. Because the observed curves do not lie on circles, they would also not be produced by holding the landmark at a fixed retinal position; rather, as an ant progresses along the route, there appears to be a gradual change in the guidance commands.
The 120/139 pairs of trajectories in which the ants reach home on the recapitulation trajectories can be used to evaluate how well the retinal image-matching model and three varieties of signpost models could explain the ants’ guidance along their routes. In 27/120 recapitulation trajectories, ants approached the feeder before continuing home (Fig. S6). In those cases, they never stopped to feed. For those 27 trajectories, only the portion after leaving the feeder vicinity, i.e., when the ant was traveling homeward, is analyzed. It is not clear from the current study what mechanism the ants in these cases used to return to the feeder—possibly some kind of image matching.
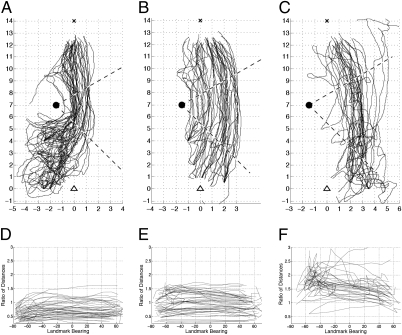
For the following analyses, “probe” points are taken every 1 m along each recapitulation trajectory. Those points within the familiar sector are then paired to “direction-matched” “reference” points on the paired sample trajectory where the landmark has the same compass bearing as at the probe points (Fig. 2D). With this point-by-point matching, the recapitulation trajectories can be categorized into three classes that show distinctive behavior. The first class is composed of the 42 recapitulation trajectories that start initially outside of the familiar sector (Fig. 3A). Before they enter the familiar sector, these trajectories show convoluted search behavior. A second class is composed of 47 recapitulation trajectories that start within the familiar sector and remain quite close to their paired sample trajectories (Fig. 3B). A useful measure to determine the proximity of recapitulation to sample trajectories at a probe point is the ratio of the distances from the probe point to the landmark and from the matched reference point to the landmark (Fig. 2D). For 31 recapitulation trajectories, the value of this recapitulation sample (R-S) distance ratio exceeds 1.75 somewhere along the route (Fig. 3C). The analysis suggests that this third, “distant” class of recapitulation trajectories shows a type of behavior different from that of the classes closer to the route.
Fig. 3.
Recapitulation trajectories divided into three classes. Trajectories that first go to the feeder are shown only after leaving the vicinity of the feeder. Dashed lines indicate sector over which convergence is analyzed. (A) Trajectories starting outside the familiar sector. (B) Trajectories within the familiar sector that remain close to the habitual route: R-S distance ratio < 1.75. (C) Trajectories for which the R-S distance ratio is >1.75. (D–F) R-S distance ratios along each pair of trajectories as a function of direction to landmark. Directions measured in degrees counterclockwise around the landmark. Zero is when the ant is level with the landmark. Trajectories divided into the same classes as for A–C.
Do Ants Use Image Matching Along the Route?
Do ants have a sequence of snapshots and use image matching to progress from snapshot location to snapshot location along a habitual route (Fig. 1B)? If so, then an ant away from its habitual route would be attracted to those snapshot locations along the route. Wherever guidance is obtained through image matching, the recapitulation trajectories should converge toward the sample trajectories. In general, however, the observed recapitulation trajectories do not appear constrained within the corridor of habitual homeward trajectories (Fig. 2B). More quantitatively, any areas of convergence could be detected using the R-S distance ratios (Fig. 3 D–F). Whenever image matching is the primary guidance cue, the R-S distance ratio would tend toward 1.
The class of distant recapitulation trajectories does show some convergence toward the sample trajectories. Of 31 trajectories, 26 are closer when the landmark is at a bearing of 30° than earlier when the landmark has a bearing of –45° (P < 0.001 binomial; Fig. 3F). This class of trajectories shows similarities to a behavior observed in the Australian desert ant Melophorus bagoti. When released for a recapitulation trajectory away from a habitual route, the Australian ants initially head approximately toward the direction of their nest, before subsequently joining the route (7). When distant from their route, both M. bagoti and C. fortis may well use something like image matching to approach or join the route or to approach the nest more directly. Because an ant's behavior off of its route can be different from its behavior on its route (7), the class of distant trajectories would not be useful in determining the guidance mechanisms along a route.
