Abstract
We show that receptor induced G protein βγ subunit translocation from the plasma membrane to the Golgi allows a receptor to initiate fragmentation and regulate secretion. A lung epithelial cell line, A549, was shown to contain an endogenous translocating G protein γ subunit and exhibit receptor-induced Golgi fragmentation. Receptor-induced Golgi fragmentation was inhibited by a shRNA specific to the endogenous translocating γ subunit. A kinase defective protein kinase D and a phospholipase C β inhibitor blocked receptor-induced Golgi fragmentation, suggesting a role for this process in secretion. Consistent with βγ translocation dependence, fragmentation induced by receptor activation was inhibited by a dominant negative nontranslocating γ3. Insulin secretion was shown to be induced by muscarinic receptor activation in a pancreatic β cell line, NIT-1. Induction of insulin secretion was also inhibited by the dominant negative γ3 subunit consistent with the Golgi fragmentation induced by βγ complex translocation playing a role in secretion.
Keywords: G protein-coupled receptors, live cell imaging, signaling, insulin
Extracellular signals act through plasma membrane localized G protein-coupled receptors (GPCRs) and G proteins to induce physiological events. The recent observation that specific βγ complexes translocate to the Golgi on activation by an extracellular signal (1–3) suggested that GPCRs on the plasma membrane may regulate the function of intracellular Golgi membranes through this translocation. Longstanding evidence has suggested that G protein subunits are present in the Golgi (4, 5) and it has been shown that the introduction of the G protein βγ complex into permeable cells or overexpression of the βγ complex in a cell results in the formation of protein kinase D (PKD) and phospholipase C β (PLCβ)-dependent secretory vesicles from the Golgi (6). But the signal and the mechanism that allows plasma membrane localized G protein subunits to reach the intracellular Golgi complex and act on it have remained unidentified.
GPCR activation is known to mediate secretory changes, an important example being the M3 muscarinic-receptor–stimulated insulin secretion from pancreatic β cells through the G protein, Gq (7). This secretory response is central to the parasympathetic acetylcholine regulation of glucose homeostasis. The specific mechanisms at the basis of M3 regulation of secretion have not been clear. Recent evidence suggests that this M3 stimulation of insulin secretion is mediated in part by PKD-mediated vesicle formation from the Golgi complex (8). Here, we examined the impact of M3-muscarinic-receptor–activated G protein βγ translocation on the Golgi complex structure and secretion of insulin. The results identify an extracellular agonist for a GPCR as a signal that can regulate Golgi fragmentation. The fragmentation is inhibited by a kinase defective PKD and an inhibitor of PLCβ showing that it is PKD and PLCβ mediated. Because PKD is known to mediate the trafficking of vesicles from the trans-Golgi network to the plasma membrane (6, 9), these results suggested that extracellular signals can regulate secretory vesicle formation from intracellular membranes through the translocation of the βγ complex from the plasma membrane to the Golgi. We then examined the role of M3-mediated βγ translocation on the secretion of insulin. A nontranslocating γ3 subunit acted in a dominant negative fashion and inhibited both M3-receptor–induced Golgi fragmentation as well as the induction of insulin secretion in a pancreatic β cell line. The results suggest that receptor-induced βγ translocation to the Golgi regulates vesicle formation and secretion.
Results and Discussion
Translocating G Protein γ Subunit Induces Redistribution of Endogenous Golgi Markers.
Six members of the G protein γ subunit family are capable of translocating as a βγ complex, from the plasma membrane to intracellular membranes—mainly the Golgi (1–3). To understand the effect of this translocation, we initially examined the effect of γ11, on the Golgi structure in HeLa cells expressing the M3 muscarinic receptor and the αq subunit. We examined the distribution of a trans-Golgi network (TGN) specific endogenous protein, TGN46 (10, 11), by immunofluorescence before and after agonist addition. TGN46 was localized to a compact region in the cell corresponding to the Golgi complex in the basal state (Fig. 1 A Upper and B). After exposure to the agonist, the TGN46 marker was extensively redistributed in small vesicles (Fig. 1 A Lower and B). This suggested that the translocated βγ induces vesicle formation from the TGN. We also examined the localization of a cis-Golgi marker, GM130 (12), using immunofluorescence in the same cells with or without agonist. GM130 was redistributed in the agonist treated cells (Fig. S1), indicating alteration of the entire Golgi structure. This exaggerated response is similar to the breakdown of the entire Golgi complex that has previously been seen when Golgi protein kinase D (PKD) was overly activated (8, 9). When distribution of TGN46 and GM130 was analyzed in cells in which γ11 was not transfected, receptor activation had no effect, showing that γ11 is necessary for fragmentation.
Fig. 1.
