Abstract
Dendritic cells (DCs) play a central role in determining the induction of T cell responses. IL-27 production by DCs favors induction of IL-10–producing regulatory T cells, whereas osteopontin (OPN) promotes pathogenic IL-17 T cell responses. The regulatory mechanisms in DCs that control these two cells types are not understood well. Here, we show that IFN-γ induces IL-27 while inhibiting OPN expression in DCs both in vitro and in vivo and that engagement of IFN-γR expressed by DCs leads to suppression of IL-17 production while inducing IL-10 from T cells. DCs modified by IFN-γ acquire IL-27–dependent regulatory function, promote IL-10–mediated T cell tolerance, and suppress autoimmune inflammation. Thus, our results identify a previously unknown pathway by which IFN-γ limits IL-17–mediated autoimmune inflammation through differential regulation of OPN and IL-27 expression in DCs.
IL-27 is a potent antiinflammatory cytokine, which belongs to the IL-12 family and is comprised of an IL-12p40–related protein, encoded by the EBV-induced gene 3 (EBI3, also known as IL27), and a unique IL-12p35–like protein, IL-27p28v (1). Initial animal studies on the biology of IL-27 suggested a role for IL-27 in the initiation of the Th1 response (2, 3); however, subsequent work using mouse models of pathogen-induced and autoimmune inflammation have indicated that IL-27 has broad inhibitory effects on Th1, Th2 subsets of T cells and APC function in mice (4, 5). In addition, we and others have shown that IL-27 is capable of inducing IL-10–producing regulatory Tr1 cells while inhibiting IL-17–producing Th17 cells both from humans and mice and acts as a negative feedback mechanism against proinflammatory immune responses (6–10).
Contrary to the function of IL-27, osteopontin (OPN) is known to have potent proinflammatory functions. OPN participates in a wide range of biological processes (11) and has been linked to autoimmune diseases including multiple sclerosis (MS) and its animal model experimental autoimmune encephalomyelitis (EAE). Mice deficient for OPN (Spp1−/−, also known as OPN−/−) show milder EAE without disease exacerbation or progression compared with WT mice (12, 13). Patients with MS have elevated levels of OPN in their serum and plasma (14, 15). OPN exacerbates EAE by skewing T cell differentiation toward IFN-γ–producing Th1 cells and IL-17–producing Th17 cells (12, 13, 16–18). In addition, OPN induces IFN-γ– and IL-17–producing T cells in patients with MS (16).
Dendritic cells (DCs) are crucial in the initiation of productive antigen-specific T cell responses and the induction of T cell tolerance (19, 20). This dual function was initially explained by the existence of specific subpopulations of DCs that preferentially trigger T cell priming, whereas other subpopulations were identified to induce T cell tolerance (21–23). The demonstration that a single DC subpopulation can elicit both T cell outcomes, led to an alternative explanation that the functional status of the DC at the time of antigen presentation, rather than its phenotypic characteristics, is critical for determining T cell responses (24). Among the factors linked to the functional status of DCs, the types of cytokines secreted by DCs can regulate the differentiation of CD4+ T cells into functional subsets including IL-17–producing pathogenic Th17 cells and IL-10–producing regulatory Tr1 cells. For example, IL-10 or IL-27 production by DCs favors generation of IL-10–producing Tr1 cells (6, 25), whereas OPN production by DCs promotes IL-17–producing Th17 cells (16, 17). However, it is unknown how IL-27 and OPN expression in DCs are modulated to regulate both IL-10 and IL-17 production by T cells.
Here, we show that IFN-γ induces IL-27 while inhibiting OPN expression in DCs both in vitro and in vivo. IFN-γ−/−-deficient mice in which EAE is induced have increased serum OPN and lower IL-27 levels in comparison with WT mice. Engagement of IFNγR expressed by DCs leads to suppression of IL-17 production and induction of IL-10 from T cells. Furthermore, IFN-γ–modified DCs ameliorate the disease severity of EAE through an IL-27–dependent mechanism. Taken together, our results identify a previously unknown pathway by which IFN-γ limits IL-17–mediated autoimmune inflammation through reciprocal DC modulation of OPN and IL-27.
Results
IFN-γ Reciprocally Regulates OPN and IL-27 Expression in DCs.
