Abstract
Arthropod-borne viruses (arboviruses) represent a global public health problem, with dengue viruses causing millions of infections annually, while emerging arboviruses, such as West Nile, Japanese encephalitis, and chikungunya viruses have dramatically expanded their geographical ranges. Surveillance of arboviruses provides vital data regarding their prevalence and distribution that may be utilized for biosecurity measures and the implementation of disease control strategies. However, current surveillance methods that involve detection of virus in mosquito populations or sero-conversion in vertebrate hosts are laborious, expensive, and logistically problematic. We report a unique arbovirus surveillance system to detect arboviruses that exploits the process whereby mosquitoes expectorate virus in their saliva during sugar feeding. In this system, infected mosquitoes captured by CO2-baited updraft box traps are allowed to feed on honey-soaked nucleic acid preservation cards within the trap. The cards are then analyzed for expectorated virus using real-time reverse transcription-PCR. In field trials, this system detected the presence of Ross River and Barmah Forest viruses in multiple traps deployed at two locations in Australia. Viral RNA was preserved for at least seven days on the cards, allowing for long-term placement of traps and continuous collection of data documenting virus presence in mosquito populations. Furthermore no mosquito handling or processing was required and cards were conveniently shipped to the laboratory overnight. The simplicity and efficacy of this approach has the potential to transform current approaches to vector-borne disease surveillance by streamlining the monitoring of pathogens in vector populations.
Keywords: arboviruses, disease control, honey, saliva, surveillance
Arthropod-borne viruses (arboviruses) are responsible for significant global morbidity and mortality, with many reemerging or appearing in new geographic regions. The continued disease burden of dengue throughout the tropics, the widespread establishment of West Nile virus (WNV) in North America following its introduction in 1999, and the chikungunya virus (CHIKV) pandemic that afflicted nations in the western Indian Ocean, India, and Italy between 2004 and 2007 graphically illustrate the impact of arboviruses on human and animal populations (1, 2). The implementation of timely and effective control strategies, such as mosquito control and/or vaccination, can be highly dependent on data generated by surveillance systems. Accordingly, a number of strategies have been employed to detect arbovirus activity, but most are based on clinical diagnosis of symptoms and/or detection of the virus or virus-specific antibodies in vertebrates or detection of the virus in arthropod vectors (3). Sentinel animals, such as chickens for WNV, St. Louis encephalitis virus, and Murray Valley encephalitis virus surveillance, or pigs for Japanese encephalitis virus surveillance, are often deployed to provide an early warning system for an impending outbreak or to detect an incursion (4–6). Virus surveillance using arthropods is based on virus isolation from, or detection of viral RNA in, mosquitoes collected with a variety of trapping techniques.
Although these surveillance strategies provide important information regarding the distribution of arboviruses, each has limitations. For instance, disease surveillance systems are hampered by a similarity of disease signs and symptoms induced by different pathogens, the potentially low clinical to subclinical disease ratio, and the recognition of cases only after an outbreak has commenced. The disadvantages of sentinel animals include the need for intensive animal husbandry and risk of injury to staff bleeding animals (7). Furthermore, sentinel animals may themselves be amplifying hosts of the virus, thus contributing to the transmission cycle, and some closely related arboviruses are difficult to distinguish using current serological tests. Finally, processing thousands of mosquitoes for virus detection is labor-intensive, especially when presorting of mosquitoes is required, and often requires a cold-chain to preserve viral integrity in arthropods collected from the field (7).
To circumvent these issues, we developed a unique surveillance strategy for the detection of arboviruses that exploits the process whereby mosquitoes expel virus in their saliva during sugar feeding (8, 9). In this system, mosquitoes attracted to and captured by specialized traps were provided with a sugar source in the form of a honey-soaked card, which preserves nucleic acids. Viral RNA, expectorated from any infected mosquito, was subsequently detected by real-time reverse-transcriptase (RT)—PCR. The novelty of this concept lies in the detection of viral RNA directly from the cards, rather than the mosquitoes, thus eliminating the costly and time-consuming analysis of mosquitoes. Furthermore, traps were designed to run continuously, and the cards preserved viral RNA for at least 7 d and inactivated live virus, demonstrating the suitability of this system for surveillance in remote areas. In this paper, we describe the laboratory development and field evaluation of the system and prove its utility in the detection of the alphaviruses, Ross River virus (RRV), Barmah Forest virus (BFV), and CHIKV, as well as the flavivirus, WNV virus (Kunjin subtype).
