Abstract
We report the results of a series of chemical, EPR, ENDOR, and HYSCORE spectroscopic investigations of the mechanism of action (and inhibition) of GcpE, E-1-hydroxy-2-methyl-but-2-enyl-4-diphosphate (HMBPP) synthase, also known as IspG, an Fe4S4 cluster-containing protein. We find that the epoxide of HMBPP when reduced by GcpE generates the same transient EPR species as observed on addition of the substrate, 2-C-methyl-D-erythritol-2, 4-cyclo-diphosphate. ENDOR and HYSCORE spectra of these transient species (using 2H, 13C and 17O labeled samples) indicate formation of an Fe-C-H containing organometallic intermediate, most likely a ferraoxetane. This is then rapidly reduced to a ferracyclopropane in which the HMBPP product forms an η2-alkenyl π- (or π/σ) complex with the 4th Fe in the Fe4S4 cluster, and a similar “metallacycle” also forms between isopentenyl diphosphate (IPP) and GcpE. Based on this metallacycle concept, we show that an alkyne (propargyl) diphosphate is a good (Ki ∼ 300 nM) GcpE inhibitor, and supported again by EPR and ENDOR results (a 13C hyperfine coupling of ∼7 MHz), as well as literature precedent, we propose that the alkyne forms another π/σ metallacycle, an η2-alkynyl, or ferracyclopropene. Overall, the results are of broad general interest because they provide new mechanistic insights into GcpE catalysis and inhibition, with organometallic bond formation playing, in both cases, a key role.
Keywords: 4Fe-4S protein, GcpE (IspG), metallacycle
Most pathogenic bacteria, plants, as well as malaria parasites (Plasmodium spp.), in contrast to humans, use the Rohmer, nonmevalonate or methyl erythritol phosphate pathway to produce isoprenoids (1, 2), so the development of inhibitors of this pathway is of interest in the context of drug (and herbicide) discovery. The structures of most of the enzymes in the pathway are now known from X-ray crystallography, but the structure of the penultimate enzyme, GcpE: E-1-hydroxy-2-methyl-but-2-enyl-4-diphosphate (1, HMBPP) synthase, EC 1.17.7.1, also known as IspG, has not yet been reported. Its mechanism of action is thus not well understood, and there is only one inhibitor (with an IC50 of ∼1.6 mM) (3). GcpE enzymes catalyze the 2H+/2e- reduction of 2-C-methyl-D-erythritol-2,4-cyclo-diphosphate (2, MEcPP) to HMBPP: 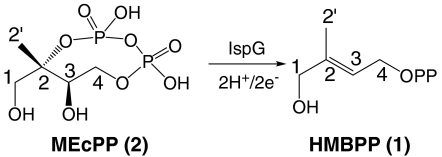 All GcpEs contain three highly conserved Cys residues that are essential for catalysis and are thought to bind to an iron-sulfur cluster (4, 5). This cluster is, based on the results of Mössbauer (4) and EPR (5) spectroscopy, thought to have a 4Fe-4S composition. There have been several catalytic mechanisms proposed for GcpE. In one, Kollas et al. (6) proposed ring-opening of the cyclo-diphosphate to form a carbocation, followed by reduction to a radical, which then underwent reduction and dehydration to form the product, HMBPP (Fig. S1A). In a second mechanism, Seemann et al. (7) proposed a similar route, but with subsequent formation of a cation radical (Fig. S1B). In a third mechanism, Brandt et al. (8) proposed a cation → radical → anion mechanism (Fig. S1C). And in a fourth mechanism, Rohdich et al. (9) proposed that MEcPP underwent an OH--assisted ring opening/ring closing to produce an oxirane 3:
All GcpEs contain three highly conserved Cys residues that are essential for catalysis and are thought to bind to an iron-sulfur cluster (4, 5). This cluster is, based on the results of Mössbauer (4) and EPR (5) spectroscopy, thought to have a 4Fe-4S composition. There have been several catalytic mechanisms proposed for GcpE. In one, Kollas et al. (6) proposed ring-opening of the cyclo-diphosphate to form a carbocation, followed by reduction to a radical, which then underwent reduction and dehydration to form the product, HMBPP (Fig. S1A). In a second mechanism, Seemann et al. (7) proposed a similar route, but with subsequent formation of a cation radical (Fig. S1B). In a third mechanism, Brandt et al. (8) proposed a cation → radical → anion mechanism (Fig. S1C). And in a fourth mechanism, Rohdich et al. (9) proposed that MEcPP underwent an OH--assisted ring opening/ring closing to produce an oxirane 3: 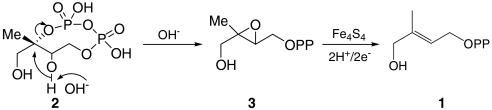 that was then reduced to the alkene, 1, via radical intermediates (Fig. S1D). The possible importance of an oxirane intermediate has also been described in recent work by Nyland et al. (10). There are, therefore, numerous mechanistic possibilities that have been proposed, and to try to clarify the GcpE mechanism, we report here the result of a series of spectroscopic observations on the structure of the intermediate reported by Adedeji et al. (5) that forms on addition of 2 to GcpE. This leads to a new GcpE mechanism as well as the discovery of a potent GcpE inhibitor.
that was then reduced to the alkene, 1, via radical intermediates (Fig. S1D). The possible importance of an oxirane intermediate has also been described in recent work by Nyland et al. (10). There are, therefore, numerous mechanistic possibilities that have been proposed, and to try to clarify the GcpE mechanism, we report here the result of a series of spectroscopic observations on the structure of the intermediate reported by Adedeji et al. (5) that forms on addition of 2 to GcpE. This leads to a new GcpE mechanism as well as the discovery of a potent GcpE inhibitor.
