Abstract
Whole-genome transgenic RNAi libraries permit systematic genetic screens in individual tissues of Drosophila. However, there is a high incidence of nonspecific phenotypes because of off-target effects. To minimize such effects, it is essential to obtain a deeper understanding of the specificity of action of RNAi. Here, in vivo assays are used to determine the minimum, contiguous nucleotide pairing required between an siRNA and a target mRNA to generate a phenotype. We observe that as few as 16 nucleotides of contiguous homology are sufficient to attenuate gene activity. This finding provides an explanation for the high incidence of off-target effects observed in RNAi-based genetic screens. Toward improving the efficacy of RNAi-induced phenotypes in vivo, we describe siRNA expression vectors that allow coexpression of one or more siRNAs with a fluorescent reporter gene in cultured cells or transgenic flies. This expression system makes use of the small intron from the ftz segmentation gene to provide efficient processing of synthetic siRNAs from a reporter transcript. These studies provide a foundation for the specific and effective use of gene silencing in transgenic Drosophila.
Keywords: intron, miRNA, shRNA
RNA interference (RNAi) is an effective method for inhibiting gene expression in various animal models and is currently being evaluated as a potential human therapeutic (1). As such, it is essential to develop methods to minimize nonspecific phenotypes while maximizing the efficiency of selective gene silencing in whole animals.
A number of recent studies have investigated off-target silencing by small interfering RNAs (siRNAs) in cultured cell lines, but it is unclear whether the critical parameters also apply to transgenic animals (2–6). For example, it has been estimated that up to 25% of all double-stranded RNAs (dsRNAs) generated by two widely used transgenic RNAi libraries induce off-target phenotypes in Drosophila (7, 8). However, the basis for such nonspecific effects is not known. To investigate this issue, we have developed Drosophila cell culture and transgenic animal assays to determine the minimal base pairing required between an siRNA and target mRNA for effective attenuation of gene activity. This study takes advantage of several attributes of Drosophila: (i) a well-annotated genome, (ii) a robust RNAi response, and (iii) a targeted genome-integration system that allows the introduction of siRNA-expressing transgenes into defined positions of the genome, thereby minimizing position effects (9, 10).
To determine the minimal base pairing required for RNAi in vivo, we expressed modified siRNAs that retain a central core of shared nucleotides between the siRNA and target mRNAs based on previous in vitro assays (11, 12). As few as 16 contiguous nucleotides are sufficient to induce a significant reduction of endogenous dpp+ gene activity in transgenic flies.
To improve the analysis of tissue-specific phenotypes, we also describe a Drosophila siRNA expression vector that exploits the intron-mediated expression of many natural microRNAs (miRNAs) (13). Expression of artificial miRNAs (shmiRs) from a modified ftz intron fused to a fluorescent reporter gene accurately marks only those cells producing active RNAi. This intron enhances reporter gene expression ≥50-fold compared with an intron-less reporter in a Drosophila cell-culture system. Surprisingly, this enhanced expression is further amplified on insertion of one or more shmiRs within the ftz intron. Thus, there seems to be a connection between the splicing machinery, miRNA processing, and stabilization of gene expression. Together, these studies provide essential information for specific and effective silencing of gene activity in transgenic Drosophila.
Results
Sequence-Specific Requirements for RNAi-Induced Gene Silencing.
Recent studies suggest that ≥19 bp of sequence homology between dsRNAs and target mRNAs is required for gene silencing in Drosophila cell culture (reviewed in ref. 14). However, in vitro assays indicate robust RNAi activity with considerably fewer nucleotides of base pairing (11). To address this disparity and begin identifying the minimal sequence requirements for RNAi in vivo, we developed an RNAi specificity assay for Drosophila cell culture and transgenic tissues.
A previously characterized artificial miRNA-based expression system (shmiR) was used to express defined siRNAs in cultured Drosophila S2 cells and transgenic flies (15). siRNAs were expressed using a modified Drosophila pre–miR-1 stem loop that facilitates cloning and expression of defined ~21-nt RNAs, which engage both the miRNA and RNAi pathways. This pre-miR-1 expression cassette was used to examine the specificity of RNAi in Drosophila.