For those 89 trajectories with the smaller R-S distance ratios, the ratios remain approximately constant between probe points (Fig. 3 D and E). Over the central part of the route, between landmark bearings of −45° and 30°, the ratios show that as many trajectory pairs diverge as converge (converging: 20/42, Fig. 3D; 25/47, Fig. 3E; P > 0.5 binomial; Fig. S7). Thus, image matching cannot be the primary guidance mechanism that ants use to travel past the landmark when they are following their route.
Signpost Guidance: How Does an Ant Recognize Its Current Position?
To discover whether the ants might be using a signpost mechanism (Fig. 1 C and D) when on or close to their routes, the remainder of the analysis uses only those 89 pairs of trajectories in the first and second classes. The analysis compares three ways in which an ant could recognize its current position and recall the appropriate heading direction. For each model, the heading directions on an individual's sample trajectory can be used to predict its heading directions along the recapitulation trajectory.
A view of the landmark can provide an ant with two independent types of information about its fine-scale position. As the ant progresses along its route, the compass bearing from the ant toward the landmark gradually shifts. The landmark is seen in a given direction at only one position along a homeward trajectory. At the same time, the distance between the ant and the landmark also changes so that generally only two points along each trajectory share the same distance from the landmark—one point before and one after the landmark is passed. Insects could obtain the distance information from either motion parallax or the retinal (apparent) size or elevation of the landmark (13). Using the direction and distance together, it would be possible to uniquely identify any location around the landmark. However, to distinguish only between the locations on a one-dimensional route, either cue alone could be sufficient (use of distance cues would require a division between before and after the landmark). Do the ants use either or both of these sources of information from the landmark to determine where they are? The following analysis of the paired trajectories suggests that as long as they remain close to their routes, the ants use simply the direction of the landmark, ignoring small inconsistencies in its distance, as a signpost to evoke appropriate remembered heading directions.
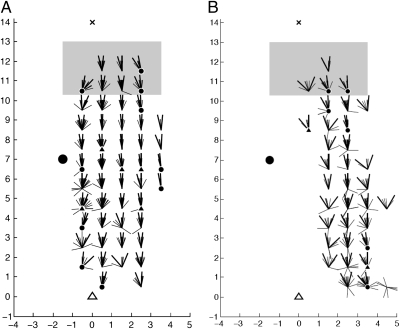
If the ants use only the direction of the landmark (the “direction-based” model), then the heading directions from the direction-matched probe and reference points should be the same. The approximately constant R-S distance ratios in Fig. 3 D and E suggest that this may be the case. A more specific test can be derived from the differences, or “residuals,” between the “actual” headings at the probe points on a recapitulation trajectory and the “predicted” headings from the matched reference points on the paired sample trajectory. These residuals at all of the probe points are shown in Fig. 4A. The residuals are grouped within 1 × 1-m grid squares and show a zero residual (i.e., a perfect match with the model predictions) as pointing upward. The shaded area shows the approximate region within which trajectories converge toward the nest (Fig. 2B). A total of 42 grid squares outside the shaded area contain at least four trajectories. At 30/42, the residuals are not significantly different from zero at 95% confidence levels (with no Bonferroni corrections). At five more, the residuals lie within 1° of the confidence intervals. The distance-based model does not predict headings on the recapitulation trajectories (i.e., the residuals show large bias or scatter) where the trajectories either enter the familiar sector or are far from the initial trajectory. However, to produce the trajectory of an ant that remains close to its route past the landmark, a simple learned mapping would be sufficient: The observed direction of the landmark at a point could cue an associated remembered heading direction. Expressed more mathematically, an ant may learn the function that relates its heading directions to the landmark bearings.
Fig. 4.
