Abstract
After lactation, weaning causes mammary epithelial cell (MEC) apoptosis. MECs express the plasma membrane calcium-ATPase 2 (PMCA2), which transports calcium across the apical surface of the cells into milk. Here we show that PMCA2 is down-regulated early in mammary involution associated with changes in MEC shape. We demonstrate that loss of PMCA2 expression raises intracellular calcium levels and sensitizes MECs to apoptosis. In contrast, overexpression of PMCA2 in T47D breast cancer cells lowers intracellular calcium and protects them from apoptosis. Finally, we show that high PMCA2 expression in breast cancers is associated with poor outcome. We conclude that loss of PMCA2 expression at weaning triggers apoptosis by causing cellular calcium crisis. PMCA2 overexpression, on the other hand, may play a role in breast cancer progression by conferring resistance to apoptosis.
Keywords: intracellular calcium
The mammary gland undergoes a dramatic cycle of development and regression during reproduction (1). The virgin gland consists of a simple system of epithelial ducts. During pregnancy, mammary epithelial cells (MECs) proliferate and form a large number of alveoli. Parturition induces full secretory differentiation and milk production. Upon weaning, the secretory cells are eliminated and the gland is remodeled to a duct system that resembles the mature nulliparous state (1). This process of mammary involution involves two sequential rounds of apoptosis. The first occurs between 12 and 24 h after weaning and causes the loss of many, but not all, secretory epithelial cells (2, 3). This phase of involution is reversible and lactation can continue if suckling is resumed within 48 h (2, 3). However, if the gland remains unemptied for longer periods of time, a second round of apoptosis associated with irreversible tissue remodeling ensues. The initial wave of apoptosis is the result of local factors associated with the accumulation of milk within alveoli (milk stasis) (2), whereas the remodeling of the mammary gland is due to the withdrawal of prolactin (2). How milk stasis causes MEC apoptosis remains unclear. Two explanations have traditionally been offered. First, it has been suggested that, in the absence of suckling, soluble factors such as TGF-β (4), serotonin (5) and LIF (6), accumulate and induce apoptosis. Second, it has been hypothesized that mechanical deformation of the MECs, caused by distension of the alveoli, triggers apoptosis (7).
Milk production requires the transport of large amounts of calcium through the epithelial cells (8). This presents challenges for cellular calcium homeostasis. Intracellular calcium levels must be maintained at low levels because acute increases in cytoplasmic calcium concentrations are involved in signal transduction (9), and persistent elevations in intracellular calcium can cause cellular dysfunction and death (10–13). In fact, many apoptotic pathways are either triggered by, or result in intracellular calcium toxicity (10–13). The plasma membrane calcium-ATPase 2 (PMCA2) plays an important role in the transport of calcium into milk (8, 14, 15). PMCA2 mRNA levels increase several hundred-fold upon functional differentiation of MECs during lactation (14–16), and this pump is responsible for the transport of 60–70% of milk calcium (14, 15). Interestingly, mammary gland PMCA2 RNA and protein levels fall dramatically upon weaning (15, 17), leading several investigators to hypothesize that the resulting disruption of calcium extrusion might lead to an accumulation of intracellular calcium and contribute to apoptosis during mammary gland involution (17, 18). In this report, we provide evidence demonstrating that milk stasis changes the shape of MECs and results in loss of PMCA2 expression. This, in turn, disrupts intracellular calcium homeostasis and contributes to the initiation of apoptosis at the onset of involution. We also report that PMCA2 can protect breast cancer cells from apoptosis and that PMCA2 expression in breast tumors correlates with clinical outcome.
Results
Milk Stasis Reduces PMCA2 Expression.
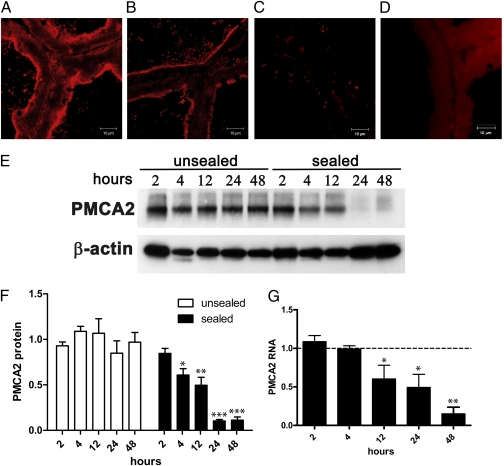
We first examined PMCA2 expression in response to milk stasis. For these studies, we sealed one teat of a lactating mouse with surgical adhesive. This causes the accumulation of milk in only that gland and triggers MEC apoptosis within 24 h (19). No apoptosis occurs in the nonsealed glands and there are no changes in systemic hormone levels (2). We measured PMCA2 protein and RNA levels in the sealed and contralateral, unsealed mammary glands by immunofluorescence staining, Western blotting, and quantitative RT-PCR (qRT-PCR) (Fig. 1). PMCA2 protein levels significantly declined as soon as 4 h after induction of milk stasis (Fig. 1 E and F), well before the onset of apoptosis in this model (2) (Fig. S1). mRNA levels fell significantly by 12 h (Fig. 1G). PMCA2 protein and mRNA levels reached their nadir by 24–48 h (Fig. 1 E–G).
