Abstract
This work tests the hypothesis that the cerebellum is critical to the perception of the timing of sensory events. Auditory tasks were used to assess two types of timing in a group of patients with a stereotyped specific degeneration of the cerebellum: the analysis of single time intervals requiring absolute measurements of time, and the holistic analysis of rhythmic patterns based on relative measures of time using an underlying regular beat. The data support a specific role for the cerebellum only in the absolute timing of single subsecond intervals but not in the relative timing of rhythmic sequences with a regular beat. The findings support the existence of a stopwatch-like cerebellar timing mechanism for absolute intervals that is distinct from mechanisms for entrainment with a regular beat.
Keywords: human, perception, absolute, relative, subsecond
The relevance of the human cerebellum to the perception of time intervals and rhythmic sequences is controversial. Involvement of the cerebellum in perceptual timing (the perception of the timing of sensory events), in addition to its role in motor timing (the timed execution of movements), has been suggested by a number of studies (1–6). One distinction that we wish to address here, which has not been made clear in previous work, is between the absolute, duration-based timing of single subsecond intervals and the relative timing of subsecond intervals based on a regular beat. Functional imaging studies suggest neural activity in the human cerebellum during the perception of the absolute duration of single time intervals (7, 8) as well as rhythmic patterns with a regular beat (9–13). However, previous lesion work to assess an obligatory cerebellar role in the perception of single time intervals has not yielded consistent results (4, 14–17). Previous lesion work to assess any obligatory role of the cerebellum in the analysis of rhythmic sequences has assessed only deficits in related motor activity, such as tapping out a beat (4, 14, 18), that do not allow clear inference about perception.
In this study, we test whether the cerebellum is a critical substrate for perceptual tasks that require the absolute, duration-based analysis of single time intervals as well as those that require the relative analysis of time intervals within rhythmic patterns based on a regular beat. Perceptual tests were conducted in the auditory domain, where accurate temporal encoding of sensory events is essential and entrainment with a beat is induced naturally. Tasks were administered to a group of 34 patients with a stereotyped cerebellar degeneration and a matched control group of 40 healthy individuals. Two absolute timing tasks tested the perception of single intervals for a variable and a fixed reference duration, respectively (Fig. 1 A and B). Three relative timing tasks tested the beat-based analysis of rhythmic sequences, including the detection of the presence of a roughly regular beat (19), a deviation from an isochronous beat (20) and a distortion of a rhythmic pattern with a metrical beat (21) (Fig. 1 C–E). The data support a cerebellar role in the absolute timing of single intervals, but not the relative timing of beat-based sequences, and distinct brain mechanisms for these two types of perceptual timing.
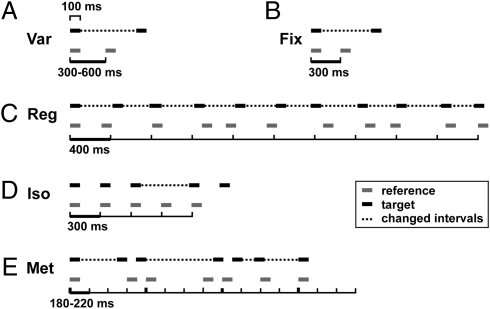
Fig. 1.
Stimuli for the five timing tasks depicting one example of one target stimulus (black) and one reference stimulus (gray) per task. Horizontal lines depict tones (200 Hz; 100 ms); ticks represent units corresponding to an underlying beat; dotted lines mark interval changes in target stimuli. (A) (Var) Variable-single interval discrimination. (B) (Fix) Fixed-single interval discrimination. (C) (Reg) Regularity detection. (D) (Iso) Isochrony-deviation detection. (E) (Met) Metrical pattern discrimination (roving of unit-duration omitted for clarity; thick ticks marking the metrical beat of 4).
Results
In all five tasks (Fig. 1), performance was measured by adaptive tracking of thresholds (Fig. S1). Group differences (Fig. 2A) and occurrence and severity of individual impairments were evaluated (Figs. 2B and S2), and correlation with motor impairment (Table S1) was assessed.
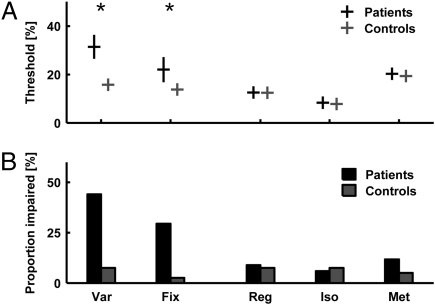
Fig. 2.
Timing thresholds group data and frequency of occurrence of individual deficits for patients (n = 34; black) compared with controls (n = 40; gray). (A) Mean timing thresholds and SEMs. Group differences were significant for Var and Fix. (B) Proportion of significant impairment at the individual level Note high values for Var and Fix, contrasted by low values for Reg, Iso, and Met. Significance level was P < 0.05.
