Abstract
Mutation of rod photoreceptor-enriched transcription factors is a major cause of inherited blindness. We identified the orphan nuclear hormone receptor estrogen-related receptor β (ERRβ) as selectively expressed in rod photoreceptors. Overexpression of ERRβ induces expression of rod-specific genes in retinas of wild-type as well as Nrl−/− mice, which lack rod photoreceptors. Mutation of ERRβ results in dysfunction and degeneration of rods, whereas inverse agonists of ERRβ trigger rapid rod degeneration, which is rescued by constitutively active mutants of ERRβ. ERRβ coordinates expression of multiple genes that are rate-limiting regulators of ATP generation and consumption in photoreceptors. Furthermore, enhancing ERRβ activity rescues photoreceptor defects that result from loss of the photoreceptor-specific transcription factor Crx. Our findings demonstrate that ERRβ is a critical regulator of rod photoreceptor function and survival, and suggest that ERRβ agonists may be useful in the treatment of certain retinal dystrophies.
Keywords: Crx, ligand, neurodegeneration, retina, development
The vertebrate retina contains two major subtypes of photoreceptors—rods and cones. Clinically, dysfunction and death of rod photoreceptors are the primary causes of most forms of inherited photoreceptor dystrophy. A number of rod-expressed transcription factors (1–7) have been identified that are required for rod photoreceptor differentiation or survival. Biochemical analysis has indicated that these factors are present at the promoters of rod-specific genes in vivo and directly activate expression of rod-specific genes (1, 8, 9). Mutation of rod-enriched transcription factors in humans can lead to rod photoreceptor dystrophy (10, 11). Failure to express normal levels of rod-specific genes thus results in rod photoreceptor degeneration, and correction of this defect may have considerable value in treating inherited blindness.
Analysis of gene expression in developing and mature rod photoreceptors has indicated that other transcription factors also show highly rod-enriched expression (12–15). Among these is estrogen-related receptor β (ERRβ), an orphan nuclear hormone receptor homologous to the classical estrogen receptor but which constitutively activates transcription in the absence of estradiol (16, 17). ERRβ is specifically expressed in differentiating and mature mouse rod photoreceptors (12, 13), with significant mRNA levels also detected in the human retina (18). Deletion of a floxed allele of ERRβ in the embryoid body using a Sox2-Cre line (hereafter referred to as ERRβ−/−) results in mice with a defect in inner ear development but no obvious retinal defects (19). Loss-of-function mutations of ERRβ have been reported in inherited forms of human deafness (20).
Given the prominent expression of ERRβ in retinal photoreceptors, we hypothesized that ERRβ might also play an important role in rod photoreceptor function. We observed that genetic or pharmacological disruption of function of ERRβ leads to rod photoreceptor degeneration. ERRβ also directly regulates expression of rod-specific genes, with ERRβ being sufficient to both drive activation of rod-specific genes in vivo and rescue rod photoreceptors of Crx−/− mice from degeneration. These data imply that synthetic agonists of ERRβ may prove useful in treating photoreceptor degeneration resulting from mutation of rod-specific transcription factors.
Results
Expression of ERRβ in Developing and Mature Retina.
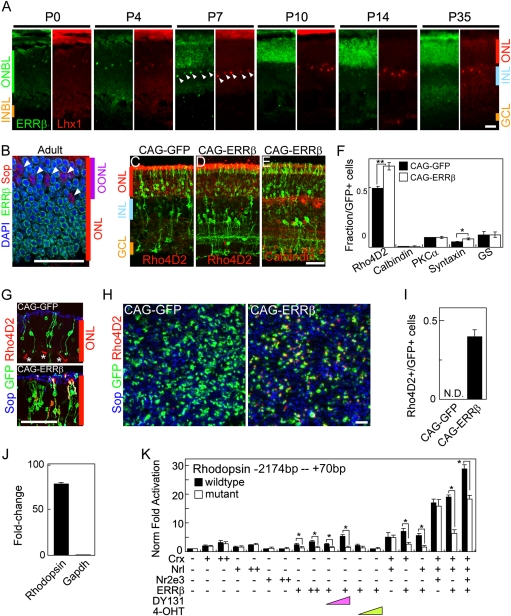
Our previous work demonstrated that ERRβ mRNA was first seen in the early postnatal mouse retina, initially showing expression in cells resembling immature horizontal cells, and from the second week of life was expressed in retinal photoreceptors (12, 13). To further clarify the distribution of ERRβ protein, we performed immunohistochemical analysis with markers of rod, cone, and horizontal cells. We found that ERRβ was coexpressed with Lhx1, a marker of horizontal cells, throughout the first week of life. However, the horizontal cell expression of ERRβ was drastically down-regulated after postnatal day (P) 10, consistent with the previously reported mRNA expression pattern (13) (Fig. 1A). Starting at P7, ERRβ immunostaining is detected across the outer nuclear layer (ONL), consistent with a photoreceptor-specific expression pattern. Immunostaining with the anti-rhodopsin antibody Rho4D2 and anti-ERRβ reveals that ERRβ and rhodopsin are colocalized at P14 (Fig. S1A). Furthermore, ERRβ and the cone-specific marker S-opsin (Fig. 1B) are not colocalized at any age examined. Finally, ERRβ protein is absent from photoreceptors of P10.5 Nrl−/− mice, although still detected in developing horizontal cells, demonstrating that ERRβ is specifically expressed in rod photoreceptors (Fig. S1B). Expression of ERRβ is still detected in photoreceptors of mice lacking functional Crx and Nr2e3, however, which retain rod photoreceptors (Fig. S1B). We thus conclude that ERRβ is selectively expressed in immature horizontal cells and in rod photoreceptors at intermediate and late stages of differentiation.