Golgi fragmentation visualized by immunofluorescence using antibodies to endogenous Golgi marker. (A) TGN marker, TGN46. HeLa cells expressing M3 receptor, αq, and GFP-γ11 probed with anti-TGN46 antibody. Representative images are shown (n > 90 from three to four independent experiments). (Left) GFP-γ11 expression, (Right) TGN46 distribution. (Upper) Basal unstimulated cells; (Lower) cells treated with 100 μM carbachol for 2.5 h. Note that in activated cells, TGN46 is completely redistributed and small vesicles can be seen (A Lower). (B) Bar graph depicting the percentage of cells (±SEM) showing compact or fragmented distribution of TGN46 based on the experiment described above in A.
To study Golgi fragmentation in live cells, we expressed galactosyl transferase, which is specific to the trans-Golgi (11) tagged with DsRed (GalT-DsRed). The effect of M3 activation on the Golgi was examined in HeLa cells, as above, with a γ11 tagged at the N terminus with YFP. We and others have shown N-terminal FP tags of the G protein β and γ subunits to be functionally active (2, 13, 14). Confocal images of the basal state of these cells showed that the trans-Golgi was compact (Fig. S2A Upper). The YFP-γ11was found in the Golgi complex in the basal state consistent with our previous finding that G protein subunits shuttle continually between the plasma membrane and the Golgi maintaining pools of subunits in both membranes in dynamic equilibrium (15). On M3 activation, trans-Golgi fragmentation was observed (Fig. S2A Lower). The γ subunit colocalized with the vesicles confirming their site of action (Fig. S2 A Lower Inset and C). To ascertain that the observed effect is mediated by the translocation process, we examined whether γ3 (1), which does not translocate on receptor activation, has any effect on Golgi fragmentation in response to receptor activation. Cells expressing γ3 did not show fragmentation of the Golgi before or after receptor activation (Fig. S2B). This result suggested that translocation of βγ to the Golgi may be a requirement for fragmentation to occur.
Identification of a Cell Line Containing an Endogenous Translocation Proficient γ Subunit.
We screened a set of mammalian cell lines for the presence of an endogenous translocating γ subunit. Because the G protein β and γ subunits are tightly bound together, the β subunit always cotranslocates with a translocating γ subunit (1). We introduced YFP-β1 into a variety of mammalian cells lines and screened them for the cotranslocation of YFP-β1 in response to the activation of an introduced M3 receptor. We identified a cell line, A549 (human alveolar epithelial cells), where YFP-β1 translocated on receptor activation and colocalized with the trans-Golgi marker GalT-DsRed (Fig. S3). We have shown using quantitative real time PCR that the predominant γ subunit type expressed in this cell line is γ11 (16), consistent with its presence in lung tissue (17).
βγ Complex Translocation Is a Requirement for Receptor-Induced Golgi Fragmentation.
We then examined the ability of the endogenous translocating γ subunit to induce Golgi fragmentation in A549 cells. The cells were transfected with M3, αq and YFP-β1 was introduced to detect translocation. To quantitate fragmentation, GalT-DsRed containing vesicles in individual cells were counted (Fig. 2 and Table 1). Cells with less than 20 vesicles were considered to contain compact Golgi and cells with more than 40 vesicles were classified as fragmented. There was no significant variation in the percentage of cells with 20–40 vesicles across experiments. In the basal state, the trans-Golgi was mostly compact (Fig. 2 and Table 1). Activation of the M3 receptor led to fragmentation of the trans-Golgi. Fragmentation was not the result of GalT expression because it was observed by immunofluorescence using TGN46 antibodies. This observation clearly demonstrated that activation of a GPCR can lead to Golgi fragmentation in a cell as long as the cell contains a translocation proficient γ subunit. The result also showed that Golgi fragmentation was not an aberrant outcome of γ subunit overexpression in cells.
Fig. 2.
Fragmentation induced by the translocation of an endogenous γ subunit. (A) Trans-Golgi fragmentation induced by M3 receptor activation in A549 cells. (Left) Basal state and (Right) receptor activated state. Images on the Left are of YFP tagged G protein subunit. Images on Right are of GalT-DsRed in the same cell. (B) Bar diagram representing the percentage of cells (± SEM) that contained the vesicle numbers shown above the plots. Total cells analyzed range from 250 to 800 from 5 to 10 independent experiments.
Table 1.