Results and Discussion
EPR, ENDOR, and HYSCORE: Clues for Catalysis.
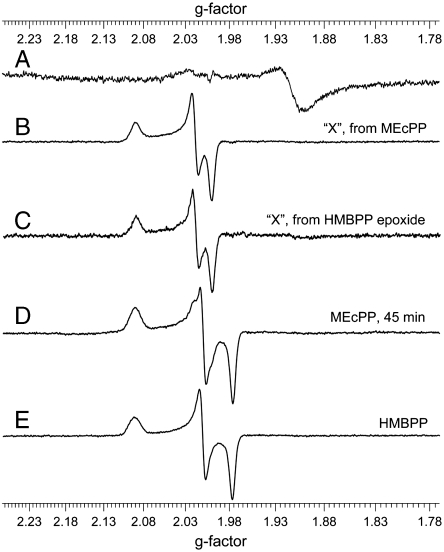
We show in Fig. 1A the 9 GHz EPR spectrum of reduced IspG and in Fig. 1B the spectrum of the transient species that we shall call “X,” formed in the presence of MEcPP. This spectrum could, in principle, arise from bound MEcPP, from a bound epoxide, from bound HMBPP, or from another reactive intermediate. To help distinguish between these possibilities, we prepared HMBPP-epoxide (a mixture of the 2R,3R and 2S,3S epoxides, formed by treating the bromohydrin of HMBPP with NH3) and investigated its effect on the GcpE EPR spectrum. Remarkably, we find that the same EPR intermediate (X) as that formed on addition of MEcPP (Fig. 1B) forms on addition of HMBPP-epoxide to Escherichia coli GcpE (Fig. 1C), and the same results are obtained with Thermus thermophilus GcpE as well (Fig. S2). This argues against the bound MEcPP possibility for the reactive intermediate, X. In addition, the observation that the spectrum of MEcPP + GcpE (at long incubation times) is identical to that found on HMBPP addition, with both E. coli (Fig. 1 D and E) and T. thermophilus GcpE (Fig. S3), rules out an HMBPP adduct as the origin of species X. The transient must, therefore, be either a bound epoxide, or another, as yet unknown, intermediate.
Fig. 1.
X-band EPR spectra of GcpE ± reactants/products. (A) EPR of E. coli GcpE reduced with 20 equivalents Na2S2O4. (B) EPR of E. coli GcpE + MEcPP, incubated for 1 min. (C) EPR of E. coli GcpE + HMBPP epoxide, incubated for 2 min. (D) EPR of E. coli GcpE + MEcPP, incubated for 45 min. (E) EPR of E. coli GcpE + HMBPP, incubated for 45 min. Frequency = 9.05 GHz, microwave power = 1 mW for A, 0.05 mW for B–E, temperature = 15 K.
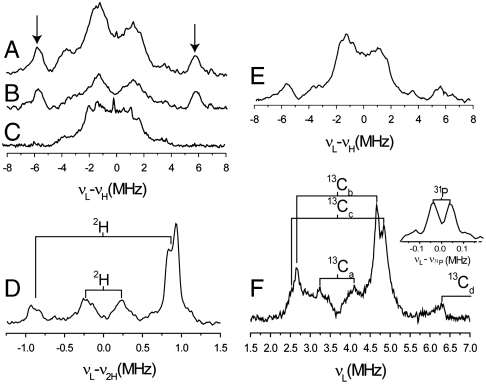
To explore this question in more detail, we next obtained the 1H ENDOR spectra of X from unlabeled MEcPP. As shown in Fig. 2A, a 1H ENDOR signal with a large hyperfine coupling (A ∼ 11.5 MHz) is observed (the smaller central couplings are due to the Cys-beta protons). This 1H ENDOR signal does not decrease on  exchange (four times, Fig. 2B) but is absent when [u-2H]-MEcPP is used, Fig. 2C, so originates from the ligand. In this deuterated sample, we also find two sets of 2H ENDOR signals in the low frequency region, Fig. 2D. The first has a large hyperfine coupling (A ∼ 1.8 MHz) with a small quadrupole splitting and corresponds to the 11.5 MHz feature found in the 1H ENDOR spectrum (Fig. 2A). The second set has a smaller coupling (A ∼ 0.5 MHz) and arises from a weaker or long-range interaction with another deuteron in the ligand. We also find that the 1H ENDOR spectrum of the intermediate X obtained by adding HMBPP epoxide (Fig. 2E) is very similar to that found with the MEcPP intermediate X (Fig. 2A).
exchange (four times, Fig. 2B) but is absent when [u-2H]-MEcPP is used, Fig. 2C, so originates from the ligand. In this deuterated sample, we also find two sets of 2H ENDOR signals in the low frequency region, Fig. 2D. The first has a large hyperfine coupling (A ∼ 1.8 MHz) with a small quadrupole splitting and corresponds to the 11.5 MHz feature found in the 1H ENDOR spectrum (Fig. 2A). The second set has a smaller coupling (A ∼ 0.5 MHz) and arises from a weaker or long-range interaction with another deuteron in the ligand. We also find that the 1H ENDOR spectrum of the intermediate X obtained by adding HMBPP epoxide (Fig. 2E) is very similar to that found with the MEcPP intermediate X (Fig. 2A).
Fig. 2.