Results
Detection of Viral RNA in Honey-Soaked Substrates Fed on by Infected Mosquitoes.
It was essential to use a substrate that preserved viral RNA for at least 7 d under field conditions and rapidly inactivated infectious virus to ensure integrity of the samples and then safe handling by field staff. Flinders Technology Associates filter paper (FTA®) cards have previously been used to inactivate and preserve viral RNA, including rabies viruses (10) and infectious bursal disease virus (11). Preliminary experiments demonstrated that both honey-soaked FTA® cards and untreated filter paper (FP) cards were able to bind RRV RNA and preserve it at 23 °C for at least 28 d (Table S1). Furthermore, the FTA® cards inactivated both RRV and WNV on contact, while infectious virus was still present on the FP cards ≤ 6 hr post inoculation (SI Text).
The ability to detect viral RNA expectorated on these honey-soaked substrates by individual infected mosquitoes was assessed. Culex annulirostris were infected with WNV or RRV via intrathoracic inoculation, and Aedes aegypti were offered an infectious CHIKV blood meal. Ten to 12 d after exposure, > 30 mosquitoes from each cohort were contained individually and allowed to feed on honey-soaked FTA® cards or FP cards for 48 hr. The honey was colored with blue food dye and internalized blue color within the mosquito confirmed whether a mosquito had fed on the card.
All three viruses were subsequently detected on the honey-soaked FTA® cards and FP cards at rates ≥70% (Table 1). There was no significant difference between the FTA® cards and FP cards for the detection of RRV (P = 0.104, Fisher’s exact test) or WNV (P = 1.000, Fisher’s exact test). The viral detection rates in saliva expectorates collected using a standard capillary tube method (12) ranged from 31% to 100% and were not significantly different from the detection rates for either of the honey-soaked substrates (P > 0.05, Fisher’s exact test), except in the case of RRV from the FP cards (P = 0.022, Fisher’s exact test).
Table 1.
Detection of West Nile, Ross River and chikungunya viruses on honey-baited substrates (FTA® cards or FP cards) and saliva collected from infected Cx. annulirostris and Ae. aegypti
| Virus | Substrate type | % substrates positive* | % surviving mosquitoes that had fed on substrate† | % positive that had fed on substrate‡ | % detection in saliva§ | ||||
| West Nile | FTA® card | 83 | (25/30) | 60 | (6/10) | 100 | (6/6) | 90 | (9/10) |
| FP card | 83 | (25/30) | 83 | (19/23) | 89 | (17/19) | 100 | (23/23) | |
| Ross River | FTA® card | 90 | (27/30) | 56 | (9/16) | 100 | (9/9) | 69 | (11/16) |
| FP card | 70 | (21/30) | 100 | (13/13) | 100 | (13/13) | 31 | (4/13) | |
| Chikungunya | FTA® card | 75 | (21/28) | 0 | (0/28) | 0 | (0/0) | 52 | (14/27) |
Mosquitoes were processed 12 days post exposure for WNV and RRV in Cx. annulirostris, and 14 days for CHIKV in Ae. aegypti.
*Percentage of the total number of substrates positive for virus (number positive/number infected mosquitoes).
†Percentage of surviving mosquitoes in which blue food dye was observed, indicating that the mosquito had imbibed honey from the substrate (number with blue dye/number surviving).
‡Percentage of mosquitoes that had imbibed honey from the substrate and expectorated virus (number positive/number that had imbibed the honey).
§Percentage of saliva expectorates from surviving mosquitoes in which viral RNA was detected by TaqMan RT-PCR (number saliva expectorates positive/number tested).
Interestingly, in some cases, virus was detected on the honey-baited substrates, although there was no evidence that the mosquito had imbibed any of the honey (i.e., blue color was not observed in the mosquito). This was particularly evident in Ae. aegypti, where despite blue dye not being observed in any mosquitoes, CHIKV RNA was detected in 75% of FTA® cards processed.