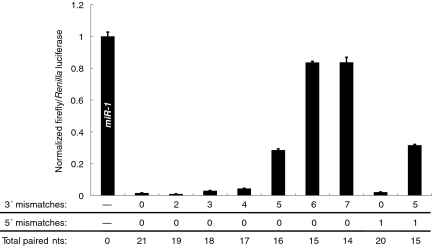
siRNAs containing an increasing number of mismatches with the firefly luciferase mRNA coding region were coexpressed along with firefly and Renilla luciferase in S2 cells. siRNAs with up to four mismatches (17 total bps) at the 3′ terminus completely silenced (≥90% knockdown) the target gene, and five 3′ mismatches (16 total bps) induced ~75% attenuation of luciferase activity in conditions of shmiR excess (8:1 shmiR to target plasmid) (Fig. 1). Whereas siRNAs with up to four 3′ mismatches retained full efficiency in target excess conditions (8:1 target to shmiR plasmids), the siRNA with five mismatches induced less than 50% silencing of reporter activity (Fig. S2). These results are consistent with previous in vitro assays using Drosophila cell-free extracts, which documented a significant decrease in RNAi cleavage rates when comparing siRNAs with four and five 3′ mismatches (11). However, there was a dramatic reduction in silencing activity when a separate shmiR directed against a different region of the luciferase mRNA contained more than three 3′ mismatches (18 total bps) (Figs. S2 and S3). Thus, the exact sequences of siRNAs or target mRNAs can influence the amount of base pairing required for efficient silencing.
Fig. 1.
Basepairing of nucleotides 2–16 between an siRNA and its target ORF can induce significant gene silencing in a Drosophila S2 cell-culture assay. A single firefly luciferase-targeting shmiR was mutated to express siRNAs harboring combinations of 3′ and 5′ mismatches with the target gene from the perspective of the siRNA (antisense or guide strand). These siRNAs were coexpressed along with firefly and Renilla luciferase in an 8:1 ratio of shmiR:luciferase plasmids. Knockdown efficiency was assayed in relation to a nonspecific control (Drosophila miR-1 expression plasmid). All siRNA sequences in relation to the target mRNA site are shown in Fig. S1.
Previous biochemical studies suggested that pairing of the 5′-most nucleotide of a siRNA is not essential for silencing (11, 16). To investigate this issue, we expressed luciferase-specific siRNAs harboring a single 5′ mismatch with or without 3′ mismatches. As predicted, mismatching the 5′ terminal nucleotide did not reduce silencing efficiency with or without mismatches at the 3′ terminus (Fig. 1). Thus, siRNAs with as few as 15 contiguous alignments (positions 2–16) can induce measurable inhibition of a reporter gene under saturating conditions.
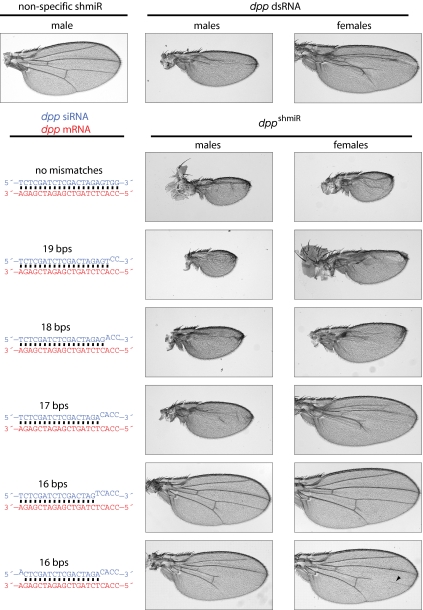
To extend this analysis to whole organisms, we examined the targeted silencing of dpp, which encodes a Bone Morphogenic Protein/Transforming Growth Factor β (BMP/TGFβ) signaling molecule that is required for patterning of the Drosophila wing (17). dpp siRNAs (perfectly paired or mismatched, as above) were selectively expressed in wing imaginal disks using the GAL4-UAS (Upstream Activation Sequence) expression system. shmiRs or dsRNAs directed against the dpp mRNA cause dose-dependent wing phenotypes, which provide accurate measurements of reduced levels of dpp gene activity (15). The ϕC31-based targeted integration system was used to introduce a single copy of various siRNA expression vectors into a specific site with the genome: 86Fb on chromosome 3(10). Targeted integration of these transgenes reduces potential position effects and allows for generally comparable expression of different siRNA variants. In addition, an X-linked driver line (A9-GAL4) enables efficient expression of UAS-responsive transgenes throughout the developing wing tissue (18). Because of dosage compensation of X-linked genes, males express transgenes at higher levels than females. Therefore, a comparison of phenotypes obtained in males and females using the A9-GAL4 driver permits the assessment of dose-dependent responses to the different dpp shmiRs (15).