Fig. 1.
ERRβ is expressed in rod photoreceptors and promotes rod-specific gene expression. (A) Developmental expression of ERRβ (green) coimmunostained with the horizontal cell marker Lhx1 (red) at P0, 4, 7, 10, 14, and 35. Expression overlaps in immature horizontal cells at P7 (white arrowheads). ONBL, outer neuroblastic layer; INBL, inner neuroblastic layer; ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. (B) Double immunostaining of ERRβ (green) and S-opsin (red) with DAPI (blue) on a P40 retinal section, demonstrating nonoverlapping expression of the two markers. OONL, outer outer nuclear layer. (C–E) Section immunohistochemistry of P14 retinas electroporated in vivo at P0 with CAG-GFP or CAG-ERRβ colabeled with Rho4D2 (C and D) or calbindin (E) antibodies. (F) Retinal cell-type composition of P14 retinas electroporated in vivo at P0 with CAG-GFP and CAG-ERRβ estimated by dissociated immunohistochemistry using cell-specific markers (Rho4D2 for rods, calbindin for horizontal cells, PKCα for rod bipolar cells, syntaxin for amacrine cells, and glutamine synthetase for Muller glia). All data are represented as mean ± SD. *P < 0.05, **P < 0.005 by Student's t test (n = 3 electroporated retinas). (G–J) ERRβ overexpression is sufficient to induce rhodopsin expression in photoreceptors of Nrl−/− mice. Section (G) and flat-mount (H) immunohistochemistry of P14 retinas electroporated in vivo at P0 with CAG-GFP and CAG-ERRβ immunostained with S-opsin (blue) and Rho4D2 (red) antibodies. Asterisks indicate background staining of blood vessels. (I) Quantification of the fraction of Rho4D2/GFP double-positive photoreceptor cells from (H). All data are represented as mean ± SD (n = 3 electroporated retinas). (J) Quantification of rhodopsin and GAPDH mRNA in P7.5 Nrl−/− retinas electroporated with CAG-ERRβ as measured by quantitative RT-PCR (n = 4 RNA preparations). Fold change in mRNA levels is expressed relative to the contralateral retina, which was not electroporated. Levels of mRNA are normalized relative to the fraction of electroporated cells (≈10% of the retinal surface, and 50% of photoreceptors within that area, were electroporated in the samples used). (K) ERRβ directly regulates rhodopsin −2174 - - +70 bp promoter construct activity. Black and white bars represent luciferase levels relative to untransfected 293T cells expressed from wild-type and a mutant promoter construct, in which the ERRβ-binding motif (AGGTCA) at the proximal promoter (-136 bp) has been mutated. Pink and green triangles represent dose treatment of DY131 and 4-OHT, respectively. + and ++ represent 15 and 30 ng, respectively. All data are represented as mean ± SD (n = 3 independent transfections). *P < 0.05 by Student's t test (n = 3 independent transfections, repeated in two separate trials). (Scale bars, 20 μm.)
Two homologs of ERRβ, ERRα and ERRγ, are also present in the retina. ERRα is broadly expressed in the mature retina, with somewhat more prominent expression in inner retina than in ONL, as previously reported by in situ hybridization analysis (13). ERRγ, on the other hand, is prominently expressed in cone photoreceptors and in a subset of inner retinal cells, with only weak expression detectable in rod photoreceptors (Fig. S1C).
Activation of Rhodopsin Expression by ERRβ.
To analyze the function of ERRβ in the developing retina, we overexpressed full-length ERRβ under the control of the ubiquitous CAG promoter as previously described (21, 22). Overexpression of ERRβ increased the fraction of cells expressing rhodopsin relative to empty vector controls at P14 (Fig. 1 C–F), although no significant increase in the fraction of cells in the ONL was detected, implying that ERRβ overexpression does not drive retinal precursors toward a rod photoreceptor fate. A modest increase in the fraction of cells expressing amacrine-specific markers and showing amacrine morphology was also observed (Fig. 1 C–F), although no increase in the fraction of cells expressing the horizontal cell marker calbindin was detected (Fig. 1 E and F).
