Abstract
Failure of remyelination is largely responsible for sustained neurologic symptoms in multiple sclerosis (MS). MS lesions contain hyaluronan deposits that inhibit oligodendrocyte precursor cell (OPC) maturation. However, the mechanism behind this inhibition is unclear. We report here that Toll-like receptor 2 (TLR2) is expressed by oligodendrocytes and is up-regulated in MS lesions. Pathogen-derived TLR2 agonists, but not agonists for other TLRs, inhibit OPC maturation in vitro. Hyaluronan-mediated inhibition of OPC maturation requires TLR2 and MyD88, a TLR2 adaptor molecule. Ablated expression of TLR2 also enhances remyelination in a lysolecithin animal model. Hyaluronidases expressed by OPCs degrade hyaluronan to hyaluronan oligomers, a requirement for hyaluronan/TLR2 signaling. MS lesions contain both TLR2+ oligodendrocytes and low-molecular-weight hyaluronan, consistent with their importance to remyelination in MS. We thus have defined a mechanism controlling remyelination failure in MS where hyaluronan is degraded by hyaluronidases into hyaluronan oligomers that block OPC maturation and remyelination through TLR2-MyD88 signaling.
Keywords: hyaluronidase, MyD88, innate immunity
In multiple sclerosis (MS), destruction of CNS myelin accounts for a majority of neurologic symptoms. MS patients typically exhibit a relapsing/remitting course in which relapses result from inflammation and demyelination and remissions result from resolution of inflammation and remyelination. Secondary progressive MS, which typically begins in patients after a decade of relapsing/remitting MS, exhibits irreversible neurologic disability.
Most chronic MS lesions show little if any remyelination. The failure of remyelination in MS theoretically could be the consequence of a deficiency in the number of oligodendrocyte progenitor cells (OPCs), absence of a promyelination signal, or the presence of inhibitory influences on OPCs. It therefore is of interest that the histopathology of the MS lesion has revealed the presence of OPCs and premyelinating oligodendrocytes in chronic MS lesions. The premyelinating oligodendrocytes extend processes that contact but fail to myelinate axons (1–4), thus suggesting failure of remyelination is caused by the loss of promyelination signals or the presence of inhibitory signals.
Recently, the glycosaminoglycan hyaluronan was identified within MS lesions and found to inhibit OPC maturation and remyelination in an MS animal model (5). The mechanism underlying this inhibition is unknown. Because hyaluronan can function as an endogenous mammalian ligand for Toll-like receptors (TLRs) 2 and 4 in innate immune cell activation (6–9), we reasoned that TLR stimulation also may be required for hyaluronan-mediated blocking of OPC maturation.
Although TLRs and the Drosophila ortholog Toll have well-described functions in innate immune cells (6–12), TLRs also have potent functions outside the immune system. Toll and TLR have diverse roles in axonal pathfinding, dorsoventral patterning, and cell-fate determination (13). In particular, TLR ligands inhibit the differentiation of several cell types. TLR2 ligands block differentiation of mesenchymal stems cells into osteogenic, adipogenic, and chondrogenic cells (14). TLR2 and TLR4 also differentially regulate hippocampal neurogenesis by unknown ligand(s) (15). Interestingly, hyaluronan also inhibits the differentiation of adipose-derived stromal cells, osteoblasts, and keratinocytes (16–19). Although hyaluronan requires TLR2 or TLR4 to activate dendritic cells, macrophages, and microglia (6–9), it is unclear whether hyaluronan also requires TLRs to modulate differentiation.
In this report, we show that TLR2 is expressed in oligodendrocytes in MS lesions. Known TLR2 ligands function similarly to hyaluronan to hold OPCs in an immature state. TLR2 is required for repressive effects of hyaluronan on OPC maturation in vitro. TLR2-null mice exhibit enhanced remyelination in the lysolecithin remyelination model. The downstream adaptor molecule MyD88 also is essential for the in vitro effect of hyaluronan. We further show that hyaluronidases expressed by OPCs first must digest high-molecular-weight (HMW) hyaluronan before low-molecular-weight (LMW) hyaluronan can block maturation. In addition, we have identified small-molecular-weight compounds that interfere with the hyaluronan/TLR2/MyD88 signal that blocks OPC maturation.
Results
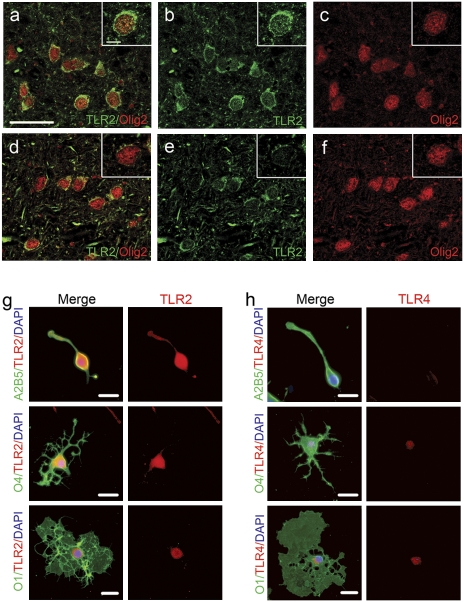
Because prior work indicated hyaluronan blocks OPC maturation (5), we hypothesized that hyaluronan triggers its effects via a hyaluronan receptor, such as TLR2 or TLR4. We first detected strong TLR2 staining throughout and very little TLR4 staining in OPCs in vitro (Fig. 1 G and H) (10, 12). OPCs also preferentially express TLR2 compared with mature oligodendrocytes. By Western blot, TLR2 immunodetection yielded one specific band with appropriate molecular weight (data not shown). We then examined TLR2 expression in oligodendrocytes in human MS proteolipid protein-negative/glial fibrillary acidic protein (GFAP)-positive chronic lesions and in normal-appearing white matter and found that TLR2 was expressed by oligodendrocytes within normal and lesioned tissue (Fig. 1 A–F). Therefore, TLR2 is present in chronic lesions and may be stimulated by hyaluronan also found in chronic lesions (Fig. 7A) (5).
Fig. 1.