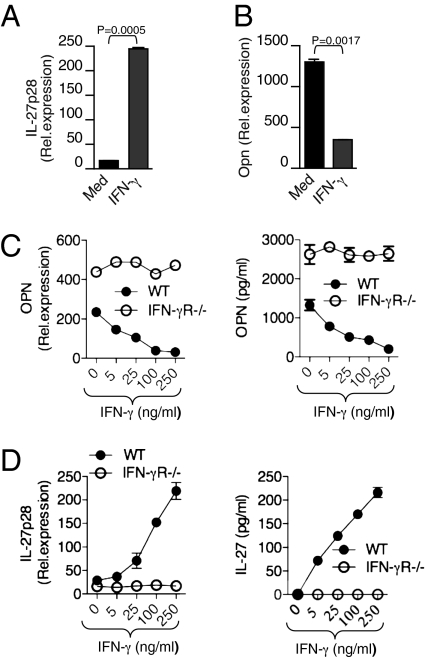
To study the effect of IFN-γ on DCs, we first examined its effect on the production of both pro- and antiinflammatory cytokines from DCs. We found that IFN-γ stimulation of DCs markedly induced IL-27 levels while inhibiting OPN expression in DCs (Fig. 1 A and B). IFN-γ stimulation did not affect other DC-associated cytokines including IL-1β, IL-6, IL-12, IL-23, and TGF-β (Fig. S1). IFN-γ inhibited OPN and induced IL-27 at both mRNA and protein levels from WT-DCs in a dose-dependent manner. IFN-γ stimulation failed to inhibit OPN or induce IL-27 expression from IFN-γR−/− DCs. In addition we found that IFN-γR−/− DCs expressed high levels of OPN in comparison with WT-DCs, whereas IL-27 levels were low in IFN-γR−/− DCs compared with WT-DCs (Fig. 1 C and D). Taken together, these results demonstrate that IFN-γ reciprocally regulates OPN and IL-27 expression in DCs.
Fig. 1.
IFN-γ reciprocally regulates OPN and IL-27 expression in DCs. (A and B) Real-time quantitative RT-PCR analysis of mRNA encoding IL-27p28 and OPN in DCs treated with or without mouse rIFN-γ (100 ng/mL). (C and D) Dose-dependent effect of IFN-γ on IL-27 and OPN expression in DCs from WT and IFN-γR−/− mice. Shown is real-time quantitative RT-PCR analysis of mRNA encoding IL-27p28 and OPN in DCs. Data are representative of five independent experiments, and the error bars represent the mean ± SD.
Altered OPN and IL-27 Expression in DCs Differentially Modulate T Cell Production of IL-17 and IL-10.
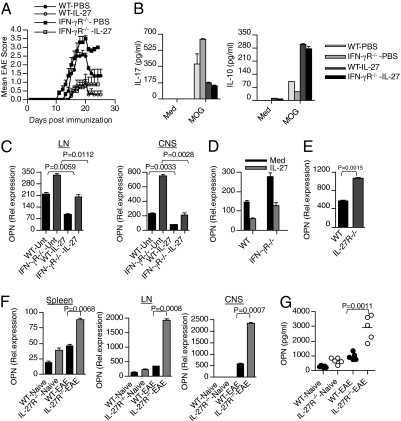
To test whether IFN-γ reciprocally regulated DC expression of OPN and IL-27 in vivo, we induced EAE in WT and IFN-γ−/− mice. We found that OPN mRNA expression was markedly increased in DCs at the peak of disease in spleen, lymph node (LN), and CNS. Furthermore, OPN levels were higher in DCs from IFN-γ−/− mice compared with WT mice (Fig. 2A). In accordance with OPN mRNA expression, IFN-γ−/− DCs expressed higher OPN at the protein level as measured by Western blot (Fig. 2B). To investigate whether IL-27 induction is also involved in the inhibitory effects of IFN-γ on EAE development in vivo, we examined IL-27 expression in DCs from WT and IFN-γ−/− mice with EAE. We found that expression of IL-27 was up-regulated in WT-DCs of mice with EAE (Fig. 2C) and that DCs from IFN-γ−/− mice with EAE had much less IL-27 expression compared with WT mice (Fig. 2C). These results demonstrate that IFN-γ up-regulates the expression of IL-27 while inhibiting OPN in DCs to negatively regulate the progression of autoimmune disease. Consistent with this observation, we found that IFN-γ−/− mice had high serum levels of OPN with little or no increase in serum IL-27 levels compared with WT mice (Fig. S2). These results clearly indicate that IFN-γ differentially modulates OPN and IL-27 expression in DCs in vivo during the course of EAE and, thus, could impact on immune responses both in the periphery and CNS.
Fig. 2.