Loss of function of the rod-specific transcription factor Nrl results in conversion of rod photoreceptors to short-wavelength cones (4), and provides an excellent model to determine whether ERRβ is sufficient to induce expression of rod-specific genes. To investigate this, we overexpressed ERRβ by electroporation of Nrl−/− retinas at P0.5. We saw robust induction of rhodopsin expression following CAG-ERRβ electroporation at P14 as measured by both section and flat-mount immunohistochemistry and by quantitative RT-PCR (Fig. 1 G–J) reminiscent of the phenotype seen following transgenic overexpression of Nr2e3 in Nrl−/− mice (23). CAG-ERRβ electroporation did not, however, result in repression of S-opsin synthesis, as is seen following Nr2e3 overexpression.
These data suggested that ERRβ might directly activate expression of rod-specific genes, perhaps in conjunction with other known rod-enriched transcription factors. To address this question, we first analyzed whether ERRβ directly activated rhodopsin expression by using a rhodopsin promoter luciferase construct (22). We observed a dose-dependent activation of luciferase expression by ERRβ, whether alone or in combination with Crx, Nrl, and/or Nr2e3 (Fig. 1K). DY131, a selective agonist of ERRβ (24), enhanced ERRβ-dependent activation of luciferase expression. In contrast, 4-hydroxytamoxifen (4-OHT), an inverse agonist of ERRβ, converted ERRβ into a potent transcriptional repressor in a dose-dependent manner. Finally, mutation of a predicted estrogen-related receptor target sequence in the proximal rhodopsin promoter significantly reduced the ERRβ-induced activation of luciferase expression. These data demonstrate that rhodopsin expression can be regulated by ERRβ and that ERRβ-dependent transcription of rhodopsin can be modulated by selective ligands of ERRβ.
Regulation of Rod Photoreceptor Survival by ERRβ.
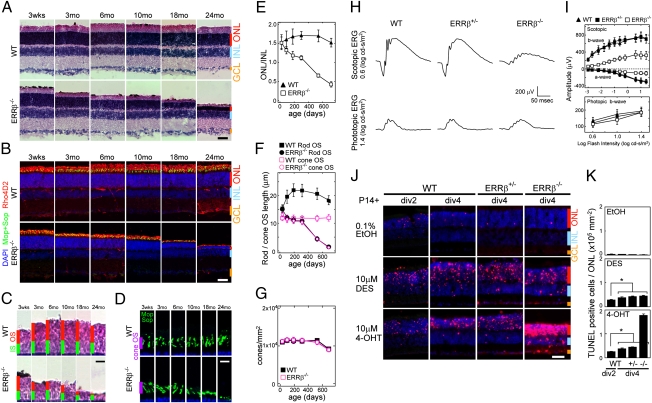
Although we did not observe any obvious morphological changes in P21 retinas of ERRβ−/− mice, we investigated whether effects on rod photoreceptor function or survival were seen at later ages. We observed a slow degeneration of rod photoreceptors in mutant mice. Starting at 3 mo of age, a decrease in the length of rod outer segments (Fig. 2 A, C, and F) was detectable by hematoxylin and eosin (H&E) staining, with a significant decrease in the number of cell bodies in the outer nuclear layer detectable by 6 mo of age (Fig. 2 A, B, and E). By 24 mo of age, the great majority of rod photoreceptors were gone, although a subset of cone photoreceptors were preserved (Fig. 2 B and C). A significant decrease in the length of cone outer segments was observed in ERRβ−/− mice by 18 mo of age (Fig. 2 D and F), although the total number of cone photoreceptor cells was not different between wild-type and mutant animals (Fig. 2G). No changes were observed in the number or structure of any inner retinal cell type (Fig. S2).
Fig. 2.
ERRβ controls rod photoreceptor survival. (A and B) H&E staining (A) and section immunohistochemistry (B) labeled with M-opsin and S-opsin (green) and Rho4D2 (red) antibodies of aged (P21, 3, 6, 10, 18, and 24 mo) mouse retinas. (C and D) High-power images of photoreceptor outer and inner segments (C) and cone outer segments (D) from A and B, respectively. (E) Quantification of ONL thickness normalized relative to the INL from A. (F) Quantification of rod and cone outer segment length from C and D, respectively. All data are represented as mean ± SD (n = 20 from three different individuals). (G) Density of cone photoreceptors estimated from B. All data are represented as mean ± SD (n = 3 retinas from different individuals). (H) Scotopic (rod) and photopic (cone) ERG responses from 10-mo-old wild-type, ERRβ+/−, and ERRβ−/− mice. The amplitude of the scotopic ERG response of ERRβ−/− mice was significantly reduced, whereas the wave forms of WT and ERRβ+/− were similar. There were no significant differences in the amplitude of the photopic ERG response. (I) Scotopic a- and b-wave amplitudes of 11 flash intensities, and photopic b-wave amplitudes of 3 flash intensities. All data are represented as mean ± SD (n = 4 animals). (J) TUNEL staining (red) with DAPI (blue) of WT, ERRβ+/−, and ERRβ−/− retinas that were harvested at P14 and explanted for 2 d in vitro (div 2) or 4 d in vitro (div 4) d exposed to 0.1% EtOH (carrier), 10 μM DES, and 10 μM 4-OHT. (K) The fraction of TUNEL-positive cells in ONL in J normalized by the area of ONL. All data are represented as mean ± SD (n = 3 independent explants). *P < 0.05 by Student's t test. [Scale bars, 20 μm (A, B, and J) and 10 μm (C and D).]