As shown previously, expression of a shmiR with full complementarity to the dpp mRNA caused a severe mutant phenotype in both males and females, as evidenced by the marked reductions in wing size and the absence of wing venation (15) (Fig. 2). The dpp mutant phenotypes obtained with this shmiR were more severe than those produced with a dsRNA inserted into a separate, highly expressive genomic location (8). Moreover, as many as four 3′ mismatches between the siRNA and dpp mRNA (17 total bps) did not significantly diminish the efficacy of gene silencing, as judged by the wing mutant phenotypes. Although less efficient, an siRNA with a single 5′ and four 3′ mismatches (16 total bps) was able to induce mild dpp phenotypes in both males and females. In contrast, no phenotype was observed when five mismatches were created at the siRNA 3′ terminus. To ensure that this effect was caused by loss of intrinsic silencing activity and not differences in shmiR processing, expression of the different dpp siRNAs was confirmed by Northern assays (Fig. S4). Overall, mismatched siRNAs are somewhat less efficient than the fully complementary siRNAs, but higher levels of the mismatched siRNAs can compensate for this reduced efficiency. These results contradict prior claims that siRNAs require at least 19 nts of pairing to induce sequence-specific gene silencing in vivo (5–8).
Fig. 2.
As few as 16 contiguous base pairs formed between an siRNA and target gene can induce dose-dependent and sequence-specific phenotypes in transgenic flies. Ectopic expression of dpp-targeting, UAS-responsive knockdown constructs was performed using the A9-GAL4 (ubiquitous wing) driver line at 25 °C. All shmiR constructs were inserted into the 86Fb (third chromosome landing site), first described by Bischof et al. (10), and the dpp dsRNA was expressed from the attP2 landing site (8, 10). For each dppshmiR condition, the siRNA is shown in blue, whereas the target region is in red. Superscript bases represent mismatched sequence between the siRNA and target site. A modest defect in L4 wing vein patterning is denoted by the black arrowhead (lower right panel).
Efficient Induction of RNAi Through Intron-Mediated shmiR Expression.
A drawback to the available transgenic Drosophila RNAi libraries is their inability to mark those cells that selectively express siRNAs. This effectively restricts the interpretation of RNAi-induced phenotypes in complex tissues, because it is unclear which cells lack target gene expression. Here, we have adopted several advances in vertebrate small RNA expression systems to circumvent this limitation by expressing shmiRs and a reporter gene from the same transcript (19, 20).
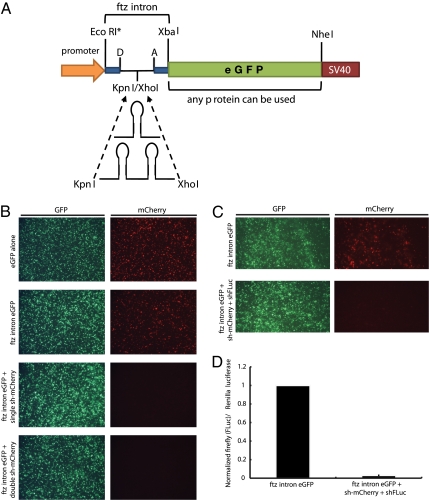
To visualize expression of defined shmiRs in various cells and tissues, an intron-mediated expression system was developed (Fig. 3A). This system takes advantage of the fact that miRNAs can be processed from an intron before splicing of the premRNA, allowing simultaneous shmiR expression and mature mRNA formation from a single transcriptional event (21–23). To assess and optimize this system, we screened separate small introns from Drosophila using a conventional expression vector (pAct5c, which uses the fly actin5C promoter) for proper splicing and reporter gene expression in S2 cells (Fig. S5). From this screen, it was determined that the ftz intron, placed 5′ of the eGFP reporter gene, produced optimal levels of green fluorescence compared with an intron-less control. The ftz intron was, therefore, modified to include a central cloning site, which enables inclusion of defined shmiRs.
Fig. 3.
Effective delivery of one or more shmiR sequences from a single ftz intron in Drosophila cells. (A) Cartoon of the modified ftz-intron transgenic system. A KpnI/XhoI cloning site has been introduced into a nonconserved region of the intron to permit insertion of mono- or bicistronic shRNA (miRNA or siRNA)-expressing constructs derived from pNE3-based vectors (15). The entire ftz-intron reporter cassette can be removed from the transgenic vectors through EcoRI/NheI double digestion. An EcoRI site is not present in the cell-culture expression vectors. (B) eGFP alone or eGFP with a 5′ ftz intron (ftz intron eGFP) was coexpressed with mCherry in Drosophila S2 cells using the strong actin5C promoter (upper two rows). In addition, a single mCherry-targeting shmiR or tandem, unique mCherry-targeting shmiRs were expressed from within the 5′ ftz intron eGFP construct (lower two rows). (C) An empty 5′ ftz eGFP transgene or a transgene containing a tandem shmiR construct (one mCherry and one firefly luciferase shmiR) in the 5′ ftz eGFP construct was expressed, along with equal concentrations of mCherry as well as Renilla and firefly luciferase. Knockdown of mCherry is shown. (D) The same cells photographed in C were assayed for firefly or Renilla luciferase activity.