Field Trials.
A CO2-baited updraft box trap was developed to collect and house mosquitoes, and it incorporated a mechanism by which the FTA® cards or FP cards could be offered to trapped mosquitoes (Fig. 1 A–C). In a field efficacy trial conducted in Cairns, far north Queensland, Australia, updraft box traps collected 1.72 times as many mosquitoes than CO2-baited Centers for Disease Control miniature light traps (Table S2), and 77–95% of collected mosquitoes fed on the honey-soaked substrates while in the trap (Tables S3 and S4).
Fig. 1.
Updraft box trap use to collect and house mosquitoes. (A) Trap components and direction of airflow depicted by solid arrows. Mosquitoes are sucked into the trap at the bottom, then become trapped within the plastic box (B) where they can feed on honey-soaked FTA® cards (C). The front of the trap has been removed for photographic purposes.
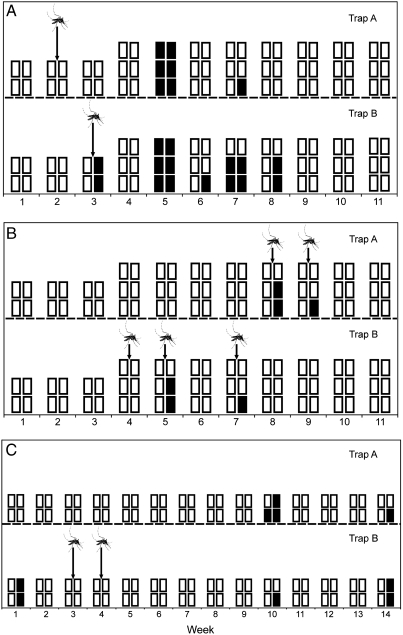
Longitudinal field trials to detect arboviruses were conducted at two locations with history of RRV and BFV activity: near Bunbury in Western Australia (WA) and near Cairns (13–15). Mosquitoes and FTA® cards were removed weekly and forwarded to the laboratory for RRV and BFV detection using real-time RT-PCR. Over the 11-week trial period in WA, RRV was detected on FTA® cards removed from at least one trap in five separate weeks (Fig. 2A). Overall, 22 FTA® cards from seven different trap collections and two pools of mosquitoes, removed from traps that simultaneously yielded positive FTA® cards, were positive. When analyzed for BFV, FTA® cards from four separate traps were positive for BFV, while eight mosquito pools from a total of five trap collections were positive (Fig. 2B).
Fig. 2.
Temporal depiction of the detection of arboviruses in honey-soaked FTA® cards and mosquito pools collected from duplicate CO2-baited updraft box traps in the field: (A) Ross River virus and (B) Barmah Forest virus from the Leschenault Peninsula near Bunbury in Western Australia, and (C) Ross River virus from Cairns, far north Queensland. Each square represents a single FTA® card that was processed in each week, and black squares indicate a card from which viral RNA was detected. Detections of viral RNA in mosquitoes removed from the updraft box traps at the same time as the FTA® cards are represented by a mosquito.
To assess the level of virus activity at the WA study sites, CO2-baited light traps were used to trap mosquitoes that were then processed for RRV and BFV using a cell culture enzyme immunoassay. A total of 3,994 mosquitoes in 230 pools were processed from six fortnightly collections obtained from two sites near the trial locations. No RRV was isolated, but three isolates of BFV were obtained, with one obtained on week five and two on week seven, which coincided with virus detection on FTA® cards.
In Cairns field trials, RRV was detected in three of the 14 weeks from nine FTA® cards from a total of five traps (Fig. 2C). Two mosquito pools yielded RRV (one each in weeks three and four). BFV was not detected in any mosquito pool or from FTA® cards in Cairns.
Discussion
Monitoring viral prevalence in mosquito vectors or vertebrate hosts provides vital data regarding the activity and spread of emerging and reemerging arboviruses for biosecurity purposes. However, current surveillance methods are laborious, expensive, and vary in their sensitivity and specificity, which greatly reduces their efficacy as a surveillance system. The unique system described here circumvents the issues with sentinel animals and eliminates the need to handle or sort mosquitoes prior to virus detection, which is a major limitation of current arthropod-based systems (7). This will result in a reduction in the turnaround time between sample collection and output of results, thus enhancing its application as an early warning system. Finally, the utility of the system was proven under both temperate (WA) and tropical (Cairns) climatic conditions.