X-band ENDOR of T. thermophilus GcpE + MEcPP/HMBPP-epoxide reaction intermediate X. (A) 1H Davies ENDOR of GcpE + MEcPP. (B) 1H Davies ENDOR of GcpE + MEcPP, in D2O buffer. (C) 1H Davies ENDOR of GcpE + [u-2H]-MEcPP. (D) 2H Mims ENDOR of GcpE + [u-2H]-MEcPP, difference spectrum (2H-labeled—unlabeled). (E) 1H Davies ENDOR of GcpE + HMBPP epoxide. (F) 13C Mims ENDOR of GcpE + [u-13C]-MEcPP, difference spectrum (13C-labeled—unlabeled). The inset is the unsubtracted ENDOR spectrum of the labeled sample showing the 31P feature. Frequency = 9.66 GHz; spectra were collected at g2 (B0 = 342.2 mT) at 20 K. τ-averaging was used for collecting Mims ENDOR spectra as follows: D , 10 spectra at 8 ns steps with an initial τ = 248 ns; F, 12 spectra at 8 ns steps with an initial τ = 248 ns.
The 13C ENDOR spectrum of the reaction intermediate X obtained from [u-13C]-MEcPP (Fig. 2F) shows several sets of peaks: One displays a small hyperfine coupling (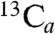 , A ∼ 0.84 MHz); two sets have medium couplings (
, A ∼ 0.84 MHz); two sets have medium couplings (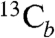 , A ∼ 2.3 MHz;
, A ∼ 2.3 MHz; 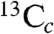 , A ∼ 2.3 MHz); while one weak peak (at 6.3 MHz) could originate from the low frequency part of a much larger coupling (
, A ∼ 2.3 MHz); while one weak peak (at 6.3 MHz) could originate from the low frequency part of a much larger coupling (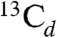 , A ∼ 19 MHz). The latter would have |A/2| > νL so would be centered at |A/2|, with two peaks separated by 2νL (7.3 MHz at 342.2 mT), in which case the high frequency peak should appear at 13.6 MHz and would be obscured by the very strong 1H peaks.
, A ∼ 19 MHz). The latter would have |A/2| > νL so would be centered at |A/2|, with two peaks separated by 2νL (7.3 MHz at 342.2 mT), in which case the high frequency peak should appear at 13.6 MHz and would be obscured by the very strong 1H peaks.
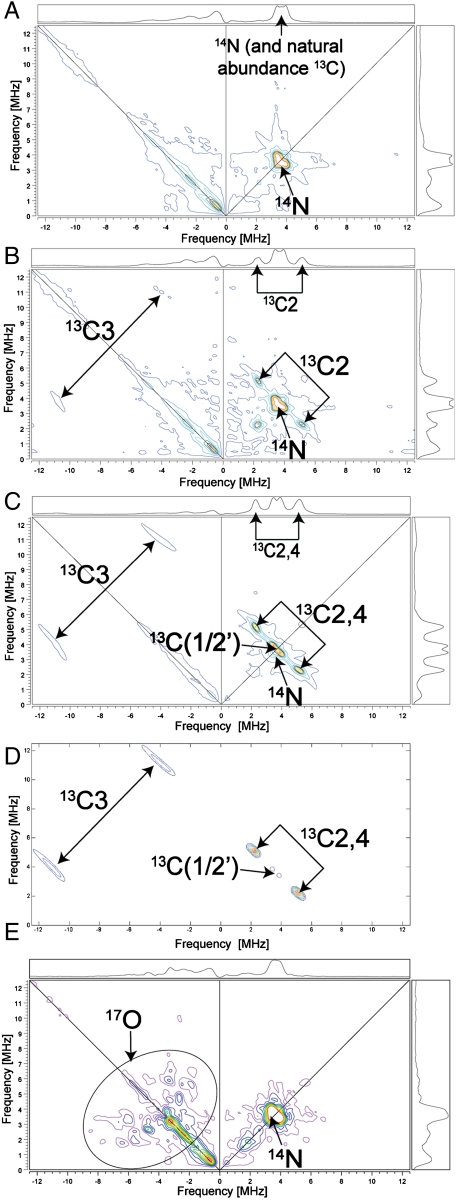
To help confirm these observations, we next obtained HYSCORE (hyperfine sublevel correlation) spectra (Fig. 3 A–C; expanded spectra are shown in Fig. S4). HYSCORE is a two dimensional form of pulsed EPR spectroscopy (11). In the (+/+) quadrant (on the right) of a HYSCORE spectrum, peaks due to weak interactions (|A/2| < νL) are on the antidiagonal, while strong hyperfine interactions (|A/2| > νL) are in the (+/-) quadrant (on the left), again on the antidiagonal. With unlabeled MEcPP, we see primarily the peak due to 14N (most likely from protein backbone nitrogens (12), together with a natural abundance 13C background (Fig. 3A), but with a ∼30% 13C-enriched [2,3-13C]-MEcPP (prepared biosynthetically from [2-13C]-glucose and randomly 13C-enriched only at C2,3) (13), there are now two additional sets of peaks: at (2.29, 5.14; 5.14, 2.29 MHz) in the (+/+) quadrant, and at (-4, 11; -11, 4 MHz) in the (+/-) quadrant, Fig. 3B. The former correspond to the peaks with medium couplings seen in ENDOR (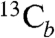 or
or 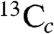 in Fig. 2F), while the latter correspond to the 13C ENDOR peak with the large hyperfine coupling (
in Fig. 2F), while the latter correspond to the 13C ENDOR peak with the large hyperfine coupling (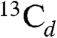 in Fig. 2F). With a [u-13C]-MEcPP labeled sample (having ∼100% 13C), the signals are much stronger (Fig. 3C), and we now see that there is a third set of signals having a very small coupling (corresponding to
in Fig. 2F). With a [u-13C]-MEcPP labeled sample (having ∼100% 13C), the signals are much stronger (Fig. 3C), and we now see that there is a third set of signals having a very small coupling (corresponding to 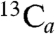 seen in the ENDOR spectrum, Fig. 2F), plus, the cross-peaks with medium couplings in the (+/+) quadrant and the cross-peaks with the large coupling in the (+/-) quadrant are more intense. These results further narrow down the possible structures for the reactive intermediate X. Specifically, the GcpE-bound epoxide 4 is unlikely because nearly equal hyperfine couplings would be expected for C2 and C3, in sharp contrast to the large difference seen experimentally. But can we make other suggestions as to the nature of X, based on these spectroscopic results?