IFN-γ deficiency alters OPN and IL-27 expression in DCs during EAE. (A) Real-time RT-PCR analysis of OPN in DCs isolated from spleen, LN, and CNS of naïve and EAE-bearing WT and IFN-γ−/− mice (n = 5–6 per group). (B) OPN protein expression was determined by Western blot analysis using anti-OPN Abs. (C) IL-27 expression is elevated in WT-DCs during the course of EAE. Real-time RT-PCR analysis of IL-27 in DCs isolated from spleen, LN, and CNS of naïve and EAE-bearing WT and IFN-γ−/− mice (n = 5–6 per group).
Polarization of T cells to Th1, Th2, or Th17 phenotypes is a critical feature of cell–mediated immunity and is influenced by production of cytokines by DCs. We and others have shown that DC-expressed OPN induces IL-17 while inhibiting IL-10 production from T cells (17, 20). In contrast, IL-27 has been shown to inhibit IL-17 while inducing IL-10 production from T cells both in humans and mice (6–13). Because we observed IFN-γ deficiency led to altered OPN and IL-27 expression in DCs during EAE, we investigated whether DC-derived OPN and IL-27 had a role in T cell differentiation, specifically T cell production of IL-17 and IL-10. To test this hypothesis, we induced EAE in WT and IFN-γ−/− mice with myelin oligodendrocyte glycoprotein (MOG) and, at day 15, postimmunization isolated splenic CD11c+ DCs. Naïve splenic DCs were used as controls. We cocultured naïve DCs and DCs derived from immunized mice from WT and IFN-γ−/− mice with MOG-specific TCR transgenic CD4+ T cells (2D2) and measured MOG Ag-specific IL-17 and IL-10 production. We found that T cells cultured with DCs from IFN-γ−/− mice showed increased IL-17 levels compared with T cells cultured with WT DCs (Fig. 3A). When IL-10 secretion was quantified, significant differences were observed between T cells cultured with DCs from IFN-γ−/− mice and WT controls. In accordance with low IL-27 expression, IFN-γ−/− DCs induced less IL-10 production by T cells whereas T cells cultured with DCs from WT mice show an increase in IL-10 levels (Fig. 3A). We then investigated whether the IFN-γ activation of DCs could alter the balance of IL-10 and IL-17 production from T cells through differential regulation of OPN and IL-27 in DCs. To test this hypothesis, the in vivo isolated DCs from EAE mice were pretreated with IFN-γ and cultured with 2D2 T cells in the presence of MOG. As shown in Fig. 3B, IFN-γ–treated DCs both from WT and IFN-γ−/− mice, which express IFNγR, inhibited IL-17 production while increasing IL-10 production from T cells. However, this effect was not observed when a neutralizing antibody to IL-27 was included in the culture. Taken together, our results demonstrate that IFN-γ signaling events in DCs play a major role in negative regulation of Th17 development and induction of IL-10 production from T cells.
Fig. 3.
Altered OPN and IL-27 expression in DCs differentially modulate T cell production of IL-17 and IL-10. (A) Total CD4+ T cells isolated from 2D2 mice were cocultured with CD11c+ DCs isolated from naïve and MOG35–55-immunized WT and IFN-γ−/− mice. Supernatants from cultures were harvested 72 h after initiation of cultures and assayed by ELISA for IL-17 and IL-10. (B) IFN-γ treated DCs suppress IL-17 production while inducing IL-10 production from 2D2 T cells. WT and IFN-γ−/− DCs isolated from EAE mice were stimulated with IFN-γ for 12 h and were then cultured with 2D2 T cells in the presence of MOG peptide for 72 h. After 72 h, IL-17 and IL-10 production by CD4+ T cells was determined by ELISA. (C) OPN-induced IL-17 production or IL-10 inhibition from CD4+ T cells was abrogated by the addition of IL-27. CD4+ T cells were activated with anti-CD3 and anti-CD28 mAb (0.3 μg/mL) in the presence of 1 μg/mL mouse rOPN. In some culture conditions, 100 ng/mL of mouse rIL-27 was added together with 1 μg/mL mouse rOPN. Supernatants from cultures were analyzed for cytokines IL-17 and IL-10 by ELISA. Data are representative of three independent experiments, and the error bars represent the mean ± SD.
