Abstract
Lithium has been the gold standard in the treatment of bipolar disorder (BPD) for 60 y. Like lithium, glycogen synthase kinase 3 (GSK-3) inhibitors display both antimanic-like and antidepressant-like effects in some animal models. However, the molecular mechanisms of both lithium and GSK-3 inhibitors remain unclear. Here we show that the GSK-3 inhibitor AR-A014418 regulated α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA)-induced GluR1 and GluR2 internalization via phosphorylation of kinesin light chain 2 (KLC2), the key molecule of the kinesin cargo delivery system. Specifically, AMPA stimulation triggered serine phosphorylation of KLC2 and, subsequently, the dissociation of the GluR1/KLC2 protein complex. This suggests that GSK-3 phosphorylation of KLC2 led to the dissociation of AMPA-containing vesicles from the kinesin cargo system. The peptide TAT-KLCpCDK, a specific inhibitor for KLC2 phosphorylation by GSK-3β, reduced the formation of long-term depression. Furthermore, the TAT-KLCpCDK peptide showed antimanic-like effects similar to lithium's on amphetamine-induced hyperactivity, a frequently used animal model of mania. It also induced antidepressant-like effects in the tail suspension and forced swim tests, two commonly used animal models of depression. Taken together, the results demonstrated that KLC2 is a cellular target of GSK-3β capable of regulating synaptic plasticity, particularly AMPA receptor trafficking, as well as mood-associated behaviors in animal models. The kinesin cargo system may provide valuable novel targets for the development of new therapeutics for mood disorders.
Keywords: α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate receptor, internalization, kinesin light chain 2
The mood stabilizer lithium has been the most commonly used treatment for bipolar disorder (BPD) for 60 y. It is effective in treating the acute manic and depressive episodes associated with this illness, as well as in reducing the recurrence of mood episodes (1). However, the mechanism of action whereby lithium exerts its mood-stabilizing effects remains unknown. Currently available treatments are insufficient for many patients suffering from mood disorders, and even those patients who do respond to available antidepressants or mood stabilizers often experience a significant therapeutic lag before clinical benefits appear (1). Thus, the major challenge in BPD research is to find the common, convergent, functional mechanisms associated with BPD to develop urgently needed, effective, and truly novel therapeutics.
Interest in the multifunctional serine/threonine kinase glycogen synthase kinase 3 (GSK-3) as a potential target for drug development in BPD (2, 3) stems from findings that it plays a fundamental role in a broad variety of functions, including synaptic plasticity, cell proliferation, cell differentiation, and cell adhesion (4). Notably, lithium inhibits GSK-3 (2), and lithium and other GSK-3 inhibitors demonstrate both antimanic-like and antidepressant-like efficacy in animal models of mood-associated behaviors, particularly in the amphetamine (AMPH)-induced locomotion and forced swim tests (5, 6).
Accumulating studies from our laboratory and those of other investigators have shown that glutamatergic synaptic plasticity, particularly α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate receptor (AMPAR) trafficking, may be a convergent point in the treatment of mood disorders (7–10). For instance, rats receiving hippocampal infusions of AMPA-specific inhibitors exhibited significant reductions in manic-like behaviors assessed through the AMPH-induced locomotion (7). In contrast, administration of antidepressant agents—such as the tricyclic antidepressant (TCA) imipramine or the mood stabilizers lamotrigine and riluzole—enhanced surface AMPAR expression and phosphorylation of GluR1S845 in hippocampal neurons in vitro and in vivo (8). AMPAR potentiators have also been reported to have antidepressant-like effects in animal models of depression (10). GSK-3 has been shown to modulate synaptic plasticity by promoting the induction of one of the major forms of synaptic plasticity in the brain, N-methyl-D-aspartate (NMDA) receptor-dependent long-term depression (LTD) (11). However, it remains unclear how GSK-3 regulates AMPAR delivery.
In this study, we sought to determine how GSK-3 modulates AMPAR trafficking and the role of this regulation on AMPAR trafficking in animal models of mood-associated behaviors. This series of experiments uncovered the cellular mechanism whereby GSK-3 inhibitors stabilize AMPAR trafficking, namely via the dephosphorylation of kinesin light chain 2 (KLC2) that, in turn, affects mood-associated behaviors in animal models.
Results
GSK-3 Inhibitors Reduce AMPAR Internalization.