Fig. 1.
PMCA2 is rapidly down-regulated by milk stasis. Mammary glands were harvested at 2, 4, 12, 24, and 48 h after teat-sealing to induce milk stasis. The unsealed, contralateral gland served as a control. Compared with the control gland (A), immunofluorescent staining for PMCA2 was less intense at 4 h (B), and nearly undetectable at 48 h (C). (D) Shows staining with 5 μg/mL rabbit IgG (Vector Labs) as a negative control. Western blotting with 15 μg of membrane protein per gland (E) revealed decreasing PMCA2 protein with time in the sealed glands, whereas PMCA2 in the unsealed glands did not decrease. Quantitation of Western blots (F) showed that PMCA2 protein levels significantly decreased by 4 h and continued to decrease to a minimum by 24 h. In unsealed glands, PMCA2 levels did not significantly differ between time points. PMCA2 protein levels were normalized to β-actin protein expression and all time-points in the unsealed glands were averaged together to establish a baseline. The mean PMCA2 levels at each individual time point were then represented as the fraction of baseline levels. In the sealed glands, PMCA2 levels at increasing times after sealing (F) are significantly different by one-way ANOVA (P < 0.0001) and the posttest for linear trend was significant at P = 0.0001, r2 = 0.8213. Likewise, PMCA2 RNA levels, as measured by qRT-PCR were normalized to Gapd expression and all data points from the unsealed glands were averaged to establish a baseline. Shown in (G) are PMCA2 mRNA levels from the sealed glands represented as the fraction of baseline expression. PMCA2 gene expression differed significantly with time (P = 0.0018) with a significant linear trend between time after sealing and PMCA2 expression (P = 0.0001, r2 = 0.7663). Bars show the mean ± SEM for each group. In (F), at 2, 12, and 24 h, n = 4; at 4 h n = 6; and at 48 h, n = 5. In (G) n = 3 at all time points. Significant differences from the 2 h time point, by the Newman-Keuls posttest, are denoted by * P < 0.05, **P < 0.01, and ***P < 0.001.
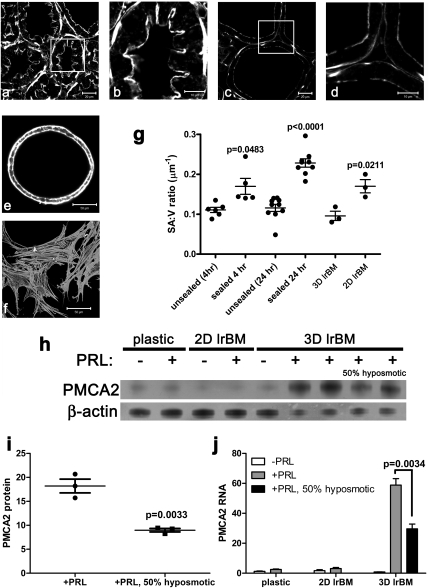
The accumulation of milk within the alveoli of the sealed gland was associated with obvious changes in cell shape, highlighted in Fig. 2 A–D by phalloidin staining of the actin cytoskeleton. During lactation (Fig. 2 A and B), epithelial cells are columnar in shape, demonstrate prominent infolding of their apical surfaces at cell junctions, and possess a thick network of apical subcortical actin. After teat sealing, the cells became flattened, the infolding between cells disappeared, and the apical actin network was thinner (Fig. 2 C and D). This “stretching” of the epithelial cells was reflected by a progressive increase in their surface area to volume ratio (SA:V) (Fig. 2G). Given the association between cell shape and PMCA2 levels in vivo, we next examined the relationship between PMCA2 expression and cell shape in MECs in vitro. MECs grown in 3D culture on a laminin-rich reconstituted basement membrane (lrBM) organize into spheres of secretory epithelial cells known as mammospheres, whereas MECs grown on plastic or at a high density on a thin layer of lrBM form flattened monolayers. Both integrin and prolactin signaling contribute to the differentiation of MECs in vivo and on lrBM (19, 20). However, as seen in Fig. 2 H and J, PMCA2 RNA and protein expression were low in primary mammary epithelial cells grown in monolayer on plastic or on a thin layer of lrBM, regardless of the presence or absence of prolactin. In the absence of prolactin, the cells grown in 3D culture expressed somewhat more PMCA2, but in the presence of prolactin, MECs in 3D culture expressed high levels of PMCA2. Cells grown on thin lrBM in 2D had prominent stress fibers, very little cortical actin, and a flat morphology, with a high SA:V ratio. However, in 3D, they had prominent cortical actin and were more columnar with a lower SA:V ratio. These data suggest that cell shape and/or deformation interact with prolactin and integrin signaling to regulate PMCA2 expression. To determine if mechanical deformation of MECs could directly influence PMCA2 levels, we examined the effects of hyposmotic swelling (21). As shown in Fig. 2 H–J, exposure of MECs in 3D culture to hyposmotic media significantly reduced PMCA2 protein and mRNA levels. Thus, in three separate experimental models, the teat-sealing model of milk stasis, 3D vs. 2D culture of mammary epithelial cells, and hyposmotic swelling, PMCA2 levels were reduced by stretching of the MECs, suggesting that cell shape may regulate PMCA2 expression.