seen in the ENDOR spectrum, Fig. 2F), plus, the cross-peaks with medium couplings in the (+/+) quadrant and the cross-peaks with the large coupling in the (+/-) quadrant are more intense. These results further narrow down the possible structures for the reactive intermediate X. Specifically, the GcpE-bound epoxide 4 is unlikely because nearly equal hyperfine couplings would be expected for C2 and C3, in sharp contrast to the large difference seen experimentally. But can we make other suggestions as to the nature of X, based on these spectroscopic results? 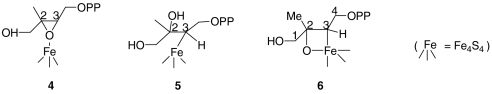
Fig. 3.
HYSCORE spectra and simulation for T. thermophilus GcpE + MEcPP/HMBPP-epoxide reaction intermediate X. (A) GcpE + unlabeled MEcPP. (B) As A but with MEcPP enriched with (∼30%) 13C at C2 and/or C3. (C) As A but with [u-13C]-HMBPP, 100% 13C-labeled. The C1,2’ and 4 assignments are tentative. (D) Simulated 13C spectrum with: C3 Aii = [13,13,20] MHz; C2,4 Aii = [2.3,2.3,3.8] MHz; and C(1 or 2’) Aii = [0.25,0.25,0.7] MHz. (E) GcpE + [2,3-17O]-HMBPP epoxide. Frequency = 9.64 GHz, spectra were collected at ∼g2 (B0 = 341.5 mT) at 18 K with τ = 136 ns. Expansions of the experimental spectra are shown in Fig. S4.
Organometallic Intermediates in GcpE Catalysis.
The large (∼16 MHz, Fig. 3D) 13C hyperfine coupling observed in the [2,3-13C]- and [u-13C]-MEcPP samples suggested to us that X might contain an Fe-C bond in which the directly bonded carbon originates from C2 or C3 of MEcPP. This, when taken together with the 11.5 MHz 1H ENDOR result, suggests that the strongly coupled 1H is most likely directly bonded to the carbon that also has the large 13C hyperfine coupling. The only proton in MEcPP that satisfies this constraint is H3, which then leads to two new candidates for X: the η1-alkyl complex 5 and the ferraoxetane 6. The difference between these two models is that in one case there is an Fe-O bond, while in the other, this bond is absent. To determine whether Fe-O bonding is present, we obtained the HYSCORE spectrum of X prepared from [2,3-17O]-HMBPP epoxide (Fig. 3E). Features due to 17O hyperfine/quadrupole interactions are clearly seen in the (+/-) quadrant, favoring the ferraoxetane 6 as the putative reaction intermediate X, although at present it is not certain whether it forms from 2 and 3 in a consecutive or parallel manner.
The possibility that the ferraoxetane 6 is a reaction intermediate is intriguing because many metallaoxatanes are known as stable species (14, 15), and in the case of Fe interacting with oxirane itself, the 1,2-ferraoxetane has been observed using matrix isolation (16). This species is more stable than is Fe + oxirane (17), and on warming, the ferraoxetane undergoes a [2 + 2] dissociation to ethylene and FeO (16). The involvement of more complex metallaoxetanes in epoxide deoxygenation was proposed early on by Sharpless (18) (and would be essentially the opposite reaction to Sharpless epoxidation), and such species might be involved in oxirane deoxygenation by GcpE as well as by model Fe4S4 clusters (19). If the ferraoxetane does represent a reactive intermediate, a possible overall reaction mechanism is as shown at the top of the following Scheme, in which 6 is converted to the π/σ metallacycle 7 and product 1: 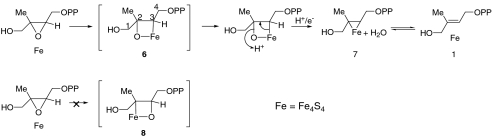 Initial formation of isomer 6 over the alternative species, 8, seems likely, because as discussed earlier, the large 1H hyperfine coupling is consistent with a 2-bond rather than a 3-bond interaction of the iron-sulfur cluster with H3. Ring formation might also proceed via radical intermediates, but the EPR and ENDOR results we do see provide no evidence for any stable, carbon-based radicals, which would have g-values at about the free electron g-value. But is there any evidence that the HMBPP metallacycle 7 actually forms? And if it does, can this help us find new inhibitors?
Initial formation of isomer 6 over the alternative species, 8, seems likely, because as discussed earlier, the large 1H hyperfine coupling is consistent with a 2-bond rather than a 3-bond interaction of the iron-sulfur cluster with H3. Ring formation might also proceed via radical intermediates, but the EPR and ENDOR results we do see provide no evidence for any stable, carbon-based radicals, which would have g-values at about the free electron g-value. But is there any evidence that the HMBPP metallacycle 7 actually forms? And if it does, can this help us find new inhibitors?