To investigate the ability of inserted shmiRs to be processed and expressed from the ftz intron, a single or tandem shmiR that targets an mCherry reporter mRNA was placed within the eGFP fusion transcript and expressed in Drosophila S2 cells (24). When coexpressed with mCherry, each of these constructs produced complete and consistent knockdown of red fluorescence without diminishing the green fluorescence produced from the eGFP reporter gene (Fig. 3B). Moreover, it is sometimes desirable to knock down more than one gene from a single RNAi expression vector to assess combinatorial gene function (25). By linking shmiRs that target unique mRNAs (mCherry and firefly luciferase), we were able to induce potent and coincident knockdown of both genes using a single tandem-intronic shmiR construct (Fig. 3 C and D).
In mammalian systems, it has been shown that intronic premiRNA hairpins are excised before splicing (21, 22). This precise order of processing is essential for intron-based RNAi to be useful for genome-wide screens. For example, it is possible that siRNA sequences could introduce a fortuitous splice-acceptor site within the shmiR hairpin. This could compromise expression of siRNAs if the intron is spliced before miRNA processing. To address this potential caveat, we introduced two strong splice acceptors (from the ftz and w genes) in the context of an intronic shmiR, expressed these constructs in S2 cells, and tested for proper splicing of the transcripts by RT-PCR. All transcripts were spliced properly, whether they contained intronic shmiRs with or without splice-acceptor sites, confirming that intronic miRNAs are generally processed before splicing in Drosophila (Fig. S6). Therefore, it does not seem that ectopic splice sites influence intron-mediated shmiR processing and expression.
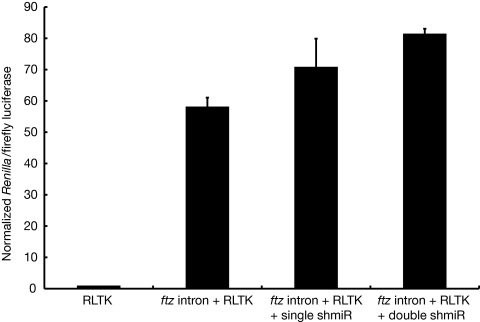
Throughout the course of these experiments, we noted that expression transcripts containing a 5′ ftz intron produced consistently brighter reporter fluorescence than their intron-less counterparts. Whereas introns have been known to enhance associated transcript expression in many systems, including Drosophila, a native Drosophila intron that boosts gene expression has not yet been described (26–28). To see whether the 5′ ftz intron was indeed affecting expression of a reporter gene, we fused the ftz intron—with or without single and tandem shmiRs—5′ of Renilla luciferase in the pAct5c expression vector. Using a quantitative dual-luciferase assay, we found that the ftz intron provided a reproducible, ≥50-fold increase in reporter expression (Fig. 4). Inclusion of shmiRs within the ftz intron led to a further, albeit modest, increase in expression, with tandem hairpins boosting expression more than single hairpins. We discuss this result in more detail below.
Fig. 4.
An associated 5′ ftz intron provides a substantial increase in reporter gene expression, with or without internal shmiRs, in Drosophila cells. Equal DNA concentrations of Renilla luciferase (RLTK), 5′ ftz RLTK, 5′ ftz RLTK with a single intronic mCherry shmiR, or 5′ ftz RLTK with tandem intronic mCherry shmiRs, along with firefly luciferase, were transfected into S2 cells. All constructs were expressed with the actin5C promoter.
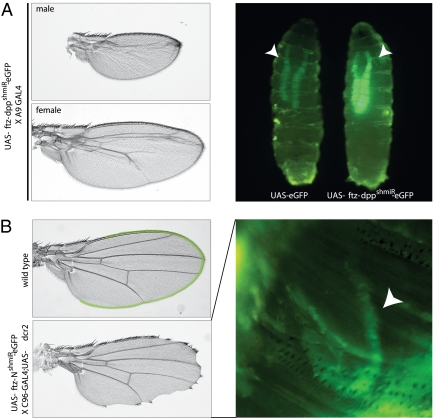
To assess the efficiency of intronic shmiR constructs for gene silencing in transgenic flies, we again used the GAL4-UAS expression system. We adapted the pattB-UAST vector to express the 5′ ftz intron-eGFP transcript from a GAL4-inducible Hsp70 promoter. Here, a dpp shmiR was processed from a 5′ ftz intron within the eGFP reporter gene after selective transcription in wing discs (A9-GAL4 driver). Overall, the wing phenotypes obtained with an intron-derived dpp shmiR were found to be comparable with those obtained with optimal dsRNAs but slightly less efficient than the same dpp shmiR expressed from its own transcript (Fig. 5A Left). It is possible that the ftz intron augments the stability of the GFP mature mRNA without increasing the rate of pre-mRNA synthesis and augmenting siRNA biogenesis (Discussion). Nonetheless, the use of the ftz intron improves the coexpression of siRNAs and GFP (or derivatives) so that it is roughly comparable with previously described shmiR-alone expression vectors.