To directly demonstrate the effect of OPN and IL-27 on T cells, we stimulated CD4+ T cells with rOPN in the presence or absence of rIL-27. We found that CD4+ T cells activated in the presence of OPN produced increased amounts of IL-17 and less IL-10 (Fig. 3C). However, the addition of IL-27 inhibited OPN-induced IL-17 while abrogating the inhibitory effect of OPN on IL-10 from T cells (Fig. 3C). Because we observed IFN-γ deficiency leads to increased OPN and low IL-27 levels in DCs during EAE, we next examined whether T cells from IFN-γ−/− mice exhibited changes in IL-17 and IL-10 expression. In accordance with increased OPN expression in DCs, T cells from IFN-γ−/− mice had high levels of IL-17 expression in spleen, LN, and CNS (Fig. 4A). When IL-10 levels were measured, we also found striking differences between T cells from WT and IFN-γ−/− mice. In accordance with increased IL-27 levels in DCs, T cells from WT mice expressed higher levels of IL-10 in comparison with T cells from IFN-γ−/− mice in spleen, LN, and CNS (Fig. 4B). Moreover, we observed higher secretion of IL-17 and less IL-10 production by ex vivo-restimulated LN cells from IFN-γ−/− mice than by cells from WT mice (Fig. 4C). Taken together, our results demonstrate that IFN-γ activation of DCs reciprocally regulates T cell IL-17 and IL-10 production through differential induction of OPN and IL-27.
Fig. 4.
IFN-γ deficiency differentially regulates IL-17 and IL-10 expression in T cells. (A and B) Real-time RT-PCR analysis of IL-17 and IL-10 in spleen, LN, and CNS-derived T cells were isolated from WT and IFN-γ−/− mice with or without EAE (n = 8 per group). (C) LN cells derived from WT and IFN-γ−/− mice were activated in vitro with MOG35–55 (20 μg/mL) for 72 h, and cell-free supernatants from cultures were assayed for IL-17 and IL-10.
IFN-γ–Modified DCs Attenuate EAE in an IL-27–dependent Manner.
To determine whether IFN-γ–modified DCs have enhanced regulatory function in vivo, we activated DCs with or without mouse recombinant IFN-γ, pulsed them with MOG, and transferred these cells into syngeneic naïve mice. We then immunized the mice with MOG peptide and monitored disease progression. We found that pretreatment with IFN-γ–modified DCs markedly reduced clinical severity of EAE compared with mice treated with control DCs (Fig. 5A). Furthermore, the effect of IFN-γ–modified DCs on reducing the clinical severity of EAE was abrogated in the mice that received neutralizing IL-27 antibody (Fig. 5A). We then analyzed cytokine production in splenocytes of mice given IFN-γ–treated DCs or control DCs after ex vivo restimulation with MOG. We found large amounts of IL-17 and small amounts of IL-10 in splenocytes of mice given control DCs (Fig. 5B). In contrast, transfer of IFN-γ–treated DCs resulted in less secretion of IL-17 and increased synthesis of IL-10 in splenocytes of recipient mice (Fig. 5B). Coinjection of IFN-γ–treated DCs with anti-IL-27 antibody resulted in higher secretion of IL-17 and less IL-10 production by ex vivo-restimulated splenocytes than by cells from mice that received IFN-γ–treated DCs. These results demonstrate a dominant tolerogenic effect of IFN-γ–treated DCs (Fig. 5B). To further examine the contribution of IL-27 to this tolerogenic effect, we injected IFN-γ-treated DCs or control DCs into WT and IL-27R−/− mice. We found that IFN-γ–modified DCs reduced clinical severity only in WT mice. Disruption of IL-27 abrogated the tolerogenic effect of IFN-γ–treated DCs, which were not able to limit disease severity (Fig. 5C). In accordance with the disease score, disruption of the IL-27 pathway abrogated the disease-modifying effect of DCs by modulating T cell cytokine secretion (Fig. 5D). In the clinical setting of MS, therapeutic intervention is often started after the onset of the symptoms. Therefore, it is important to investigate whether a treatment regimen, which is effective EAE prevention, can also reverse established disease. Thus, we tested the efficacy of IFN-γ–modified DCs on EAE after the onset of clinical symptoms. We found that treatment with IFN-γ–modified DCs after the onset of EAE (score of ≥1.5) resulted in rapid clinical recovery from EAE (Fig. 5E). These results indicate an essential role for IFN-γ in driving DCs that blunt Th17 responses and halt autoimmune inflammation through mechanisms involving IL-27 and IL-10.
Fig. 5.