The relative simplicity of this system will facilitate the deployment of a large number of traps, resulting in a wider geographical area that can be included in the surveillance program. Once a virus of interest has been detected, more comprehensive trapping in a given location can be implemented to obtain more information on virus ecology. For instance, further trapping for virus isolation can incriminate mosquito vectors and allow for calculation of infection rates (16, 17), while blood meal analysis may provide an important indication of vertebrate hosts that may be involved in transmission cycles (18, 19). To increase the capacity for this system to detect unexpected or novel arboviruses/pathogens, universal primer sets and/or probes could be employed in the molecular assays (20). Alternatively, a multiplexed assay may further enhance the capabilities of this system to rapidly detect a greater range of pathogens.
Sugar is an important energy source to facilitate mosquito flight, mating, and egg production (21). We developed the surveillance system to exploit the phenomenon whereby mosquitoes expectorate virus while they sugar feed (9). Honey was used as the sugar source on FTA® cards and FP cards because it possesses a number of unique biochemical properties. Honey is composed primarily of sugars, predominately glucose and fructose (22), which are attractive to mosquitoes (21). Indeed, observed feeding rates of trapped mosquitoes on the honey-baited FTA® cards during the field trial (as indicated by the presence of blue dye in the mosquito abdomen) were 42% and 73% in WA and Cairns, respectively, although there was variation in rates between species. However, the results of the laboratory experiments suggest that virus is expectorated in the saliva irrespective of whether the mosquitoes imbibe the honey or not. This is not surprising, because probing by an infected mosquito is sufficient to transmit the virus to a susceptible host (23). Therefore, the presence of blue dye in the mosquito abdomen may underestimate the true expectoration rate for some mosquito species.
Once dispensed onto the FTA® cards, the honey remained moist for the weekly trapping period, ensuring that trapped mosquitoes maintained access to liquid sugars. This was particularly important because feeding and survival rates are significantly lower when mosquitoes are maintained on substrates coated in dried sugar, compared with substrates that remain moistened with sugar solution. Another advantage of honey, and, in particular, Manuka honey, is its antibacterial properties (24, 25), which could reduce the inhibitory effects of RNAses produced by bacteria. Combined with the proprietary chemicals on the cards, this potentially helped to preserve viral RNA expectorated by mosquitoes in the field trial.
Although the utility of the sugar-baited system for detecting arboviruses has been demonstrated here, there are many other potential applications for this strategy. Sugar feeding, primarily in the form of nectar, is widespread within mosquito vectors (20), and thus our technique could be expanded to include other pathogens such as Plasmodium. For instance, should Anopheles spp. expel Plasmodium sporozoites during sugar feeding and if Plasmodium DNA was likewise preserved on the FTA® cards, this system could be used as a surveillance tool for malaria. Additionally, this system could be adapted for surveillance of pathogens transmitted by other hematophagous arthropods that are attracted to similar traps, such as Culicoides spp, which are vectors of bluetongue virus (26). Finally, honey-soaked FTA® cards or FP cards could also be used to demonstrate pathogen transmission in the laboratory, bypassing the need to use animal models (27) or methods that sacrifice the mosquito, such as in vitro saliva collection (12).
Materials and Methods
Laboratory Experiments.
Honey-soaked substrates.
As substrates we used FTA® cards (Whatman International Ltd, Maidstone, UK), which are filter papers impregnated with proprietary chemicals to retain and preserve RNA and DNA. Untreated filter paper (FP) cards (Bio-Rad, Hercules, CA) were used for comparison.
Manuka honey (Manuka, bee vital active 5+; Capillano, Richlands, Australia) was selected as food source due to its antibacterial and antifungal properties (24, 25). Because quarantine restrictions prevented the use of manuka honey in Western Australia, Medihoney™ Antibacterial Medical Honey™ (Medihoney®, Richlands, Australia) was used as the honey bait in Western Australia. Blue food coloring (Queen, Alderley, Queensland, Australia) was added to honey to differentiate between mosquitoes that had imbibed the honey and those that were unfed. The blue color was visible in the mosquito crop, diverticula, and midgut post-feeding. Blue honey-soaked FTA® cards and/or FP cards were used in all experiments. Dry substrates without honey were used as negative controls.