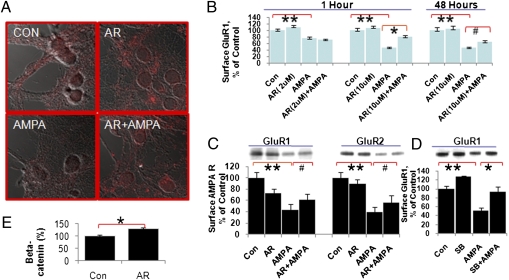
Because GSK-3 is necessary for LTD expression, and because AMPAR endocytosis is the essential step in LTD expression, we first investigated whether GSK-3 is required for AMPAR internalization using two independent assays: a surface immunostaining assay and a biotinylation assay. Consistent with previous reports (12), we found that the GSK-3 inhibitor AR-A014418 enhanced β-catenin levels in cultured hippocampal neurons (Fig. 1E). In cultured hippocampal neurons, we found that AMPA induced AMPAR internalization, as demonstrated by a significant reduction of GluR1 on the neuronal surface (Fig. 1 A–C). This AMPA-induced GluR1 receptor internalization was significantly inhibited by the GSK-3 inhibitor AR-A014418 in a time- and dose-dependent manner (Fig. 1B). After 1 h of treatment with AR-A014418 (10 μM), AMPA-induced GluR1 receptor internalization was inhibited by 48% in the group treated with 10 μM AR-A014418. This effect was sustained up to 48 h (Fig. 1B). Low-dose treatment with AR-A014418 (2 μM) had no significant effect (Fig. 1B).
Fig. 1.
GSK-3 inhibitors blocked AMPA-induced internalization in hippocampal neurons. (A) Surface GluR1 immunostaining after AR-A014418 (10 μM, 1 h) followed by AMPA (100 μM, 30 min) in hippocampal neurons. (B) Quantification of surface GluR1 immunostaining after AR-A014418 followed by AMPA treatment in hippocampal neurons (one-way ANOVA, Tukey's multiple comparison test, **P < 0.001, *P < 0.01, N = 2–3, n = 160–286; Student's t test, unpaired, two-tailed, #P = 0.0496, n = 21–32 per group). (C) The GSK-3 inhibitor AR-A014418 blocked AMPA-induced GluR1 and GluR2 internalization in hippocampal neurons (one-way ANOVA, Tukey's multiple comparison test, N = 3, n = 32–39, **P < 0.001; Student's t test, paired, two-tailed, n = 10; for GluR1, #P = 0.022; for GluR2, #P = 0.045). (D) The GSK-3 inhibitor SB-216763 inhibited AMPA-induced internalization of GluR1 in hippocampal neurons (one-way ANOVA, Tukey's multiple comparison test, N = 6, n = 55, **P < 0.001, *P < 0.01). (E) AR-A014418 treatment significantly enhanced β-catenin levels in cultured hippocampal neurons (Student's t test, Con: n = 8; AR: n = 8, *P < 0.05).
Next, we confirmed this result using a biotinylation assay. AMPA treatment led to GluR1/2 internalization, as revealed by reduced GluR1 and GluR2 levels on the neuronal surface to 44.1 ± 9.1% and 39.5 ± 9.1% of control, respectively. In hippocampal neurons [12–14 days in vitro (DIV)], AMPA-induced reductions of surface GluR1 and GluR2 were significantly inhibited by treatment with 10 μM AR-A014418 for 1 h. Surface GluR1 and GluR2 levels were 62.2 ± 9.2% and 56.7 ± 13.0% of control, respectively, after AR-A014418 treatment followed by AMPA stimulation (Fig. 1C). To further confirm the results, we tested another small-molecule GSK-3 inhibitor, SB-216763. We found that SB-216763 (1 μM) also significantly inhibited AMPA-induced reductions of surface GluR1 (50.6 ± 6.7%) in cultured hippocampal neurons to 93.3 ± 11.9% of control (Fig. 1D).
NMDA-induced AMPAR internalization is one of the key mechanisms for LTD formation. Therefore, we tested whether or not the GSK-3 inhibitor AR-A014418 blocked NMDA-induced AMPAR internalization. We found that AR-A014418 significantly inhibited NMDA-induced GluR1 and GluR2 internalization (Fig. S1 A and B). Further investigations to determine whether this was a common mechanism for AMPAR internalization in chemically induced AMPAR internalization revealed that insulin-induced GluR1 and GluR2 internalization (13) was blocked by GSK-3 inhibitors (Fig. S1 C and D). These data suggest that GSK-3 inhibitors significantly inhibited AMPA-, NMDA-, and insulin-induced GluR1/2 internalization, suggesting that GSK-3 is an important modulator of AMPA synaptic strength.
GSK-3 Phosphorylates KLC2 to Dissociate AMPA from the Kinesin Cargo System.