IFN-γ–modified DCs attenuate EAE in an IL-27–dependent manner. (A) DCs were left untreated (DC-Unt) or treated with IFN-γ (DC-IFN-γ) and pulsed with MOG and were then transferred into syngeneic WT mice. As indicated, some mice were coinjected with neutralizing anti-IL-27 antibody together with IFN-γ-treated DCs. Five days later, the DC primed mice were immunized with MOG and monitored for EAE. (B) LN cells obtained 18 d after immunization from mice described in (A) above, were restimulated with MOG35–55 (20 μg/mL) for 72 h. Cell-free supernatants were assayed for IL-17 and IL-10 by ELISA. (C) Clinical scores of EAE in WT and IL-27R−/− mice injected with IFN-γ–treated or –untreated DCs before MOG immunization. (D) LN cells from IFN-γ-modified DC-treated and control DC-treated WT and IL-27R−/− mice were activated in vitro with MOG35–55 (20 μg/mL) for 72 h, and cell-free culture supernatants were assayed for IL-17 and IL-10 by ELISA. (E) Clinical scores of EAE in WT mice immunized with MOG and treated with MOG-pulsed DCs modified with IFN-γ or their unmodified counterparts. Arrow indicates time of DC injection (clinical score of ≥1.5).
IL-27 Treatment Inhibits Clinical Severity of EAE in IFN-γR–Deficient Mice.
Our results thus far suggest that IFN-γ suppresses EAE development via induction of IL-27. To further investigate this observation, we asked whether IL-27 treatment in vivo could reverse the severe EAE phenotype observed in IFNγR−/− mice. To test this hypothesis, WT and IFN-γR−/− mice were administered recombinant mouse IL-27 after immunization with MOG. Consistent with recent studies (10), we found that IL-27 could inhibit EAE development in WT mice (Fig. 6A). Strikingly, injection of IL-27 suppressed the severe EAE phenotype observed in IFN-γR−/− mice compared with PBS controls (Fig. 6A). In addition, LN cells from both IL-27-treated WT and IFN-γR−/− mice produced much less IL-17 and higher IL-10 when restimulated with MOG antigen (Fig. 6B). Furthermore, we found that IL-27 treatment inhibited OPN levels in DCs from both WT and IFN-γR−/− mice (Fig. 6C). Because we observed that IFN-γ–induced IL-27 expression was accompanied by reduced OPN levels in DCs, we examined whether IL-27 had any inhibitory effects on OPN expression in DCs. In accordance with the in vivo inhibition of OPN, we found that IL-27 significantly inhibited OPN expression in DCs both from WT and IFN-γR−/− mice (Fig. 6D). In addition we found that bone marrow-derived DCs from IL-27R−/− mice expressed higher levels of OPN than DCs from WT mice (Fig. 6E). Moreover, DCs isolated from spleen, LN, and CNS of IL-27R−/− mice had very high levels of OPN compared with WT mice (Fig. 6F), and serum OPN levels were significantly higher in IL-27R−/− mice in comparison with WT mice (Fig. 6G). These findings indicate that the reduced OPN expression observed in DCs from IL-27–treated mice is due to the inhibitory effect of IL-27 on OPN. Thus, IL-27–mediated resolution of inflammation is mediated both through induction of IL-10 and inhibition of IL-17 from CD4+ T cells and by inhibition of OPN expression in DCs. Taken together, these results demonstrate that IFN-γ serves a protective role in Th17-mediated CNS inflammation via IL-27 induction.
Fig. 6.
IL-27 treatment inhibits clinical severity of EAE in IFN-γR−/− mice. (A) IL-27 inhibits clinical severity of EAE in WT and IFN-γR−/− mice. WT and IFN-γR−/− mice (n = 6) were immunized with MOG peptide emulsified in complete freund's adjuvant. Recombinant IL-27 (0.25 μg per mouse) was administered by s.c. to immunized WT and IFN-γR−/− mice every other day from day 2 until day 18. (B) LN cells from IL-27-treated and control mice were activated in vitro with MOG35–55 (20 μg/mL) for 72 h, and cell-free culture supernatants were assayed for IL-17 and IL-10 by ELISA. (C) Real-time RT-PCR analysis of OPN in DCs isolated from LN and CNS of WT and IFN-γR−/− mice (n = 5–6 per group) treated with or without IL-27. (D) Real-time RT-PCR analysis of OPN in DCs isolated from spleen of naïve WT and IFN-γR−/− mice treated with or without rIL-27 (100 ng/mL). (E) Real-time RT-PCR analysis of OPN in DCs isolated from spleen of naïve WT and IL-27R−/− mice. (F) Real-time RT-PCR analysis of OPN in DCs isolated from spleen, LN, and CNS of naïve and EAE bearing WT and IL-27R−/− mice (n = 6 per group). (G) ELISA of serum OPN from WT and IL-27R−/− mice with or without EAE.