Both single-interval timing tasks (Var, Fix; Fig. 1 A and B) demonstrated significant deficits in the patients compared with the control group. The larger difference in thresholds was found for the reference interval having a variable duration (significant at the level of P < 0.001, t test on log-transformed data; effect size 0.87; Fig. 2A). The difference for a reference of fixed duration was also significant, with a smaller effect size (significant at the level of P < 0.05, t test on log-transformed data; effect size 0.40; Fig. 2A). In accordance with the group-level analysis, single-subject inference based on z scores demonstrated a frequency of occurrence of significant impairments with P < 0.05 in 44% of the patients for the timing of single intervals with variable reference duration and 29% for fixed-reference duration (Fig. 2B).
The relative timing of beat-based rhythmic patterns was systematically assessed at three levels of perceptual analysis: the detection of a regular beat (Fig. 1C, Reg), the detection of a deviation from an isochronous beat (Fig. 1D, Iso), and the detection of a distortion in a metrical beat pattern (Fig. 1E, Met). No group level deficit in performance was found for any of the three tasks (not significant at the level of P < 0.05; Reg, Mann–Whitney U test; Iso, Welch's t test; Met, Mann–Whitney U test; Fig. 2A). The frequency of occurrence of individual deficits was low overall and comparable to that in controls: Significant impairments for the detection of a roughly regular beat, the deviation from an isochronous beat, and the distortion in a metrical beat were found in 9%, 6% and 12%, respectively (Fig. 2B).
No correlations were found between performance in perceptual timing tasks and the duration or severity of motor symptoms (Fig. S2 and Table S1). Impairments tended not to occur in presymptomatic individuals, and were substantial in some severely affected individuals, but there was no consistent relationship at the group level.
Discussion
This work tested the role of the human cerebellum as a critical substrate for the perceptual timing of single intervals based on their absolute duration and that of rhythmic sequences with a regular beat based on the relative durations of intervals. The findings are based on reliable measures of individual performance for both types of timing in a large and homogeneous group of patients with a specific type of cerebellar degeneration (SCA-6). A significant deficit in the patients compared with the matched control group was demonstrated for the timing of single intervals, but not for rhythmic sequences based on a regular beat. The data suggest impairment in a mechanism for absolute timing and preservation of mechanisms of relative timing.
Deficit in Absolute Duration-Based Timing.
The significant deficit in the timing of single intervals in the patients compared with controls is consistent with previous neuropsychological reports of deficits or trends to deficits in related tasks (16, 17, 22, 23). Some inconsistency of the effect in previous studies may reflect the heterogeneity of lesions in these populations of stroke patients (14, 15). The current study assessed 34 individuals with a genetically determined, isolated disorder of the cerebellum that advances in a stereotyped way from the superior to the inferior part (24). Subjects were recruited on the basis of their diagnosis with SCA-6 and included irrespective of the severity of motor symptoms. Substantial group effects were shown despite the inclusion of asymptomatic subjects. The absence of correlations with motor symptoms may be due to insufficient power or dissociation of neural substrates for motor tasks relevant to ataxia and perception within the cerebellum. The group-level and individual deficits for the fixed-interval task, where the reference interval is presented repeatedly throughout the test, compare well with the moderate effects reported in previous studies (4, 14, 16). The larger effect size and proportion of individual deficits in more than 40% of the patients in the variable-interval task, in which the duration of the reference interval changes from trial to trial, support the specific involvement of the cerebellum in the perceptual analysis of unrepeated intervals. In this task, the duration of each interval must be timed anew with no possibility of creating an internal long-term reference: The task is a more exacting test of the perception of absolute time. Cerebellar integrity seems crucial to the normal functioning of this stopwatch-like timing mechanism (25). This notion is consistent with cerebellar activation in normal individuals being stronger for the perception of novel compared with familiar sequences of intervals (11).
One alternative explanation for the difference in performance between patients and controls could be based on cognitive demands and the recent implication of the cerebellum in cognition (26–29). This appears unlikely, as the single-interval timing tasks are less demanding than those of beat-based timing, and as the cognitive deficits demonstrated in patients with cerebellar damage were specific to aspects of executive function or attention (27–29) and would not lead to the present dissociation.