Mosquitoes.
Colonized Cx. annulirostris were obtained from the Australian Army Malaria Institute, Brisbane, Australia. The colony originated from adults collected from the Boondall Wetlands, Australia, in 1998 and were > F50 generation. Aedes aegypti eggs were collected from ovitraps set in Cairns, Australia, in 2009, and F2 adults were used in the experiments.
Viruses.
West Nile virus (Kunjin subtype; 2002–1412) was isolated from Cx. annulirostris collected from Burketown in 2002 and had been passaged twice in C6/36 (Aedes albopictus) cells and once in porcine stable-equine kidney (PS-EK) cells. Ross River virus (389A) was isolated from mosquitoes collected from Cairns in 1998 and passaged three times in C6/36 cells. Chikungunya virus was isolated from a patient from Mauritius who had visited Melbourne, Australia, in 2006 and was passaged three times in African green monkey kidney (Vero) cells. The WNV, RRV, and CHIKV stocks had final titers of 108, 109, and 108 Vero tissue culture infectious dose (TCID)50/mL, respectively.
Mosquito infection.
Five to 7 d old Cx. annulirostris were intrathoracically inoculated with 0.2 μL of a suspension containing 105.0 TCID50/mL of WNV or RRV diluted in growth media (GM; Opti-MEM (Gibco, Invitrogen Corporation, Grand Island, NY) containing 3% foetal bovine serum (FBS), and antibiotics and antimycotics). Aedes aegypti were exposed to CHIKV diluted in washed defibrinated sheep blood maintained at 37 °C within a membrane feeding apparatus. Following virus exposure, all mosquitoes were maintained at 28 °C, 75% relative humidity and 12 h∶12 h light:day cycle.
Exposure of mosquitoes to honey-baited substrates.
After 10 d for WNV and RRV or 12 d for CHIKV, individual mosquitoes were placed in separate 50 mL vials. A 1 cm2 square section of honey-soaked FTA® card or FP card was placed over a hole that had been cut in the lid of each vial. After 48 h, all mosquitoes, whether dead or alive, were microscopically examined for visible blue food dye, indicative of feeding on the substrates. To compare the honey-bait system with a standard in vitro capillary tube technique (12), saliva from surviving mosquitoes was collected in capillary tubes containing GM + 20% FBS. All FTA® cards, FP cards, mosquitoes and saliva were stored at -80 °C.
Virus Assay.
Individual mosquitoes were homogenized in 1 mL of GM + 3% FBS, filtered through a 0.2 μm filter (Pall Corporation, Ann Arbor, MI), before being inoculated in duplicate onto confluent Vero cell monolayers within a 96 well microtiter plate. Plates were incubated at 37 °C and 5% CO2 for 7 d. Plates were then examined for cytopathic effect and fixed in PBS/acetone. Infection was confirmed using a cell culture enzyme immunoassay and the monoclonal antibodies, 10A1 for WNV, and B10 for RRV and CHIKV (28).
The FTA® cards and filter papers were placed into a 5 mL tube, and 1 mL of GM + 3% FBS was added. Samples were kept on ice and vortexed every 5 min for 20 min (11). The eluate was either extracted immediately or stored at -80 °C to await analysis.
Viral RNA was extracted from the FTA® card and FP card eluates, and saliva expectorates with a Bio Robot Universal System (Qiagen, Hilden, Germany) using the QIAamp® Virus BioRobot® MDx Kit (Qiagen, Clifton Hill, Australia) according to manufacturer’s instructions. Viral RNA was detected using real-time TaqMan RT-PCR assays specific for WNV, CHIKV, and RRV (29–31).
Field Trials.
Study locations.