Fig. 2.
PMCA2 levels are associated with cell shape. Unsealed (A and B) and sealed (C and D) mammary glands, as well as 3D and 2D cultures of MMEC (E and F, respectively) were stained with Alexa-488-phalloidin to visualize microfilaments. Morphometric measurements (G) revealed that the surface area to volume ratio (SA:V) was significantly higher in sealed mammary glands and that the epithelial layer became thinner as milk accumulated over 24 h. Each point in G represents the measurement of the alveoli in one individual field-of-view. One-way ANOVA gave an overall P < 0.0001, and the Newman-Keuls multiple comparison posttest showed significant differences between unsealed and sealed glands (at 4 and 24 h) and between sealed glands at 4 vs. 24 h (unsealed 4 h n = 6; sealed 4 h, n = 5; unsealed 24 h, n = 10; sealed 24 h, n = 9). In addition, MMEC grown in 2D on lrBM were thinner than those grown in 3D (P = 0.0211, n = 3). The Student's t test P values are shown above the bars in graph g. Western blotting of 100 μg (lanes 1–5) or 50 μg (lanes 6–9) of total protein (H) and qRT-PCR (J) show that maximal PMCA2 expression in MMEC requires prolactin (PRL) and culture in 3D on lrBM. PMCA2 levels were reduced (P = 0.0033 for protein, P = 0.0034 for RNA) by mechanical deformation of 3D MMEC cultures with 50% hyposmotic treatment for 4 h (H–J). For protein, +PRL, n = 3; +PRL with 50% hyposmotic media, n = 3. For RNA, plastic –PRL, n = 7; plastic +PRL, n = 8; 2D lrBM −PRL, n = 8; 2D lrBM +PRL, n = 8; 3D lrBM −PRL, n = 3; 3D lrBM +PRL, n = 6; 3D lrBM +PRL with 50% hyposmotic media, n = 3. The mean ± SEM values are shown, with individual data points for G and I.
Lack of PMCA2 Precipitates MEC Apoptosis.
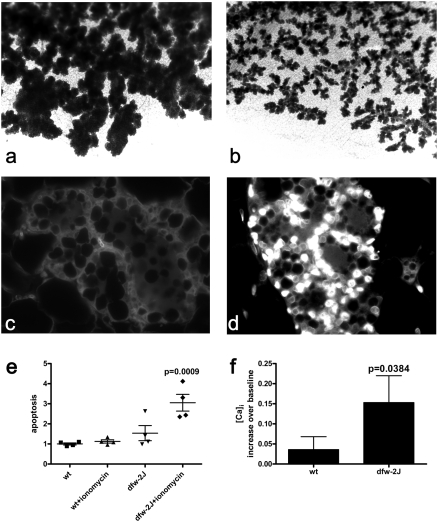
Next, we examined the effect of PMCA2 deficiency on mammary gland development in mice harboring the deafwaddler-2J (dfw-2J), null mutation in the Atp2b2 gene (encoding PMCA2) (22). Although PMCA2 is expressed at low levels by ductal epithelial cells, development of the mammary gland was intact until late pregnancy, when PMCA2 levels normally increase in both ductal and alveolar cells. As shown in Fig. 3 A and B, toward the end of pregnancy, the alveolar structures in dfw-2J glands appeared smaller than those in control littermates, a difference that persisted through lactation. Despite the reduction in the alveolar mass, dfw-2J mice lactated and supported their pups (15). Dfw-2J MECs proliferated and differentiated normally during pregnancy (Fig. S2), but TUNEL staining revealed widespread apoptosis at P18 (Fig. 3 C and D). In dfw-2J glands, 30.1 ± 10.0% of cells were TUNEL-positive, although only 2.6 ± 1.2% of cells in control glands were positive. Along with apoptosis, early involution is marked by characteristic changes in the epithelial expression of several transcription factors (23, 24). As shown in Fig. S3, these include the loss of nuclear phospho-Stat5a (25) and the induction of nuclear phospho-Stat3 (26) and NFκB (24). Similar to lactating glands, at P18, normal MECs expressed nuclear phospho-Stat5a but did not express nuclear phospho-Stat3 or NFκB. However, at P18, dfw-2J epithelial cells lost nuclear phospho-Stat5a and gained nuclear phsopho-Stat3 and NFκB, mimicking normal cells during involution. Thus, loss of PMCA2 led to premature changes in the expression of phosph-Stat5a, phospho-Stat3, and NFκB, accompanied by wide-spread MEC apoptosis, mimicking the initial phase of involution at the end of pregnancy.
Fig. 3.