TLR2 expression in MS lesions. (A–C) MS lesions and control brain specimens were stained for TLR2. Strong staining for TLR2 (green) was observed in oligodendrocytes within MS lesions that colocalizes to Olig2+ (red) cells). (D–F) In contrast, white matter from control subjects had only faint TLR2 immunostaining of oligodendrocytes. (Scale bars, 25 μm; Insets, scale bars, 5 μm.) (G) Purified OPCs or oligodendrocytes at various stages of maturation were stained for TLR2 (red) and A2B5, O4, or O1 (green). (H) There was faint TLR4 staining to cell bodies of OPCs or oligodendrocytes. (Scale bars, 20 μm.)
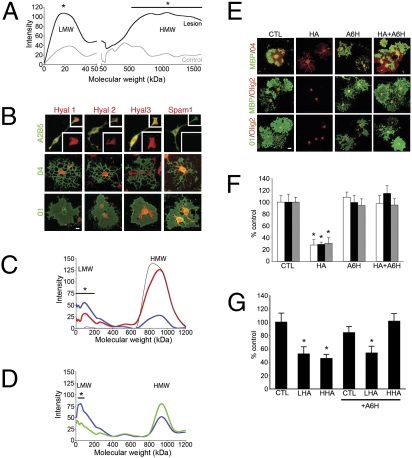
Fig. 7.
Hyaluronidases expressed by oligodendrocytes are essential for hyaluronan effects on oligodendrocyte progenitor maturation. Tissue from MS lesions or control white matter was homogenized and electrophoresed. Hyaluronan (HA) was detected by staining with 3,3′-dimethyl-9-methyl-4,5,4'5′-dibenzothiacarbocyanine and comparison with lanes of aliquoted specimens from identical starting tissue treated with hyaluronidase (Hyal) and β-glucuronidase for 1 h. (A) Densitometric graph of intensity staining versus kDa molecular weight. Differences (asterisks) were observed between MS lesion samples and control white matter hyaluronan at 20 kDa and 500–1500 kDa by t test (P < 0.05). (B) OPCs and oligodendrocytes were stained for hyaluronidases 1–3 and SPAM1 (red) in conjunction with oligodendrocyte markers A2B5, O4, and O1 (green) to determine maturational expression of these hyaluronidases. (C) Hyaluronan-containing medium was exposed to cultured OPCs (red line) or to no cells (thin black line) for 2 d. Conditioned medium then was electrophoresed and stained for hyaluronan as above. Also shown is hyaluronan (blue line) that remained cell associated after medium was removed and cells were washed with PBS. For cell associated hyaluronan, there was more LMW hyaluronan and less HMW hyaluronan compared with conditioned medium (red line). *, P < 0.05 by t test between OPC-exposed hyaluronan (red line) and unexposed hyaluronan (black line). After 10 μM A6H was added to OPC cultures, OPCs were exposed to hyaluronan for 2 d. Cell-associated hyaluronan then was collected and detected as above. (D) Molecular weight distribution of cell-associated hyaluronan from cultures exposed to 10 μM A6H (green line) and control cultures (blue line). *, P < 0.05 by t test in comparison with untreated control. (E) OPCs were treated with 25 μg/mL hyaluronan (CTL) and/or 10 μM A6H, cultured for 2 d, and stained for MBP, O1, O4, and olig2. (F) A6H treatment completely blocked the effect of hyaluronan on OPC maturation. (G) Rat OPCs were treated with PBS or 25 μg/mL HMW or LMW hyaluronan with or without 10 mM A6H for 2 d and stained for O1 and olig2. Percent O1+/olig2+ cells was calculated, showing that A6H blocked effects of HMW but not LMW hyaluronan on OPC maturation. *, P < 0.05 by t test in comparison with control (CTL). (Scale bars in B and D, 20 μm.)
Little is known about the underlying mechanism through which hyaluronan blocks OPC maturation. Through in vitro studies, we found that hyaluronan does not alter oligodendrocyte cell death or proliferation to give the appearance of impaired maturation (Fig. 2 D and E). Instead, we found hyaluronan blocks OPC maturation directly in a dose-dependent manner (Fig. 2C). Our hyaluronan preparations were free of contaminating proteins, DNA, or RNA that could account for the effects on OPC maturation (Fig. 6 A–H).
Fig. 2.
Hyaluronan directly blocks OPC maturation in vitro. (A) Purified OPCs were treated with 25 μg/mL hyaluronan (HA), 10 μg/mL lipoteichoic acid (LTA), 10 μg/mL zymosan (ZYM), 10 μg/mL peptidoglycan (PGN), 10 ng/mL LPS, or 500 ng/mL flagellin (FLA). (B) After 2 d culture with 10 ng/mL CNTF and 15 μM triiodothyronine, cells were stained for oligodendrocyte markers. Representative cell staining is shown for each condition. Note MBP and O1 staining intensity was adjusted similarly for all cells examined. Percent mature oligodendrocytes [percent 01+/olig2+ (white bars), percent MBP+/olig2+ (black bars), and percent MBP+/O4+, (gray bars)] was calculated, showing significant differences with untreated cells only for TLR2 ligands but not TLR4 or TLR5 ligands. (C) After exposure to hyaluronan for 48 h, rat OPCs were fixed and stained for O1/olig2 (white bars) MBP/olig2 (black bars), or MBP/O4 (gray bars). The percent O1+/olig2+ cells, MBP+/olig2+ cells, and MBP+/O4+ cells was calculated for exposures to PBS, 5 μg/mL hyaluronan, and 25 μg/mL hyaluronan. Using Live/Dead methods and BrdU immunostaining, respectively, the percent cell death (D) and percent BrdU+ cells (E) was measured after OPCs were exposed for 48 h to PBS, 25 μg/mL hyaluronan, 10 μg/mL LTA, 10 μg/mL ZYM, or 10 μg/mL PGN. Error bars represent SE. *, P < 0.05 by t test in comparison with control. (Scale bars, 20 μ.)