Field trials were undertaken on the Leschenault Peninsula near Bunbury in Western Australia from October 14 to December 24, 2008, and at a site near Cairns in far north Queensland from January 21 to April 28, 2009. The Leschenault Peninsula is a narrow stretch of land between the Indian Ocean and the Leschenault Inlet, approximately 18 km north of the city of Bunbury in the southwest of Western Australia. It is characterized by coastal heath, tuart tree (Eucalyptus gomphocephala), and peppermint tree (Agonis flexuosa) woodland and tidal salt marshes (Sarcocornia sp.). The site near Cairns is bordered by sugar cane fields, tidal salt marshes, and Melaleuca swamps, and it is in close proximity to waste transfer and sewerage treatment facilities.
Trap design.
An updraft box trap was designed that combines an updraft fan to maximize mosquito collections (32) and a plastic box to contain captured mosquitoes (Fig. 1A). A 32 x 21 x 19 cm clear plastic container with removable lid was used to hold mosquitoes and provide access to the honey-soaked FTA® cards (Fig. 1 B and C). A computer cooling fan (12 V, 0.27 A brushless motor) was fitted to a 44 cm section of 10 cm diameter polyvinylchloride (PVC) pipe that was inserted into the middle of the box. A 19 cm diameter, 8 cm deep clear plastic bowl was fitted to the other end of the PVC pipe to enhance mosquito capture. A section of wire fitted to the bowl was used to hold the outlet tube from a CO2 cylinder 10 cm below the bowl, the minimal distance that released gas would not be sucked back into the trap (32). CO2 was released at 450–500 mL/ min using an adjustable regulator with a 0.25 mm orifice (Pacific Biologics, Scarborough, Australia). Four 8 cm diameter holes were cut into the portion of the PVC pipe inside the holding compartment. A plastic baffle inside the PVC pipe shunted mosquitoes through the unscreened bottom two holes into the holding compartment. The upper two holes allowed the air to exhaust through the top of the trap. These upper holes were covered with 1 mm fly screen to prevent mosquitoes from being drawn back into the pipe from the holding compartment. To prevent access by ants when deployed in the field, petroleum jelly was applied to the suspending chain, battery cable, and CO2 outlet line.
To produce the honey-baited FTA® card, blue honey was dispensed onto the wound pad of a Primapore Dressing (Smith & Nephew, 8.3 × 6 cm) and the FTA® card was laid over the honey and left to absorb overnight. Traps were run continuously during the study period, and FTA® cards and mosquitoes were removed weekly. Upon collection, FTA® cards were placed between layers of Parafilm M (Alcan Packaging, Neenah, WI) within a snap-lock bag, and mosquitoes were placed in 50 mL jars, before being dispatched by overnight courier to the laboratory in Brisbane, Australia, for analysis.
Mosquitoes were sorted into pools of ≤ 500, to which five beads and 5 mL of GM + 3% FBS was added. Pools were homogenized, clarified by centrifugation and filtered through a 0.8/0.2 μm dual filter (Pall Corporation, Ann Arbor, MI), before being stored at -80 °C. The FTA® cards were cut into small pieces, before being eluted as described previously. The FTA® card eluates and mosquito pools were extracted and analyzed using an in-house BFV and the RRV real-time TaqMan RT-PCR assays. Pools collected during concurrent trapping were processed for virus isolation using the methods of Johansen and others (33).
Supplementary Material
Acknowledgments.
The authors are grateful for the advice and assistance of Alyssa Pyke, Cassie Jansen, and Frederick Moore. We also thank Haydn Jones for assistance in the field, and laboratory staff in the Arbovirus Surveillance and Research Laboratory who conducted the concurrent trapping and virus isolation work in WA. In addition, we thank Donna Mackenzie, Stephen Frances, and Petrina Johnson for providing mosquitoes for the laboratory experiments, and Julian Druce for providing the CHIKV isolate. This work was funded by the Australian Biosecurity Cooperative Research Centre for Emerging Infectious Disease. Additional funding for the Western Australian study was provided by the Western Australian Department of Health and a University of Western Australia Small Research Grant.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1002040107/-/DCSupplemental.