To determine whether photoreceptor function was lost, we measured the electroretinogram (ERG) of these mice at 10 and 24 mo of age. By 10 mo, a dramatic reduction in the scotopic a and b wave was observed, whereas the photopic b wave was not reduced (Fig. 2 H and I). The time to peak of the photopic b wave of ERRβ−/− mice was also delayed, as previously reported in mice showing progressive rod dystrophy (25). At 24 mo of age, a severe deterioration of the scotopic response was seen, along with a secondary reduction in the photopic ERGs (Fig. S3). No abnormalities in either scotopic or photopic ERGs are observed at P33, in line with the normal cellular morphology observed at this age (Fig. 2A and Fig. S2). These data imply that ERRβ expression is necessary for rod photoreceptor function and survival.
We next examined whether pharmacological inhibition of ERRβ directly regulates rod photoreceptor survival. Diethylstilbestrol (DES) functions as an inverse agonist of ERRα, ERRβ, and ERRγ (17), whereas 4-OHT is an inverse agonist for ERRβ and ERRγ but not ERRα (16). Beginning at P14, we treated retinal explants with ERRβ inverse agonists. By 2 d postexplanting, we observed selective rod photoreceptor degeneration in retinas treated with either 10 μM DES or 4-OHT, but not with 0.1% EtOH carrier, with prominent levels of TUNEL staining detected (Fig. 2 J and K). The increase in TUNEL staining was selective for rod photoreceptors, with no effect on cone or bipolar cell viability observed (Fig. S4). Surprisingly, an increase in TUNEL staining not only in photoreceptor cells but also in the inner retina of 4-OHT-treated retinas is observed in ERRβ−/− retinas relative to wild-type animals (Fig. 2 J and K). Although the reason for this observation remains unclear, this may imply that the 4-OHT-induced selective cell death of rod photoreceptors is not being entirely mediated by ERRβ, but also partially by ERRγ or an uncharacterized cellular target. The prominent cell death seen in mutant animals in cells of the inner retina, which do not express ERRβ, also suggests that ERRβ might regulate retinal cell survival through a partially non-cell-autonomous mechanism.
We next tested whether overexpression of a drug-insensitive, constitutively active ERRβ could rescue rod degeneration observed following DES or 4-OHT treatment of retinal explants. Whereas overexpression of CAG-VP16 did not protect rod photoreceptors from degeneration, overexpression of the VP16 transcriptional activator domain fused to the DNA-binding domain of ERRβ resulted in a significant level of protection of electroporated photoreceptors. Interestingly, this protection extended to nearby nonelectroporated regions. This suggests that ERRβ might regulate either expression of a secreted neuroprotective factor or else allow removal of a diffusible toxic factor (Fig. S5 A and B).
Identification of ERRβ Target Genes.
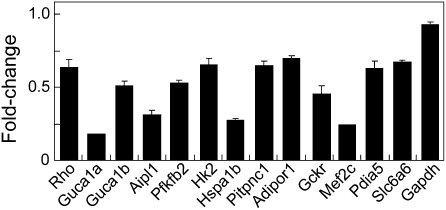
Next, we used DNA microarray analysis to determine whether ERRβ regulated expression of rod-specific genes in addition to rhodopsin. We observed 425 transcripts that were significantly down-regulated following loss of ERRβ at P21, with 303 transcripts significantly up-regulated (fold change greater or less than 1.25×; P < 0.05). We found that down-regulated genes were significantly enriched for a known rod photoreceptor-enriched expression pattern (P = 0.0004). One of the most highly down-regulated genes was the transcription factor Mef2c, which is down-regulated in Nrl−/− mice (14, 15). A number of phototransduction-associated genes, such as rhodopsin, were also significantly reduced in expression, but several genes in this group stood out as particularly strongly down-regulated. Specifically, the guanylate cyclase activator proteins Guca1a and Guca1b and the sodium/potassium/calcium rod inner segment cation exchanger Slc24a1 (Table S1) were greatly affected. In addition, we observed significant down-regulation of several genes involved in rate-limiting steps in glycolysis, including Hk2, Pfkb2, and Pfkp along with Ppara, a transcription factor known to directly regulate transcription of fatty acid-metabolizing enzymes (26). Each of these genes has previously been identified as a transcriptional target of ERRα and ERRγ in nonneuronal tissues (27), but a role for ERRβ in regulation of metabolism has thus far not been reported. Quantitative real-time PCR analysis of a representative subset of such transcripts indicated that they were indeed significantly down-regulated in P21 ERRβ−/− retinas (Fig. 3). The cellular expression pattern of a number of transcripts down-regulated in ERRβ−/− retinas was examined using in situ hybridization. We determined that some were enriched in rod photoreceptors. Others, such as Hk2, are broadly expressed, but selectively down-regulated in the photoreceptor layer of P21 ERRβ−/− mice (Fig. S6). See Dataset S1 for a full list of transcripts that are differentially expressed in the P21 ERRβ−/− retina.