Fig. 5.
Effective reporter gene expression and gene silencing mediated by ftz intron-derived shmiRs in transgenic flies. (A) Similar to Fig. 2, an intronic dpp shmiR was expressed from a UAS-responsive, 5′ ftz intron-eGFP fusion transgene (inserted into the 86Fb landing site) using the A9-GAL4 driver line. Expression of this construct induces the wing phenotypes shown on Left. At Right, green fluorescence in third instar larvae expressing UAS-eGFP alone or the dpp shmiR from a 5′ ftz intron-eGFP fusion transgene is compared. The A9-GAL4 driver promotes transgene expression in larval salivary glands (marked by white arrowheads). (B) Similar to A, a Notch-targeting shmiR (NshmiR) was expressed from within a 5′ ftz intron fused to eGFP using the wing margin-specific, C96-GAL4;UAS-dcr2 driver, which will also express extra copies of the Drosophila dcr2 gene. The expression domain of the C96 driver is false-colored (green) on top of a wild-type fly wing. Efficient knockdown of Notch with the NshmiR is shown at Left (notching of wing margin). The developing wing of a 48- to 72-h-old pupa expressing the 5′ ftz-NshmiR-eGFP transgene was photographed through the side of a clear plastic vial using a fluorescent dissecting microscope. eGFP expression in the wing margin (crescent shape) is marked by the white arrowhead.
To assay for proper expression of the intron-fused reporter transgene, we compared the level of green fluorescence generated by a UAS-ftz intron-eGFP (with dpp shmiR) line with a publicly available UAS-eGFP stock. A9-GAL4–driven expression of these transgenes in Drosophila salivary glands revealed that the intron-fused construct produced brighter fluorescence (Fig. 5A Right). This suggests that eGFP fluorescence is not reduced when the reporter and intronic shmiR are expressed from the same transgene, although the eGFP control gene is inserted at a different region of the genome. Nonetheless, fluorescence was sufficiently intense to visualize expression of a Notch shmiR in the wing margin—a thin row of cells—using a dissecting microscope without removing larvae from their vials (Fig. 5B Right) (expression performed using the C96-GAL4 driver). The Notch shmiR produces a robust mutant phenotype (Fig. 5B) that is distinct from those produced by dpp shmiRs (Fig. 5A).
Discussion
Minimizing nonspecific or off-target effects is increasingly important with the expanding use of RNAi-based genetic screens. Although several recent studies have investigated sequence-specific and nonspecific RNAi activities in cultured cells, it is difficult to relate this information to transgenic animals. To address this issue, we have developed a sensitive, transgenic Drosophila assay to determine the minimal sequence complementarity required to induce RNAi-specific phenotypes in a live animal model. As few as 16 contiguous base pairs (positions 2–17 of 21 possible base pairs) between an siRNA and target RNA are sufficient to induce sequence-specific silencing in both cultured cells and transgenic flies. Moreover, an siRNA with only 17 contiguous nucleotides of homology with its target RNA was just as efficient as an optimal dsRNA, which is the standard method for inducing RNAi in Drosophila.
Interestingly, siRNAs directed against different regions of a target RNA might have different sequence requirements for efficient silencing. For example, we observed potent silencing of the luciferase mRNA with a siRNA containing as few as 15 nucleotides of contiguous homology. In contrast, a second siRNA directed against a different region of the same target required 17 nucleotides of homology for efficient silencing. This suggests that additional parameters remain to be identified (intrinsic efficiency of an siRNA sequence, transcript secondary structure, etc.) to predict the full activities of a given siRNA. Off-target effects associated with <19 nt of homology have been noted in previous studies, but these effects were thought to be outside the range of statistical significance (5). The results presented in this study suggest that there can be meaningful cross-reactivity of siRNAs, with unknown-targets RNAs containing 16–18 nt of contiguous homology. Therefore, any siRNA with contiguous pairing between nucleotides 2 and 17 should be avoided for RNAi-based experiments with stringent specificity requirements.