Lack of PMCA2 causes apoptosis of MECs during pregnancy. Mammary glands were taken from homozygous dfw-2J mice, which lack PMCA2 expression, and WT mice on day 18 of pregnancy. Alveoli were smaller in mammary whole mounts prepared from dfw-2J mice (B) compared with WT mice (A). TUNEL staining of WT (C) and dfw-2J (D) mammary glands at P18 revealed many more apoptotic nuclei in dfw-2J glands than in WT glands. Primary MMEC were cultured on lrBM with lactogenic media and treated with 5 μM ionomycin (Invitrogen) or vehicle for 16 h before analysis of apoptosis using the Cell Death ELISAPLUS(Roche) (E). WT MMEC in 3D lrBM culture were relatively resistant to 5 μM ionomycin-induced intracellular calcium stress (E), although in dfw-2J MMEC, ionomycin induced apoptosis (P = 0.0009; n = 4). The increased apoptosis in dfw-2J MMEC treated with 5 μM ionomycin is associated with a higher intracellular calcium level when compared with WT MMEC (F) (P = 0.0384, WT n = 122 cells in two experiments, dfw-2J n = 51 cells in two experiments). Cells were imaged while perfused with standard media for approximately 2 min to determine the baseline calcium levels, followed by perfusion with media containing 5 μM ionomycin. Data are expressed as mean percent increase in fluorescence over baseline. Error bars represent the mean ± SEM.
The timing of MEC apoptosis in dfw-2J mice coincided with the functional differentiation of MECs (27), which is also the point at which PMCA2 expression normally increases (15). This is likely also the point at which calcium entry into MECs increases in preparation for the onset of milk production. Therefore, we hypothesized that the lack of PMCA2 might result in an inability to transport the increased calcium load, progressive intracellular calcium toxicity, and, ultimately, apoptosis. To test this hypothesis, we grew 3D cultures of primary mammary epithelial cells from dfw-2J or control mice and assessed their sensitivity to calcium-induced apoptosis. At baseline, apoptosis tended to be higher in dfw-2J cells than in WT cells, and calcium influx, induced by ionomycin treatment, produced a significant increase in apoptosis. In contrast, there was no increase in apoptosis in control cells exposed to ionomycin (Fig. 3E). Likewise, ionomycin precipitated a significantly greater rise in intracellular calcium concentrations in dfw-2J cells than it did in control cells (Fig. 3F). Therefore, entry of calcium into MECs induces apoptosis in the absence of PMCA2.
PMCA2 Overexpression Inhibits Apoptosis in Breast Cancer Cells.
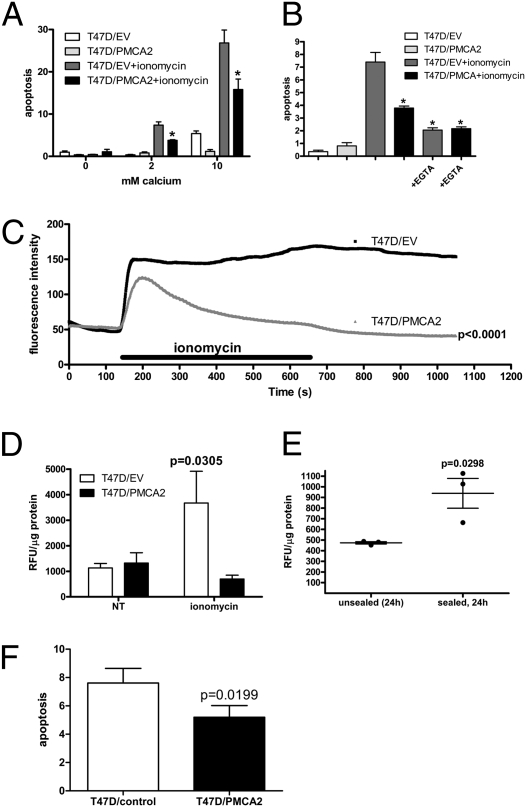
If PMCA2 levels regulate sensitivity to calcium-induced apoptosis in normal breast cells, then PMCA2 overexpression might protect breast cancer cells from apoptosis. We chose to investigate whether increased PMCA2 expression alters the sensitivity of T47D breast cancer cells to calcium-induced apoptosis. Although these cells express higher levels than normal cells, T47D cells express lower baseline levels of PMCA2 than several other standard cell lines. T47D cells were transfected with a construct, encoding mouse PMCA2b/w, to generate a stable cell line, T47D/PMCA2 (Fig. S4). A control cell line contained empty vector (T47D/EV). In the absence of extracellular calcium, ionomycin treatment caused little apoptosis in either T47D/PMCA2 or T47D/EV cells. In T47D/EV cells, ionomycin induced a progressive increase in apoptosis at 2 mM calcium and 10 mM calcium. However, at both 2 mM and 10 mM calcium, PMCA2 overexpression significantly reduced apoptosis in response to ionomycin in T47D/PMCA2 cells. The addition of EGTA to the culture media reduced ionomycin-induced apoptosis in T47D/EV and T47D/PMCA2 cells, confirming that apoptosis was dependent on the entry of extracellular calcium into the cells.