Fig. 3.
Quantitative RT-PCR analysis of transcripts down-regulated in P21 ERRβ−/− mice. RNA from whole dissected retinas was used, and the results were normalized to β-actin levels. Signal levels represent mRNA levels in mutant retinas normalized to those of age-matched wild-type retinas (n = 3 RNA preparations). Sequences for primers used for are shown in Table S2.
ERRβ Activation Promotes Rod Photoreceptor Survival.
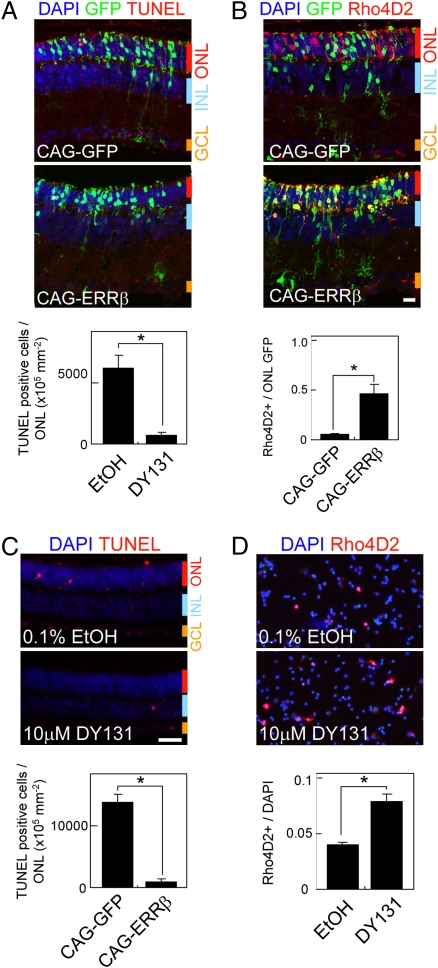
Finally, we investigated whether enhancing ERRβ function could rescue defects caused by genetic disruption of other rod-expressed transcription factors. Because mutations in Crx are the most common disease-associated defect of this sort, we determined whether enhancing ERRβ function through either overexpression or pharmacological manipulation could rescue defects in rod-specific gene expression and rod survival found in Crx−/− mice (28). Although ERRβ expression is reduced in Crx−/− mice, it is still detectable in rod photoreceptors (Fig. S1B). Electroporation of CAG-ERRβ in Crx−/− mice resulted in a substantial decrease in cell death in photoreceptor cells at P21, along with a restoration of rod-specific gene expression in electroporated cells (Fig. 4 A and B). In retinas electroporated with ERRβ-CAG, the protection from apoptosis extended to nearby photoreceptors that were not GFP-positive, although induction of rhodopsin expression was confined to GFP-positive cells, reminiscent of the cell-non-autonomous cell protection observed in DES- and 4-OHT-treated retinas that were electroporated with ERRβ-VP16. In addition, treatment of retinal explants obtained from Crx−/− mice with DY131, a selective agonist for ERRβ/γ, resulted in a substantial decrease in the fraction of TUNEL-positive cells in the ONL, along with a significant increase in the expression of rhodopsin (Fig. 4 C and D). These data suggest that manipulation of ERRβ activity may protect rod photoreceptors from dysfunction and death in mutants where expression of rod-specific transcripts is compromised.
Fig. 4.
ERRβ overexpression and the ERR-specific agonist DY131 rescue defects in rod function in Crx−/− mice. (A) Retinal sections of P33 Crx−/− retinas electroporated in vivo at P0 with CAG-GFP and CAG-ERRβ that were TUNEL-positive (A) and immunostained with Rho4D2 (B). The fractions of TUNEL-positive cells and Rho4D2-positive electroporated cells in ONL are shown in the lower panel. (C) TUNEL staining of P7 + 5 d in vitro (div5) Crx−/− retinal explants exposed to 0.1% EtOH (carrier) and 10 μM DY131. The fraction of TUNEL-positive cells in ONL was normalized by the area of ONL. All data are represented as mean ± SD (n = 3). (D) Dissociated cell immunohistochemistry using P7 + div5 Crx−/− retinal explants. The fraction of Rho4D2-positive cells is shown. All data are represented as mean ± SD (n = 3 retinas). *P < 0.05 by Student's t test. (Scale bars, 20 μm.)