The cerebellar substrate for interval perception might have a number of precise anatomical locations. Previous functional imaging studies have not demonstrated clear differences between cerebellar activation patterns for absolute and relative perceptual timing (7, 8, 10, 11, 30). Repetitive transcranial magnetic stimulation targeting the medial and right cerebellum can produce similar acute deficits in absolute time-interval perception (31), demonstrating consistent effects of both chronic and acute cerebellar dysfunction. One previous neuropsychological report of a perceptual timing deficit implicated superior parts of the cerebellum, in particular, the right lateral hemisphere (14). The present study demonstrates a specific deficit in absolute time perception in a group with SCA-6 that experiences stereotyped progression of the cerebellar degeneration from the superior to the inferior parts, especially affecting the vermis (24). Our data are consistent with previous suggestions of a particular role of the superior cerebellum in absolute timing, and suggest a particular importance of the midline cerebellum.
Preservation of Relative, Beat-Based Timing Mechanisms.
All three tests of relative timing of rhythmic sequences based on a regular beat were unaffected in the patients at the group level. The first task, requiring the detection of regularity, was based on isochronous sequences that become increasingly irregular (19) and was unaffected with the exception of a few individuals. If the detection of a regular beat depended on accurate single-interval timing in a hierarchical fashion, one would expect to find a deficit in the regularity task also. The lack of impairment suggests that this task depends on a mechanism distinct from the stopwatch-type device that we suggest for single intervals. The second task, the detection of a deviation from an isochronous beat, was also unimpaired at the group level. Thresholds were lower than those for single intervals or within sequences with no regular beat, as reported in previous studies (20, 32, 33), with the drop in thresholds being even larger in the patients than in the controls. The third task, assessing the ability of subjects to use metrical sequences to achieve greater precision in rhythmic timing tasks (21), was also unimpaired at the group level.
All three tasks could be accomplished by one or more mechanisms that are different from the timing of single intervals. Unlike single-interval mechanisms being based on absolute duration, those additional mechanisms would use the presence of a regular beat as an alternative reference frame for the timing of intervals relative to the beat. Psychophysical data from previous studies in normals support a dissociation between mechanisms for duration-based as opposed to beat-based timing of intervals and rhythmic sequences (20, 21, 32, 34–38). The preservation of relative timing tasks is not consistent with the idea that the cerebellum provides a single mechanism subserving both single-interval and beat-based timing; rather, the data suggest one or more mechanisms of perceptual entrainment with a regular beat that are independent of single-interval timing and can occur despite cerebellar damage. The preservation of beat-based perception here is consistent with that of sensorimotor transduction elsewhere (39), and with a perceptual mechanism of entrainment that depends on a neural substrate that is unaffected in the present study.
We cannot rule out the possibility that focal lesions in parts of the cerebellum that were unaffected in the patients in the present study might impair beat-based rhythm perception. The present study demonstrates an obligatory role of the cerebellum in absolute, subsecond timing but does not exclude any possible role in relative timing involving mechanisms that are relatively spared by the degenerative process. We would point out, however, that the study includes subjects with advanced disease and widespread atrophy who do not show individual deficits in beat-based rhythm perception. The preservation of performance contrasts with expectations based on cerebellar activation in fMRI studies of normal controls, and suggests that such activity might not be an obligatory aspect of relative time perception (9, 11, 13, 40).
Cerebellar Contribution to the Timing Network of the Brain.
This work suggests a differential involvement of the cerebellum as a part of the perceptual timing network of the brain. A “cerebellar clock” has previously been promoted as a device that both measures absolute interval duration and entrains with a regular beat of a sequence of intervals at the subsecond level (20, 41–43). The present data support a role of the cerebellum in the former but not the latter. We argue for a role for the cerebellum as a stopwatch mechanism with specific and obligatory involvement in absolute time-interval perception (25), in which the cerebellar circuitry would provide the suitable neural machinery (44–46). This is in accordance with the notion of “event timing” and the multiple-timer model suggested by Ivry et al. (20, 42, 43).
The preserved beat-based perception suggests a distinct neural substrate from the proposed cerebellar stopwatch mechanism for single-interval timing. Possible substrates include a distinct cerebellar subregion. However, the preservation of beat-based perception even in patients with extensive cerebellar damage would argue against this. Alternatively, beat-based perception may depend on distinct parts of the timing network beyond the cerebellum such as the basal ganglia or cerebral cortex (10, 30, 47, 48). Anatomical connections between the cerebellum and the basal ganglia and prefrontal cortex (via the thalamus) (46, 49–51) could support efficient integrated network processing. Recent functional data further suggest specialized loops or zones of cortico-cerebellar connectivity, including one for sensori-motor functions involving cerebellar lobules V–VII (52, 53).
This study addresses mechanisms of perceptual timing, and any link between perceptual and motor timing (54) needs to be made with caution. Previous patient studies suggested an obligatory cerebellar role in the motor timing of individual movements in isolation and in the context of a regular beat (4, 14, 18). Deficits in perceptual timing were here only demonstrated for single intervals in isolation and not within the context of a regular beat. Overall the data could support a common cerebellar mechanism in the timing of individual motor or perceptual events. The present data further suggest that beat-based perceptual timing can function independently of an impairment in this mechanism; they do not allow a direct inference on a common mechanism of entrainment for motor and perceptual timing.