Although our study focused on siRNA pairing to the coding region of target mRNAs, partial complementarity of siRNAs with 3′ UTRs can also produce unintended gene silencing (2, 4). Significant attenuation in gene expression can be obtained by the pairing of seed residues (nucleotides 2–8) in mammalian cell culture (4). It is currently unknown whether this also occurs in Drosophila cell culture or transgenic flies. However, it should be noted that small RNAs that enter either the miRNA or RNAi pathways in flies (through Ago1 and Ago2, respectively) can repress translation when bound to a target 3′ UTR (29).
Drosophila has become a workhorse for genome-wide RNAi-based genetic screens. Therefore, it is desirable to optimize the methods for producing RNAi-induced phenotypes. To that end, we have developed an intron-based system to coexpress defined siRNAs and a reporter gene from a single transcript. This system provides the ability to label only those cells producing RNAi, akin to marking clones for mosaic analyses (30). Furthermore, the ftz intron-based siRNA expression method seems to be at least as efficient as existing dsRNA knockdown systems in transgenic Drosophila.
The intron-based expression system is versatile, in that it enables simultaneous silencing of multiple target genes and visualization with any reporter gene such as eGFP or mCherry (31). Also, this system may find additional use for controlling RNAi specificity or gene replacement. It should be possible to coexpress an siRNA against an endogenous gene, along with a cDNA or BAC, that is refractory to the siRNA. Furthermore, enhanced reporter gene expression was observed when its transcript contained a 5′ ftz intron in cell-culture assays. Although 5′ intron positioning has been shown to stimulate gene expression in various organisms—a phenomenon termed intron-mediated enhancement (IME)—this is evidence of an endogenous intron that enhances gene expression in Drosophila (28, 32). To our surprise, this reporter expression was boosted further when the intron contained a processed shmiR, suggesting a link between intronic-miRNA processing and stabilization of the premRNA for subsequent splicing.
Although the ftz intron was able to augment expression of GFP in S2 cells, the intron did not enhance siRNA-mediated disruption of dpp+ activity in transgenic wings. Indeed, the same shmiR sequence is slightly more potent when expressed from its own transcript compared with expression from the ftz intron vector (compare Figs. 4 and 5). This effect may be caused by differences in miRNA processing or loading when comparing the two types of shmiR-harboring transcripts. Also, the expression cassette of the ftz-intron reporter construct produces a transcript ~3-fold longer than the shmiR-alone transcript. Thus, more of the shmiR-alone primary transcript can be produced per unit of time compared with the ftz-intron reporter variant. Nonetheless, the efficacy of gene silencing is similar with the shmiR-alone expression vector and the linked vector containing the ftz intron and GFP coding sequence. Thus, the ftz intron permits the use of GFP for marking cells without seriously compromising the efficiency of siRNA-mediated silencing.
The basic mechanisms underlying IME are not completely known (32), although introns have been shown to augment gene expression by stabilizing mRNAs and increasing translation (33, 34). That is, introns seem to boost the amount of mature mRNA, but it is unclear whether shmiR biogenesis is also augmented because of increases in premRNA expression. The ftz-intron expression vector might provide a useful platform to examine IME and further improve gene-silencing methodologies. Such methods might also take advantage of recently developed quantitative reporter assays (35).
Materials and Methods
shmiR Design and Cloning.
All shmiRs were designed as described (15). The 71-nt DNA oligonucleotides (oligos) used for shmiR cloning were designed using a web-based program (flybuzz.berkeley.edu/cgi-bin/constructhairpin.cgi) and were synthesized by Integrated DNA Technologies (www.idtdna.com). Oligos designated with HB or NE were ligated into the pHB vector or pattB-NE3 vectors, respectively. Oligo sequences for each construct are provided in SI Text and Tables S1–S4.
The Drosophila miR-1 locus was PCR cloned from yw genomic DNA using the primers described in SI Text. All PCR cloning was performed using the Expand High Fidelity PCR system (Roche) and the pCR2.1-TOPO TA cloning system (Invitrogen).
Intron-Mediated shmiR Expression Design and Cloning.