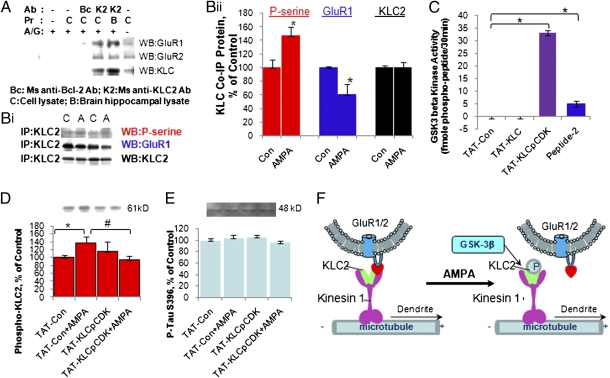
Notably, the kinesin cargo system performs fast anterograde transport of AMPA-containing membrane-bound vesicles to the processes in neurons (14). Therefore, we hypothesized that KLC2 phosphorylation by GSK-3β might be the key step for GluR1 dissociation from the kinesin cargo system for anterograde transport. We assessed the interaction between KLC2 and GluR1 using a coimmunoprecipitation assay and found that mouse anti-KLC2 antibody coimmunoprecipitated with the GluR1 and GluR2 molecule in protein samples from hippocampal cell culture and hippocampal tissue from rat brain (Fig. 2A). The same amount of mouse anti-B-cell lymphoma 2 (Bcl-2) IgG worked as a negative control (Fig. 2A).
Fig. 2.
KLC2 phosphorylation by GSK-3 led to dissociation of GluR1 from KLC2. (A) Coimmunoprecipitation of GluR1 and GluR2 with KLC. (B, i and ii) AMPA (A) enhanced serine phosphorylation of KLC2 and reduced KLC2/GluR1 complex compared with control (C) (Student's t test, unpaired, two-tailed, N = 2–4, n = 5–9; for p-serine, P = 0.0287; for GluR1, P = 0.040; for KLC2, P = 0.8514). (C) GSK-3β phosphorylated peptide TAT-KCLpCDK (one-way ANOVA, N = 2, n = 24, Tukey's multiple comparison test, **P < 0.001). (D) TAT-KLCpCDK significantly inhibited serine phosphorylation of KLC2 induced by AMPA (Student's t test, unpaired, two-tailed, N = 2–3, n = 5–9; Tat-Con versus Tat-Con + AMPA, *P = 0.045; TAT-Con + AMPA versus TAT-KLCpCDK + AMPA, #P = 0.024). (E) TAT-KLCpCDK had no effect on phosphorylation of Tau S396. (F) Regulation of GluR1/2 receptor trafficking by GSK-3 via KLC2 phosphorylation.
To test whether or not the phosphorylation of KLC2 occurred during AMPA-induced internalization, levels of phosphoserine of KLC2 after AMPA treatment were determined by immunoprecipitation of KLC2 followed by Western blot analysis of anti-phosphoserine antibody. We found that serine phosphorylation of KLC2 was significantly increased after AMPA treatment up to 147 ± 12.5% (Fig. 2B i and ii). KLC2 levels that immunoprecipitated down remained unchanged (Fig. 2B i and ii). In addition, we found that coimmunoprecipitation of GluR1 with KLC2 was significantly decreased to 64.8 ± 12.9% after AMPA stimulation (Fig. 2B i and ii). This suggests a dissociation of GluR1-containing vesicles from the kinesin cargo system (Fig. 2F).
To further confirm that KLC2 is a substrate of GSK-3, we designed and synthesized three TAT peptides—TAT-KLCpCDK, TAT-KLC, and TAT-Con—all of which possess a leading TAT peptide to facilitate transportation through the blood–brain barrier and cell membrane. The TAT-KLCpCDK peptide has the sequence form KLC2 (601–622) with phosphorylation on its CDK-5 kinase site to provide a docking site for the GSK-3β enzyme (Fig. 2C). This study tested these peptides as substrates for the recombinant GSK-3 enzyme using an in vitro enzyme assay; the commercially available GSK-3 substrate peptide II served as a positive control for GSK-3β enzyme activity. We found that the recombinant GSK-3 kinase was able to phosphorylate the TAT-KLCpCDK peptide as well as peptide II, but not the TAT-KLC or TAT-Con peptides (Fig. 2C). This suggests that KLC2 is the GSK-3 substrate present in hippocampal neurons. Notably, CDK-5 site phosphorylation is necessary for GSK-3 to phosphorylate KLC2 as a substrate.
If the KLCpCDK site is the phosphorylation site of KLC2 by GSK-3 and is required for GSK-mediated AMPAR endocytosis, then we would predict that the TAT-KLCpCDK peptide would competitively inhibit both AMPA-induced KLC2 phosphorylation and AMPAR endocytosis. We first investigated the inhibition of KLC2 phosphorylation by this TAT-KLCpCDK peptide and found that TAT-KLCpCDK significantly inhibited AMPA-induced increases in KLC2 serine phosphorylation from 138 ± 14.6% down to 94.5 ± 9.8% of control (Fig. 2D). However, this peptide did not affect other GSK-3β phosphorylation sites, such as Tau (serine 396) (Fig. 3E). This suggests that TAT-KLCpCDK is a relatively specific GSK-3β inhibitor and inhibits KLC2 phosphorylation.