Regulation of Golgi fragmentation by GPCR (muscarinic M3 receptor) activation through translocation of G protein βγ subunit in A549 cells
| cDNA | Status | Other treatment | Cells* | % cells with >40 vesicles | V/C ratio† | Fragment‡ |
| β1 | Basal | − | 222 | 12.9 ± 4.22 | 0.18 | − |
| β1 | Activated | − | 156 | 56.5 ± 5.85 | 2.51 | +++ |
| γ3 | Basal | − | 384 | 17.3 ± 1.89 | 0.27 | − |
| γ3 | Activated | − | 262 | 13.9 ± 2.55 | 0.21 | − |
| γ4 | Basal | − | 288 | 12.5 ± 1.93 | 0.17 | − |
| γ4 | Activated | − | 328 | 17.3 ± 1.07 | 0.26 | − |
| γ11 | Basal | − | 877 | 10.3 ± 1.26 | 0.14 | − |
| γ11 | Activated | − | 653 | 50.2 ± 3.00 | 2.07 | +++ |
| γ3 mutant | Basal | − | 134 | 15.6 ± 1.82 | 0.25 | − |
| γ3 mutant | Activated | − | 196 | 62.8 ± 2.69 | 3.32 | +++ |
| γ11 | Activated | Acetylcholine§ | 341 | 47.0 ± 1.52 | 1.65 | ++ |
| γ11 | Inactivated | Carbachol removed¶ | 170 | 37.0 ± 0.43 | 0.84 | ± |
| γ11 | Activated║ | − | 294 | 53.7 ± 1.66 | 2.55 | +++ |
| γ11+PKD1-KD** | Activated | − | 607 | 18.6 ± 1.45 | 0.29 | − |
| γ11+PKD1†† | Activated | − | 354 | 30.6 ± 4.28 | 0.65 | ± |
| γ11 | Activated | U73122‡‡ | 377 | 15.6 ± 1.32 | 0.22 | − |
| γ11 | Activated | U73343§§ | 173 | 53.9 ± 4.82 | 2.10 | +++ |
| β1 | Basal | γ11-shRNA | 177 | 20.8 ± 1.55 | 0.36 | − |
| β1 | Activated | γ11-shRNA | 167 | 24.6 ± 2.43 | 0.43 | − |
| β1 | Basal | Control shRNA | 100 | 22.7 ± 5.78 | 0.32 | − |
| β1 | Activated | Control shRNA | 133 | 56.4 ± 5.64 | 1.97 | ++ |
−, < 20% cells or V/C ratio < 0.5; ±, 20–40% cells or V/C ratio 0.5–1.0; ++, 40–50% cells or V/C ratio 1.0–2.0; +++, > 50% cells or V/C ratio > 2.0.
*Total cells from at least 3 independent experiments. Cells expressing YFP tagged G protein subunits were scored for GalT distribution. All cells expressed M3 receptor and αq.
†V/C ratio is ratio of percentage cells with > 40 vesicles over percentage of cells with < 20 vesicles.
‡Based on percentage of cells with > 40 vesicles or V/C ratio.
§Natural ligand for muscarinic receptor (carbachol is derivative for the same).
¶Incubated without carbachol for 12–16 h after treatment with carbachol.
║Control set for the experiments with PKD and PLCβ experiments.
**Kinase dead mutant of PKD1 previously shown to inhibit secretion.
††Overexpression of PKD1 can lead to sequestration of βγ with a proportion of the complex incapable of interaction with downstream partners due to high concentrations resulting in a decrease in fragmentation compared with a control that is not expressing PKD.
‡‡PLCβ inhibitor.
§§Inactive analog of U73122 (PLCβ inhibitor).
To ensure that fragmentation was not the result of introducing multiple constructs, we expressed a M3-αq fusion in which CFP-αq was tethered to the C terminus of M3 (16) in A549 cells and observed that the Golgi fragmented after M3 activation (Fig. S4).
To confirm the role of translocation in Golgi fragmentation, we expressed the nontranslocating γ3 subunit in A549 cells. γ3 was expected to sequester endogenous β subunit and inhibit translocation, thus acting in a dominant negative manner. When A549 cells were transfected with γ3 and YFP-β1, translocation of YFP-β1 was not detectable on receptor activation demonstrating that the introduced γ3 acted as a dominant negative. Consistent with this dominant negative property, receptor activation had no effect on Golgi structure in A549 cells expressing γ3 (Fig. 2 and Table 1). To ensure that this effect was not peculiar to γ3, the effect of another nontranslocating γ subunit, γ4, was examined. γ4 also inhibited fragmentation induced by M3 activation (Table 1). βγ translocation is thus a prerequisite for Golgi fragmentation.
Fragmentation is not dependent on a particular agonist because acetylcholine had the same effect on the Golgi in these cells (Fig. S5A). Fragmentation also requires the receptor because in the absence of M3 the agonist had no effect.
A gain of function mutant of γ3 provided additional support for βγ translocation playing a role in Golgi fragmentation. We mutated five residues at the C terminus of γ3, enabling it to translocate on receptor activation to the Golgi (Fig. S6). The mutant γ3 subunit also induced trans-Golgi fragmentation in A549 cells, confirming that fragmentation is a result of βγ translocation and is not dependent on the type of γ subunit (Fig. S6).
Knockdown of Endogenous γ11 Subunit Using shRNA Prevents Receptor-Activation–Dependent Golgi Fragmentation.