HMBPP (and IPP) Form Metallacycles with GcpE.
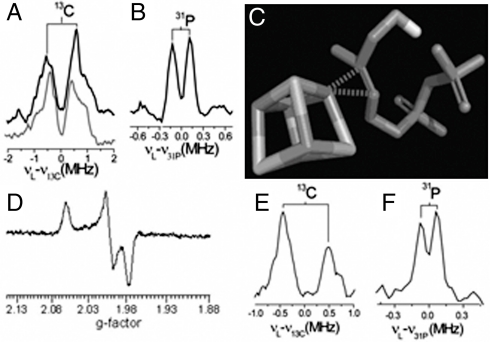
We next consider the question as to whether the HMBPP product does in fact bind to the Fe4S4 cluster in GcpE. Because HMBPP is, chemically, just a substituted ethylene or allyl alcohol, and because ethylene and allyl alcohol are known to form π (or π/σ) η2-alkenyl “metallacycle” complexes with a nitrogenase FeMo cofactor (which is thought to contain a Fe3MoS3X cubane-like structure) (20, 21), we reasoned that HMBPP bound to GcpE might also form a π (or π/σ) complex with the unique fourth Fe in the Fe4S4 cluster. As noted above, we find that there are very large changes in the EPR spectra of GcpE on addition of HMBPP (or MEcPP, at ∼45 min) (Fig. 1 A, D, and E), indicating major changes in the cluster’s electronic structure. To test the metallacycle hypothesis further, we obtained the ENDOR spectrum of [u-13C]-HMBPP bound to T. thermophilus GcpE, Fig. 4 A and B. As can be seen in Fig. 4A, there are ENDOR resonances due to 13C hyperfine coupling (Fig. 4A, A ∼ 1.4 MHz), together with a small 31P coupling (Fig. 4B, A ∼ 0.3 MHz), consistent with the idea that HMBPP is bound to GcpE, forming a π (or π/σ) η2-alkenyl metallacycle, as illustrated schematically in Fig. 4C. This type of interaction also occurs with isopentenyl diphosphate (IPP), because as shown in Fig. 4D, a narrow line spectrum (with g = 2.065, 1.995 and 1.975) is obtained for GcpE (from T. thermophilus) in the presence of IPP, and 13C and 31P ENDOR signals are found on binding of  -IPP, Fig. 4 E and F. So, HMBPP and IPP, both of which contain alkene groups, interact with the reduced Fe4S4 cluster in GcpE, forming, we propose, η2-alkenyl π (or π/σ) metallacycles, basically the same type of structure as seen with ethylene or allyl alcohol (the HMBPP “parent” molecules) bound to the nitrogenase FeMo cofactor. Notably, this type of π-complex formation is also found with reduced LytB (22), the last enzyme in the nonmevalonate pathway. And because there is no 1-OH group in IPP, alkoxide binding as in LytB (23) is not essential for interaction with the Fe4S4 cluster.
-IPP, Fig. 4 E and F. So, HMBPP and IPP, both of which contain alkene groups, interact with the reduced Fe4S4 cluster in GcpE, forming, we propose, η2-alkenyl π (or π/σ) metallacycles, basically the same type of structure as seen with ethylene or allyl alcohol (the HMBPP “parent” molecules) bound to the nitrogenase FeMo cofactor. Notably, this type of π-complex formation is also found with reduced LytB (22), the last enzyme in the nonmevalonate pathway. And because there is no 1-OH group in IPP, alkoxide binding as in LytB (23) is not essential for interaction with the Fe4S4 cluster.
Fig. 4.
X-band EPR and ENDOR spectra of GcpE (and IspH) + HMBPP/IPP. (A) 13C ENDOR of GcpE + [u-13C]-HMBPP, difference spectrum (13C-labeled—unlabeled). The bottom inset is the A. aeolicus IspH E126A mutant + [u-13C]-HMBPP (from ref. 22). (B) 31P ENDOR of GcpE + [u-13C]-HMBPP. (C) Schematic illustration of HMBPP bound to an Fe4S4 cluster illustrating metallacycle formation. (D) EPR of GcpE + IPP. (E) 13C ENDOR of GcpE + [4-13C]-IPP, difference spectrum (13C-labeled–unlabeled). (F) 31P ENDOR of GcpE + [4-13C]-IPP. EPR frequency = 9.05 GHz; ENDOR frequency = 9.66 GHz; B0 was selected as the field where the maximum EPR signal intensity was obtained: A and B, 347.9 mT; E and F, 344.4 mT. τ-averaging (64 spectra at 8 ns step with initial τ = 200 ns) were used for collecting the Mims ENDOR spectra.
Discovery of Potent GcpE Inhibition Involving a Metallacycle (π or π/σ) Complex.
The results described above, in which we find evidence for organometallic (π or π/σ) complexes, led to new ideas for GcpE inhibitors. In their work on alkyne reductions by model Fe4S4 clusters, Itoh et al. (24) discovered that diphenylacetylene was reduced to cis-stilbene. This is reminiscent of the reduction of acetylene to ethylene catalyzed by [Fe4S4(SPh)4]2-/3-/HOAc/Ac2O reported by McMillan et al. (25), where it was suggested that the reaction involved formation of a π or π/σ complex in which acetylene binds to one of the Fe atoms, with species (resonance hybrids) such as 9a, 9b being formed. Direct evidence for π complex formation between an alkyne and an Fe4S4 cluster has been observed by Tanaka et al. (26), who found that the C ≡ C vibrational Raman frequency of acetylene decreased by ≈60 cm-1 when bound to [Fe4S4(SPh)4]3- and was accompanied by the loss of one PhS- ligand (as determined by UV-VIS spectroscopy). 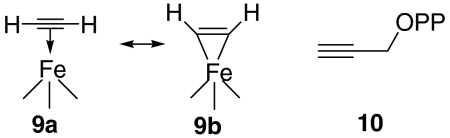 Taken together, this literature as well as our ENDOR results suggested to us that because alkenes and alkynes can bind to Fe4S4 clusters, species such as 10, propargyl diphosphate, might be GcpE inhibitors, forming η2-alkynyl complexes, just as they do with LytB (22).