Overexpression of PMCA2 also altered intracellular calcium concentrations in response to ionomycin. Confocal calcium imaging of fluo-4 showed a sustained rise in intracellular calcium levels in control T47D/EV cells treated with ionomycin (Fig. 4C). This was associated with obvious blebbing of the plasma membrane in many cells, consistent with an apoptotic response. However, in T47D/PMCA2 cells, ionomycin caused only a transient rise in intracellular calcium concentrations (Fig. 4C). One of the mechanisms through which elevations in intracellular calcium is thought to cause apoptosis is by activating the calcium-dependant proteolytic enzyme calpain (13). Therefore, we examined calpain activation in T47D/PMCA2 and T47D/EV cells exposed to calcium and ionomycin. As shown in Fig. 4D, calpain activity was elevated in T47D/EV cells under these conditions, but not in T47D/PMCA2 cells. Interestingly, calpain activity was also elevated during early involution induced by teat sealing. As shown in Fig. 4E, there was little calpain activity in the unsealed gland where PMCA2 levels were high. However, in the sealed (involuting) gland, where PMCA2 levels were low, calpain was activated at 24 h. Therefore, PMCA2 overexpression lowers intracellular calcium levels and prevents calpain activation and apoptosis in T47D breast cancer cells. Finally, it has been suggested that some chemotherapeutic agents, including docetaxel, induce apoptosis, in part by increasing intracellular calcium levels (28). As shown in Fig. 4F, T47D/PMCA2 cells also demonstrated resistance to apoptosis in response to docetaxel as compared with T47D/EV cells.
Fig. 4.
PMCA2 overexpression protects T47D breast cancer cells from calcium-mediated apoptosis. T47D/EV control cells or T47D/PMCA2 cells, overexpressing PMCA2, were treated with 5 μM ionomycin for 16 h in the presence of 0, 2, or 10 mM extracellular CaCl2 before assessment of apoptosis by the Cell Death ELISAPLUS (A). Increasing extracellular calcium increased ionomycin-induced apoptosis in T47D/EV and T47D/PMCA2 cells. However, overexpression of PMCA2 significantly reduced apoptosis. At 2 mM and 10 mM calcium, the overall one-way ANOVA P values were <0.0001. The Student's t test gave a P = 0.0187 at 2 mM calcium and P = 0.0308 at 10 mM calcium for the reduction of apoptosis in T47D/PMCA2 vs. T47D/EV cells with ionomycin. At 2 mM calcium, 5 mM EGTA was able to prevent the apoptosis induced by ionomycin (B). The one-way ANOVA (P < 0.0001 overall, n = 4 for all groups) with Newman-Keuls multiple comparison test showed that EGTA significantly (P < 0.05) reduced apoptosis caused by ionomycin in T47D/EV (P = 0.0064 by t test) and T47D/PMCA2 cells (P = 0.0003 by t test). Significant reductions in apoptosis, compared with ionomycin-treated T47D/EV cells is denoted by * (Newman-Keuls posttest, P < 0.05, n = 4) in A and B. (C) Overexpression of PMCA2 changed the magnitude and shape of the intracellular calcium response to ionomycin treatment in T47D cells (P < 0.0001 by paired t test of the average of 82 T47D/EV and 87 T47D/PMCA2 cells from three independent experiments). (D) Calpain activity, indicative of calcium-mediated apoptosis, was increased significantly by ionomycin in T47D/EV cells (P = 0.0305 one-way ANOVA, P < 0.05 Newman-Keuls posttest, n = 3), whereas overexpression of PMCA2 prevented the increase in calpain activity in T47D/PMCA2 cells (P > 0.05 in Newman-Keuls posttest). Calpain activity is also significantly increased at 24 h after the onset of involution (P = 0.0298 by Student's t test, n = 3) in sealed vs. unsealed lactating mammary glands (E). (F) PMCA2 overexpression in T47D/PMCA2 cells reduced apoptosis in response to docetaxel treatment (5 ng/mL for 40 h) as compared with control T47D/EV cells (n = 9, P = 0.0199; four replicates in each of nine independent experiments). Bars and lines represent the mean ± SEM.
PMCA2 Expression Predicts Breast Cancer Outcome.