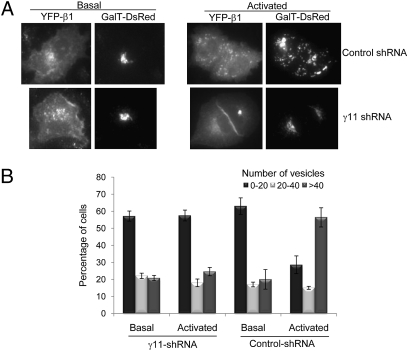
To further confirm the involvement of γ11 in the Golgi fragmentation process, we suppressed the expression of γ11 RNA in A549 cells using a specific shRNA. We screened shRNAs specific to γ11 from the Broad Institute TRC shRNA library (18) and identified an shRNA that was effective at knocking down γ11 RNA level by 70–80% as detected by quantitative real time PCR expression analysis in A549 cells. In the receptor-activation–dependent Golgi fragmentation assay, A549 cells expressing γ11 specific shRNA had significantly lower fragmentation compared with cells expressing a nontarget shRNA control (Fig. 3). Receptor-activation–dependent Golgi fragmentation thus requires the presence of γ11.
Fig. 3.
Inhibition of receptor-induced Golgi fragmentation by γ11 shRNA. A549 cells expressing M3 receptor, αq, YFP-β1, GalT-DsRed, and γ11-shRNA or nontarget control shRNA as indicated were used. Cells were stimulated with 100 μM carbachol for 2.5 h. Cells were imaged as described in Material and Methods. (A) Images of cells show presence of compact or fragmented Golgi. (Left) Basal state and (Right) receptor activated state. Images on Left are of YFP tagged G protein subunit. Images on Right are of GalT-DsRed in the same cell. (B) Bar diagram represents the percentage of cells that contained the vesicle numbers shown above the plots. Total cells analyzed range from 100 to 200 from three to five independent experiments.
M3-Induced Golgi Fragmentation Is Reversible.
An important property of GPCR signaling is that it can be switched on and off in a cell allowing for the modulation of physiological effects. Because βγ translocation is reversible on receptor deactivation (1, 2, 19), we examined whether the Golgi fragmentation seen here is similarly reversible. A549 cells expressing M3, αq, and γ11 were first exposed to the agonist and then the agonist was washed away and fresh medium was added. Cells were imaged before agonist addition, after agonist addition and after agonist removal and cultured for 16–18 h in the absence of agonist. The number of cells containing compact Golgi increased significantly after 16–18 h of removal of the agonist (Fig. S5B and Table 1). This suggests that the action of the translocating βγ subunits does not lead to the irreversible breakdown of the trans-Golgi and is reversible.
Endogenous GPCRs Are Capable of Inducing Golgi Fragmentation.
To ascertain that the Golgi fragmentation observed here is not due to overexpression of a GPCR or peculiar to M3 receptors, we examined the effect of an endogenous receptor on Golgi structure. Using a Fluo-4–based calcium release assay we identified histamine receptors in HeLa cells (Fig. S7A). CFP-γ11 was introduced and cells were stimulated with 100 μM histamine. Cells were fixed and stained for the distribution of TGN46 after 3 h of stimulation by immunofluorescence. When compared with basal cells where TGN46 is present in a distinct compact region (Fig. S7B), the agonist-stimulated cells showed clear redistribution of TGN46 marker (Fig. S7 B and C) similar to that observed in cells in which an introduced M3 receptor was activated (Fig. 1A). These results showed that an endogenous receptor can induce Golgi fragmentation. It also showed that the fragmentation observed in earlier experiments is not due to M3 receptor overexpression.
Golgi Fragmentation Is Independent of Microtubule Reorganization.
Microtubule depolymerization leads to Golgi disassembly (20). To examine the involvement of microtubules in the βγ-mediated fragmentation, we coexpressed CFP-tubulin in A549 cells with M3 receptor and YFP-γ11. In the basal state, where the Golgi was compact, normal tubulin distribution was observed (Fig. S8A). We observed a distinct clearing of CFP-tubulin from the nuclear region when depolymerization of microtubules occurs in response to nocodazole treatment (Fig. S8B) (21, 22). Microtubule depolymerization, was accompanied by GalT-DsRed redistribution indicating Golgi fragmentation (Fig. S8B). We could thus detect nocodazole-induced microtubule depolymerization and Golgi fragmentation in these live A549 cells. When the cells were treated with carbachol, Golgi fragmentation was observed, but no detectable changes were seen in the distribution of CFP-tubulin (Fig. S8C). This result showed that M3-receptor–induced Golgi fragmentation is not mediated by microtubule depolymerization.
M3 Induced Golgi Fragmentation Through Translocating G Protein βγ Complexes Is Mediated by Protein Kinase D and Phospholipase C β.