Taken together, this literature as well as our ENDOR results suggested to us that because alkenes and alkynes can bind to Fe4S4 clusters, species such as 10, propargyl diphosphate, might be GcpE inhibitors, forming η2-alkynyl complexes, just as they do with LytB (22).
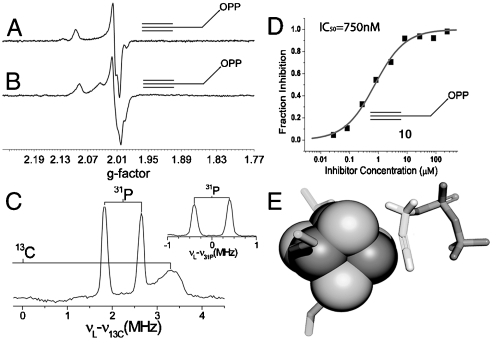
The EPR spectrum of propargyl diphosphate 10 bound to E. coli GcpE exhibited a narrow line spectrum (Fig. 5A) clearly distinct from that observed in the absence of the ligand (Fig. 1A). A similar result was obtained for T. thermophilus GcpE + 10 (Fig. 5B). There is, therefore, a major change in the electronic structures of both clusters on binding this acetylenic compound, and the results of an ENDOR experiment using 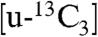 -10 (Fig. 5C) indicates a large (A ∼ 7 MHz) 13C hyperfine interaction. As with HMBPP and IPP binding, these results suggest formation of a π (or π/σ) complex, a ferracyclopropene. Notably, the 13C hyperfine couplings in the alkyne are much larger than those found in any of the alkene complexes (∼1–3.7 MHz) (20, 22), suggesting stronger binding with the alkyne. This could be due solely to the fact that alkynes are better donors/acceptors than are alkenes, but in this particular system it might also be due to the presence of “resonance” forms that could stabilize alkyne bonding—not dissimilar to the presence of resonance in, e.g., the cyclopropenyl cation [which like some metallacycles (27), is aromatic]. Consistent with the stronger binding affinity suggested by these spectroscopic results, we find that propargyl diphosphate (10) is a competitive GcpE inhibitor with an IC50 ∼ 750 nM (Ki ∼ 330 nM) (Fig. 5D) binding we propose as the π (or π/σ) complex (Fig. 5E) and is ∼1,000× more potent than previously reported GcpE inhibitors (3).
-10 (Fig. 5C) indicates a large (A ∼ 7 MHz) 13C hyperfine interaction. As with HMBPP and IPP binding, these results suggest formation of a π (or π/σ) complex, a ferracyclopropene. Notably, the 13C hyperfine couplings in the alkyne are much larger than those found in any of the alkene complexes (∼1–3.7 MHz) (20, 22), suggesting stronger binding with the alkyne. This could be due solely to the fact that alkynes are better donors/acceptors than are alkenes, but in this particular system it might also be due to the presence of “resonance” forms that could stabilize alkyne bonding—not dissimilar to the presence of resonance in, e.g., the cyclopropenyl cation [which like some metallacycles (27), is aromatic]. Consistent with the stronger binding affinity suggested by these spectroscopic results, we find that propargyl diphosphate (10) is a competitive GcpE inhibitor with an IC50 ∼ 750 nM (Ki ∼ 330 nM) (Fig. 5D) binding we propose as the π (or π/σ) complex (Fig. 5E) and is ∼1,000× more potent than previously reported GcpE inhibitors (3).
Fig. 5.
Inhibition of GcpE by an alkynyl diphosphate 10 also involves metallacycle formation. (A) 9.05 GHz EPR of E. coli GcpE + 10 equivalents propargyl diphosphate 10 and 20 equivalents sodium dithionite. (B) 9.05 GHz EPR of T. thermophilus GcpE + 10 equivalents propargyl diphosphate 10 and 20 equivalents sodium dithionite. (C) ENDOR of T. thermophilus GcpE + [u-13C] propargyl diphosphate 10. The inset is the ENDOR spectrum of GcpE + unlabeled 10, showing only the 31P signals. Frequency = 9.66 GHz; Spectra were collected at 15 K at B0 = 342.4 mT, where the maximum EPR signal intensity was obtained. τ-averaging (64 spectra at 8 ns steps with initial τ = 200 ns) was used. (D) E. coli GcpE inhibition by propargyl diphosphate, IC50 = 750 nM (Ki ∼ 330 nM). (E) Schematic illustration (based on LytB + 10 docking calculation) (22) of how propargyl diphosphate might bind to GcpE, forming an η2-alkynyl complex.