Accuracy of direction-based predictions. For all probe points within each 1-m grid square that contains at least four probe points, segments show residuals between actual and predicted headings. Shown originating at the center of grid squares, with perfect coincidence (zero residual) pointing upwards. Larger lines indicate 95% confidence intervals for mean residuals within grid squares. Circles or triangles indicate where a zero residual lies outside confidence intervals: triangles by <1°, circles by >1°. To be conservative, confidence intervals are calculated only from heading directions in the upper two (nestward) quadrants and without any Bonferroni corrections. (A) R-S distance ratio < 1.75. (B) R-S distance ratio > 1.75.
For the class of distant trajectories, the residuals (Fig. 4B) tend to show both more scatter and greater bias than those at the same grid squares in Fig. 4A. This difference between the classes again suggests that the ants use different guidance mechanisms when they are away from their route.
Insects often use information associated with the distances from landmarks. When searching for a nest or feeder, insects use the retinal sizes or elevations of landmarks to provide distance information (18, 31). Wood ants approaching a landmark along a short route in the absence of a sun-based compass seem to determine their position from the angular distance between an edge and the center of gravity of a landmark (25). Might the desert ants be using some such measure of the landmark's retinal size or distance, rather than its direction, to determine their positions along a route? To test this alternative possibility, predicted heading directions can be obtained from reference points that share the same distance to the landmark as the probe points. Where the recapitulation trajectory does not lie on the sample trajectory, the predictions from those “distance-matched” reference points will also depend on how the heading directions themselves are encoded. One possibility is that the heading directions are encoded as compass directions (Fig. 5A), using either celestial polarization patterns or distant panorama cues (21). Alternatively, the heading directions could be encoded as angles from the line of sight towards the landmark (Fig. 5B), which could be achieved by keeping the landmark at a particular retinal position (8, 25). Note that the predictions obtained from the direction-matched reference points (Fig. 2D) did not depend on how the heading directions are encoded.
Fig. 5.
Distance-based predictions. (A and B) Matching probe points to reference points that have the same distance from the landmark. Lines from probe points show predicted heading directions. (A) Heading directions encoded as compass directions. (B) Heading directions encoded as position of landmark on retina. (C and D) Residuals between actual and predicted headings using encodings in A and B, respectively. (E and F) Proportion of trajectories that lie between direction-matched and distance-matched predictions. Proportion is shown as size of radius. Circle is dashed where proportion is >0.5. (E) Heading encoded as compass direction. (F) Heading encoded as retinal position of landmark.
Different subsets of probe points can be matched to distance-based versus direction-based reference points (compare Figs. 4 and 5). With the distance matching, predicted headings can be obtained outside as well as inside the familiar sector. As can be seen from Fig. 5 C and D, even within the familiar sector, the residuals from both of the distance-based models are generally less accurate, and more scattered, than from the direction-based model. To compare the distance-based models with the direction-based model, while avoiding any spatial autocorrelation along a trajectory, the mean absolute difference between predictions and trajectories for each model was calculated across all common probe points along a recapitulation trajectory. The direction-based predictions were significantly better than predictions from either the distance to compass heading (63/89 trajectories, P < 6 × 10−5, binomial) or the distance to retinal heading (75/89 trajectories P < 2 × 10−11, binomial).
Do Ants on the Route Use Only One Dimension of Available Positional Cues?
Might ants actually be using a combination of both the direction and distance to the landmark to determine their position along the route? If this were the case, then a weighted average between compass-based and distance-based predictions should be more accurate than simply the direction-based predictions. Such an averaging would imply that the recapitulation trajectories should lie between the two predictions. Figure 5 E and F show the proportion of recapitulation trajectories in each grid square for which the distance- and direction-based predictions lie on either side of the actual recapitulation trajectories. In fact, at all points near the route, the distance- and direction-based predictions generally lie on the same side of the actual trajectories (solid-outlined circles). Thus, when an ant is on its route, it appears not to use the distance information; rather, the ant uses only a single dimension of the cues that are available to determine its fine-grain position.