Protein kinase D is a serine/threonine kinase that has been shown to be localized to the Golgi complex and to mediate fragmentation that leads to the formation of cargo laden vesicles destined for transport to the plasma membrane (6, 23). The G protein βγ complex has been shown to activate PKD1 (24). We examined whether the Golgi fragmentation detected here is mediated by PKD.
A PKD1 mutant (K618N) that is kinase defective inhibits protein transport from the trans-Golgi network to the cell surface (25). We examined the effect of this kinase defective PKD1 on M3-induced Golgi breakdown in A549 cells. Kinase defective PKD1 strongly inhibited fragmentation induced by M3 activation (Fig. 4 A and C and Table 1). The wild-type PKD also had a weaker inhibitory effect (Table 1), likely due to the higher level of overexpressed PKD-βγ11 complex being unable to access limiting levels of downstream molecules. In control experiments, the kinase defective PKD had no effect on receptor-induced βγ11 translocation (Fig. S9A) and did not affect M3 activation of a downstream effector, PLCβ (Fig. S9B). The effect of the kinase defective PKD is not therefore due to the inhibition of βγ translocation or M3 receptor activity. The effect of the kinase defective PKD also suggests that the translocated βγ complex regulates the fission of secretory vesicles that traffic from the trans-Golgi network to the plasma membrane. It does not affect the formation of vesicles that traffic from the trans-Golgi to endosomes because it has been shown previously that PKD is involved only in vesicle trafficking to the plasma membrane (25).
Fig. 4.
Effect of a kinase defective PKD and a PLCβ inhibitor on Golgi fragmentation in A549 cells. (Left) YFP-γ11 subunit and (Right) GalT-DsRed in the same cell. (A) Effect of a kinase dead PKD1 mutant after receptor activation in the presence of γ11. Images were captured after M3 receptor activation. (B) Effect of U73122 (PLCβ) inhibitor and its inactive analog U73343 after receptor activation in the presence of γ11. (C) Bar diagrams depicting the effect of receptor activation in the presence of various inhibitors. The cells expressing the indicated G protein subunits tagged with YFP were scored for the expression pattern of GalT-DsRed as described in Fig. 2C. Total cells analyzed range from 250 to 500 in from three to six independent experiments. A549 cells expressing M3 receptor, αq, YFP-γ11 were used. The cells were activated with carbachol for 2.5 h (U73122, PLCβ inhibitor; U73343, inactive analog for PLCβ inhibitor).
Evidence for the ability of G protein βγ complexes to activate phospholipase C β and the regulation of PKD by diacylglycerol (DAG), which is the product of phospholipase C β has lead to the proposal that PKD regulation by G protein βγ complexes in the Golgi fragmentation process is routed through PLCβ (6). Recent evidence demonstrating the ability of a PLCβ inhibitor, U73122 to curtail Golgi fragmentation by the βγ complex in permeabilized cells supports this suggestion (26). We examined here whether the Golgi fragmentation induced by extracellular signals through βγ translocation is affected by the same PLCβ inhibitor; U73122. A549 cells expressing γ11 were treated with U73122 for 30 min before the activation of M3. When these cells were activated with carbachol, we did not see an increase in fragmentation of the Golgi compared with untreated cells (Fig. 4 B and C and Table 1). This result is consistent with a role for βγ translocation in PLCβ–dependent secretory vesicle formation. When an innocuous analog of PLCβ inhibitor, U73343 (27), was used in place of U73122, in experiments identical to those above, fragmentation of the Golgi was observed after agonist treatment (Fig. 4 B and C and Table 1). This result showed that the effect of U73122 is specific.
M3-Receptor–Induced Increase in Insulin Secretion Is Mediated by Translocating βγ.
Acetycholine stimulation of the M3 receptor increases the secretion of insulin from pancreatic β cells and plays a prominent role in glucose homeostasis. Although the up-regulation of insulin secretion by the M3 receptor is known to involve Ca2+ release and protein kinase C activation, the precise mechanisms that mediate the secretion of insulin have been unclear. We examined here the possibility that a mechanism at the basis of M3 stimulation of insulin secretion is the translocation of the βγ complex to the Golgi and resultant vesicle formation. First, we tested the response of the mouse pancreatic β cell line, NIT-1, to M3 receptor stimulation with carbachol. A significant induction of insulin secretion was observed on M3 activation (Fig. S10A). Next, we examined the expression status of G protein γ subunit types in these cells using real time PCR. The results showed that NIT-1 cells express three translocation proficient γ subunits, γ5, γ10, and γ13 (Fig. S10B). Unlike in the case of A549 cells, γ11 was expressed at a very low level. This expression profile suggested that multiple γ subunits would have to be knocked down to examine the effect of the translocation proficient γ subunit types in NIT-1 cells. We therefore chose to introduce the nontranslocating γ3 subunit into NIT-1 cells to help sequester endogenous β and act in a dominant negative manner as in the case of A549 cells where γ3 inhibits receptor translocation of β1 and Golgi fragmentation (Fig. 2B). When agonist activated insulin secretion was examined in these γ3 transfected NIT-1 cells in comparison with mock transfected cells, muscarinic-receptor–induced insulin secretion was significantly lowered (Fig. 5A). To ensure that this inhibition of insulin secretion in the presence of γ3 did not occur because γ3 inhibited signaling from the M3 receptor in some nonspecific manner, we examined Ca2+ release from γ3 transfected NIT-1 cells and control cells in response to different concentrations of the agonist, carbachol. We found no reduction in Ca2+ release response in γ3 cells compared with control. These results clearly show that reduction in translocation proficient βγ leads to a reduction in receptor-stimulated secretion. It also suggests strongly that the receptor-induced Golgi fragmentation induced by βγ translocation is at the basis of secretion.