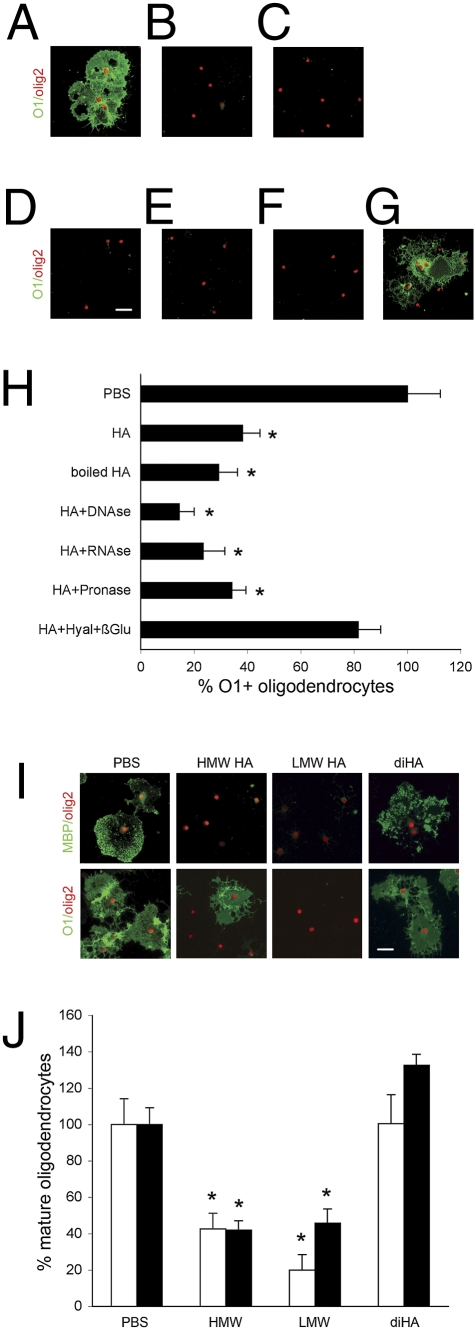
Fig. 6.
Both HMW and LMW hyaluronan block OPC maturation in vitro. Hyaluronan (HA) was treated by DNase, RNase, pronase E, or hyaluronidase (Hyal) with β-glucuronidase (β-glu) for 1 h. After enzymatic treatment, samples were boiled for 30 min. OPCs then were treated with the resulting mixtures at a final concentration of 25 μg/mL hyaluronan for 2 d and stained with O1 (green) and olig2 (red). Shown are representative figures of OPCs treated with PBS (A), hyaluronan (B), boiled hyaluronan (C), DNase-treated hyaluronan (D), RNase-treated hyaluronan (E), pronase E treated hyaluronan (F), and hyaluronidase/β-glucuronidase–treated hyaluronan (G). Percent mature oligodendrocytes was measured and compared with control PBS treatment (H). Only treatment with hyaluronidase and β-glucuronidase neutralized the effect of hyaluronan on OPC maturation. OPCs were exposed to 25 μg/mL 870-kDa hyaluronan (HMW hyaluronan), 25 μg/mL 0.8-kDa hyaluronan (LMW hyaluronan), 25 μg/mL hyaluronan disaccharide (diHA), or PBS for 48 h. (I) Representative figures are shown for each treatment and MBP or O1 (green) and olig2 (red) staining. (J) The percent O1+/olig2+ cells (white bars) and MBP+/olig2+ cells (black bars) was calculated for PBS and hyaluronan exposures. Error bars represent SE. *, P < 0.05 by t test in comparison with control. (Scale bars, 20 μm.)
Because of the abundance of TLR2 expression by OPCs, we tested whether known TLR2 agonists, including lipoteichoic acid, zymosan, and peptidoglycan, as well as hyaluronan, would have similar blocking effects on OPC maturation in vitro. All TLR2 agonists blocked OPC maturation, whereas LPS and flagellin (TLR4 and TLR5 agonists, respectively) had no effect (Fig. 2 A and B). Differences in maturation were not a product of cell death or alterations in total olig2+ cell numbers (data not shown). We next tested whether TLR2-blocking antibodies disrupted the effect of hyaluronan on OPC maturation. TLR2-blocking antibodies, but not TLR4- or CD44-blocking antibodies, ablated the effects of hyaluronan on OPC maturation (Fig. 3 A and B).
Fig. 3.
Hyaluronan (HA) acts through TLR2 to block OPC maturation. (A) OPCs were exposed to 25 μg/mL hyaluronan alone or with 10 μg/mL neutralizing antibodies to TLR2, CD44, or TLR4 with controls of nonimmune mouse IgG or nonimmune rat IgG. (B) After 2 d in culture with 10 ng/mL CNTF and 15 μM T3, cells were assessed for O1/olig2 staining. Compared with untreated cells, significant differences in the percent O1/olig2 mature oligodendrocytes were observed with hyaluronan with or without each antibody treatment except for TLR2-blocking antibodies. Mixed glia derived from strain-matched postnatal day 0 (P0) wild-type pups (C) or TLR2-null pups (D) were cultured for 14 d and then grown in oligodendrocyte medium for 4 d. Cells were stained for O1 (green), olig2 (red), and DAPI (blue). (E) The density of O1+ cells and olig2+ cells was measured, standardized by wild-type levels. (White bars, wild-type; black bars, TLR2-null.) (F) Wild-type mixed glia were stained by biotinylated hyaluronan-binding protein (HABP), FITC-linked streptavidin (green), and DAPI (blue) after culture for 14 d and then were grown in oligodendrocyte medium for 4 d. FITC-linked streptavidin (green) detected endogenous hyaluronan in wild-type mixed glia cultures. (G) After chondroitinase treatment to remove hyaluronan, mixed glia were washed, stained for hyaluronan, and found to exhibit virtually no hyaluronan staining. (Scale bars, 20 μm in A and 100 μm in C, D, F, and G.)
Astrocytes and microglia within cultured wild-type, TLR2-null, or MyD88-null mixed glia produce hyaluronan (Fig. 3 F and G) (data not shown). OPCs are known to mature slowly in wild-type mixed glia cultures, perhaps because of the presence of endogenously produced hyaluronan. Using mixed glia, we tested whether TLR2-null OPCs would mature more rapidly than wild-type OPCs. After cells were cultured for 2 wk in glia medium and then for 4 d in oligodendrocyte medium, we found that there were many more O1+ oligodendrocytes in TLR2-null mixed glial cultures than in wild-type cultures (Fig. 3 C–E). In contrast, olig2+ oligodendrocyte numbers were not different in the two culture types, indicating differences in olig2+ cell densities do not account for the increased density of mature oligodendrocytes.