The occurrence of impairment may relate to views of the cerebellum as a “universal” basic sensory acquisition controller (1) and as a part of a network for processing magnitude (55), in this case time or duration. Both views are congruent with the present argument for a cerebellar role in the absolute, duration-based perceptual timing of single intervals but not to the relative, beat-based timing within rhythmic sequences. The involvement of the postulated cerebellar mechanism of absolute, subsecond timing in other perceptual modalities is supported by previous reports of the effect of cerebellar TMS on duration-based somatosensory timing (56) and cerebellar activations in visual timing (7, 11).
Studies of lesions of the other components, including the basal ganglia and prefrontal cortex, and in other modalities will allow further tests of the dissociation between duration-based and beat-based timing.
Materials and Methods
Subjects.
The patient group included 34 individuals (12 males) with genetically diagnosed spinocerebellar ataxia type 6 (SCA-6) (Fig. 3).The patients’ age range was 45–81 y (mean 64 ± 10 SD); estimated premorbid IQs (57) and verbal IQs (58) were in the normal range (premorbid, mean 101 ± 7 SD; verbal, mean 105 ±11 SD). Duration of symptoms was self-reported and ranged from 0 to 20 y (mean 8.8 ± 5.8 SD), severity of motor impairment assessed on a locally developed scale from 1 to 5; patient details are listed in Table S2. SCA-6 is an autosomal-dominant disease caused by a loss-of-function mutation in a voltage-gated calcium channel that specifically affects the Purkinje cells of the cerebellum. Degeneration starts at the superior–anterior end and progresses inferior–posteriorly in a stereotyped manner (24). The matched control group included 40 subjects (18 males) of 45–81 y (mean 64 ± 9 y). There were no group differences in age, years of education, estimated premorbid IQ, or hearing sensitivity (Table 1).
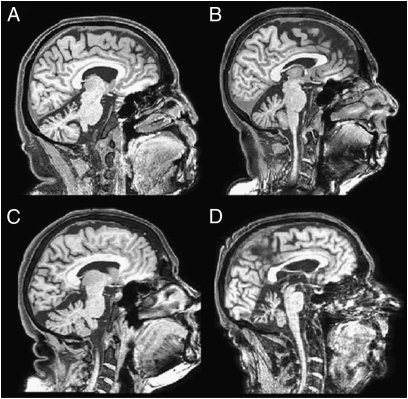
Fig. 3.
Structural MRI scans of SCA-6–induced cerebellar degeneration in four patients. (A) P27: male, 60 y, 3 y postonset, x 4. (B) P32: female, 62 y, 6 y postonset, x +4. (C) P07: female, 69 y, 10 y postonset, x −7. (D) P13: male, 74 y, 20 y postonset, x −3. x coordinates according to MNI convention.
Table 1.
Control and patient group descriptive statistics
| Age, y | Education, y | Estimated premorbid IQ | Audiogram, dB hearing loss | |
| Controls | 64.1 ± 9.0 | 10.0 ± 3.0* | 103.0 ± 9.7* | 19.4 ± 11.0 |
| Patients | 64.1 ± 10.1 | 11.0 ± 1.4* | 102.0 ± 4.9* | 20.6 ± 7.9 |
| Difference | NS | NS | NS | NS |
Values are group mean ± SD in cases of normal distributions, and group medians ± mean deviations from the median for non-normal distributions (*tested by Lilliefors modification of the Kolmogorov–Smirnoff test for composite normality, significance level, P = 0.05). No significant group differences were found at the level of P < 0.05, tested by the independent two-sample t test or Mann–Whitney U test in cases of normal and non-normal distributions, respectively. IQ, intelligence quotient; NS, not significant.
Setup and Stimuli.
All stimuli were composed of 200-Hz pure tones, 100 ms in duration with 20 ms gating times. Stimuli were created using Matlab 6.5 (Mathworks) with a 44.1-kHz sampling rate and 16-bit resolution, delivered at 70 dB rms Sound Pressure Level via an external soundcard (Edirol Audio Capture UA-3FX) and closed headphones (Sennheiser HD265 linear).
Procedure.