Discussion
Our results identify a previously unknown pathway by which IFN-γ limits IL-17–mediated autoimmune inflammation through DC modulation of OPN and IL-27. In EAE, it was initially thought that CD4+ T cells mediating autoimmunity had a Th1 phenotype characterized by the production of IFN-γ (26). However, this traditional view has been challenged by studies describing more EAE in IFN-γ–deficient animals (27). Evidence now indicates that T cells critical for EAE are characterized by the production of IL-17. In addition, IL-17 expression has been detected in the target tissue in human autoimmune diseases including MS, rheumatoid arthritis, and psoriasis (28). IL-17–deficient mice develop EAE with delayed onset and reduced severity (29), and anti-IL-17 antibody prevents chemokine expression in the brain and the subsequent development of EAE (30). Furthermore, T cell infiltration and inflammation in the brain in EAE occur only when Th17 cells outnumber Th1 cells (31).
IFN-γ plays an important role in Th17 cell biology. IFN-γ−/− mice immunized with collagen in CFA develop severe arthritis, and T cells from such mice secrete increased amounts of IL-17 after collagen restimulation in vitro (32). Along the same line, after mycobacterial infection, IFN-γ−/− mice had larger numbers of IL-17–producing T cells than WT mice (33). In addition, a disease-ameliorating effect of IFN-γ has been observed in lethal autoimmune myocarditis (34). Nonetheless, although endogenous IFN-γ is protective in animal models of arthritis and in EAE, the mechanisms that orchestrate the antiinflammatory effects of IFN-γ in controlling autoimmune inflammation are poorly understood. Here, we show that IFN-γ induces IL-27 while inhibiting OPN expression in DCs and that engagement of IFNγR expressed by DCs leads to suppression of IL-17 production while inducing IL-10 from T cells. In accordance with these in vitro results, DCs from IFN-γ−/− mice expressed very high levels of OPN and low levels of IL-27 in comparison with DC from WT mice. T cells cultured with IFN-γ−/− DCs produced higher IL-17 and lower IL-10, whereas T cells cultured with DCs from WT mice produced lower IL-17 and higher IL-10. This difference in T cell production of IL-17 and IL-10 correlated with the pattern of OPN and IL-27 expression by DCs that are modulated by IFN-γ. We have reported that OPN-induced IL-17 production by CD4 T cells via β3-integrin receptor and inhibited IL-10 via CD44 receptor (16). We have also shown that OPN-induced IL-17 production is associated with an increase in the IL-17 lineage transcription factor RORγt expression. IL-27–mediated inhibition of IL-17 has been shown through the down-regulation of RORγt (7). It appears that IL-27 overrides the effect of OPN in activated T cells by down-regulating RORγt expression and indirectly affects IL-10 via the CD44 receptor. Thus, our results demonstrate that there is a reciprocal regulation in the induction of pathogenic cytokine IL-17 and protective cytokine IL-10 in the immune system depending on the cytokine secretion by the innate immune system.
Previous studies have shown that the differentiation of Th17 cells is suppressed by IFN-γ. In vitro, treatment with IFN-γ-neutralizing antibody leads to increased frequency of Th17 cells, whereas exogenous IFN-γ reduces Th17 populations (35). Our results identify a previously unknown pathway by which IFN-γ limits IL-17–mediated autoimmune inflammation through DC modulation of OPN and IL-27. The negative impact of IFN-γ on IL-17 production from T cells was mediated through inhibition of OPN and induction of IL-27 from DCs that, in turn, leads to suppression of IL-17 from T cells. In addition, Th17 generation was also suppressed by expression of the IFN-αR receptor on DCs and macrophages, thus demonstrating the potential similarities between IFN-γ– and IFN-α/β–dependent suppression of the Th17 response (17, 36).
The nature of specialized DCs that selectively dampen effector T cell-driven immunity is not well understood. Our results suggest a dominant function for IFN-γ–modified DCs to dampen autoimmunity through the induction of IL-10 and/or inhibition of IL-17 production from T cells in an IL-27–dependent manner. It has been shown that IL-27R−/− mice develop exacerbated EAE, owing to high IL-17 or low IL-10 production from T cells (8–10). Consistent with these findings, the tolerogenic effect of IFN-γ-modified DCs we observed was completely abrogated in IL-27R−/− mice. The increased expression of IL-27 in WT vs. IFN-γ–deficient mice correlates with increased IL-10 expression in T cells during EAE, which suggests that IFN-γ contributes to dampening inflammatory responses and driving the resolution of autoimmune pathology.