Conclusions
The results we have described above are of interest for several reasons. First, we find that the EPR spectrum of the reactive intermediate X formed on MEcPP addition is the same as that found on HMBPP-epoxide addition, indicating that both MEcPP and HMBPP-epoxide form the same intermediate. Second, we propose a tentative structure for this intermediate: an Fe-C-H containing (organometallic) species, most likely a ferraoxetane, based on the results of 1H, 2H, 13C, and 17O ENDOR and HYSCORE experiments. Third, we propose that HMBPP, as well as IPP, form π (or π/σ) metallacycle complexes, ferracyclopropanes, based on the EPR and ENDOR results and precedent. Fourth, we find that an alkynyl diphosphate, propargyl diphosphate, is a good (IC50 = 750 nM) GcpE inhibitor lead that also forms a metallacycle (a π or π/σ, η2-alkynyl) complex with the Fe4S4 cluster, based on the large hyperfine coupling seen in its ENDOR spectrum and on literature precedent for acetylene/Fe4S4 cluster interactions in model systems. These results lead to the idea that, in the future, it may be possible to develop related compounds as novel drugs targeting isoprenoid biosynthesis and that organometallic complex formation may play a role in reactions catalyzed by other Fe4S4-containing proteins containing “unique,” fourth Fe atoms.
Methods
Pulsed ENDOR/HYSCORE spectra were obtained on a Bruker ElexSys E-580-10 FT-EPR X-band EPR spectrometer using a Bruker RF amplifier (150 W, 100 kHz–250 MHz, for pulsed ENDOR experiments) and an Oxford Instruments CF935 cryostat. Mims pulsed ENDOR used a three-pulse sequence (π/2mw-τ-π/2mw - T - π/2mw-τ-echo; π/2mw = 16 ns, with πRF applied during T). τ-averaging was used to reduce the blind spots that arise from the τ-dependent oscillations. Davies pulsed ENDOR used a three-pulse sequence (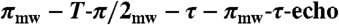 ; π/2mw = 48 ns, with πRF applied during T). HYSCORE used a four-pulse sequence (π/2mw - τ-π/2mw - t1 - πmw - t2 - π/2mw - echo; π/2mw = 16 ns), 256 points for both t1 and t2, each at 20 ns steps. Time-domain data were baseline corrected using a third order polynomial, then Hamming windowed, followed by zero-filling and 2D-Fourier transformation. The HYSCORE spectrum was simulated using the EasySpin program package (28).
; π/2mw = 48 ns, with πRF applied during T). HYSCORE used a four-pulse sequence (π/2mw - τ-π/2mw - t1 - πmw - t2 - π/2mw - echo; π/2mw = 16 ns), 256 points for both t1 and t2, each at 20 ns steps. Time-domain data were baseline corrected using a third order polynomial, then Hamming windowed, followed by zero-filling and 2D-Fourier transformation. The HYSCORE spectrum was simulated using the EasySpin program package (28).
Additional details on protein purification and reconstitution, enzyme assays, EPR/ENDOR/HYSCORE sample preparation, and compound syntheses are reported in SI Methods.
Supplementary Material
Acknowledgments.
We are grateful to Pinghua Liu for providing his E. coli GcpE expression system and to H. Jomaa and J. Wiesner for providing their T. thermophilus GcpE expression systems. We thank Mark J. Nilges for helpful discussions and assistance with the ENDOR and pulsed EPR experiments, and Thomas B. Rauchfuss, John Hartwig, Wilfred van der Donk, Yonghui Zhang, Evert Duin, and Sergei Dikanov, for their helpful suggestions. This work was supported by the United States Public Health Service (National Institutes of Health Grant GM65307).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1000264107/-/DCSupplemental.
References
- 1.Rohmer M. From molecular fossils of bacterial hopanoids to the formation of isoprene units: Discovery and elucidation of the methylerythritol phosphate pathway. Lipids. 2008;43:1095–1107. doi: 10.1007/s11745-008-3261-7. [DOI] [PubMed] [Google Scholar]
- 2.Rohmer M, Grosdemange-Billiard C, Seemann M, Tritsch D. Isoprenoid biosynthesis as a novel target for antibacterial and antiparasitic drugs. Curr Opin Invest Drugs. 2004;5:154–162. [PubMed] [Google Scholar]
- 3.Van Hoof S, et al. Synthesis of analogues of (E)-1-hydroxy-2-methylbut-2-enyl 4-diphosphate, an isoprenoid precursor and human gamma delta t cell activator. J Org Chem. 2008;73:1365–1370. doi: 10.1021/jo701873t. [DOI] [PubMed] [Google Scholar]
- 4.Seemann M, et al. Isoprenoid biosynthesis in chloroplasts via the methylerythritol phosphate pathway: The (E)-4-hydroxy-3-methylbut-2-enyl diphosphate synthase (GcpE) from Arabidopsis thaliana is a [4Fe-4S] protein. J Biol Inorg Chem. 2005;10:131–137. doi: 10.1007/s00775-004-0619-z. [DOI] [PubMed] [Google Scholar]
- 5.Adedeji D, et al. Possible direct involvement of the active-site [4Fe-4S] cluster of the GcpE enzyme from Thermus thermophilus in the conversion of MEcPP. FEBS Lett. 2007;581:279–283. doi: 10.1016/j.febslet.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 6.Kollas AK, et al. Functional characterization of GcpE, an essential enzyme of the non-mevalonate pathway of isoprenoid biosynthesis. FEBS Lett. 2002;532:432–436. doi: 10.1016/s0014-5793(02)03725-0. [DOI] [PubMed] [Google Scholar]