We used a lysolecithin injection animal model to study whether TLR2 is required for the previously characterized hyaluronan-mediated block in remyelination (5). First, examination of wild-type and TLR2-null mice did not show any obvious differences in myelination in adult mice or during development (Fig. 4 and Fig. S1). We observed only that at postnatal day 7 TLR2-null mice had myelin basic protein (MBP)-positive cells in the corpus callosum, whereas C57BL6 mice did not. Finding no substantial effect on developmental myelination was not surprising given preliminary reports that hyaluronan synthase-null mice have no CNS phenotype. Using the lysolecithin model, we found that remyelination was approximately the same in wild-type and TLR2-null mice treated with lysolecithin only (Fig. 4 A, C, and E). We thus confirmed that hyaluronan blocks remyelination in wild-type mice (Fig. 4 A–G). In contrast, when lysolecithin and hyaluronan were injected into TLR2-null mice, we found enhanced remyelination, indicating that hyaluronan is unable to block remyelination in TLR2-null mice (Fig. 4 B and D–G). This effect is not caused by alterations in oligodendrocyte numbers, because we observed similar levels of olig2+ cells in wild-type and TLR2-null corpus callosum after exposure to lysolecithin and hyaluronan (Fig. 4 F and G).
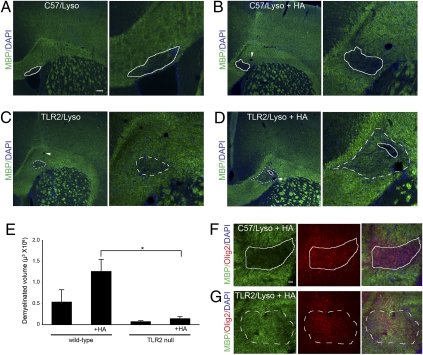
Fig. 4.
TLR2 is required for remyelination after lysolecithin-induced demyelination. Brains of C57BL6 (C57) (A and B) or TLR2-null (C and D) mice were injected with lysolecithin (Lyso) with (B and D) or without (A and C) 100 μg/mL hyaluronan (HA). (A–D) After 14 d, brains were fixed, sectioned, and stained for MBP (green) and DAPI (blue). Hyaluronan blocked remyelination in wild-type mice, but remyelination was more complete in TLR2-null mice. Solid lines represent completely demyelinated areas; dashed lines represent partially remyelinated areas. (E) Demyelinated volumes were measured (n = 3–5) and found to be significantly smaller in TLR2-null mice than in C57BL6 mice only when hyaluronan also was coinjected. Error bars represent SE. *, P < 0.05 by t test. (F and G) Staining for MBP (green) and olig2 (red) indicates that oligodendrocyte numbers are not appreciably different between lysolecithin/hyaluronan-treated wild-type (F) and TLR2-null (G) mice. Arrowheads indicate blood vessels. (Scale bars, 100 μm in A–D and 2 5 μm in F and G.)
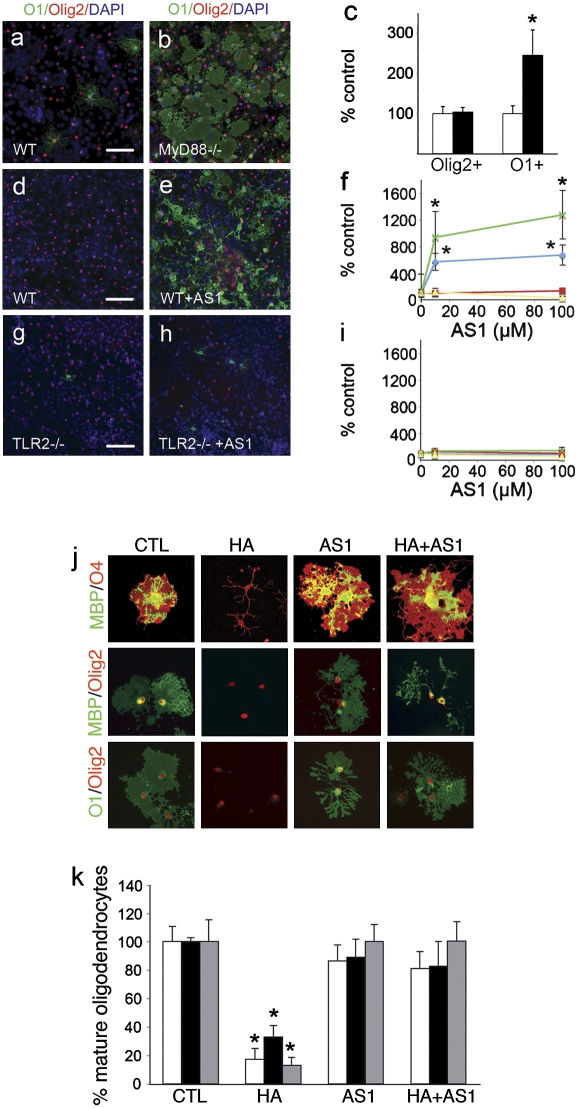
Next, we sought to determine if the downstream signal machinery required for TLR2 function is required for hyaluronan blockade of OPC maturation. Without inducing significant cell death (data not shown), we found that a small-molecular-weight inhibitor of MyD88 (AS1) (Fig. 5 J and K) blocked effects of hyaluronan on OPC maturation. Using wild-type mixed glia, we found a dose-dependent enhancing effect of AS1 on OPC maturation that was not caused by differences in olig2+ densities (Fig. 5 D–F). In contrast, AS1 had no effect on OPC maturation in TLR2-null or MyD88-null cultures (Fig. 5 F–H, yellow and red lines, respectively), indicating that AS1 requires the presence of both TLR2 and MyD88 for its effect. In addition, similar differences in OPC maturation were seen in MyD88-null mixed glia and in wild-type mixed glia (Fig. 5 A–C), suggesting that MyD88 expression also impairs OPC maturation in mixed glial cultures.