An additional mechanism to limit immune responses is the negative feedback of inflammatory process by inflammatory cytokines. DC expressed OPN promotes induction of IFN-γ in addition to IL-17 from T cells both in humans and mice (19). Our results demonstrate that OPN induces a negative feedback loop whereby IFN-γ limits IL-17 production by T cells through the induction of IL-27 and inhibition of OPN from DCs, and that this feedback loop is perturbed by the absence of IFN-γ (Fig. S3). The role of IFN-γ in antagonizing the function of IL-17, a critical pathogenic cytokine in autoimmune conditions, might be paradoxical for IFN-γ, a proinflammatory cytokine. However, it is now recognized that IFN-γ has regulatory function in limiting inflammation. At the height of inflammation during the course of an autoimmune pathology, the immune system is mobilized to restrict excess inflammation, and it appears that IFN-γ acts as a master upstream regulator of both inflammatory and regulatory pathways (37, 38). Our study identifies an important IFN-γ–dependent self-regulatory process that serves to control immune system homeostasis through an OPN/IL-27 axis in dendritic cells.
Materials and Methods
DCs and CD4+ T cells were purified from spleens and LNs by positive selection using CD11C+ and CD4+ microbeads, respectively (Miltenyi Biotec). Bone marrow-derived DCs were generated by using a described method (39). For in vitro assays, DCs were activated with recombinant mouse IFN-γ for 12–60 h. Splenocytes or LN cells were activated with MOG 35–55 peptide (20 μg/mL) for 60–72 h. For DC-T cell cocultures, total CD4+ T cells isolated from MOG-specific TCR-transgenic mice (2D2) were cocultured with CD11c+ DC isolated from naïve and MOG35-55-immunized WT and IFN-γ−/− mice. Cocultures were performed at a 1:3 DC/T cell ratio in U-bottom 96-well plates. Cytokines were measured by using real-time quantitative RT-PCR, and specific ELISA and Western blotting were performed with primers and antibodies as listed in SI Materials and Methods. For EAE induction, mice were injected s.c. with 100 mg of MOG35–55 peptide emulsified in CFA (Difco), supplemented with 5 mg/mL Mycobacterium tuberculosis and injected twice i.v. with 200 ng of pertussis toxin. Clinical assessment of EAE was performed as described in SI Materials and Methods. See SI Materials and Methods for full methods.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants NS038037, AI043458, and NS23132 and the Nancy Davis Foundation. G.M is supported by a postdoctoral fellowship from National Multiple Sclerosis Society, New York. A.M. is supported by the National Research Service Award Fellowship Grant F32AI075761 from the National Institute of Allergy and Infectious Diseases.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1002099107/-/DCSupplemental.
References
- 1.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: Related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- 2.Pflanz S, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 3.Takeda A, et al. Cutting edge: Role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol. 2003;170:4886–4890. doi: 10.4049/jimmunol.170.10.4886. [DOI] [PubMed] [Google Scholar]
- 4.Yoshimoto T, Yoshimoto T, Yasuda K, Mizuguchi J, Nakanishi K. IL-27 suppresses Th2 cell development and Th2 cytokines production from polarized Th2 cells: A novel therapeutic way for Th2–mediated allergic inflammation. J Immunol. 2007;179:4415–4423. doi: 10.4049/jimmunol.179.7.4415. [DOI] [PubMed] [Google Scholar]
- 5.Wang S, Miyazaki Y, Shinozaki Y, Yoshida H. Augmentation of antigen-presenting and Th1-promoting functions of dendritic cells by WSX-1(IL-27R) deficiency. J Immunol. 2007;179:6421–6428. doi: 10.4049/jimmunol.179.10.6421. [DOI] [PubMed] [Google Scholar]
- 6.Awasthi A, et al. A dominant function for interleukin 27 in generating interleukin 10–producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 7.Murugaiyan G, et al. IL-27 is a key regulator of IL-10 and IL-17 production by human CD4+ T cells. J Immunol. 2009;183:2435–2443. doi: 10.4049/jimmunol.0900568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Batten M, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17–producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 9.Stumhofer JS, et al. Interleukin 27 negatively regulates the development of interleukin 17–producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 10.Fitzgerald DC, et al. Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. J Immunol. 2007;179:3268–3275. doi: 10.4049/jimmunol.179.5.3268. [DOI] [PubMed] [Google Scholar]
- 11.Denhardt DT, Noda M, O'Regan AW, Pavlin D, Berman JS. Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest. 2001;107:1055–1061. doi: 10.1172/JCI12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chabas D, et al. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science. 2001;294:1731–1735. doi: 10.1126/science.1062960. [DOI] [PubMed] [Google Scholar]