Fig. 5.
Regulation of secretion by receptor-mediated translocation of the G protein βγ complex. (A) Muscarinic receptor stimulation of insulin secretion in NIT-1 cells is inhibited by a dominant negative nontranslocating γ subunit. NIT-1 pancreatic β cells were mock transfected (control) or transfected with the nontranslocating γ3 subunit (γ3). Insulin secreted by NIT-1 cells with and without receptor activation was assayed as described in Materials and Methods. The fold induction in insulin secreted from various cells was calculated with respect to basal levels from control cells (n > 6); **P ≤ 0.001. (B) Effect of dominant negative γ3 on trans-Golgi fragmentation in NIT-1 cells. NIT-1 cells were transiently transfected with YFP alone or YFP-γ3 and GalT-DsRed for 24 h. The cells were then imaged using Andor Revolution–Leica spinning disk confocal microscope system. The 3D images of the trans-Golgi were obtained by Z stacking GalT-DsRed expressing cells using Andor IQ software. (Left) Confocal image of cells expressing YFP, (Right) collapsed Z-stack image of the GalT-DsRed expression from the same cell. The total number of cells showing either compact or fragmented Golgi were counted and are depicted in the bar diagram, n = 3 (>50 cells each experiment). (C) Model for regulation of Golgi structure and secretion by an extracellular signal. GPCR stimulation by an agonist leads to the translocation of the βγ complex to the Golgi from the plasma membrane where it mediates secretory vesicle traffic from the trans-Golgi network to the plasma membrane (arrow).
We then examined the structure of the trans-Golgi in NIT1 cells. Even in the basal state, the cells showed extensive fragmentation of the trans-Golgi when visualized with GalT-DsRed as a marker (Fig. 5B Top). This basal level fragmentation precluded an assay to examine carbachol-stimulated fragmentation. When the dominant negative nontranslocating γ3 subunit was introduced into these cells, a significantly compact trans-Golgi structure was observed (Fig. 5B Lower) in most cells, consistent with the effect of γ3 on fragmentation induced by the M3 receptor activation in A549 cells (Fig. 2A). The effect of γ3 was not due to a generalized effect of G protein γ subunits because introduction of γ10 or γ11 did not reduce basal fragmentation in NIT1 cells. Basal level insulin secretion from these control and γ3 expressing NIT-1 cells was similar, suggesting that trans-Golgi fragmentation is essential for muscarinic-receptor–stimulated insulin secretion but not basal level secretion. The inhibition of receptor-stimulated insulin secretion and basal fragmentation by γ3 suggests that the basal level Golgi fragmentation facilitates rapid induction of insulin secretion on receptor activation.
Overall, these results show that extracellular signals can regulate the structure and function of the Golgi. Although there has also been long standing evidence for G protein subunits being associated with the Golgi complex (4, 5) and the introduction of the G protein βγ complex into cells inducing secretory vesicle formation in the Golgi (6), the signal that regulates fragmentation and the molecular mechanism that allows GPCRs and G protein subunits to reach the Golgi to act on it have not been identified. The results here show that an extracellular signal can regulate Golgi structure and function by translocating the G protein βγ complex from the plasma membrane to the Golgi (Fig. 5C). Consistent with a role for the GPCR-induced fragmentation changes in secretion observed here, Golgi fragmentation has been observed in secretory pancreatic acinar cells activated with a GPCR agonist, cholecystokinin (28). Our results are also consistent with the ablation of p38Δ enzyme in pancreatic β cells, which leads to overactivation of PKD, increased insulin secretion, and fragmentation of the entire Golgi complex (8). The receptor-induced translocation of the G protein βγ complex may thus serve as an unexpected mechanism at the basis of the regulation of secretion.
The direct involvement of translocation proficient γ subunits in the Golgi fragmentation and secretory process as reported here also provides clues for the function of the large family of G protein subunits whose structures are highly conserved across mammalian species (29, 30). The ability of G protein subunits to directly translocate and regulate Golgi structure and its function on receptor activation may provide a more specific mode of regulation in GPCR signaling in contrast to action through classical small molecule second messengers such as IP3 and cAMP released at the plasma membrane.