Fig. 5.
MyD88 function is critical for hyaluronan effects on oligodendrocyte progenitor maturation. Mixed glia from strain-matched wild-type pups (A) or MyD88-null pups (B) were cultured for 14 d and then grown in oligodendrocyte medium, for 4 d. Cells were stained for O1 (green), olig2 (red), and DAPI (blue). (C) The density of olig2+ cells and O1+ cells was measured and standardized by wild-type levels (white bars, wild type; black bars MyD88 null). (D–I) C57BL6 wild-type (D–F and I), TLR2-null (F–I), MyD88-null (F and I), or MyD88 control wild-type (F and I) mixed glia were cultured for 14 d and exposed for 4 d to vehicle control (D and G) or 100 μM AS1 (E and H) in oligodendrocyte medium. Cells were stained for olig2 (red), O1 (green), and DAPI (blue). The density of olig2+ cells (I) and O1+ cells (F) was measured, standardized by untreated levels, and graphed (TLR2-null, yellow line; C57BL6, blue line; MyD88, red line; MyD88 control wild-type, green line). (J) OPCs were incubated with 25 μg/mL hyaluronan, 100 μM AS1, or both, allowed to mature for 2 d, and stained for oligodendrocyte markers as shown. (K) Effects of hyaluronan (HA) on oligodendrocyte progenitor maturation were blocked completely by 100 μM AS1. Error bars represent SE. *, P < 0.05 by t test in comparison with control (CTL). (Scale bars, 100 μm in A–E, G, and H and 20 μm in J.)
Although only HMW hyaluronan is thought to block OPC maturation (5), only LMW hyaluronan appears to stimulate TLR2 and TLR4 (7–9). Thus, we also investigated whether the molecular weight of hyaluronan is crucial to its effects on OPC maturation. We found that both LMW hyaluronan and HMW hyaluronan block OPC maturation (Fig. 6 I and J). Only when hyaluronan is degraded to disaccharide is no effect on maturation observed (Fig. 6 I and J).
Because both HMW and LMW hyaluronan block OPC maturation, and only LMW hyaluronan stimulates TLR2 and TLR4 in other cell types (7–9), we suspected that HMW hyaluronan must be processed to a LMW form to be able to inhibit OPC maturation via TLR2. Indeed, treating HMW hyaluronan with hyaluronidase alone did not neutralize the effect of hyaluronan on OPC maturation (data not shown). Only hyaluronidase and β-glucuronidase neutralized HMW hyaluronan effects on OPCs (Fig. 6 A–H).
To test whether HMW hyaluronan is processed by OPCs, we electrophoresed conditioned medium containing HMW hyaluronan to assay for hyaluronan molecular weight. When OPCs were present, the major HMW hyaluronan peak was reduced in size. In addition, there was a concomitant increase in LMW hyaluronan, ranging from ~20–200 kDa, indicating degradation of HMW hyaluronan (Fig. 7C). Further, after washing with PBS, cells were trypsinized, analyzed for adherent hyaluronan, and found to contain proportionately higher levels of LMW hyaluronan than HMW hyaluronan. This finding suggested that the degrading activity associated with OPCs is cell-associated such as through cell-associated hyaluronidases.
If OPCs degrade hyaluronan in vitro, LMW hyaluronan should be present in MS lesions as well, a condition that would be necessary if TLR2/hyaluronan signaling blocks OPC maturation and remyelination in MS. Therefore, we compared the molecular weight of hyaluronan in MS lesions and in white matter controls. Interestingly, we found that two peaks of hyaluronan were significantly elevated in MS lesions compared with control: a broad peak from 500–1500 kDa and a well-defined 20-kDa peak (Fig. 7A). Because hyaluronidases are known to generate a stable intermediate degradation product of 20 kDa (20), the presence of a 20-kDa form of hyaluronan in MS lesions suggests the presence of hyaluronidase activity within lesions. Thus, we found compelling indirect evidence that HMW hyaluronan is processed enzymatically into LMW hyaluronan in vitro and in MS lesions in vivo. In addition, we found that OPCs degraded HMW hyaluronan in vitro (Fig. 7C) to a molecular weight range of 20–200 kDa. Based on these data, we predicted that OPCs would express hyaluronidases.
Two hyaluronidases, Hyal1 and Hyal3, are expressed in brain (21, 22). Hyal2 is expressed in brain only during development, whereas sperm adhesion molecule 1 (SPAM1) has not been detected in brain as yet (23, 24). We found that Hyal1, Hyal2, Hyal3, and SPAM1 are expressed by oligodendrocytes in vitro (Fig. 7B). Although SPAM1 appears to be weakly expressed primarily by O1+ oligodendrocytes, Hyals1–3 are expressed preferentially in the growth cones of OPCs, suggesting a role for these hyaluronidases in OPC migration, a hypothesis we currently are exploring.
Because functional redundancy of OPC hyaluronidases may make knockdown approaches difficult, we used a small-molecular-weight hyaluronidase inhibitor, ascorbate 6-hexadecanoate (A6H) (25), to determine whether hyaluronidases are important to hyaluronan/TLR2-dependent signaling. We treated OPCs with 10 μM A6H for 10 min, then added 100 μg/mL HMW hyaluronan, and cultured the OPCs for 2 d. By electrophoresing cell-associated hyaluronan, we found that A6H significantly inhibits HMW hyaluronan degradation (Fig. 7D). We then exposed OPCs to A6H and/or HMW hyaluronan for 2 d and determined the effect on OPC maturation. Although HMW hyaluronan blocked OPC maturation, the addition of A6H to HMW hyaluronan allowed OPC maturation to proceed normally (Fig. 7 E and F). A6H alone had no effect. In addition, A6H blocked only HMW hyaluronan but not LMW hyaluronan effects on OPC maturation (Fig. 7G). We also confirmed this effect of A6H using mixed glia that produce HMW hyaluronan endogenously (data not shown) (Fig. 3 F and G). Therefore, hyaluronidases appear essential to degrade HMW hyaluronan into LMW hyaluronan to stimulate TLR2.