Discussion
Our findings indicate that ERRβ is a rod photoreceptor-specific transcription factor that regulates the expression of multiple rod-specific genes and is required for survival of this cell type. Targeted disruption of ERRβ leads to a slow and selective degeneration of rod photoreceptors, preceded by loss of rod outer segments. Furthermore, pharmacological inhibition of ERRβ/γ leads to a selective degeneration of rod photoreceptors, which can be rescued by overexpression of constitutively active ERRβ. Selective down-regulation of a subset of rod-specific genes is also observed in mutant mice. A working model for the action of ERRβ in regulation of rod photoreceptor-specific gene expression and rod survival based on these findings is shown in Fig. S7. ERRβ—like Crx, Nrl, and Nr2e3—directly activates rhodopsin expression. Although ERRβ expression is reduced in mice mutant for both Crx and Nrl, it is unclear whether these factors directly regulate ERRβ transcription (14, 15). However, our finding that ERRβ overexpression in Nrl−/− animals induces photoreceptor expression implies that ERRβ can function independently of this gene to regulate rod-specific gene transcription. Like Crx, loss of function of ERRβ leads to both dysfunction and degeneration of rod photoreceptors, although with substantially slower kinetics (28). Unlike in Nrl- and Nr2e3-deficient retinas, no ectopic activation of cone-specific genes is observed in ERRβ−/− animals.
ERRβ has been previously shown to regulate cell specification and survival at a number of different stages of development. This role in development is distinct from the role of its homologs ERRα and ERRγ, which act primarily to regulate cellular metabolism rather than differentiation (29). Targeted mutation of ERRβ leads to defects in trophoblast cell differentiation and survival (30), which result in embryonic lethality. Epiblast-specific deletion of ERRβ with Sox2-Cre leads to a defect in specification of endolymph-producing cells in the developing inner ear, resulting in deafness and vestibular defects (19). This defect is mirrored in humans carrying point mutants in the ERRβ gene, who show early-onset hearing loss (20). Our findings extend the known functions of ERRβ to the regulation of sensory neuron specification and survival, and further suggest that individuals lacking ERRβ may turn out to suffer from late-onset rod photoreceptor dystrophy, a defect which may remain undetected owing to the relative youth of the patients investigated thus far.
The rod photoreceptor degeneration observed in ERRβ−/− retinas manifests itself very slowly. It is not yet clear how ERRβ loss of function leads to rod degeneration. Although expression of a number of genes that regulate phototransduction is reduced in ERRβ−/− mice, the most dramatic reduction is seen for Guca1a and Guca1b, which are the rate-limiting regulators of cGMP production in photoreceptor outer segments, and Slc24a1, the rod inner segment sodium/potassium/calcium transporter. Inner segment cation transport and cGMP production are by far the largest users of free energy in rod photoreceptors (31). Selective defects are also seen in enzymes that regulate free energy generation in photoreceptors, notably enzymes regulating glycolysis and the pentose phosphate pathway, including Hk2, Pfkb2, and AldoA, each of which is photoreceptor-enriched and down-regulated in Nrl−/− mice (14, 15). Rod photoreceptors exhibit a high level of aerobic glycolysis, which acts to both regenerate cGMP in rod outer segments as well as generate high levels of NADPH via the pentose phosphate pathway for chromophore reduction. Selective defects in both functions have been proposed to lead to photoreceptor degeneration (32, 33). A general rescue of photoreceptor metabolism may be one reason why non-cell-autonomous protection of photoreceptors is observed in both DES- and 4-OHT-treated retinas overexpressing ERRβ-VP16, and also in Crx−/− mice overexpressing ERRβ (Fig. 4 C and D).
Our studies suggest that pharmacological manipulation of retinal ERRβ activity may serve as a therapeutically useful tool for preventing rod photoreceptor degeneration in mutations that result in loss-of-function defects in transcription factors such as Crx, Nrl, and Nr2e3. These proteins regulate rod-specific gene expression and collectively comprise 3–5% of all identified forms of autosomal-dominant retinitis pigmentosa and 1–2% of Leber congenital amaurosis (34, 35). DY131, a selective agonist of ERRβ and ERRγ (24), enhances expression of rod-specific genes and inhibits rod degeneration when applied to retinas of Crx−/− mice, whereas overexpression of ERRβ in photoreceptors of these mice achieves the same result. Selective ocular drug delivery is routinely used in treatment of a broad range of ophthalmic disorders, and takes advantage of the fact that the eye represents a relatively self-contained system that restricts the spread of drugs and resulting side effects on other organs. Our findings suggest that ERRβ agonists may prove useful in treating select inherited forms of rod photoreceptor dystrophy.
Materials and Methods
Animals.
Timed pregnant CD-1 and C57BL/6 mice were purchased from Charles River Breeding Laboratories and Jackson Laboratory, respectively. ERRβ−/− mice were generated as previously described (19), with homozygous mutant mice generated by breeding to the Sox2-Cre line (36). All experimental procedures were preapproved by the Institutional Animal Care and Use Committee of Johns Hopkins University School of Medicine.
In Vivo Electroporation, Immunohistochemistry, and in Situ Hybridization.
In vivo electroporation was performed at P0 as previously described (21). Protocols for section immunohistochemistry, dissociated cell immunohistochemistry, and in situ hybridization were performed essentially as previously described (13, 22). H&E staining solution (Sigma) was used according to the manufacturer's recommended protocol.
Electroretinogram.
ERGs were recorded as previously described (37).
Retinal Explants and TUNEL Staining.