Materials and Methods
Cell Imaging and Immunofluorescence.
Constructs and cell lines are described in SI Materials and Methods. Live cell imaging was performed essentially as described (1). M3 muscaranic receptor was activated with 100 μM carbachol for 2.5 h at 37 °C before imaging to analyze the effect of receptor activation. U73122 (Sigma) was dissolved in DMSO and was added to a final concentration of 10 μM and incubated for 30 min at 37 °C before carbachol addition to activate the M3 receptor. Images were captured using a Nikon Eclipse TE2000-U inverted epifluorescence microscope using a 60× Plan Apo oil immersion objective (1.4 N.A.) unless otherwise mentioned. Confocal images were acquired using an Andor Revolution-Leica spinning disk confocal microscope. Basal and agonist-treated cells were probed with the TGN46 specific antibody (Sigma). Additional details of live cell imaging, immunofluorescence, and Ca2+ release assay are in SI Materials and Methods.
shRNA Knockdown and Quantitative Real-Time PCR Analysis.
To screen for efficacy, plasmids encoding the shRNAs were transfected into A549 cells, RNA was isolated after 48 h and real time PCR was performed to evaluate reduction in gene expression levels (details in SI Materials and Methods).
Insulin Secretion Assay.
Twenty-four hours after transfection, NIT-1 cells were washed with HBSS containing Ca2+/Mg2+ and 3 mM glucose, and 100 μM carbachol was added to activate the M3 receptor. Cells were incubated for 3 h after which the medium was assayed for insulin by ELISA. Additional details are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank T. Mohanakumar (Washington University School of Medicine, St. Louis, MO) for access to insulin assays and M. Gautam and S. Kornfeld for valuable discussions. This work was supported by National Institutes of Health Grants GM69027 and GM080558 (to N.G.) and an American Heart Association postdoctoral fellowship (to D.K.S.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1003042107/-/DCSupplemental.
References
- 1.Saini DK, Kalyanaraman V, Chisari M, Gautam N. A family of G protein betagamma subunits translocate reversibly from the plasma membrane to endomembranes on receptor activation. J Biol Chem. 2007;282:24099–24108. doi: 10.1074/jbc.M701191200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akgoz M, Kalyanaraman V, Gautam N. Receptor-mediated reversible translocation of the G protein betagamma complex from the plasma membrane to the Golgi complex. J Biol Chem. 2004;279:51541–51544. doi: 10.1074/jbc.M410639200. [DOI] [PubMed] [Google Scholar]
- 3.Saini DK, Chisari M, Gautam N. Shuttling and translocation of heterotrimeric G proteins and Ras. Trends Pharmacol Sci. 2009;30:278–286. doi: 10.1016/j.tips.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson BS, Komuro M, Farquhar MG. Cellular variations in heterotrimeric G protein localization and expression in rat pituitary. Endocrinology. 1994;134:233–244. doi: 10.1210/endo.134.1.8275939. [DOI] [PubMed] [Google Scholar]
- 5.Gleeson PA, Lock JG, Luke MR, Stow JL. Domains of the TGN: Coats, tethers and G proteins. Traffic. 2004;5:315–326. doi: 10.1111/j.1398-9219.2004.00182.x. [DOI] [PubMed] [Google Scholar]
- 6.Bard F, Malhotra V. The formation of TGN-to-plasma-membrane transport carriers. Annu Rev Cell Dev Biol. 2006;22:439–455. doi: 10.1146/annurev.cellbio.21.012704.133126. [DOI] [PubMed] [Google Scholar]
- 7.Gautam D, et al. Metabolic roles of the M3 muscarinic acetylcholine receptor studied with M3 receptor mutant mice: A review. J Recept Signal Transduct Res. 2008;28:93–108. doi: 10.1080/10799890801942002. [DOI] [PubMed] [Google Scholar]
- 8.Sumara G, et al. Regulation of PKD by the MAPK p38delta in insulin secretion and glucose homeostasis. Cell. 2009;136:235–248. doi: 10.1016/j.cell.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bossard C, Bresson D, Polishchuk RS, Malhotra V. Dimeric PKD regulates membrane fission to form transport carriers at the TGN. J Cell Biol. 2007;179:1123–1131. doi: 10.1083/jcb.200703166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prescott AR, Lucocq JM, James J, Lister JM, Ponnambalam S. Distinct compartmentalization of TGN46 and beta 1,4-galactosyltransferase in HeLa cells. Eur J Cell Biol. 1997;72:238–246. [PubMed] [Google Scholar]