Fig. 3.
TAT-KLCpCDK inhibited LTD and AMPAR internalization in hippocampal neurons. (A) TAT-KLCpCDK peptide significantly inhibited AMPA-induced internalization in cultured hippocampal neurons (N = 3, n = 56, one-way ANOVA, Bonferroni's multiple comparison test, **P < 0.01; Student's t test, unpaired, two-tailed, n = 11 per group, #P = 0.0489). (B) The effect of TAT-KLCpCDK on the formation of LTD in hippocampal slices from rats compared with TAT-Con (Student's t test, unpaired, two-tailed, n = 6–7 cells, *P = 0.00011). (C) The effect of TAT-KLCpCDK on the formation of LTP in hippocampal slices from rats (n = 6).
TAT-KLCpCDK Inhibits Formation of LTD and AMPAR Internalization.
We then examined whether the specific peptide inhibitor TAT-KLCpCDK affected AMPAR internalization. After treatment with TAT-KLCpCDK (80 μM) for 1 h, the neurons were stimulated by AMPA (100 μM) and surface GluR1 levels were determined by biotinylation assay. Surface GluR1 levels were significantly reduced in the control and TAT-Con-treated groups after AMPA (100 μM) treatment (by 31.1 ± 7.6% and 53.7 ± 10.6%, respectively). TAT-KLCpCDK peptide significantly inhibited AMPA-induced internalization of surface GluR1, bringing surface GluR1 levels to 95.2 ± 10.8% (Fig. 3A).
Previous studies found that, in rats, GSK-3 inhibitors inhibited LTD formation in hippocampal slices (11). Because AMPAR internalization is involved in LTD formation, we used whole-cell recordings to investigate whether TAT-KLCpCDK peptide, a relatively specific peptide for GSK-3β phosphorylation of KLC2, blocked LTD formation in rat hippocampal slices. LTD was successfully induced in CA1 neurons from hippocampal slices. Treatment with TAT-KLCpCDK completely blocked LTD induction compared with the group treated with TAT-Con peptide (Fig. 3B). We further investigated the involvement of GSK-3β in long-term potentiation (LTP) formation. Consistent with previous findings (11), we determined that acute treatment of hippocampal CA1 neurons with TAT-KLCpCDK had no effect on LTP formation (Fig. 3C). Thus, TAT-KLCpCDK peptide, which is a specific inhibitor of KLC2 phosphorylation by GSK-3, significantly blocked AMPA-induced GluR1 internalization as well as LTD formation.
TAT-KLCpCDK Had Antidepressant-like and Antimanic-like Effects in Animal Models.
Finally, we reasoned that if GSK inhibitors exert their antimanic-like and antidepressant-like effects by inhibiting GSK-mediated KCL2 phosphorylation and AMPAR endocytosis/LTD, then TAT-KLCpCDK might mimic the effects of these GSK inhibitors and affect mood-associated behaviors in rodents. To test this hypothesis, we conducted tail suspension, forced swim, and AMPH-induced activity tests in mice.
In mice, TAT-KLCpCDK peptide (20 mg/mL, 6 μL/d, 120 μg peptide/d, duration 14 d), TAT-Con peptide (20 mg/mL, 6 μL/d, 120 μg peptide/d, duration 14 d), or vehicle (saline, 6 μL/d, duration 14 d) was infused into the third ventricle by minipump (Materials and Methods). We found that, in mice, both application of AR-A014418 (30 μmol/kg) and TAT-KLCpCDK peptide infusion enhanced surface GluR1 (cross-linked, MW ~430 kDa) levels in the hippocampal region (Fig. 4Ai and SI Materials and Methods). After 8 d of treatment, TAT-KLCpCDK significantly reduced immobility time in the tail suspension test by 49% compared with the TAT-Con-treated group (P < 0.05; Fig. 4Aii). To further confirm this result, we conducted the forced swim test and found that, after 8–10 d of treatment, TAT-KLCpCDK also significantly reduced immobility time compared with the TAT-Con group (Fig. 4Aiii).
Fig. 4.