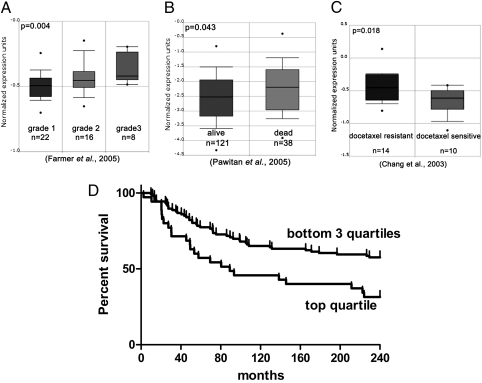
Resistance to apoptosis is an important hallmark of malignant progression (29). Given that PMCA2 alters the sensitivity of MECs and breast cancer cells to apoptosis, we next examined whether PMCA2 levels correlated with clinical-pathological features of human breast cancer. Using the Oncomine database and data mining tools (30), we observed that high PMCA2 mRNA expression was associated with higher tumor grade (31) (Fig. 5A) and lower 5-y survival (32) (Fig. 5B). Reflecting our results in vitro (Fig. 4F), we also found that PMCA2 expression correlated with resistance to docetaxel in patients (33) (Fig. 5C). We next stained a tissue microarray (TMA), consisting of 652 primary breast cancers, for PMCA2 using automated quantitative analysis (AQUA). The characteristics of the patient cohort are shown in Table S1. PMCA2 levels correlated positively with higher tumor grade, lymph node involvement and HER2-positive status (Table S2). Typical prognostic clinical markers, including tumor size, nodal status, nuclear grade, and hormone receptor (ER/PR) status, were significantly associated with survival by univariate analysis (Table S3). PMCA2 expression also correlated inversely with survival by univariate analysis (P = 0.0089). Using the Cox proportional hazards model, multivariate analysis (Table S4) showed that tumor size, nodal status, and PMCA2 scores were independently associated with survival (P = 0.0489). The association of PMCA2 expression with survival was more significant for younger premenopausal women (under age 50; see Table S1 for characteristics of this cohort), with a Kaplan-Meier P value of 0.0120. As a continuous variable, PMCA2 levels were associated with survival in women under 50, but the relative risk was low (Cox continuous P = 0.0037, 1.042 relative risk by univariate analysis; P = 0.0027, 1.047 relative risk by multivariate analysis). However, when expression was divided by quartiles, PMCA2 levels became strongly predictive of survival. Fig. 5D shows the Kaplan-Meier plot for survival in women under 50 y of age, plotting tumor PMCA2 levels by quartiles. As shown, younger women with tumors in the highest quartile of PMCA2 expression had a striking decrease in survival. The quartile with the highest PMCA2 expression in the <50-y-old cohort had a relative mortality risk of 2.475 (P = 0.0015) by univariate analysis and 2.817 (P = 0.0009) by multivariate analysis compared with the rest of the cohort. Applying the Cox proportional hazards model to just the women under 50 y of age, multivariate analysis suggested that only PMCA2 remained independently associated with survival (P = 0.0009). All other markers lost significance, suggesting that PMCA2 levels were more predictive of patient survival in younger women than standard prognostic markers used in the clinic. Finally, because PMCA2 levels were independent of estrogen or progesterone receptor status but correlated with HER2 status (Table S2), we examined whether PMCA2 correlated more strongly with survival in HER2-positive patients. In the entire cohort, PMCA2 levels were no longer predictive of survival in patients with HER2-negative tumors but tended to be predictive of mortality in HER2-positive tumors (P = 0.08). However, in just the women under 50, the predictive value of PMCA2 was independent of HER2 status.
Fig. 5.
High PMCA2 expression predicts poor outcome in human breast cancer cases. (A–C) Using the Oncomine database and data mining tools (www.oncomine.org), we found associations between PMCA2 expression (ATP2B2 gene) and increasing breast tumor grade (A, P = 0.004, Pearson correlation), death within 5 y of breast cancer diagnosis (B, P = 0.043, t test) and resistance to docetaxel treatment (C, P = 0.018, t test). (D) Association between mortality and the upper and lower three quartiles of PMCA2 expression in a subgroup of women with breast cancer under age 50. Compared with the bottom three quartiles for PMCA2 AQUA scores, the top quartile for PMCA2 expression had significantly lower survival (Kaplan-Meier analysis, P = 0.0120; Cox continuous P = 0.0037; lower three quartiles, n = 106; top quartile, n = 35).
Discussion
Mammary gland involution is triggered by milk stasis and is one of the most striking examples of coordinated cell death in nature. Our findings suggest that an important event in initiating apoptosis is a marked change in the shape of the MECs, which, in turn, is associated with a prompt decrease in PMCA2 levels within the cells. The regulation of PMCA2 levels by milk stasis provides an elegant method of coupling the secretory process of lactation to the cell death machinery required for involution. In essence, by down-regulating PMCA2, prolonged distension of the alveoli overloads the calcium-buffering capacity of the cells causing a sustained increase in intracellular calcium and apoptosis. One of the effects of elevated intracellular calcium in MECs appears to be activation of calpain activity. Given that Stat5 and Stat3 are substrates for calpain (34, 35), and given that loss of PMCA2 is associated with loss of nuclear phospho-Stat5 and the activation of Stat3, it is likely that increases in intracellular calcium levels at weaning contribute to the alterations in these transcription factors.
In addition to protecting MECs from calcium toxicity during lactation, we find that PMCA2 activity also protects breast cancer cells from apoptosis. This may explain why elevated PMCA2 expression is associated with larger tumors, higher grade, and nodal metastases, and predicts for poor outcome in breast cancer patients. Interestingly, PMCA2 expression correlated with HER2 expression among tumors in the TMA cohort. Because HER2-positive tumors were more common among younger women, this observation may hold a clue to the particularly striking association between high PMCA2 levels and death in women under 50 y old. Although PMCA2 expression tended to predict mortality in women with HER2-positive tumors in the entire cohort, the association was not quite statistically significant (P = 0.08). These results suggest that PMCA2 may be especially important in the biology of premenopausal breast cancer and/or in HER2-positive tumors. Further studies will be required to determine if there are biological interactions between ErbB2 signaling and PMCA2 in breast tumors.
Methods
Milk Stasis-Induced Involution.