References
- 1.Gould EA, Solomon T. Pathogenic flaviviruses. Lancet. 2008;371:500–509. doi: 10.1016/S0140-6736(08)60238-X. [DOI] [PubMed] [Google Scholar]
- 2.Staples JE, Breiman RF, Powers AM. Chikungunya fever: An epidemiological review of a re-emerging infectious disease. Clin Infect Dis. 2009;49:942–948. doi: 10.1086/605496. [DOI] [PubMed] [Google Scholar]
- 3.Eisen L, Beaty BJ, Morrison AC, Scott TW. Proactive vector control strategies and improved monitoring and evaluation practices for dengue prevention. J Med Entomol. 2009;46:1245–1255. doi: 10.1603/033.046.0601. [DOI] [PubMed] [Google Scholar]
- 4.Reisen WK, et al. Persistent West Nile virus transmission and the apparent displacement of St. Louis encephalitis virus in southeastern California, 2003–2006. J Med Entomol. 2008;45:494–508. doi: 10.1603/0022-2585(2008)45[494:pwnvta]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanna JN, et al. Japanese encephalitis in north Queensland, Australia, 1998. Med J Aust. 1999;170:533–536. doi: 10.5694/j.1326-5377.1999.tb127878.x. [DOI] [PubMed] [Google Scholar]
- 6.Broom AK, Lindsay MD, Harrington SA, Smith DW. Investigation of the southern limits of Murray Valley encephalitis activity in Western Australia during the 2000 wet season. Vector-Borne Zoonot Dis. 2002;2:87–95. doi: 10.1089/153036602321131887. [DOI] [PubMed] [Google Scholar]
- 7.Ritchie SA, et al. Operational trial of a remote mosquito trap system for Japanese encephalitis virus surveillance in the Torres Strait, Australia. Vector-Borne Zoonot Dis. 2007;7:497–506. doi: 10.1089/vbz.2006.0643. [DOI] [PubMed] [Google Scholar]
- 8.Doggett SL, Klowden MJ, Russell RC. Are vector competence experiments competent vector experiments? Arbovirus Res Aust. 2001;8:126–130. [Google Scholar]
- 9.van den Hurk AF, et al. Expectoration of flaviviruses during sugar feeding by mosquitoes (Diptera: Culicidae) J Med Entomol. 2007;44:845–850. doi: 10.1603/0022-2585(2007)44[845:eofdsf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 10.Picard-Meyer E, Barrat J, Cliquet F. Use of filter paper (FTA®) technology for sampling, recovery and molecular characterisation of rabies viruses. J Virol Methods. 2007;140:174–182. doi: 10.1016/j.jviromet.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Purvis LB, Villegas P, Perozo F. Evaluation of FTA paper and phenol for storage, extraction and molecular characterization of infectious bursal disease virus. J Virol Methods. 2006;138:66–69. doi: 10.1016/j.jviromet.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 12.Aitken THG. An in vitro feeding technique for artificially demonstrating virus transmission by mosquitoes. Mosq News. 1977;37:130–133. [Google Scholar]
- 13.Lindsay M, et al. An outbreak of Ross River virus disease in southwestern Australia. Emerg Infect Dis. 1996;2:117–120. doi: 10.3201/eid0202.960206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindsay MDA, Johansen CA, Broom AK, Smith DW, Mackenzie JS. Emergence of Barmah Forest virus in Western Australia. Emerg Infect Dis. 1995;1:22–26. doi: 10.3201/eid0101.950104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harley DO, Ritchie SA, Phillips DA, van den Hurk AF. Mosquito isolates of Ross River virus from Cairns, Queensland, Australia. Am J Trop Med Hyg. 2000;62:561–565. doi: 10.4269/ajtmh.2000.62.561. [DOI] [PubMed] [Google Scholar]
- 16.Nasci RS, et al. West Nile virus isolates from mosquitoes in New York and New Jersey, 1999. Emerg Infect Dis. 2001;7:626–630. doi: 10.3201/eid0704.010404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ritchie SA, et al. Isolation of Japanese encephalitis virus from Culex annulirostris in Australia. Am J Trop Med Hyg. 1997;56:80–84. doi: 10.4269/ajtmh.1997.56.80. [DOI] [PubMed] [Google Scholar]