Figure 5F shows that, in some grid squares further from the route, the recapitulation trajectories do lie between the direction-based and the distance-based retinal headings (dashed circles). These locations are predominantly those where the direction-based predictions are less successful (Fig. 4). This pattern, along with the convergence shown by the “distant” trajectories (Fig. 3F), suggests that the ants may use both distance and direction information when they are further from the route. At the same time, it also hints that the headings may be encoded as the retinal positions of prominent features (8, 13, 15, 25).
Discussion
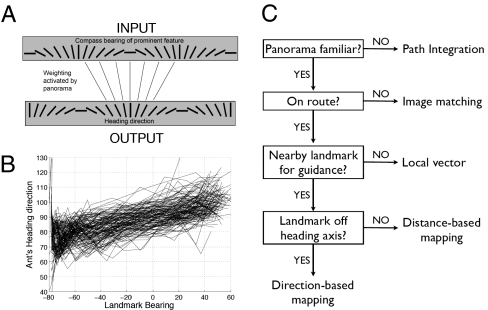
There is growing evidence that insects partition their routes into segments defined by distinctive panoramas (12, 16, 32) and that they use panoramas to recognize routes (7) and locations (14, 15). This process of place recognition is likely to use as much information, including the retinal image sizes of landscape features, as is required for a reliable identification of a familiar area. Honeybees and desert ants also tend to use both the retinal image sizes or elevations of features and their directions when searching for a goal at the end of a route (18, 33). Both types of information could also be used to approach a route (Figs. 3F and 4F). So why should some of this information not be used when traveling along a route? The answer may be that ants can exploit the 1D nature of habitual routes to minimize their computational costs during travel. In the present case, the panorama specific to the route could activate something as simple as a 1D mapping between the compass direction of the most prominent local feature and its desired position on the retina (Fig. 6A). Curiously, the paths that the ants follow past the landmark produce an approximately linear relationship between landmark bearings and heading directions (Fig. 6B). It may well be that a simple mapping is easy to learn (34) and that the learning process biases the shapes of these routes.
Fig. 6.
Direction-based mapping. (A) How direction-based mapping might be encoded in the brain. Critical sensory input could be compass direction of most prominent feature on skyline, and output could be where to hold that feature on the retina. A route segment could then be encoded as a set of synaptic weightings. Input and output experienced while following path integration and other guidance mechanisms would provide training data for supervised learning. (B) Heading directions along initial trajectories as function of compass bearings from ant to landmark. These relationships should reflect proposed mappings of ants. (C) Suggested schematic for the process of choosing a guidance mechanism for a path segment. This would occur once an insect has processed the landscape panorama.
Moment-to-moment guidance can often use quite different cues and computations from those used for processes of recognition (14, 35, 36). Such a separation between recognition and guidance could permit hierarchical models of navigation, such as that schematized in Fig. 6C. Scene recognition that uses both direction and distance cues need occur only at locations where there are significant changes in panoramic context. When a familiar panorama indicates that an individual is sufficiently close to a habitual route (in the present case, when the landmark lies in the appropriate direction and at the approximately appropriate distance), it can activate a guidance memory that controls movement along the associated route segment. To monitor its fine-grain progress along the segment, an insect could then use a simpler cue that does not involve repeating the complete scene recognition process. Where the path is not guided by a landmark, the guidance memory will be a local vector that encodes a constant remembered heading direction and uses a local odometer to measure progress along the segment (12, 32). When ants use a landmark along a route, the present findings suggest that ants may use a simple input–output mapping. When heading towards a landmark, the distance (but not the direction) to the landmark can provide a good indication of position along a segment. Wood ants approaching a landmark indeed appear to use a simple measure associated with the distance to the landmark (25). When passing to the side of a landmark, the distance to the landmark provides a less good measure of position along a route segment (Fig. 5C) than does the direction (Fig. 2D). Results here show that the direction to the landmark then appears to provide the input to the mapping. By involving scene recognition only when required, and then using simple route-segment guidance memories, even a complex habitual route (6) could be followed rapidly and reliably, and possibly with relatively low computational costs.