Effects of TAT-KLCpCDK peptide in animal models of depression and mania. (Ai) The GSK-3 inhibitor AR-A014418 and TAT-KLCpCDK both enhanced surface expression of GluR1 (AR control n = 5; AR-treated, n = 6, Student's t test, paired, P = 0.028; TAT-Con, n = 6, TAT-KLCpCDK, n = 8; Student's t test, unpaired, P = 0.014). (Aii) TAT-KLCpCDK infusion significantly reduced immobility time in the tail suspension test (one-way ANOVA, Bonferroni's multiple comparison test, n = 35, *P < 0.05). (Aiii) TAT-KLCpCDK infusion significantly reduced immobility time in the forced swim test (one-way ANOVA, Tukey's multiple comparison test, n = 55, *P < 0.01). (Bi) AR-A014418 blocked Sp-cAMP–induced insertion of GluR1 into the neuronal surface. Hippocampal neurons (12 DIV) were treated with AR-A014418 (10 μM) for 1 h and Sp-cAMP (50 μM) for an additional 30 min. A biotinylation assay was performed to determine surface GluR1 and GluR2 levels (one-way ANOVA, Bonferroni's multiple comparison test, N = 2, n = 40, **P < 0.001, *P < 0.05). (Bii) TAT-KLCpCDK peptide significantly inhibited AMPH-induced hyperactivity (repeated-measures ANOVA [F(1,13) = 11.4, P = 0.005]). (Biii) Time course of locomotor activity before or after AMPH. (Biv) Difference in distance traveled before and after AMPH (n = 7–8 animals per group, Student's t test, unpaired, two-tailed, *P = 0.005).
Previous studies have shown that dopamine D1 receptor stimulation enhances GluR1 surface expression by activating cyclic adenosine monophosphate (cAMP) (15). We therefore postulated that GSK-3 inhibitors could also block dopamine/cAMP-induced insertion of GluR1 into the neuronal surface. To test this hypothesis, hippocampal neurons were pretreated with AR-A014418 for 1 h; Sp-cAMP was then added for 30 min. Indeed, AR-A014418 significantly inhibited the insertion of GluR1 receptors into the neuronal membrane (from 144 ± 9.9% to 74.2 ± 13.0%; Fig. 4Bi).
Following this experiment, we tested the effect of TAT-KLCpCDK peptide on AMPH-induced hyperactivity. Baseline activity of the animals was recorded for 1 h before AMPH (3 mg/kg, i.p.) injection. As expected, AMPH significantly increased activity levels [F(1,13) = 140.1, P < 0.001]. Treatment with TAT-KLCpCDK peptide caused a nonsignificant but slight elevation in baseline locomotor activity [F(1,13) = 0.0, P = 0.956] (Fig. 4Biii). A significant interaction was noted between GSK inhibition and AMPH treatment analyzed by multiple-measures analysis of variance (ANOVA) [F(1,13) = 11.4, P = 0.005]. This interaction showed that the effects of AMPH on locomotor activity were significantly lower in the TAT-KLCpCDK-treated group than in the TAT-Con-treated animals (Fig. 4 Bii and Biii). The difference in total distance traveled per hour before and after AMPH treatment (enhanced distance = distance post-AMPH minus distance pre-AMPH) was also significantly reduced by 45% in the TAT-KLCpCDK-treated group compared with the TAT-Con group (P < 0.05, Student's t test, unpaired) (Fig. 4Biv).
Discussion
Taken together, the findings from this series of experiments identify phosphorylation of KLC2 by GSK-3β as an intracellular signaling pathway for regulating AMPAR trafficking. In addition, we demonstrate that this GSK-3β/KLC2/AMPAR signaling pathway may contribute to the molecular mechanisms associated with the mood-associated behavioral effects of GSK-3 inhibitors observed in animal models.
GSK-3 Uses the Kinesin Cargo System to Regulate AMPAR Trafficking.
Regulation of either receptor insertion or internalization rapidly changes the number of these receptors expressed on the neuronal membrane surface and synapses, thereby playing an important role in mediating certain forms of synaptic plasticity, including LTP and LTD (16–18). In this study, we found that AMPAR internalization requires GSK-3 activation (Fig. 1). This is a common signaling pathway for AMPAR trafficking; AMPAR internalization is induced by several different mechanisms, including AMPA, NMDA, and insulin, and all require GSK-3 activation (Fig. 1 and Fig. S1). GSK-3β is highly expressed in the mature brain and has been implicated in many diseases (19, 20). One recent study showed that GSK-3β mediates LTD formation (11). Therefore, the fact that in the present study a GSK-3 inhibitor inhibited NMDA-induced GluR1/2 internalization may provide a molecular mechanism for the regulation of LTD by GSK-3 (Fig. S1). This GSK-3/KLC2/AMPAR signaling pathway may provide a mechanism for the modulation of synaptic plasticity by many cellular signaling pathways, growth factors, and neurotransmitters and is involved in learning and memory, as well as the pathophysiology of some psychiatric conditions (9, 21, 22).