- 18.Apperson CS, et al. Host-feeding habits of Culex and other mosquitoes (Diptera: Culicidae) in the Borough of Queens in New York City, with characters and techniques for identification of Culex mosquitoes. J Med Entomol. 2002;39:777–785. doi: 10.1603/0022-2585-39.5.777. [DOI] [PubMed] [Google Scholar]
- 19.Jansen CC, et al. Blood sources of mosquitoes collected from urban and peri-urban environments in eastern Australia with species-specific molecular analysis of avian blood meals. Am J Trop Med Hyg. 2009;81:849–857. doi: 10.4269/ajtmh.2009.09-0008. [DOI] [PubMed] [Google Scholar]
- 20.Scaramozzino N, et al. Comparison of Flavivirus universal primer pairs and development of a rapid, highly sensitive heminested reverse transcription-PCR assay for detection of flaviviruses targeted to a conserved region of the NS5 gene sequences. J Clin Microbiol. 2001;39:1922–1927. doi: 10.1128/JCM.39.5.1922-1927.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foster WA. Mosquito sugar feeding and reproductive energetics. Annu Rev Entomol. 1995;40:443–474. doi: 10.1146/annurev.en.40.010195.002303. [DOI] [PubMed] [Google Scholar]
- 22.Anklam E. A review of the analytical methods to determine the geographical and botanical origin of honey. Food Chem. 1998;63:549–562. [Google Scholar]
- 23.Gould DJ, Barnett HC, Suyemoto W. Transmission of Japanese encephalitis virus by Culex gelidus Theobald. T Roy Soc Trop Med H. 1962;56:429–435. doi: 10.1016/0035-9203(62)90018-4. [DOI] [PubMed] [Google Scholar]
- 24.Weston RJ, Brocklebank LK, Lu Y. Identification and quantitative levels of antibacterial components of some New Zealand honeys. Food Chem. 2000;70:427–435. [Google Scholar]
- 25.Lusby PE, Coombes AL, Wilkinson JM. Bactericidal activity of different honeys against pathogenic bacteria. Arch Med Res. 2005;36:464–467. doi: 10.1016/j.arcmed.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 26.Mellor PS, Boorman J, Baylis M. Culicoides biting midges: Their role as arbovirus vectors. Annu Rev Entomol. 2000;45:307–340. doi: 10.1146/annurev.ento.45.1.307. [DOI] [PubMed] [Google Scholar]
- 27.van den Hurk AF, et al. Vector competence of Australian mosquitoes (Diptera: Culicidae) for Japanese encephalitis virus. J Med Entomol. 2003;40:82–90. doi: 10.1603/0022-2585-40.1.82. [DOI] [PubMed] [Google Scholar]
- 28.Broom AK, et al. Identification of Australian arboviruses in inoculated cell cultures using monoclonal antibodies in ELISA. Pathology. 1998;30:286–288. doi: 10.1080/00313029800169456. [DOI] [PubMed] [Google Scholar]
- 29.Pyke AT, et al. Detection of Australasian flavivirus encephalitic viruses using rapid fluorogenic TaqMan RT-PCR assays. J Virol Methods. 2004;117:161–167. doi: 10.1016/j.jviromet.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 30.van den Hurk AF, Hall-Mendelin S, Pyke AT, Smith GA, Mackenzie JS. Vector competence of Australian mosquitoes for chikungunya virus. Vector-Borne Zoonot Dis. doi: 10.1089/vbz.2009.0106. Ahead of print. doi:10.1089/vbz.2009.0106. [DOI] [PubMed] [Google Scholar]
- 31.Hall RA, Prow N, Pyke A. Molecular diagnostics for Ross River virus. In: Liu D, editor. Molecular Detection of Human Viral Pathogens. Boca Raton, FL: CRC Press; 2010. Chapter 75. In press. [Google Scholar]
- 32.Ritchie SA, Zborowski P, Banks D, Walsh I, Davis J. Efficacy of novel updraft traps for the collection of mosquitoes in Cairns, Australia. J Am Mosq Control Assoc. 2008;24:520–527. doi: 10.2987/5698.1. [DOI] [PubMed] [Google Scholar]
- 33.Johansen CA, et al. Surveillance of arboviruses in mosquitoes from the southwest of Western Australia between 2000 and 2004. Arbovirus Res Aust. 2005;9:159–163. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.