- 11.Schaub BE, Berger B, Berger EG, Rohrer J. Transition of galactosyltransferase 1 from trans-Golgi cisterna to the trans-Golgi network is signal mediated. Mol Biol Cell. 2006;17:5153–5162. doi: 10.1091/mbc.E06-08-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura N, et al. Characterization of a cis-Golgi matrix protein, GM130. J Cell Biol. 1995;131:1715–1726. doi: 10.1083/jcb.131.6.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azpiazu I, Gautam N. A fluorescence resonance energy transfer-based sensor indicates that receptor access to a G protein is unrestricted in a living mammalian cell. J Biol Chem. 2004;279:27709–27718. doi: 10.1074/jbc.M403712200. [DOI] [PubMed] [Google Scholar]
- 14.Rebois RV, et al. Heterotrimeric G proteins form stable complexes with adenylyl cyclase and Kir3.1 channels in living cells. J Cell Sci. 2006;119:2807–2818. doi: 10.1242/jcs.03021. [DOI] [PubMed] [Google Scholar]
- 15.Chisari M, Saini DK, Kalyanaraman V, Gautam N. Shuttling of G protein subunits between the plasma membrane and intracellular membranes. J Biol Chem. 2007;282:24092–24098. doi: 10.1074/jbc.M704246200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chisari M, Saini DK, Cho JH, Kalyanaraman V, Gautam N. G protein subunit dissociation and translocation regulate cellular response to receptor stimulation. PLoS ONE. 2009;4:e7797. doi: 10.1371/journal.pone.0007797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morishita R, Ueda H, Kato K, Asano T. Identification of two forms of the gamma subunit of G protein, gamma10 and gamma11, in bovine lung and their tissue distribution in the rat. FEBS Lett. 1998;428:85–88. doi: 10.1016/s0014-5793(98)00498-0. [DOI] [PubMed] [Google Scholar]
- 18.Moffat J, et al. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell. 2006;124:1283–1298. doi: 10.1016/j.cell.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 19.Azpiazu I, Akgoz M, Kalyanaraman V, Gautam N. G protein betagamma11 complex translocation is induced by Gi, Gq and Gs coupling receptors and is regulated by the alpha subunit type. Cell Signal. 2006;18:1190–1200. doi: 10.1016/j.cellsig.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang W, Storrie B. Scattered Golgi elements during microtubule disruption are initially enriched in trans-Golgi proteins. Mol Biol Cell. 1998;9:191–207. doi: 10.1091/mbc.9.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang P, Stearns T. Delta-tubulin and epsilon-tubulin: Two new human centrosomal tubulins reveal new aspects of centrosome structure and function. Nat Cell Biol. 2000;2:30–35. doi: 10.1038/71350. [DOI] [PubMed] [Google Scholar]
- 22.Fry AM, Meraldi P, Nigg EA. A centrosomal function for the human Nek2 protein kinase, a member of the NIMA family of cell cycle regulators. EMBO J. 1998;17:470–481. doi: 10.1093/emboj/17.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prestle J, Pfizenmaier K, Brenner J, Johannes FJ. Protein kinase C mu is located at the Golgi compartment. J Cell Biol. 1996;134:1401–1410. doi: 10.1083/jcb.134.6.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jamora C, et al. Gbetagamma-mediated regulation of Golgi organization is through the direct activation of protein kinase D. Cell. 1999;98:59–68. doi: 10.1016/S0092-8674(00)80606-6. [DOI] [PubMed] [Google Scholar]
- 25.Liljedahl M, et al. Protein kinase D regulates the fission of cell surface destined transport carriers from the trans-Golgi network. Cell. 2001;104:409–420. doi: 10.1016/s0092-8674(01)00228-8. [DOI] [PubMed] [Google Scholar]
- 26.Díaz Añel AM. Phospholipase C beta3 is a key component in the Gbetagamma/PKCeta/PKD-mediated regulation of trans-Golgi network to plasma membrane transport. Biochem J. 2007;406:157–165. doi: 10.1042/BJ20070359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin W, Lo TM, Loh HH, Thayer SA. U73122 inhibits phospholipase C-dependent calcium mobilization in neuronal cells. Brain Res. 1994;642:237–243. doi: 10.1016/0006-8993(94)90927-x. [DOI] [PubMed] [Google Scholar]
- 28.Dahan S, Anderson KL, Weller S, Krueger E, McNiven MA. Agonist-induced vesiculation of the Golgi apparatus in pancreatic acinar cells. Gastroenterology. 2005;129:2032–2046. doi: 10.1053/j.gastro.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Downes GB, Gautam N. The G protein subunit gene families. Genomics. 1999;62:544–552. doi: 10.1006/geno.1999.5992. [DOI] [PubMed] [Google Scholar]
- 30.Yang W, Hildebrandt JD. Genomic analysis of G protein gamma subunits in human and mouse - the relationship between conserved gene structure and G protein betagamma dimer formation. Cell Signal. 2006;18:194–201. doi: 10.1016/j.cellsig.2005.04.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.