The timing tasks included two interval timing tasks and three beat-based timing tasks. All tasks used an adaptive, two alternative forced-choice procedure following a two-down–one-up tracking algorithm (59) (Fig. S1). Each test consisted of 60 trials, preceded by a minimum of three practice trials to familiarize the subject with the task. Each trial contained one target and one reference stimulus in randomized order, and the task of the subject was to indicate the position of the target. Target positions were randomized at equal probabilities, with order fixed across subject. Interstimulus and intertrial intervals were 1,500 ms each. Subjects communicated their responses by pressing corresponding buttons on a response box (controls) or pointing to corresponding circles on paper (patients) to avoid motor difficulties with button presses. Response time was not limited, but subjects were encouraged to make the decisions quickly. The difference between target and reference was at suprathreshold levels initially and decreased after two responses correct in a row and increased after each incorrect one. A larger step size was used up to the fourth reversal (change between increase and decrease) and after that a smaller one. Thresholds were calculated as the mean of the last six reversals, estimating the 70.7% correct point of the psychometric function (59). The total time needed was less than 2 h, including a pure tone audiogram for 0.125, 0.25, 0.5, 1, 2, and 4 kHz.
Tasks of Absolute Timing of Single Intervals.
Variable interval timing (Var).
In the variable interval task, subjects were required to discriminate a longer target interval against a shorter reference interval (Fig. 1A). Intervals were marked by pairs of tones; the reference interval had a variable interonset-interval of 300, 360, 420, 480, 560, or 600 ms in duration (at equal probabilities in pseudorandomized order fixed across subjects). The target interval had a silent gap between the flanking tones that was longer than the reference by 90% of the silent reference interval initially and adaptively adjusted in steps of 12% and 6%.
Fixed-interval timing (Fix).
As in the variable-interval task, subjects were required to discriminate a longer target against a shorter reference interval, with the only difference that the interonset-interval of the reference was fixed at 300 ms in this task (Fig. 1B). The target interval was longer by 30% initially and adaptively adjusted in steps of 4% and 1.33%.
Tasks of Relative Timing of Beat-Based Patterns.
Regular beat detection (Reg).
In the beat detection task, subjects had to discriminate a more regular target sequence against a less regular reference sequence based on an underlying beat and despite the introduction of an increasing amount of irregularity (Fig. 1C). Both sequences consisted of 11 tones and were based on a regular beat of a 400 ms interonset-interval. The beat could easily be detected in the target sequence initially as it was perfectly regular (isochronous). The reference sequence was highly irregular as each time interval was shortened or lengthened at random by 30% on average, making the underlying regular beat imperceptible (19). The mean irregularity in the target, starting at 0% initially, was adaptively increased in steps of 4% and 2.5% until the underlying beat could not be detected and the target could not be reliably discriminated against the reference anymore.
Isochrony deviation detection (Iso).
Subjects were required to detect a lengthening of the third interval in an otherwise isochronous, five-tone sequence with a regular interonset-interval of 300 ms (20) (Fig. 1D). This task was physically identical to that using a fixed single interval (B, Fix), with the added context of an isochronous beat. The initial difference in interval length was 20%; adaptive step sizes were 2% and 1%.
Metrical pattern discrimination (Met).
Subjects were required to detect a relative change in the timing of a rhythmic sequence of seven tones with a metrical beat (Fig. 1E) based on two levels of periodicity (21). The duration of the lower level periodicity was 200 ms on average (roved from 180 to 220 ms in 4-ms steps). The higher-level metrical beat of 4 was induced by a regular occurrence of temporally induced accents on every fourth unit (with a periodicity of 800 ms on average; marked by thick ticks). The target contained a change in the relative timing of the intervals within the sequence that resulted in a distortion of the metrical beat pattern. The change in pattern was introduced by changes in duration in the four long intervals of 2x one and 2x two silent units, where one of each was lengthened and one shortened, all by the same percentage. The intervals containing silent units were thus no longer integer multiples of the underlying unit and the pattern would sound “wrong.” The change amounted to 65% initially, and was adaptively adjusted in steps of 12% and 6%.
Statistical Data Analysis.
Group-level comparison.
Distribution of data samples was tested by the Lilliefors modification of the Kolmogorov–Smirnoff test for composite normality. Between-group comparisons were carried out by Student's or Welch's t test for normally distributed samples (original or logged) with equal or unequal variance, or the Mann--Whitney U Test for nonnormally distributed samples of equal dispersion (no unequal dispersion occurred). The significance level was P = 0.05. Effect sizes were calculated taking into account sample size.
Single-subject inference.
Single-subject inference was based on z score transformation of patients’ thresholds in relation to the control group while taking into account the effect of age. A complete list of age-dependency correlation coefficients based on the use of Pearson product-moment or Spearman rank correlation test for normal and nonnormal distributions, respectively, is given in Table S2.
Age-dependence of thresholds was modeled by linear regression of the control data:
where x is the individual threshold, β1 and β0 are the regression coefficients, and ε is the error of the regression model between threshold and age.