Phosphorylation of KLC2 by GSK-3 as a Unique Signal for AMPAR Trafficking.
In cells, the kinesin cargo system is the molecular motor operating anterograde vesicular transport (e.g., the transport of synaptic vesicle components to axons and neurotransmitter receptors to dendrites), and the dynein cargo system for the retrograde transport of vesicles from the synapse to the cell body (23, 24). The kinesin heavy chain KIF5 can interact directly with GluR2-interacting protein 1, an AMPAR subunit, to steer the AMPA-containing vesicles to dendrites (14). On the other hand, AMPAR internalization uses the dynein cargo system for retrograde transport of the AMPAR-containing vesicles to the cell body (25–27). Here we identified KLC2 as a member of the “anterograde motor complex” for AMPA GluR1/2 trafficking. In the axon, phosphorylation of KLC2 on a specific GSK-3 site by GSK-3 results in the dissociation of KLC with the membrane-bound vesicles (28).
In this context, we observed that AMPA treatment led to serine phosphorylation of KLC2, which caused the dissociation and/or disassembly of AMPA-containing vesicles from the kinesin cargo system. It is possible that GSK-3-released kinesin binding for microtubules is also important for internalization, because dynein (for retrograde transport) (13, 29) competes with kinesin (for anterograde transport) for the microtubule binding site (24). The end result would be that disassembling AMPA-containing vesicles with the kinesin cargo system would facilitate the dynein cargo system for internalization of clathrin-coated AMPA-containing vesicles. In addition, we found that a GSK-3 inhibitor inhibited cAMP-induced insertion into membrane surfaces, suggesting that it might inhibit AMPAR recycling. Hence, GSK-3 inhibition resulted in increased surface GluR1 levels, which could be interpreted as a combination of internalization and recycling inhibition.
GSK-3 Regulation of AMPAR Trafficking as a Mechanism for Affecting Mood-Associated Behaviors in Animal Models.
The present study showed that TAT-KLCpCDK, a peptide that specifically inhibits the phosphorylation of KLC2, possessed both antidepressant-like and antimanic-like properties in animal models of mood-associated behaviors, thus mimicking lithium's effect on these behaviors. Numerous studies have shown that in addition to its clinical antimanic effects, in animal models lithium has robust antidepressant-like effects in the forced swim and tail suspension tests (30). These antidepressant-like effects can be blocked by the AMPAR inhibitor GYKI52446 (31). Here we found that, like lithium (31), in vivo administration of AR-A014418 and TAT-KLCpCDK enhanced AMPA surface localization in the hippocampus. Previous studies have shown that the nucleus accumbens (NAc) is a critical part of the reward circuit for psychostimulants in the mesocorticolimbic system. The NAc receives dopaminergic input from the ventral tegmental area and glutamate input from regions including the prefrontal cortex, amygdala, and hippocampus (32). In addition, recent studies found that the hippocampus is involved in dopaminergic signaling circuits, and that the ventral subiculum of the hippocampus modulates dopaminergic neuronal activity (33, 34). Rats receiving hippocampal infusion of the AMPA-specific antagonists GYKI 52466, Tat-S845 (which inhibits GluR1 phosphorylation at S845), or TAT-TGL (which inhibits AMPA synaptic localization) exhibited significant reductions in AMPH-induced locomotion (7). In the present study, the inhibition of AMPAR internalization in hippocampal neurons by GSK-3 may have contributed to the increased surface GluR1 levels in hippocampal neurons.
Although lithium has multiple effects on signaling cascades—for instance, it affects the function of the inositol monophosphatase, protein kinase A, protein kinase C, GSK-3, and mitogen activated protein kinase cascades (35)—our findings suggest that the GSK-3 mechanism noted here may at least partially explain lithium's effects on mood-associated behaviors.
In summary, this study identified the phosphorylation of KLC2 by GSK-3β as an intracellular signaling pathway for regulating AMPAR trafficking and mood-associated behaviors in animal models. Our study raises the possibility that modulating the kinesin cargo system and, subsequently, AMPAR trafficking, may be a valuable novel mechanism for developing new therapeutics to treat BPD and other mood disorders.
Materials and Methods
Hippocampal Neuronal Culture Preparation.
Cultures of hippocampal neurons were prepared as previously described with minor modifications (36). Detailed methods are provided in SI Materials and Methods.
Fluorescent Immunostaining for Surface GluR1.
Surface immunostaining was performed as previously described (36). Detailed methods are provided in SI Materials and Methods.
Peptide Design and Synthesis.