Supplementary Material
Acknowledgments
I thank Tom Collett for practical assistance, and Tom Collett, John Endler, Ken Cheng, Randy Gallistel, Leandra Bucher, and anonymous referees for helpful comments on the manuscript. Funding was provided by the Biotechnology and Biological Sciences Research Council and the Engineering and Physical Sciences Research Council.
Footnotes
*This Direct Submission article had a prearranged editor.
The author declares no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1001401107/-/DCSupplemental.
References
- 1.Biro D, Meade J, Guilford T. Familiar route loyalty implies visual pilotage in the homing pigeon. Proc Natl Acad Sci USA. 2004;101:17440–17443. doi: 10.1073/pnas.0406984101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collett M, Collett TS. In: Spatial Aspects of Foraging in Ants and Bees, Invertebrate Neurobiology. North G, Greenspan RJ, editors. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 2007. pp. 467–502. [Google Scholar]
- 3.Chang Q, Gold PE. Switching memory systems during learning: Changes in patterns of brain acetylcholine release in the hippocampus and striatum in rats. J Neurosci. 2003;23:3001–3005. doi: 10.1523/JNEUROSCI.23-07-03001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eichenbaum H, Stewart C, Morris RG. Hippocampal representation in place learning. J Neurosci. 1990;10:3531–3542. doi: 10.1523/JNEUROSCI.10-11-03531.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collett TS, Dillmann E, Giger A, Wehner R. Visual landmarks and route following in desert ants. J Comp Physiol. 1992;170:435–442. [Google Scholar]
- 6.Kohler M, Wehner R. Idiosyncratic route-based memories in desert ants, Melophorus bagoti: How do they interact with path-integration vectors? Neurobiol Learn Mem. 2005;83:1–12. doi: 10.1016/j.nlm.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Narendra A. Homing strategies of the Australian desert ant Melophorus bagoti. II. Interaction of the path integrator with visual cue information. J Exp Biol. 2007;210:1804–1812. doi: 10.1242/jeb.02769. [DOI] [PubMed] [Google Scholar]
- 8.Collett TS, Collett M, Wehner R. The guidance of desert ants by extended landmarks. J Exp Biol. 2001;204:1635–1639. doi: 10.1242/jeb.204.9.1635. [DOI] [PubMed] [Google Scholar]
- 9.Cartwright BA, Collett TS. Landmark learning in bees—experiments and models. J Comp Physiol. 1983;151:521–543. [Google Scholar]
- 10.Zeil J. Orientation flights of solitary wasps (Cerceris, Sphecidae, Hymenoptera). 2. Similarities between orientation and return flights and the use of motion parallax. J Comp Physiol. 1993;172:207–222. [Google Scholar]
- 11.Åkesson S, Wehner R. Visual navigation in desert ants Cataglyphis fortis: Are snapshots coupled to a celestial system of reference? J Exp Biol. 2002;205:1971–1978. doi: 10.1242/jeb.205.14.1971. [DOI] [PubMed] [Google Scholar]
- 12.Collett M, Collett TS. Local and global navigational coordinate systems in desert ants. J Exp Biol. 2009;212:901–905. doi: 10.1242/jeb.024539. [DOI] [PubMed] [Google Scholar]
- 13.Collett M. Spatial memories in insects. Curr Biol. 2009;19:R1103–R1108. doi: 10.1016/j.cub.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Collett TS, Kelber A. The retrieval of visuo-spatial memories by honeybees. J Comp Physiol. 1988;163:145–150. doi: 10.1007/BF00612004. [DOI] [PubMed] [Google Scholar]
- 15.Graham P, Cheng K. Ants use the panoramic skyline as a visual cue during navigation. Curr Biol. 2009;19:935–937. doi: 10.1016/j.cub.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 16.Collett M, Harland D, Collett TS. The use of landmarks and panoramic context in the performance of local vectors by navigating honeybees. J Exp Biol. 2002;205:807–814. doi: 10.1242/jeb.205.6.807. [DOI] [PubMed] [Google Scholar]