Individual patients’ z scores were calculated as a function of age:
where θ is the individual threshold, 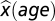 is the age-dependent expected threshold, and std(ε) is the SD of the error of the regression model.
is the age-dependent expected threshold, and std(ε) is the SD of the error of the regression model.
The significance level was P = 0.05, equivalent to a score of z > 1.65.
Supplementary Material
Acknowledgments
We thank Sukhbinder Kumar and Martin O'Gorman for advice with data analysis and Sundeep Teki for comments on the manuscript. This work was supported by Wellcome Trust Grant WT061136MA (to T.D.G.). F.C. is funded by an Ataxia UK Ph.D. studentship. P.F.C. and T.D.G. are both Wellcome Trust Senior Fellows in Clinical Science.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. W.T.T. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0910473107/-/DCSupplemental.
References
- 1.Gao JH, et al. Cerebellum implicated in sensory acquisition and discrimination rather than motor control. Science. 1996;272:545–547. doi: 10.1126/science.272.5261.545. [DOI] [PubMed] [Google Scholar]
- 2.Glickstein M. The cerebellum and motor learning. Curr Opin Neurobiol. 1992;2:802–806. doi: 10.1016/0959-4388(92)90137-a. [DOI] [PubMed] [Google Scholar]
- 3.Hore J, Wild B, Diener HC. Cerebellar dysmetria at the elbow, wrist, and fingers. J Neurophysiol. 1991;65:563–571. doi: 10.1152/jn.1991.65.3.563. [DOI] [PubMed] [Google Scholar]
- 4.Ivry R, Keele SW. Timing functions of the cerebellum. J Cogn Neurosci. 1989;1:136–152. doi: 10.1162/jocn.1989.1.2.136. [DOI] [PubMed] [Google Scholar]
- 5.Ivry RB, Keele SW, Diener HC. Dissociation of the lateral and medial cerebellum in movement timing and movement execution. Exp Brain Res. 1988;73:167–180. doi: 10.1007/BF00279670. [DOI] [PubMed] [Google Scholar]
- 6.Thach WT, Goodkin HP, Keating JG. The cerebellum and the adaptive coordination of movement. Annu Rev Neurosci. 1992;15:403–442. doi: 10.1146/annurev.ne.15.030192.002155. [DOI] [PubMed] [Google Scholar]
- 7.Lewis PA, Miall RC. Distinct systems for automatic and cognitively controlled time measurement: Evidence from neuroimaging. Curr Opin Neurobiol. 2003;13:250–255. doi: 10.1016/s0959-4388(03)00036-9. [DOI] [PubMed] [Google Scholar]
- 8.Lewis PA, Miall RC. Remembering the time: A continuous clock. Trends Cogn Sci. 2006;10:401–406. doi: 10.1016/j.tics.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Chen JL, Penhune VB, Zatorre RJ. Tapping in synchrony to auditory rhythms: Effect of temporal structure on behavior and neural activity. Ann N Y Acad Sci. 2005;1060:400–403. doi: 10.1196/annals.1360.044. [DOI] [PubMed] [Google Scholar]
- 10.Grahn JA, Brett M. Rhythm and beat perception in motor areas of the brain. J Cogn Neurosci. 2007;19:893–906. doi: 10.1162/jocn.2007.19.5.893. [DOI] [PubMed] [Google Scholar]
- 11.Penhune VB, Zattore RJ, Evans AC. Cerebellar contributions to motor timing: A PET study of auditory and visual rhythm reproduction. J Cogn Neurosci. 1998;10:752–765. doi: 10.1162/089892998563149. [DOI] [PubMed] [Google Scholar]
- 12.Rao SM, Mayer AR, Harrington DL. The evolution of brain activation during temporal processing. Nat Neurosci. 2001;4:317–323. doi: 10.1038/85191. [DOI] [PubMed] [Google Scholar]
- 13.Thaut MH. Neural basis of rhythmic timing networks in the human brain. Ann N Y Acad Sci. 2003;999:364–373. doi: 10.1196/annals.1284.044. [DOI] [PubMed] [Google Scholar]
- 14.Harrington DL, Lee RR, Boyd LA, Rapcsak SZ, Knight RT. Does the representation of time depend on the cerebellum? Effect of cerebellar stroke. Brain. 2004;127:561–574. doi: 10.1093/brain/awh065. [DOI] [PubMed] [Google Scholar]
- 15.Ivry RB, Spencer RM. Evaluating the role of the cerebellum in temporal processing: Beware of the null hypothesis. Brain. 2004;127:E13. doi: 10.1093/brain/awh226. [DOI] [PubMed] [Google Scholar]
- 16.Mangels JA, Ivry RB, Shimizu N. Dissociable contributions of the prefrontal and neocerebellar cortex to time perception. Brain Res Cogn Brain Res. 1998;7:15–39. doi: 10.1016/s0926-6410(98)00005-6. [DOI] [PubMed] [Google Scholar]