Briefly, reporter genes [eGFP, mCherry, or Renilla luciferase (RLTK)] were PCR cloned using primer sequences provided in SI Text. Reporters were placed into either the pAc5.1-B (Invitrogen) or pattB-UAST cell culture or transgenic expression vectors, respectively (10). Next, regions spanning the Drosophila ftz or tomosyn (housing miR-970) introns were cloned in two parts to allow for inclusion of an intron-localized KpnI/XhoI multiple cloning site. This cloning site is optimized for directional insertion of shmiRs originating from either the pNE3 or pattB-NE3 vectors described in ref. 15. The tomosyn intron was cloned to exclude the premiR-970 hairpin from the final construct. These modified introns were placed upstream (5′) of the reporter gene in each base vector, creating the pAc5.1/pattB—ftzin/tomoin—eGFP/mCherry vector collection. Complete sequence data for the intron + reporter cassettes are available by request.
S2 Cell-Culture and Transient Transfection Assays.
General protocols for S2 cell transfection have been described previously (15). All constructs were cloned into pAc5.1-B. Assays were performed in 24-well plates. Specifically, for shmiR excess conditions, 400 ng shmiR expression plasmid were transfected along with 50 ng GL3 and 50 ng RLTK Luciferase (Promega) expression plasmids (8:1 shmiR to target ratio). For target excess conditions, 400 ng of GL3 expression plasmid were transfected along with 50 ng shmiR and 50 ng RLTK expression plasmids (8:1 target to shmiR ratio). Cells were collected 48 h after transfection, and luminescence was measured in a 20/20 Glomax luminometer (Promega) with a Dual Luciferase Assay kit (Promega).
Fly Genetics.
The dsRNA-dpp (Valium10-based; #25782), C96-GAL4;UAS-Dcr2 (#25757), and UAS-eGFP (#5430) fly stocks were obtained from the Bloomington Stock Center (8). All dppshmiR fly stocks were generated by BestGene Inc. using standard methods. Specifically, all ϕC31-based expression vectors were integrated into the 86FB site on chromosome III (#24749; Bloomington Stock Center) (10). At least three independent lines were generated for each construct. For wing knockdown assays, males of the dsRNA or shmiR expression stocks were crossed to virgin A9-GAL4 females (#8716; Bloomington Stock Center). Crosses for each construct were set up in parallel, and the flies were raised at 25 °C in a temperature-controlled incubator. Male and female progeny were collected 2 or 3 d after hatching. Wings were dissected and mounted as described (15).
Northern blot analysis was performed using third instar larvae as described (15). Probe sequences are described in SI Text.
Supplementary Material
Acknowledgments
We thank members of the Levine Laboratory and Heather Melichar for support and critical comments on the manuscript. This work was supported by National Institutes of Health Grant GM34431 (to M.L.) and an American Cancer Society Postdoctoral Fellowship (to B.H.)
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1006689107/-/DCSupplemental.
References
- 1.de Fougerolles A, Vornlocher HP, Maraganore J, Lieberman J. Interfering with disease: A progress report on siRNA-based therapeutics. Nat Rev Drug Discov. 2007;6:443–453. doi: 10.1038/nrd2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birmingham A, et al. 3′ UTR seed matches, but not overall identity, are associated with RNAi off-targets. Nat Methods. 2006;3:199–204. doi: 10.1038/nmeth854. [DOI] [PubMed] [Google Scholar]
- 3.Jackson AL, et al. Expression profiling reveals off-target gene regulation by RNAi. Nat Biotechnol. 2003;21:635–637. doi: 10.1038/nbt831. [DOI] [PubMed] [Google Scholar]
- 4.Jackson AL, et al. Widespread siRNA “off-target” transcript silencing mediated by seed region sequence complementarity. RNA. 2006;12:1179–1187. doi: 10.1261/rna.25706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kulkarni MM, et al. Evidence of off-target effects associated with long dsRNAs in Drosophila melanogaster cell-based assays. Nat Methods. 2006;3:833–838. doi: 10.1038/nmeth935. [DOI] [PubMed] [Google Scholar]
- 6.Ma Y, Creanga A, Lum L, Beachy PA. Prevalence of off-target effects in Drosophila RNA interference screens. Nature. 2006;443:359–363. doi: 10.1038/nature05179. [DOI] [PubMed] [Google Scholar]