- 13.Jansson M, Panoutsakopoulou V, Baker J, Klein L, Cantor H. Cutting edge: Attenuated experimental autoimmune encephalomyelitis in eta-1/osteopontin-deficient mice. J Immunol. 2002;168:2096–2099. doi: 10.4049/jimmunol.168.5.2096. [DOI] [PubMed] [Google Scholar]
- 14.Comabella M, et al. Plasma osteopontin levels in multiple sclerosis. J Neuroimmunol. 2005;158:231–239. doi: 10.1016/j.jneuroim.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Vogt MH, et al. Osteopontin levels and increased disease activity in relapsing-remitting multiple sclerosis patients. J Neuroimmunol. 2004;155:155–160. doi: 10.1016/j.jneuroim.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Murugaiyan G, Mittal A, Weiner HL. Increased osteopontin expression in dendritic cells amplifies IL-17 production by CD4+ T cells in experimental autoimmune encephalomyelitis and in multiple sclerosis. J Immunol. 2008;181:7480–7488. doi: 10.4049/jimmunol.181.11.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shinohara ML, Kim JH, Garcia VA, Cantor H. Engagement of the type I interferon receptor on dendritic cells inhibits T helper 17 cell development: Role of intracellular osteopontin. Immunity. 2008;29:68–78. doi: 10.1016/j.immuni.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinman L. A molecular trio in relapse and remission in multiple sclerosis. Nat Rev Immunol. 2009;9:440–447. doi: 10.1038/nri2548. [DOI] [PubMed] [Google Scholar]
- 19.Banchereau J, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 20.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 21.Huang FP, et al. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J Exp Med. 2000;191:435–444. doi: 10.1084/jem.191.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munn DH, et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297:1867–1870. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 23.Scheinecker C, McHugh R, Shevach EM, Germain RN. Constitutive presentation of a natural tissue autoantigen exclusively by dendritic cells in the draining lymph node. J Exp Med. 2002;196:1079–1090. doi: 10.1084/jem.20020991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belz GT, et al. The CD8alpha(+) dendritic cell is responsible for inducing peripheral self-tolerance to tissue-associated antigens. J Exp Med. 2002;196:1099–1104. doi: 10.1084/jem.20020861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wakkach A, et al. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity. 2003;18:605–617. doi: 10.1016/s1074-7613(03)00113-4. [DOI] [PubMed] [Google Scholar]
- 26.Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 27.Ferber IA, et al. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- 28.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 29.Komiyama Y, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 30.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stromnes IM, Cerretti LM, Liggitt D, Harris RA, Goverman JM. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat Med. 2008;14:337–342. doi: 10.1038/nm1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chu CQ, Swart D, Alcorn D, Tocker J, Elkon KB. Interferon-gamma regulates susceptibility to collagen-induced arthritis through suppression of interleukin-17. Arthritis Rheum. 2007;56:1145–1151. doi: 10.1002/art.22453. [DOI] [PubMed] [Google Scholar]
- 33.Cruz A, et al. Cutting edge: IFN-gamma regulates the induction and expansion of IL-17–producing CD4 T cells during mycobacterial infection. J Immunol. 2006;177:1416–1420. doi: 10.4049/jimmunol.177.3.1416. [DOI] [PubMed] [Google Scholar]
- 34.Afanasyeva M, et al. Impaired up-regulation of CD25 on CD4+ T cells in IFN-gamma knockout mice is associated with progression of myocarditis to heart failure. Proc Natl Acad Sci USA. 2005;102:180–185. doi: 10.1073/pnas.0408241102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harrington LE, et al. Interleukin 17–producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 36.Guo B, Chang EY, Cheng G. The type I IFN induction pathway constrains Th17–mediated autoimmune inflammation in mice. J Clin Invest. 2008;118:1680–1690. doi: 10.1172/JCI33342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang J. Yin and yang interplay of IFN-gamma in inflammation and autoimmune disease. J Clin Invest. 2007;117:871–873. doi: 10.1172/JCI31860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu X, Ivashkiv LB. Cross-regulation of signaling pathways by interferon-gamma: implications for immune responses and autoimmune diseases. Immunity. 2009;31:539–550. doi: 10.1016/j.immuni.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murugaiyan G, Agrawal R, Mishra GC, Mitra D, Saha B. Differential CD40/CD40L expression results in counteracting antitumor immune responses. J Immunol. 2007;178:2047–2055. doi: 10.4049/jimmunol.178.4.2047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.