- 17.Nichelli P, Alway D, Grafman J. Perceptual timing in cerebellar degeneration. Neuropsychologia. 1996;34:863–871. doi: 10.1016/0028-3932(96)00001-2. [DOI] [PubMed] [Google Scholar]
- 18.Spencer RM, Zelaznik HN, Diedrichsen J, Ivry RB. Disrupted timing of discontinuous but not continuous movements by cerebellar lesions. Science. 2003;300:1437–1439. doi: 10.1126/science.1083661. [DOI] [PubMed] [Google Scholar]
- 19.Madison G, Merker B. On the limits of anisochrony in pulse attribution. Psychol Res. 2002;66:201–207. doi: 10.1007/s00426-001-0085-y. [DOI] [PubMed] [Google Scholar]
- 20.Foxton JM, Nandy RK, Griffiths TD. Rhythm deficits in ‘tone deafness’. Brain Cogn. 2006;62:24–29. doi: 10.1016/j.bandc.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Grube M, Griffiths TD. Metricality-enhanced temporal encoding and the subjective perception of rhythmic sequences. Cortex. 2009;45:72–79. doi: 10.1016/j.cortex.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Ivry RB. The representation of temporal information in perception and motor control. Curr Opin Neurobiol. 1996;6:851–857. doi: 10.1016/s0959-4388(96)80037-7. [DOI] [PubMed] [Google Scholar]
- 23.Malapani C, Dubois B, Rancurel G, Gibbon J. Cerebellar dysfunctions of temporal processing in the seconds range in humans. Neuroreport. 1998;9:3907–3912. doi: 10.1097/00001756-199812010-00026. [DOI] [PubMed] [Google Scholar]
- 24.Butteriss D, Chinnery P, Birchall D. Radiological characterization of spinocerebellar ataxia type 6. Br J Radiol. 2005;78:694–696. doi: 10.1259/bjr/73834093. [DOI] [PubMed] [Google Scholar]
- 25.Buhusi CV, Meck WH. Relativity theory and time perception: Single or multiple clocks? PLoS ONE. 2009;4:e6268. doi: 10.1371/journal.pone.0006268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121:561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- 27.Cooper FE, et al. The contribution of the cerebellum to cognition in spinocerebellar ataxia type 6. Behav Neurol. doi: 10.3233/BEN-2010-0265. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gottwald B, Mihajlovic Z, Wilde B, Mehdorn HM. Does the cerebellum contribute to specific aspects of attention? Neuropsychologia. 2003;41:1452–1460. doi: 10.1016/s0028-3932(03)00090-3. [DOI] [PubMed] [Google Scholar]
- 29.Gottwald B, Wilde B, Mihajlovic Z, Mehdorn HM. Evidence for distinct cognitive deficits after focal cerebellar lesions. J Neurol Neurosurg Psychiatry. 2004;75:1524–1531. doi: 10.1136/jnnp.2003.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen JL, Penhune VB, Zatorre RJ. Listening to musical rhythms recruits motor regions of the brain. Cereb Cortex. 2008;18:2844–2854. doi: 10.1093/cercor/bhn042. [DOI] [PubMed] [Google Scholar]
- 31.Lee KH, et al. The role of the cerebellum in subsecond time perception: Evidence from repetitive transcranial magnetic stimulation. J Cogn Neurosci. 2007;19:147–157. doi: 10.1162/jocn.2007.19.1.147. [DOI] [PubMed] [Google Scholar]
- 32.Friberg A, Sundberg J. Time discrimination in a monotonic, isochronous sequence. J Acoust Soc Am. 1995;98(5):2524–2531. [Google Scholar]
- 33.Hirsh IJ, Monahan CB, Grant KW, Singh PG. Studies in auditory timing: 1. Simple patterns. Percept Psychophys. 1990;47:215–226. doi: 10.3758/bf03204997. [DOI] [PubMed] [Google Scholar]
- 34.Deutsch D. Recognition of durations embedded in temporal patterns. Percept Psychophys. 1986;39:179–186. doi: 10.3758/bf03212489. [DOI] [PubMed] [Google Scholar]
- 35.Handel S, Oshinsky JS. The meter of syncopated auditory polyrhythms. Percept Psychophys. 1981;30:1–9. doi: 10.3758/bf03206130. [DOI] [PubMed] [Google Scholar]
- 36.Patel AD, Iversen JR, Chen Y, Repp BH. The influence of metricality and modality on synchronization with a beat. Exp Brain Res. 2005;163:226–238. doi: 10.1007/s00221-004-2159-8. [DOI] [PubMed] [Google Scholar]
- 37.Povel DJ, Essens P. Perception of temporal patterns. Music Percept. 1985;2:411–440. doi: 10.3758/bf03207132. [DOI] [PubMed] [Google Scholar]