- 17.Collett TS, Baron J. Biological compasses and the coordinate frame of landmark memories in honeybees. Nature. 1994;368:137–140. [Google Scholar]
- 18.Wehner R, Räber F. Visual spatial memory in desert ants, Cataglyphis bicolor. Experientia. 1979;35:1569–1571. [Google Scholar]
- 19.Judd SPD, Collett TS. Multiple stored views and landmark guidance in ants. Nature. 1998;392:710–714. [Google Scholar]
- 20.Dyer FC. Memory and sun compensation by honey-bees. J Comp Physiol. 1987;160:621–633. [Google Scholar]
- 21.Towne WF, Moscrip H. The connection between landscapes and the solar ephemeris in honeybees. J Exp Biol. 2008;211:3729–3736. doi: 10.1242/jeb.022970. [DOI] [PubMed] [Google Scholar]
- 22.Voss C. Über das Formensehen der roten Waldameise (Formica rufa-Gruppe) Z Vgl Physiol. 1967;55:225–254. [Google Scholar]
- 23.Graham P, Durier V, Collett TS. The binding and recall of snapshot memories in wood ants (Formica rufa L.) J Exp Biol. 2004;207:393–398. doi: 10.1242/jeb.00771. [DOI] [PubMed] [Google Scholar]
- 24.Collett TS, Baron J, Sellen K. On the encoding of movement vectors by honeybees. Are distance and direction represented independently? J Compar Physiol. 1996;179:395–406. [Google Scholar]
- 25.Harris RA, Graham P, Collett TS. Visual cues for the retrieval of landmark memories by navigating wood ants. Curr Biol. 2007;17:93–102. doi: 10.1016/j.cub.2006.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolf H, Wehner R. Pinpointing food sources: Olfactory and anemotactic orientation in desert ants, Cataglyphis fortis. J Exp Biol. 2000;203:857–868. doi: 10.1242/jeb.203.5.857. [DOI] [PubMed] [Google Scholar]
- 27.Collett M, Collett TS. How do insects use path integration for their navigation? Biol Cybern. 2000;83:245–259. doi: 10.1007/s004220000168. [DOI] [PubMed] [Google Scholar]
- 28.Wehner R, Srinivasan MV. In: The Neurobiology of Spatial Behaviour. Jeffery KJ, editor. Oxford: Oxford University Press; 2003. pp. 9–30. [Google Scholar]
- 29.Wehner R, Michel B, Antonsen P. Visual navigation in insects: Coupling of egocentric and geocentric information. J Exp Biol. 1996;199:129–140. doi: 10.1242/jeb.199.1.129. [DOI] [PubMed] [Google Scholar]
- 30.Capaldi EA, Dyer FC. The role of orientation flights on homing performance in honeybees. J Exp Biol. 1999;202:1655–1666. doi: 10.1242/jeb.202.12.1655. [DOI] [PubMed] [Google Scholar]
- 31.Cartwright BA, Collett TS. How honey-bees know their distance from a near-by visual landmark. J Exp Biol. 1979;82:367–372. [Google Scholar]
- 32.Srinivasan MV, Zhang SW, Bidwell NJ. Visually mediated odometry in honeybees. J Exp Biol. 1997;200:2513–2522. doi: 10.1242/jeb.200.19.2513. [DOI] [PubMed] [Google Scholar]
- 33.Lehrer M, Collett TS. Approaching and departing bees learn different cues to the distance of a landmark. J Comp Physiol. 1994;175:171–177. [Google Scholar]
- 34.Braun DA, Aertsen A, Wolpert DM, Mehring C. Motor task variation induces structural learning. Curr Biol. 2009;19:352–357. doi: 10.1016/j.cub.2009.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Milner AD, Goodale MA. The Visual Brain in Action. Cambridge, MA: Oxford University Press; 1995. [Google Scholar]
- 36.Poulet JFA, Hedwig B. Auditory orientation in crickets: Pattern recognition controls reactive steering. Proc Natl Acad Sci USA. 2005;102:15665–15669. doi: 10.1073/pnas.0505282102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.