Animal experiments were approved by Yale University's Institutional Animal Care and Use Committee. The teat-sealing model was performed as described by Li et al. (2). PMCA2 mRNA and protein levels were measured by qRT-PCR and Western blotting, as previously described (15). Immunostaining, morphometric measurements, and culture of primary mouse mammary epithelial cells are described in SI Text.
Analysis of Deafwaddler-2J, PMCA2-Deficient Mice.
Mammary whole mounts were prepared as previously described (36). Apoptotic nuclei were labeled using the DeadEnd Fluorometric TUNEL System (Promega). Apoptosis was measured in 3D cultures of MMEC using the Cell Death ELISAPLUS (Roche). Intracellular calcium concentrations were imaged in MMEC on glass coverslips in lactogenic media by fluo-4 (Invitrogen) live-cell confocal microscopy using a Zeiss LSM 510NLO, and analyzed with Zeiss LSM and Volocity image analysis software (Improvision/Perkin-Elmer).
Overexpression of PMCA2 in T47D Breast Cancer Cells.
The generation of T47D cells overexpressing mouse PMCA2 is detailed in SI Text.
Breast Cancer Tissue Microarray Analysis.
The breast carcinoma tissue microarrays were constructed as previously described (37). Tissue microarray analysis and statistical methods are described in SI Text.
Supplementary Material
Acknowledgments
We thank Al Mennone for technical assistance and David Tuck for helpful discussions. This work was funded by National Institutes of Health Grant DK069542 (to J.W.), Department of Defense Grant BC062952 (to J.V.), and Susan G. Komen for the Cure Grant KG081429 (to J.V.).
Footnotes
Conflict of interest statement: G.C. serves as a consultant for HistoRx Inc. D.L.R. and R.C. are founders, stockholders, and consultants to HistoRx Inc.
This article is a PNAS Direct Submission.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. GU734816).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0911186107/-/DCSupplemental.
References
- 1.Watson CJ. Involution: Apoptosis and tissue remodelling that convert the mammary gland from milk factory to a quiescent organ. Breast Cancer Res. 2006;8:203. doi: 10.1186/bcr1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li ML, et al. Mammary-derived signals activate programmed cell death during the first stage of mammary gland involution. Proc Natl Acad Sci USA. 1997;94:3425–3430. doi: 10.1073/pnas.94.7.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baxter FO, Neoh K, Tevendale MC. The beginning of the end: Death signaling in early involution. J Mammary Gland Biol Neoplasia. 2007;12:3–13. doi: 10.1007/s10911-007-9033-9. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen AV, Pollard JW. Transforming growth factor beta3 induces cell death during the first stage of mammary gland involution. Development. 2000;127:3107–3118. doi: 10.1242/dev.127.14.3107. [DOI] [PubMed] [Google Scholar]
- 5.Matsuda M, et al. Serotonin regulates mammary gland development via an autocrine-paracrine loop. Dev Cell. 2004;6:193–203. doi: 10.1016/s1534-5807(04)00022-x. [DOI] [PubMed] [Google Scholar]
- 6.Schere-Levy C, et al. Leukemia inhibitory factor induces apoptosis of the mammary epithelial cells and participates in mouse mammary gland involution. Exp Cell Res. 2003;282:35–47. doi: 10.1006/excr.2002.5666. [DOI] [PubMed] [Google Scholar]
- 7.Quaglino A, Salierno M, Pellegrotti J, Rubinstein N, Kordon EC. Mechanical strain induces involution-associated events in mammary epithelial cells. BMC Cell Biol. 2009;10:55. doi: 10.1186/1471-2121-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.VanHouten JN, Wysolmerski JJ. Transcellular calcium transport in mammary epithelial cells. J Mammary Gland Biol Neoplasia. 2007;12:223–235. doi: 10.1007/s10911-007-9057-1. [DOI] [PubMed] [Google Scholar]
- 9.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: Dynamics, homeostasis and remodelling. Natl Rev. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 10.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Natl Rev. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 11.Demaurex N, Distelhorst C. Cell biology. Apoptosis—the calcium connection. Science. 2003;300:65–67. doi: 10.1126/science.1083628. [DOI] [PubMed] [Google Scholar]
- 12.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: The calcium-apoptosis link. Natl Rev. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 13.Gil-Parrado S, et al. Ionomycin-activated calpain triggers apoptosis. A probable role for Bcl-2 family members. J Biol Chem. 2002;277:27217–27226. doi: 10.1074/jbc.M202945200. [DOI] [PubMed] [Google Scholar]
- 14.Reinhardt TA, Lippolis JD, Shull GE, Horst RL. Null mutation in the gene encoding plasma membrane Ca2+-ATPase isoform 2 impairs calcium transport into milk. J Biol Chem. 2004;279:42369–42373. doi: 10.1074/jbc.M407788200. [DOI] [PubMed] [Google Scholar]