- 7.Seemann M, et al. Isoprenoid biosynthesis through the methylerythritol phosphate pathway: The (E)-4-hydroxy-3-methylbut-2-enyl diphosphate synthase (GcpE) is a [4Fe-4S] protein. Angew Chem Int Ed Engl. 2002;41:4337–4339. doi: 10.1002/1521-3773(20021115)41:22<4337::AID-ANIE4337>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 8.Brandt W, et al. A proposed mechanism for the reductive ring opening of the cyclodiphosphate MEcPP, a crucial transformation in the new DXP/MEP pathway to isoprenoids based on modeling studies and feeding experiments. Chembiochem. 2004;5:311–323. doi: 10.1002/cbic.200300743. [DOI] [PubMed] [Google Scholar]
- 9.Rohdich F, et al. The deoxyxylulose phosphate pathway of isoprenoid biosynthesis: Studies on the mechanisms of the reactions catalyzed by IspG and IspH protein. Proc Natl Acad Sci USA. 2003;100:1586–1591. doi: 10.1073/pnas.0337742100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nyland RL, II, Xiao Y, Liu P, Freel Meyers CL. IspG converts an epoxide substrate analogue to (E)-4-hydroxy-3-methylbut-2-enyl diphosphate: Implications for IspG catalysis in isoprenoid biosynthesis. J Am Chem Soc. 2009;131:17734–17735. doi: 10.1021/ja907470n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schweiger A, Jeschke G. Principles of pulse electron paramagnetic resonance. Oxford: Oxford Univ Press; 2001. [Google Scholar]
- 12.Dikanov SA, Samoilova RI, Kappl R, Crofts AR, Huttermann J. The reduced [2Fe-2S] clusters in adrenodoxin and arthrospira platensis ferredoxin share spin density with protein nitrogens, probed using 2D ESEEM. Phys Chem Chem Phys. 2009;11:6807–6819. doi: 10.1039/b904597j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisenreich W, Bacher A, Arigoni D, Rohdich F. Biosynthesis of isoprenoids via the non-mevalonate pathway. Cell Mol Life Sci. 2004;61:1401–1426. doi: 10.1007/s00018-004-3381-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashmi AS, Hutchings GJ. Gold catalysis. Angew Chem Int Ed Engl. 2006;45:7896–7936. doi: 10.1002/anie.200602454. [DOI] [PubMed] [Google Scholar]
- 15.Calhorda MJ, et al. Rhodaoxetane—Synthesis, structure, and theoretical evaluation. Organometallics. 1993;12:3316–3325. [Google Scholar]
- 16.Kafafi ZH, Hauge RH, Billups WE, Margrave JL. Infrared-spectroscopy and photochemistry of iron ethylene-oxide in cryogenic matrices—The FTIR spectrum of vinyliron hydroxide. J Am Chem Soc. 1987;109:4775–4780. [Google Scholar]
- 17.Backvall JE, Bokman F, Blomberg MRA. Metallaoxetanes as possible intermediates in metal-promoted deoxygenation of epoxides and epoxidation of olefins. J Am Chem Soc. 1992;114:534–538. [Google Scholar]
- 18.Sharpless KB, Teranishi AY, Backvall JE. Chromyl chloride oxidations of olefins—Possible role of organometallic intermediates in oxidations of olefins by oxo transition-metal species. J Am Chem Soc. 1977;99:3120–3128. [Google Scholar]
- 19.Itoh T, Nagano T, Sato M, Hirobe M. Deoxygenation of oxiran compounds to olefins by [Fe4S4(SC6H5)4]2- in the presence of NaBH4. Tetrahedron Lett. 1989;30:6387–6388. [Google Scholar]
- 20.Lee HI, et al. An organometallic intermediate during alkyne reduction by nitrogenase. J Am Chem Soc. 2004;126:9563–9569. doi: 10.1021/ja048714n. [DOI] [PubMed] [Google Scholar]
- 21.Pelmenschikov V, Case DA, Noodleman L. Ligand-bound S = 1/2 FeMo-cofactor of nitrogenase: Hyperfine interaction analysis and implication for the central ligand X identity. Inorg Chem. 2008;47:6162–6172. doi: 10.1021/ic7022743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang W, et al. Bioorganometallic mechanism of action, and inhibition, of IspH. Proc Natl Acad Sci USA. 2010;107:4522–4527. doi: 10.1073/pnas.0911087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gräwert T, et al. Probing the reaction mechanism of IspH protein by X-ray structure analysis. Proc Natl Acad Sci USA. 2010;107:1077–1081. doi: 10.1073/pnas.0913045107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Itoh T, Nagano T, Hirobe M. [Fe4S4(SR)4]2- catalytic reduction of diphenylacetylene to cis-stilbene in the presence of NaBH4. Tetrahedron Lett. 1980;21:1343–1346. [Google Scholar]
- 25.McMillan RS, Renaud J, Reynolds JG, Holm RH. Biologically related iron-sulfur clusters as reaction centers. Reduction of acetylene to ethylene in systems based on [Fe4S4(SR)4]3- J Inorg Biochem. 1979;11:213–227. doi: 10.1016/s0162-0134(00)80019-7. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka K, Nakamoto M, Tsunomori M, Tanaka T. Raman-spectra of the adducts of reduced species of [Fe4S4(SPh)4]2- and [Mo2Fe6S8(SPh)9]3- with acetylene. Chem Lett. 1987:613–616. [Google Scholar]
- 27.Periyasamy G, Burton NA, Hillier IH, Thomas JM. Electron delocalization in the metallabenzenes: A computational analysis of ring currents. J Phys Chem A. 2008;112:5960–5972. doi: 10.1021/jp7106044. [DOI] [PubMed] [Google Scholar]
- 28.Stoll S, Schweiger A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J Magn Reson. 2006;178:42–55. doi: 10.1016/j.jmr.2005.08.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.