- 7.Dietzl G, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- 8.Ni JQ, et al. A Drosophila resource of transgenic RNAi lines for neurogenetics. Genetics. 2009;182:1089–1100. doi: 10.1534/genetics.109.103630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams MD, et al. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- 10.Bischof J, Maeda RK, Hediger M, Karch F, Basler K. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc Natl Acad Sci USA. 2007;104:3312–3317. doi: 10.1073/pnas.0611511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haley B, Zamore PD. Kinetic analysis of the RNAi enzyme complex. Nat Struct Mol Biol. 2004;11:599–606. doi: 10.1038/nsmb780. [DOI] [PubMed] [Google Scholar]
- 12.Martinez J, Tuschl T. RISC is a 5′ phosphomonoester-producing RNA endonuclease. Genes Dev. 2004;18:975–980. doi: 10.1101/gad.1187904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 14.Perrimon N, Mathey-Prevot B. Matter arising: Off-targets and genome-scale RNAi screens in Drosophila. Fly (Austin) 2007;1:1–5. doi: 10.4161/fly.3601. [DOI] [PubMed] [Google Scholar]
- 15.Haley B, Hendrix D, Trang V, Levine M. A simplified miRNA-based gene silencing method for Drosophila melanogaster. Dev Biol. 2008;321:482–490. doi: 10.1016/j.ydbio.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang G, Reinhart BJ, Bartel DP, Zamore PD. A biochemical framework for RNA silencing in plants. Genes Dev. 2003;17:49–63. doi: 10.1101/gad.1048103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spencer FA, Hoffmann FM, Gelbart WM. Decapentaplegic: A gene complex affecting morphogenesis in Drosophila melanogaster. Cell. 1982;28:451–461. doi: 10.1016/0092-8674(82)90199-4. [DOI] [PubMed] [Google Scholar]
- 18.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 19.Du G, Yonekubo J, Zeng Y, Osisami M, Frohman MA. Design of expression vectors for RNA interference based on miRNAs and RNA splicing. FEBS J. 2006;273:5421–5427. doi: 10.1111/j.1742-4658.2006.05534.x. [DOI] [PubMed] [Google Scholar]
- 20.Lin SL, Ying SY. Gene silencing in vitro and in vivo using intronic microRNAs. Methods Mol Biol. 2006;342:295–312. doi: 10.1385/1-59745-123-1:295. [DOI] [PubMed] [Google Scholar]
- 21.Kataoka N, Fujita M, Ohno M. Functional association of the Microprocessor complex with the spliceosome. Mol Cell Biol. 2009;29:3243–3254. doi: 10.1128/MCB.00360-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morlando M, et al. Primary microRNA transcripts are processed co-transcriptionally. Nat Struct Mol Biol. 2008;15:902–909. doi: 10.1038/nsmb.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim YK, Kim VN. Processing of intronic microRNAs. EMBO J. 2007;26:775–783. doi: 10.1038/sj.emboj.7601512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaner NC, et al. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol. 2004;22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- 25.Hafen E. Pushing single-gene genetic analysis up a Notch. Dev Cell. 2009;16:623–624. doi: 10.1016/j.devcel.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 26.Zieler H, Huynh CQ. Intron-dependent stimulation of marker gene expression in cultured insect cells. Insect Mol Biol. 2002;11:87–95. doi: 10.1046/j.0962-1075.2001.00312.x. [DOI] [PubMed] [Google Scholar]
- 27.Duncker BP, Davies PL, Walker VK. Introns boost transgene expression in Drosophila melanogaster. Mol Gen Genet. 1997;254:291–296. doi: 10.1007/s004380050418. [DOI] [PubMed] [Google Scholar]
- 28.Le Hir H, Nott A, Moore MJ. How introns influence and enhance eukaryotic gene expression. Trends Biochem Sci. 2003;28:215–220. doi: 10.1016/S0968-0004(03)00052-5. [DOI] [PubMed] [Google Scholar]
- 29.Iwasaki S, Kawamata T, Tomari Y. Drosophila argonaute1 and argonaute2 employ distinct mechanisms for translational repression. Mol Cell. 2009;34:58–67. doi: 10.1016/j.molcel.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- 31.Davidson MW, Campbell RE. Engineered fluorescent proteins: Innovations and applications. Nat Methods. 2009;6:713–717. doi: 10.1038/nmeth1009-713. [DOI] [PubMed] [Google Scholar]
- 32.Rose AB. Intron-mediated regulation of gene expression. Curr Top Microbiol Immunol. 2008;326:277–290. doi: 10.1007/978-3-540-76776-3_15. [DOI] [PubMed] [Google Scholar]
- 33.Nott A, Le Hir H, Moore MJ. Splicing enhances translation in mammalian cells: An additional function of the exon junction complex. Genes Dev. 2004;18:210–222. doi: 10.1101/gad.1163204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nott A, Meislin SH, Moore MJ. A quantitative analysis of intron effects on mammalian gene expression. RNA. 2003;9:607–617. doi: 10.1261/rna.5250403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Markstein M, Pitsouli C, Villalta C, Celniker SE, Perrimon N. Exploiting position effects and the gypsy retrovirus insulator to engineer precisely expressed transgenes. Nat Genet. 2008;40:476–483. doi: 10.1038/ng.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.