- 38.Rammsayer TH, Brandler S. Aspects of temporal information processing: A dimensional analysis. Psychol Res. 2004;69:115–123. doi: 10.1007/s00426-003-0164-3. [DOI] [PubMed] [Google Scholar]
- 39.Molinari M, et al. Sensorimotor transduction of time information is preserved in subjects with cerebellar damage. Brain Res Bull. 2005;67:448–458. doi: 10.1016/j.brainresbull.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 40.Xu D, Liu T, Ashe J, Bushara KO. Role of the olivo-cerebellar system in timing. J Neurosci. 2006;26:5990–5995. doi: 10.1523/JNEUROSCI.0038-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci. 2005;6:755–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- 42.Ivry RB, Richardson TC. Temporal control and coordination: The multiple timer model. Brain Cogn. 2002;48:117–132. doi: 10.1006/brcg.2001.1308. [DOI] [PubMed] [Google Scholar]
- 43.Ivry RB, Spencer RM, Zelaznik HN, Diedrichsen J. The cerebellum and event timing. Ann N Y Acad Sci. 2002;978:302–317. doi: 10.1111/j.1749-6632.2002.tb07576.x. [DOI] [PubMed] [Google Scholar]
- 44.Medina JF, Garcia KS, Nores WL, Taylor NM, Mauk MD. Timing mechanisms in the cerebellum: Testing predictions of a large-scale computer simulation. J Neurosci. 2000;20:5516–5525. doi: 10.1523/JNEUROSCI.20-14-05516.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Middleton SJ, et al. High-frequency network oscillations in cerebellar cortex. Neuron. 2008;58:763–774. doi: 10.1016/j.neuron.2008.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Voogd J, Glickstein M. The anatomy of the cerebellum. Trends Neurosci. 1998;21:370–375. doi: 10.1016/s0166-2236(98)01318-6. [DOI] [PubMed] [Google Scholar]
- 47.Chen JL, Zatorre RJ, Penhune VB. Interactions between auditory and dorsal premotor cortex during synchronization to musical rhythms. Neuroimage. 2006;32:1771–1781. doi: 10.1016/j.neuroimage.2006.04.207. [DOI] [PubMed] [Google Scholar]
- 48.Grahn JA, Brett M. Impairment of beat-based rhythm discrimination in Parkinson's disease. Cortex. 2009;45:54–61. doi: 10.1016/j.cortex.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 49.Hoshi E, Tremblay L, Féger J, Carras PL, Strick PL. The cerebellum communicates with the basal ganglia. Nat Neurosci. 2005;8:1491–1493. doi: 10.1038/nn1544. [DOI] [PubMed] [Google Scholar]
- 50.Middleton FA, Strick PL. Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science. 1994;266:458–461. doi: 10.1126/science.7939688. [DOI] [PubMed] [Google Scholar]
- 51.Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. J Neurosci. 2001;21:700–712. doi: 10.1523/JNEUROSCI.21-02-00700.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Habas C, et al. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci. 2009;29:8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O'Reilly JX, Beckmann CF, Tomassini V, Ramnani N, Johansen-Berg H. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb Cortex. 2010;20:953–965. doi: 10.1093/cercor/bhp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keele SW, Pokorny RA, Corcos DM, Ivry R. Do perception and motor production share common timing mechanisms: A correctional analysis. Acta Psychol (Amst) 1985;60:173–191. doi: 10.1016/0001-6918(85)90054-x. [DOI] [PubMed] [Google Scholar]
- 55.Walsh V. A theory of magnitude: Common cortical metrics of time, space and quantity. Trends Cogn Sci. 2003;7:483–488. doi: 10.1016/j.tics.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 56.Fierro B, et al. Role of the cerebellum in time perception: A TMS study in normal subjects. J Neurol Sci. 2007;263:107–112. doi: 10.1016/j.jns.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 57.Crawford JR, et al. Estimating premorbid IQ from demographic variables: Regression equations derived from a UK sample. Br J Clin Psychol. 1989;28:275–278. doi: 10.1111/j.2044-8260.1989.tb01377.x. [DOI] [PubMed] [Google Scholar]
- 58.Nelson HE, Willison JR. National Adult Reading Test Manual. 2 Ed. Windsor, UK: NFER-Nelson; 1991. [Google Scholar]
- 59.Levitt H. Transformed up-down methods in psychoacoustics. J Acoust Soc Am. 1971;49(Suppl 2):467–477. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.