The peptides TAT-KCLpCDK, TAT-KLC, and TAT-Con were designed and synthesized: TAT-KLCpCDK (33 aa): YGRKKRRQRR-RLSDSRTLS (GSK-3β site) SSSMDLSRRS (p) S (CDK5 site) LVG; TAT-KLC (33 aa): YGRKKRRQRRR-LSDSRTLSSSSMDLSRRSSLVG; and TAT-Con (33 aa): YGRKKRRQRRR-LSDSRTLASSSMDLSRRSALVG. Detailed methods are provided in SI Materials and Methods.
Surface Biotinylation and Western Blot Analysis of GluR1 and GluR2.
Detailed methods for performing the biotinylation assay are provided in SI Materials and Methods.
Immunoprecipitation.
Immunoprecipitation was performed as previously described with minor modifications (37). Detailed methods are provided in SI Materials and Methods.
GSK-3β Kinase Assay.
GSK-3β kinase (Upstate Biotechnology) assay was performed according to the manufacturer's protocol. Detailed methods are provided in SI Materials and Methods.
Electrophysiological Recording.
Hippocampal slices (400-μm thickness) were prepared and brain slice recording was performed as previously described (38). Detailed methods are provided in SI Materials and Methods.
Behavioral Tests.
Male Swiss CD1 mice underwent surgery to implant the minipumps with the peptides TAT-KLCpCDK or TAT-Con (20 mg/mL, 120 μg/d). Mice underwent the tail suspension test on day 8, the forced swim test on day 10, and the AMPH-induced hyperactivity test on day 12. Detailed methods are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Ioline Henter for outstanding editorial assistance and Dr. Haim Einat for scientific advice. We also gratefully acknowledge AstraZeneca Pharmaceuticals for providing the GSK-3 inhibitor AR-A014418 compound. We gratefully acknowledge the support of the Intramural Research Program of the National Institute of Mental Health.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0913138107/-/DCSupplemental.
References
- 1.Goodwin GM, Geddes JR. Latest maintenance data on lithium in bipolar disorder. Eur Neuropsychopharmacol. 2003;13(Suppl 2):S51–S55. doi: 10.1016/s0924-977x(03)00078-6. [DOI] [PubMed] [Google Scholar]
- 2.Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci USA. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serretti A, et al. Association between GSK-3β-50T/C polymorphism and personality and psychotic symptoms in mood disorders. Psychiatry Res. 2008;158:132–140. doi: 10.1016/j.psychres.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 4.Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3β in cellular signaling. Prog Neurobiol. 2001;65:391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- 5.Gould TD, Einat H, Bhat R, Manji HK. AR-A014418, a selective GSK-3 inhibitor, produces antidepressant-like effects in the forced swim test. Int J Neuropsychopharmacol. 2004;7:387–390. doi: 10.1017/S1461145704004535. [DOI] [PubMed] [Google Scholar]
- 6.O'Brien WT, et al. Glycogen synthase kinase-3β haploinsufficiency mimics the behavioral and molecular effects of lithium. J Neurosci. 2004;24:6791–6798. doi: 10.1523/JNEUROSCI.4753-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du J, et al. The role of hippocampal GluR1 and GluR2 receptors in manic-like behavior. J Neurosci. 2008;28:68–79. doi: 10.1523/JNEUROSCI.3080-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du J, et al. The anticonvulsants lamotrigine, riluzole, and valproate differentially regulate AMPA receptor membrane localization: Relationship to clinical effects in mood disorders. Neuropsychopharmacology. 2007;32:793–802. doi: 10.1038/sj.npp.1301178. [DOI] [PubMed] [Google Scholar]
- 9.Hu H, et al. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell. 2007;131:160–173. doi: 10.1016/j.cell.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 10.Li X, et al. Antidepressant-like actions of an AMPA receptor potentiator (LY392098) Neuropharmacology. 2001;40:1028–1033. doi: 10.1016/s0028-3908(00)00194-5. [DOI] [PubMed] [Google Scholar]
- 11.Peineau S, et al. LTP inhibits LTD in the hippocampus via regulation of GSK3β. Neuron. 2007;53:703–717. doi: 10.1016/j.neuron.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 12.Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- 13.Man HY, et al. Regulation of AMPA receptor-mediated synaptic transmission by clathrin-dependent receptor internalization. Neuron. 2000;25:649–662. doi: 10.1016/s0896-6273(00)81067-3. [DOI] [PubMed] [Google Scholar]
- 14.Setou M, et al. Glutamate-receptor-interacting protein GRIP1 directly steers kinesin to dendrites. Nature. 2002;417:83–87. doi: 10.1038/nature743. [DOI] [PubMed] [Google Scholar]