P14 retinas were dissected and explanted as previously described (21). The culture media containing 0.1% EtOH (carrier), 10 μM DES (Sigma), 10 μM 4-OHT (Sigma), and 10 μM DY131 (Tocris Bioscience) were changed every 2 d.
Full experimental procedures for this study can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank T. Shimogori, J. Nathans, and members of the Blackshaw lab for their comments on the manuscript, P. Heite for assistance with luciferase assays, and K. Miki and P. Campochario for assistance with ERG recording. This work was supported by a W. M. Keck Distinguished Young Scholar in Medical Research Award (to S.B.), National Institutes of Health Grants EY012543 and EY02687, a Lew Wasserman Merit Award from Research to Prevent Blindness (to S.C.), and a Knights Templar Pediatric Ophthalmology Research Award (to A.O.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Primary microarray data files have been deposited in the Gene Expression Omnibus (GEO) database (accession no. GSE21944).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1000102107/-/DCSupplemental.
References
- 1.Chen S, et al. Crx, a novel Otx-like paired-homeodomain protein, binds to and transactivates photoreceptor cell-specific genes. Neuron. 1997;19:1017–1030. doi: 10.1016/s0896-6273(00)80394-3. [DOI] [PubMed] [Google Scholar]
- 2.Furukawa T, Morrow EM, Cepko CL. Crx, a novel otx-like homeobox gene, shows photoreceptor-specific expression and regulates photoreceptor differentiation. Cell. 1997;91:531–541. doi: 10.1016/s0092-8674(00)80439-0. [DOI] [PubMed] [Google Scholar]
- 3.Haider NB, et al. Mutation of a nuclear receptor gene, NR2E3, causes enhanced S cone syndrome, a disorder of retinal cell fate. Nat Genet. 2000;24:127–131. doi: 10.1038/72777. [DOI] [PubMed] [Google Scholar]
- 4.Mears AJ, et al. Nrl is required for rod photoreceptor development. Nat Genet. 2001;29:447–452. doi: 10.1038/ng774. [DOI] [PubMed] [Google Scholar]
- 5.Nishida A, et al. Otx2 homeobox gene controls retinal photoreceptor cell fate and pineal gland development. Nat Neurosci. 2003;6:1255–1263. doi: 10.1038/nn1155. [DOI] [PubMed] [Google Scholar]
- 6.Jia L, et al. Retinoid-related orphan nuclear receptor RORβ is an early-acting factor in rod photoreceptor development. Proc Natl Acad Sci USA. 2009;106:17534–17539. doi: 10.1073/pnas.0902425106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J, et al. Rb regulates proliferation and rod photoreceptor development in the mouse retina. Nat Genet. 2004;36:351–360. doi: 10.1038/ng1318. [DOI] [PubMed] [Google Scholar]
- 8.Cheng H, et al. Photoreceptor-specific nuclear receptor NR2E3 functions as a transcriptional activator in rod photoreceptors. Hum Mol Genet. 2004;13:1563–1575. doi: 10.1093/hmg/ddh173. [DOI] [PubMed] [Google Scholar]
- 9.Peng GH, Chen S. Chromatin immunoprecipitation identifies photoreceptor transcription factor targets in mouse models of retinal degeneration: New findings and challenges. Vis Neurosci. 2005;22:575–586. doi: 10.1017/S0952523805225063. [DOI] [PubMed] [Google Scholar]
- 10.Chen S, et al. Functional analysis of cone-rod homeobox (CRX) mutations associated with retinal dystrophy. Hum Mol Genet. 2002;11:873–884. doi: 10.1093/hmg/11.8.873. [DOI] [PubMed] [Google Scholar]
- 11.Rivolta C, Berson EL, Dryja TP. Dominant Leber congenital amaurosis, cone-rod degeneration, and retinitis pigmentosa caused by mutant versions of the transcription factor CRX. Hum Mutat. 2001;18:488–498. doi: 10.1002/humu.1226. [DOI] [PubMed] [Google Scholar]
- 12.Blackshaw S, Fraioli RE, Furukawa T, Cepko CL. Comprehensive analysis of photoreceptor gene expression and the identification of candidate retinal disease genes. Cell. 2001;107:579–589. doi: 10.1016/s0092-8674(01)00574-8. [DOI] [PubMed] [Google Scholar]
- 13.Blackshaw S, et al. Genomic analysis of mouse retinal development. PLoS Biol. 2004;2:e247. doi: 10.1371/journal.pbio.0020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corbo JC, Myers CA, Lawrence KA, Jadhav AP, Cepko CL. A typology of photoreceptor gene expression patterns in the mouse. Proc Natl Acad Sci USA. 2007;104:12069–12074. doi: 10.1073/pnas.0705465104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akimoto M, et al. Targeting of GFP to newborn rods by Nrl promoter and temporal expression profiling of flow-sorted photoreceptors. Proc Natl Acad Sci USA. 2006;103:3890–3895. doi: 10.1073/pnas.0508214103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tremblay GB, Bergeron D, Giguere V. 4-Hydroxytamoxifen is an isoform-specific inhibitor of orphan estrogen-receptor-related (ERR) nuclear receptors β and γ. Endocrinology. 2001;142:4572–4575. doi: 10.1210/endo.142.10.8528. [DOI] [PubMed] [Google Scholar]