Discussion
This report identifies TLR2 as the hyaluronan receptor responsible for mediating the repressive effects of hyaluronan on OPC maturation and remyelination. CD44 clearly is not required, because CD44 is expressed at low levels, if at all, by OPCs, CD44 overexpression does not further block remyelination (5), and CD44-blocking antibodies have no effect in vitro. In contrast, TLR2 is expressed at high levels in OPCs within MS lesions. In addition, TLR2 and MyD88 were found to be essential for hyaluronan-induced inhibition of OPC maturation in vitro. Finally, remyelination in a lysolecithin model was more robust in TLR2-null mice. Therefore, hyaluronan functions through the TLR2 pathway to inhibit OPC maturation and remyelination.
TLR2 is expressed by a variety of cell types, including microglia, astrocytes, neurons, and oligodendrocytes (10–12, 31). Thus, it is possible that TLR2 influences OPC maturation and remyelination through a variety of TLR2+ cell types. Nevertheless, using purified OPCs, we found that hyaluronan directly stimulates TLR2 on OPCs to inhibit maturation. In addition, the identification of the pathway through which hyaluronan functions is significant intrinsically. Thus, therapeutics designed to block this pathway may be an important next step in advancing MS clinical care.
A prior report found the major peak of hyaluronan in chronic MS lesions was about 1,000 kDa in size and that HMW but not LMW hyaluronan blocks OPC maturation and remyelination (5). This finding suggested HMW hyaluronan, but not LMW hyaluronan, impedes remyelination in MS. In contrast, only LMW hyaluronan appears to stimulate TLR2 expressed by other cell types (7–9), a finding that may be at odds with the hypothesis that TLR2 and MyD88 are essential for HMW hyaluronan effects on OPC maturation. Thus, we speculated that HMW hyaluronan is processed to a LMW form of hyaluronan before TLR2 stimulation. In contrast to a previous report (5), we found that hyaluronan molecular weight did not influence OPC maturation. In addition, peaks of LMW hyaluronan were found in MS lesions, suggesting enzymatic degradation of hyaluronan in vivo. Using a known hyaluronidase inhibitor, A6H, we then found A6H blocks HMW hyaluronan degradation and HMW hyaluronan effects on OPC maturation in vitro. Thus, we have identified a pathway in which OPC hyaluronidases first process HMW hyaluronan to LMW hyaluronan, allowing TLR2 stimulation and blockade of OPC maturation.
This work identifies possible approaches to improving remyelination in MS. First, we show that inhibitors of TLR2 and its signaling pathway are effective in blocking hyaluronan inhibitory effects, resulting in enhanced OPC maturation in vitro. Second, hyaluronidase inhibition also successfully blocks hyaluronan effects and increases OPC maturation. Approaches like these may be of great importance in developing treatments for impaired remyelination in MS, a major source of disability in this common neurologic disorder.
Methods
Animals.
C57BL/6 mice were obtained from Charles River Laboratories, and Sprague-Dawley rats were obtained from Taconic. Animals were maintained and bred in the Harvard Institutes of Medicine animal housing facility under specific pathogen-free conditions and following all institutional guidelines for care and research of vertebrate animals. MyD88−/− mice, a generous gift of S. Akira (Osaka University, Osaka, Japan), were used for comparison with wild-type mice with identical genetic background. TLR2−/− mice (Jackson Laboratories) were used for comparison with C57BL/6 mice as controls (12).
Purified Oligodendrocyte Cultures.
Mouse mixed glia were generated as detailed (26). Cells were grown in DMEM with 10% FBS (mixed glia medium) for the first 14-d culture and in DMEM with 0.025% BSA and N2 (Invitrogen) for the subsequent 4 d. Rat oligodendrocytes were purified from postnatal day 0 rat forebrain as detailed in prior publications by our group (26). Purified OPCs were cultured in DMEM with 0.025% BSA and N2. All oligodendrocyte cultures were assayed for microglial and astrocyte contamination by cell type-specific immunocytochemistry. Typically cultures contained <2% microglia and <5% astrocytes as assayed by isolectin B4 (IB4) and GFAP staining, respectively. Oligodendrocytes purified initially were ~100% A2B5+ and 0% O1+. To induce differentiation, we cultured oligodendrocytes in DMEM supplemented with 10 ng/mL ciliary neurotrophic factor, 15 nM 3,3′,5-triiodothyronine, N2 (Invitrogen), and 0.025% BSA. After 2 d, >60% of oligodendrocytes were MBP+ and O1+. For some experiments, cells were exposed to 10 μg/mL blocking antibodies to TLR2 (T2.5; Biolegend), CD44 (IM7; Biolegend), or TLR4 (HTA125; Biolegend). Nonimmune mouse and rat IgG was purchased from Jackson ImmunoResearch. None of the antibodies used contained sodium azide. Hydrocinnamoyl-L-valyl pyrrolidone was purchased from Calbiochem, and A6H was purchased from Sigma.
Immunocytochemistry.
Cells were fixed in 4% paraformaldehyde in PBS at room temperature, rinsed with PBS, and treated for 1 h with blocking solution (PBS supplemented with 0.1% Triton ×100 and 1% BSA). Sections were incubated overnight at 4 °C with the primary antibody diluted in blocking solution. Cells were stained with antibodies to TLR2 (1:500) (Abcam), TLR4 (1:200) (Santa Cruz), olig2 (1:10,000) (a gift from D. Rowitch, University of California, San Francisco, CA), MBP (1:500) (Santa Cruz), GFAP (1:400) (Millipore), IB4 (1:50) (Invitrogen), Hyal1-3 (Santa Cruz), SPAM1 (Novus), and/or A2B5, O4, or O1 (ATCC). Subsequently, cells were rinsed and incubated with the relevant secondary antibodies (Cy3 or Alexa-488) (Jackson ImmunoResearch) for 1 h at room temperature. Immunofluorescence images were obtained by a Nikon Eclipse 660 Microscope and a SPOT-cooled CCD digital camera (Diagnostic Instruments).
Maturation Assay.