- 15.Sun X, Zhao Y, Wolf ME. Dopamine receptor stimulation modulates AMPA receptor synaptic insertion in prefrontal cortex neurons. J Neurosci. 2005;25:7342–7351. doi: 10.1523/JNEUROSCI.4603-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beattie EC, et al. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat Neurosci. 2000;3:1291–1300. doi: 10.1038/81823. [DOI] [PubMed] [Google Scholar]
- 17.Lee HK, Barbarosie M, Kameyama K, Bear MF, Huganir RL. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature. 2000;405:955–959. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]
- 18.Shi SH, et al. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- 19.Beaulieu JM, Gainetdinov RR, Caron MG. Akt/GSK3 signaling in the action of psychotropic drugs. Annu Rev Pharmacol Toxicol. 2009;49:327–347. doi: 10.1146/annurev.pharmtox.011008.145634. [DOI] [PubMed] [Google Scholar]
- 20.Kannoji A, Phukan S, Sudher Babu V, Balaji VN. GSK3β: A master switch and a promising target. Expert Opin Ther Targets. 2008;12:1443–1455. doi: 10.1517/14728222.12.11.1443. [DOI] [PubMed] [Google Scholar]
- 21.Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carlezon WA, Jr, Nestler EJ. Elevated levels of GluR1 in the midbrain: A trigger for sensitization to drugs of abuse? Trends Neurosci. 2002;25:610–615. doi: 10.1016/s0166-2236(02)02289-0. [DOI] [PubMed] [Google Scholar]
- 23.Karcher RL, Deacon SW, Gelfand VI. Motor-cargo interactions: The key to transport specificity. Trends Cell Biol. 2002;12:21–27. doi: 10.1016/s0962-8924(01)02184-5. [DOI] [PubMed] [Google Scholar]
- 24.Mizuno N, et al. Dynein and kinesin share an overlapping microtubule-binding site. EMBO J. 2004;23:2459–2467. doi: 10.1038/sj.emboj.7600240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carroll RC, et al. Dynamin-dependent endocytosis of ionotropic glutamate receptors. Proc Natl Acad Sci USA. 1999;96:14112–14117. doi: 10.1073/pnas.96.24.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaskolski F, Mayo-Martin B, Jane D, Henley JM. Dynamin-dependent membrane drift recruits AMPA receptors to dendritic spines. J Biol Chem. 2009;284:12491–12503. doi: 10.1074/jbc.M808401200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petrini EM, et al. Endocytic trafficking and recycling maintain a pool of mobile surface AMPA receptors required for synaptic potentiation. Neuron. 2009;63:92–105. doi: 10.1016/j.neuron.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morfini G, Szebenyi G, Elluru R, Ratner N, Brady ST. Glycogen synthase kinase 3 phosphorylates kinesin light chains and negatively regulates kinesin-based motility. EMBO J. 2002;21:281–293. doi: 10.1093/emboj/21.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim CH, Lisman JE. A labile component of AMPA receptor-mediated synaptic transmission is dependent on microtubule motors, actin, and N-ethylmaleimide-sensitive factor. J Neurosci. 2001;21:4188–4194. doi: 10.1523/JNEUROSCI.21-12-04188.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Donnell KC, Gould TD. The behavioral actions of lithium in rodent models: Leads to develop novel therapeutics. Neurosci Biobehav Rev. 2007;31:932–962. doi: 10.1016/j.neubiorev.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gould TD, et al. Involvement of AMPA receptors in the antidepressant-like effects of lithium in the mouse tail suspension test and forced swim test. Neuropharmacology. 2008;54:577–587. doi: 10.1016/j.neuropharm.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carlezon WA, Jr, Thomas MJ. Biological substrates of reward and aversion: A nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56(Suppl 1):122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 34.Lodge DJ, Grace AA. Hippocampal dysfunction and disruption of dopamine system regulation in an animal model of schizophrenia. Neurotox Res. 2008;14:97–104. doi: 10.1007/BF03033801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manji HK, et al. Enhancing neuronal plasticity and cellular resilience to develop novel, improved therapeutics for difficult-to-treat depression. Biol Psychiatry. 2003;53:707–742. doi: 10.1016/s0006-3223(03)00117-3. [DOI] [PubMed] [Google Scholar]
- 36.Du J, et al. Modulation of synaptic plasticity by antimanic agents: The role of AMPA glutamate receptor subunit 1 synaptic expression. J Neurosci. 2004;24:6578–6589. doi: 10.1523/JNEUROSCI.1258-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Du J, et al. Dynamic regulation of mitochondrial function by glucocorticoids. Proc Natl Acad Sci USA. 2009;106:3543–3548. doi: 10.1073/pnas.0812671106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Wu J, Rowan MJ, Anwyl R. Conditions for the induction of long-term potentiation and long-term depression by conjunctive pairing in the dentate gyrus in vitro. J Neurophysiol. 1997;78:2569–2573. doi: 10.1152/jn.1997.78.5.2569. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.