- 17.Tremblay GB, et al. Diethylstilbestrol regulates trophoblast stem cell differentiation as a ligand of orphan nuclear receptor ERR β. Genes Dev. 2001;15:833–838. doi: 10.1101/gad.873401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharon D, Blackshaw S, Cepko CL, Dryja TP. Profile of the genes expressed in the human peripheral retina, macula, and retinal pigment epithelium determined through serial analysis of gene expression (SAGE) Proc Natl Acad Sci USA. 2002;99:315–320. doi: 10.1073/pnas.012582799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J, Nathans J. Estrogen-related receptor β/NR3B2 controls epithelial cell fate and endolymph production by the stria vascularis. Dev Cell. 2007;13:325–337. doi: 10.1016/j.devcel.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Collin RW, et al. Mutations of ESRRB encoding estrogen-related receptor β cause autosomal-recessive nonsyndromic hearing impairment DFNB35. Am J Hum Genet. 2008;82:125–138. doi: 10.1016/j.ajhg.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuda T, Cepko CL. Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proc Natl Acad Sci USA. 2004;101:16–22. doi: 10.1073/pnas.2235688100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Onishi A, et al. Pias3-dependent SUMOylation directs rod photoreceptor development. Neuron. 2009;61:234–246. doi: 10.1016/j.neuron.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng H, et al. In vivo function of the orphan nuclear receptor NR2E3 in establishing photoreceptor identity during mammalian retinal development. Hum Mol Genet. 2006;15:2588–2602. doi: 10.1093/hmg/ddl185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu DD, Forman BM. Identification of an agonist ligand for estrogen-related receptors ERRβ/γ. Bioorg Med Chem Lett. 2005;15:1311–1313. doi: 10.1016/j.bmcl.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 25.Jaissle GB, et al. Evaluation of the rhodopsin knockout mouse as a model of pure cone function. Invest Ophthalmol Vis Sci. 2001;42:506–513. [PubMed] [Google Scholar]
- 26.Aoyama T, et al. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor α (PPARα) J Biol Chem. 1998;273:5678–5684. doi: 10.1074/jbc.273.10.5678. [DOI] [PubMed] [Google Scholar]
- 27.Giguère V. Transcriptional control of energy homeostasis by the estrogen-related receptors. Endocr Rev. 2008;29:677–696. doi: 10.1210/er.2008-0017. [DOI] [PubMed] [Google Scholar]
- 28.Furukawa T, Morrow EM, Li T, Davis FC, Cepko CL. Retinopathy and attenuated circadian entrainment in Crx-deficient mice. Nat Genet. 1999;23:466–470. doi: 10.1038/70591. [DOI] [PubMed] [Google Scholar]
- 29.Tremblay AM, Giguere V. The NR3B subgroup: An ovERRview. Nucl Recept Signal. 2007;5:e009. doi: 10.1621/nrs.05009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo J, et al. Placental abnormalities in mouse embryos lacking the orphan nuclear receptor ERR-β. Nature. 1997;388:778–782. doi: 10.1038/42022. [DOI] [PubMed] [Google Scholar]
- 31.Okawa H, Sampath AP, Laughlin SB, Fain GL. ATP consumption by mammalian rod photoreceptors in darkness and in light. Curr Biol. 2008;18:1917–1921. doi: 10.1016/j.cub.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Punzo C, Kornacker K, Cepko CL. Stimulation of the insulin/mTOR pathway delays cone death in a mouse model of retinitis pigmentosa. Nat Neurosci. 2009;12:44–52. doi: 10.1038/nn.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aslanukov A, et al. RanBP2 modulates Cox11 and hexokinase I activities and haploinsufficiency of RanBP2 causes deficits in glucose metabolism. PLoS Genet. 2006;2:e177. doi: 10.1371/journal.pgen.0020177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daiger SP, Bowne SJ, Sullivan LS. Perspective on genes and mutations causing retinitis pigmentosa. Arch Ophthalmol. 2007;125:151–158. doi: 10.1001/archopht.125.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.den Hollander AI, Roepman R, Koenekoop RK, Cremers FP. Leber congenital amaurosis: Genes, proteins and disease mechanisms. Prog Retin Eye Res. 2008;27:391–419. doi: 10.1016/j.preteyeres.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 36.Hayashi S, Tenzen T, McMahon AP. Maternal inheritance of Cre activity in a Sox2Cre deleter strain. Genesis. 2003;37:51–53. doi: 10.1002/gene.10225. [DOI] [PubMed] [Google Scholar]
- 37.Okoye G, et al. Increased expression of brain-derived neurotrophic factor preserves retinal function and slows cell death from rhodopsin mutation or oxidative damage. J Neurosci. 2003;23:4164–4172. doi: 10.1523/JNEUROSCI.23-10-04164.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.