After cell fixation, we stained cells for markers of different maturation stages of oligodendrocytes. As a general marker for all oligodendrocytes, Olig2 or O4 was used. For OPCs, we used A2B5 and morphology. For mature oligodendrocytes, we used O1 and MBP. By fluorescence microscopy, we quantified percent maturation as percent O1+/Olig2+, MBP+/Olig2+, or MBP+/O4+, a well-established approach used by many others (5, 27, 28). Cells were counted in six or more 20× fields per experimental arm.
Cell Viability Assay.
Cell viability after different treatments was determined using the Live/Dead assay (Invitrogen), following the manufacturer's instructions. Cells were counted in six 20× fields per experimental arm.
Proliferation Assay.
Cells were incubated with 2.5 mM BrdU (Sigma) for 2d. Fixed cells were stained with anti-BrdU antibody (1:100; Abcam) and the nuclear stain DAPI (Invitrogen). We quantified proliferation as the percent BrdU+ cell nuclei versus total nuclei. Cells were counted in 12 20× fields per experimental arm.
Lysolecithin Injections.
Mice were anesthetized with i.p. injection of 0.5 mg/g Avertin (Sigma). After placing mice in a stereotaxic frame, we drilled a burr hole at the stereotaxic coordinates of 5.5 mm anterior to the lambda and 1 mm lateral to the bregma (5, 29). We then injected 2 μL 4% lysolecithin in PBS or control 2.5 mm deep from the skull surface. Because lysolecithin does not induce hyaluronan expression (5), we injected 4% lysolecithin (Sigma) and 100 μg/mL hyaluronan in another set of mice. We reinjected the same volume and concentration of hyaluronan or PBS control 5 d and 10 d after the initial injection. Brains were harvested 8 and 14 d after initial injection. For perfusion and fixation, mice were perfused intracardially with 4% paraformaldehyde after Avertin anesthesia. Brains were cryoprotected in 20% sucrose overnight and sectioned at 20 microns by cryostat.
Human Tissue.
The use of human tissues was approved by the Human Subjects Committee at the Cleveland Clinic and the Beth Israel Deaconess Medical Center. For immunohistochemistry, three brains from individuals with MS (mean duration of disease, 24.3 y; range, 19–29 y; mean age, 53.0 y; range 47–63 y) and age-matched control brains were studied. For hyaluronan studies, chronic plaques (Luxol fast blue-negative, reduced Olig2+ cell numbers, and few to no HLA+ cells) from nine individuals with MS (mean age, 64.7 y; range, 54–78 y) and nine age-matched controls (mean age, 69.1 y; range, 58–76 y) were used. Demyelinated and normal-appearing white matter was snap frozen in isopentane and stored at −80 °C.
Immunohistochemistry.
Frozen sections were mounted on glass slides, postfixed in ice-cold acetone, blocked with 20% mixture of 1:1 normal goat and human serum (for human sections) or 1% BSA and 3% normal donkey serum (for mouse sections), and incubated in primary antibodies at 4 °C. Primary antibodies included TLR2-specific goat polyclonal antibody (Abcam), MBP goat polyclonal antibody(Santa Cruz), Olig2-specific rabbit polyclonal antibody (Millipore), and proteolipid protein-specific rat monoclonal antibody. FITC- and Texas Red-conjugated secondary antibodies (Jackson ImmunoResearch) were used to detect primary antibodies. Fluorescently labeled sections were scanned with a Leica SP5 confocal microscope (Leica) or with a Nikon Eclipse 660 Microscope and a SPOT-cooled CCD digital camera. Autofluorescence was removed from confocal images using ImageJ.
Hyaluronan Electrophoresis.
Tissue dissected from MS lesions and control brain white matter was Dounce homogenized in PBS. Immediately thereafter, protein content was measured, and equal amounts of sample by protein were electrophoresed in 1% agarose gels (30). To determine the total amount of hyaluronan in each sample, samples were treated with 1 mg/mL pronase E (Sigma) for 1 h and then boiled. Aliquots derived from each sample were either treated or not treated with 5 U/mL hyaluronidase (Worthington) and 5 U/mL β-glucuronidase (Sigma) for 1 h before electrophoresis in parallel. The difference between hyaluronidase/β-glucuronidase–treated and untreated samples was taken to represent the hyaluronan content. Gels were stained by the cationic dye 3,3′-dimethyl-9-methyl-4,5,4'5′-dibenzothiacarbocyanine (Stains-All; Sigma).
Statistical Analysis.
Statistical analysis was conducted using Macintosh-based Statview and Excel software (Microsoft). Differences among control and experimental groups were analyzed by t test or ANOVA in conjunction with appropriate post hoc tests. We calculated the significance of each treatment as a P value, and when depicted in a graph, P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Drs. Cynthia Hayne and Lawrence Sherman for helpful discussions. This work was supported by National Multiple Sclerosis Society Grants RG 4116-A-2 (to J.A.S.) and RG 3977A3/1 (to T.V.) and National Institutes of Neurological Disorders and Stroke Grants NS038475 and 1R01NS066007-01 (to T.V.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1006496107/-/DCSupplemental.