- 15.VanHouten JN, Neville MC, Wysolmerski JJ. The calcium-sensing receptor regulates plasma membrane calcium adenosine triphosphatase isoform 2 activity in mammary epithelial cells: A mechanism for calcium-regulated calcium transport into milk. Endocrinology. 2007;148:5943–5954. doi: 10.1210/en.2007-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reinhardt TA, Filoteo AG, Penniston JT, Horst RL. Ca(2+)-ATPase protein expression in mammary tissue. Am J Physiol Cell Physiol. 2000;279:C1595–C1602. doi: 10.1152/ajpcell.2000.279.5.C1595. [DOI] [PubMed] [Google Scholar]
- 17.Reinhardt TA, Lippolis JD. Mammary gland involution is associated with rapid down regulation of major mammary Ca2+-ATPases. Biochem Biophys Res Commun. 2009;378:99–102. doi: 10.1016/j.bbrc.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Lee WJ, Monteith GR, Roberts-Thomson SJ. Calcium transport and signaling in the mammary gland: Targets for breast cancer. Biochim Biophys Acta. 2006;1765:235–255. doi: 10.1016/j.bbcan.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Akhtar N, Streuli CH. Rac1 links integrin-mediated adhesion to the control of lactational differentiation in mammary epithelia. J Cell Biol. 2006;173:781–793. doi: 10.1083/jcb.200601059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alcaraz J, et al. Laminin and biomimetic extracellular elasticity enhance functional differentiation in mammary epithelia. EMBO J. 2008;27:2829–2838. doi: 10.1038/emboj.2008.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Groulx N, Boudreault F, Orlov SN, Grygorczyk R. Membrane reserves and hypotonic cell swelling. J Membr Biol. 2006;214:43–56. doi: 10.1007/s00232-006-0080-8. [DOI] [PubMed] [Google Scholar]
- 22.Street VA, McKee-Johnson JW, Fonseca RC, Tempel BL, Noben-Trauth K. Mutations in a plasma membrane Ca2+-ATPase gene cause deafness in deafwaddler mice. Nat Genet. 1998;19:390–394. doi: 10.1038/1284. [DOI] [PubMed] [Google Scholar]
- 23.Watson CJ, Neoh K. The Stat family of transcription factors have diverse roles in mammary gland development. Semin Cell Dev Biol. 2008;19:401–406. doi: 10.1016/j.semcdb.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 24.Clarkson RWE, et al. NF-kappaB inhibits apoptosis in murine mammary epithelia. J Biol Chem. 2000;275:12737–12742. doi: 10.1074/jbc.275.17.12737. [DOI] [PubMed] [Google Scholar]
- 25.Santos SJ, Haslam SZ, Conrad SE. Estrogen and progesterone are critical regulators of Stat5a expression in the mouse mammary gland. Endocrinology. 2008;149:329–338. doi: 10.1210/en.2007-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chapman RS, et al. Suppression of epithelial apoptosis and delayed mammary gland involution in mice with a conditional knockout of Stat3. Genes Dev. 1999;13:2604–2616. doi: 10.1101/gad.13.19.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neville MC, McFadden TB, Forsyth I. Hormonal regulation of mammary differentiation and milk secretion. J Mammary Gland Biol Neoplasia. 2002;7:49–66. doi: 10.1023/a:1015770423167. [DOI] [PubMed] [Google Scholar]
- 28.Piñeiro D, Martín ME, Guerra N, Salinas M, González VM. Calpain inhibition stimulates caspase-dependent apoptosis induced by taxol in NIH3T3 cells. Exp Cell Res. 2007;313:369–379. doi: 10.1016/j.yexcr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 29.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 30.Rhodes DR, et al. ONCOMINE: A cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6:1–6. doi: 10.1016/s1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farmer P, et al. Identification of molecular apocrine breast tumours by microarray analysis. Oncogene. 2005;24:4660–4671. doi: 10.1038/sj.onc.1208561. [DOI] [PubMed] [Google Scholar]
- 32.Pawitan Y, et al. Gene expression profiling spares early breast cancer patients from adjuvant therapy: Derived and validated in two population-based cohorts. Breast Cancer Res. 2005;7:R953–R964. doi: 10.1186/bcr1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang JC, et al. Gene expression profiling for the prediction of therapeutic response to docetaxel in patients with breast cancer. Lancet. 2003;362:362–369. doi: 10.1016/S0140-6736(03)14023-8. [DOI] [PubMed] [Google Scholar]
- 34.Hendry L, John S. Regulation of STAT signalling by proteolytic processing. Eur J Biochem. 2004;271:4613–4620. doi: 10.1111/j.1432-1033.2004.04424.x. [DOI] [PubMed] [Google Scholar]
- 35.Oda A, Wakao H, Fujita H. Calpain is a signal transducer and activator of transcription (STAT) 3 and STAT5 protease. Blood. 2002;99:1850–1852. doi: 10.1182/blood.v99.5.1850. [DOI] [PubMed] [Google Scholar]
- 36.Dunbar ME, et al. Temporally regulated overexpression of parathyroid hormone-related protein in the mammary gland reveals distinct fetal and pubertal phenotypes. J Endocrinol. 2001;171:403–416. doi: 10.1677/joe.0.1710403. [DOI] [PubMed] [Google Scholar]
- 37.Camp RL, Chung GG, Rimm DL. Automated subcellular localization and quantification of protein expression in tissue microarrays. Nat Med. 2002;8:1323–1327. doi: 10.1038/nm791. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.