References
- 1.Wolswijk G. Chronic stage multiple sclerosis lesions contain a relatively quiescent population of oligodendrocyte precursor cells. J Neurosci. 1998;18:601–609. doi: 10.1523/JNEUROSCI.18-02-00601.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolswijk G. Oligodendrocyte precursor cells in chronic multiple sclerosis lesions. Mult Scler. 1997;3:168–169. doi: 10.1177/135245859700300221. [DOI] [PubMed] [Google Scholar]
- 3.Wolswijk G. Oligodendrocyte precursor cells in the demyelinated multiple sclerosis spinal cord. Brain. 2002;125:338–349. doi: 10.1093/brain/awf031. [DOI] [PubMed] [Google Scholar]
- 4.Chang A, Tourtellotte WW, Rudick R, Trapp BD. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med. 2002;346:165–173. doi: 10.1056/NEJMoa010994. [DOI] [PubMed] [Google Scholar]
- 5.Back SA, et al. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med. 2005;11:966–972. doi: 10.1038/nm1279. [DOI] [PubMed] [Google Scholar]
- 6.Jiang D, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 7.Termeer C, et al. Oligosaccharides of hyaluronan activate dendritic cells via Toll-like receptor 4. J Exp Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor KR, et al. Recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on Toll-like receptor 4, CD44, and MD-2. J Biol Chem. 2007;282:18265–18275. doi: 10.1074/jbc.M606352200. [DOI] [PubMed] [Google Scholar]
- 9.Scheibner KA, et al. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol. 2006;177:1272–1281. doi: 10.4049/jimmunol.177.2.1272. [DOI] [PubMed] [Google Scholar]
- 10.Lehnardt S, et al. The Toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neurosci. 2002;22:2478–2486. doi: 10.1523/JNEUROSCI.22-07-02478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lehnardt S, et al. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci USA. 2003;100:8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehnardt S, et al. A mechanism for neurodegeneration induced by group B streptococci through activation of the TLR2/MyD88 pathway in microglia. J Immunol. 2006;177:583–592. doi: 10.4049/jimmunol.177.1.583. [DOI] [PubMed] [Google Scholar]
- 13.Rose D, Chiba A. A single growth cone is capable of integrating simultaneously presented and functionally distinct molecular cues during target recognition. J Neurosci. 1999;19:4899–4906. doi: 10.1523/JNEUROSCI.19-12-04899.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pevsner-Fischer M, et al. Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood. 2007;109:1422–1432. doi: 10.1182/blood-2006-06-028704. [DOI] [PubMed] [Google Scholar]
- 15.Rolls A, et al. Toll-like receptors modulate adult hippocampal neurogenesis. Nat Cell Biol. 2007;9:1081–1088. doi: 10.1038/ncb1629. [DOI] [PubMed] [Google Scholar]
- 16.Passi A, et al. Hyaluronan suppresses epidermal differentiation in organotypic cultures of rat keratinocytes. Exp Cell Res. 2004;296:123–134. doi: 10.1016/j.yexcr.2004.01.031. [DOI] [PubMed] [Google Scholar]
- 17.Falconi D, Aubin JE. LIF inhibits osteoblast differentiation at least in part by regulation of HAS2 and its product hyaluronan. J Bone Miner Res. 2007;22:1289–1300. doi: 10.1359/jbmr.070417. [DOI] [PubMed] [Google Scholar]
- 18.Chen PY, Huang LL, Hsieh HJ. Hyaluronan preserves the proliferation and differentiation potentials of long-term cultured murine adipose-derived stromal cells. Biochem Biophys Res Commun. 2007;360:1–6. doi: 10.1016/j.bbrc.2007.04.211. [DOI] [PubMed] [Google Scholar]
- 19.Huang L, et al. The effect of hyaluronan on osteoblast proliferation and differentiation in rat calvarial-derived cell cultures. J Biomed Mater Res A. 2003;66:880–884. doi: 10.1002/jbm.a.10535. [DOI] [PubMed] [Google Scholar]
- 20.Rai SK, et al. Candidate tumor suppressor HYAL2 is a glycosylphosphatidylinositol (GPI)-anchored cell-surface receptor for jaagsiekte sheep retrovirus, the envelope protein of which mediates oncogenic transformation. Proc Natl Acad Sci USA. 2001;98:4443–4448. doi: 10.1073/pnas.071572898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Csóka AB, et al. The hyaluronidase gene HYAL1 maps to chromosome 3p21.2-p21.3 in human and 9F1-F2 in mouse, a conserved candidate tumor suppressor locus. Genomics. 1998;48:63–70. doi: 10.1006/geno.1997.5158. [DOI] [PubMed] [Google Scholar]
- 22.Triggs-Raine B, Salo TJ, Zhang H, Wicklow BA, Natowicz MR. Mutations in HYAL1, a member of a tandemly distributed multigene family encoding disparate hyaluronidase activities, cause a newly described lysosomal disorder, mucopolysaccharidosis IX. Proc Natl Acad Sci USA. 1999;96:6296–6300. doi: 10.1073/pnas.96.11.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Müllegger J, Lepperdinger G. Degradation of hyaluronan by a Hyal2-type hyaluronidase affects pattern formation of vitelline vessels during embryogenesis of Xenopus laevis. Mech Dev. 2002;111:25–35. doi: 10.1016/s0925-4773(01)00593-7. [DOI] [PubMed] [Google Scholar]
- 24.Lepperdinger G, Strobl B, Kreil G. HYAL2, a human gene expressed in many cells, encodes a lysosomal hyaluronidase with a novel type of specificity. J Biol Chem. 1998;273:22466–22470. doi: 10.1074/jbc.273.35.22466. [DOI] [PubMed] [Google Scholar]
- 25.Botzki A, et al. L-Ascorbic acid 6-hexadecanoate, a potent hyaluronidase inhibitor. X-ray structure and molecular modeling of enzyme-inhibitor complexes. J Biol Chem. 2004;279:45990–45997. doi: 10.1074/jbc.M406146200. [DOI] [PubMed] [Google Scholar]
- 26.Sloane JA, Vartanian TK. Myosin Va controls oligodendrocyte morphogenesis and myelination. J Neurosci. 2007;27:11366–11375. doi: 10.1523/JNEUROSCI.2326-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arnett HA, et al. bHLH transcription factor Olig1 is required to repair demyelinated lesions in the CNS. Science. 2004;306:2111–2115. doi: 10.1126/science.1103709. [DOI] [PubMed] [Google Scholar]
- 28.Wang S, et al. Notch receptor activation inhibits oligodendrocyte differentiation. Neuron. 1998;21:63–75. doi: 10.1016/s0896-6273(00)80515-2. [DOI] [PubMed] [Google Scholar]
- 29.Nait-Oumesmar B, et al. Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur J Neurosci. 1999;11:4357–4366. doi: 10.1046/j.1460-9568.1999.00873.x. [DOI] [PubMed] [Google Scholar]
- 30.Lee HG, Cowman MK. An agarose gel electrophoretic method for analysis of hyaluronan molecular weight distribution. Anal Biochem. 1994;219:278–287. doi: 10.1006/abio.1994.1267. [DOI] [PubMed] [Google Scholar]
- 31.Bsibsi M, Ravid R, Gveric D, van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol. 2002;61:1013–1021. doi: 10.1093/jnen/61.11.1013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.