Abstract
Background
Magnetic resonance imaging (MRI) studies have shown decreased caudate volumes in individuals with attention deficit hyperactivity disorder (ADHD). However, most of these studies have been carried out in male children. Very little research has been done in adults, and the results obtained in children are difficult to extrapolate to adults. We sought to compare the volume of the caudate of adults with ADHD with that of healthy controls; we also compared these volumes between men and women.
Methods
We performed an MRI scan on 20 adults with ADHD (10 men and 10 women) aged 25–35 years and 20 healthy controls matched by age and sex. We used voxel-based morphometry with the DARTEL algorithm for image analyses. We used the specifically designed Friederichsen, Almeida, Serrano, Cortes Test (FASCT) to measure the severity of ADHD; both the self-reported (FASCT-SR) and the observer (FASCT-O) versions were used.
Results
The statistical parametric map showed a smaller region with low grey matter volume and a smaller concentration of grey matter in this region of the right caudate in ADHD patients than in health controls, both in the entire sample and within each sex. There was a significant correlation between the volume of this region of the caudate with the number of DSM IV-TR criteria, as well as with the total scores and most of the factors of the FASCT-SR and FASCT-O scales. A separate correlation analysis by sex gave similar results.
Limitations
The study design was cross-sectional.
Conclusion
The region of the right caudate with low grey matter volume was smaller in adults with ADHD in both sexes and was correlated with ADHD severity.
Introduction
Follow-up studies have found that 5%–66% of children with attention deficit hyperactivity disorder (ADHD) continue to have this disorder into adulthood.1 In addition, ADHD is more frequent in boys than girls.2 As of 1990, there have been many structural magnetic resonance imaging (MRI) studies involving ADHD individuals,3–5 but most research has focused on patients aged 9 to 15 years, and the majority (95%) of samples have included only male patients. Despite the homogeneity of the samples, there have been many contradictory findings, likely owing to differences in experimental methods, particularly those used to measure the encephalic structures.6
Valera and colleagues4 a meta-analysis of the data from 21 non–voxel based morphometry (VBM) studies involving patients with ADHD.4 They concluded that the regions most frequently assessed and displaying the largest differences in ADHD individuals included the cerebellar regions, the splenium of the corpus callosum, the total and right cerebral regions and the right caudate.
However, another meta-analysis that included 7 VBM studies showed that the putamen and globus pallidus were smaller in youth (9.9–15.4 yr) with ADHD.7 A more recent study that involved manual tracing and large-deformation diffeomorphic metric surface mapping in 47 children with ADHD and 66 healthy controls found that the putamen, caudate and globus pallidus were smaller and abnormally shaped in children with ADHD.5 These contradictory findings stress the need for further research.
The abnormalities in the volume of the caudate nucleus and other basal ganglia8 make sense because these regions have a role in diverse cognitive functions (e.g., language,9 learning and memory,10,11 attention12 and control of behavioural responses13,14 ). The results of diverse studies of the anatomic features of the caudate in ADHD patients have been contradictory. Some studies have reported that the right caudate nucleus is smaller in children with ADHD,4,15–17 whereas others have found that only the left caudate is smaller in ADHD individuals5 or that there is no difference.18 Another study found that the total volume of the caudate was smaller in individuals with ADHD but that this difference disappeared during adolescence.19
Structural studies involving adults with ADHD are limited, despite the fact that the prevalence of adult ADHD in the general population is 4.4%20 and only about 10% of patients with childhood ADHD achieve functional remission at 18–20 years of age.21 These findings clearly support the presence of ADHD in adults.20,22,23 There have been 4 structural MRI reports regarding morphologic abnormalities in adults with ADHD.24–27 Two studies did not report an analysis of the subcortical structures,24,25 and the other 2 studies26,27 reported that there was no difference in the volume of the caudate nucleus between adults with ADHD and healthy individuals.
It is important to mention that the latter 2 studies26,27 used nearly identical sample populations, which suggests that too few adults with ADHD have been studied to yield definite conclusions about the structural features of this disease in adults. Additionally, to the best of our knowledge, there are no published studies investigating a correlation between the severity of ADHD and structural measures of the caudate, and there have been no straightforward comparisons between adults with ADHD and controls by sex performed in an independent fashion. Therefore, we attempted to study the structural features of adults with ADHD because, although ADHD is considered a primarily childhood disorder, it is important to acknowledge the low rate of functional remission and the few available data about the structural features of ADHD in adulthood.
Our aims were to compare the concentration of grey matter and the volume of the caudate nucleus in adults with ADHD and to compare these characteristics by sex.
Methods
Participants
We included 20 unmedicated adults with ADHD, combined type, diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revised (DSM-IV-TR) criteria and the Mini International Neuropsychiatric Interview plus version 5.0 (MINI).28 We included patients aged 25–35 years, and 50% were women. We included 20 healthy individuals as controls, who were matched to the ADHD patients by age, sex, body mass index (BMI) and intelligence quotient (IQ). We excluded individuals with a current or previous neurologic or psychiatric disease other than ADHD, general medical illness, an IQ less than 85 points, a history of substance abuse or dependence or stimulant treatment, or any abnormality on the MRI scan. We also excluded patients who met the DSM-IV-TR criteria for ADHD, predominantly inattentive type, and ADHD, predominantly hyperactive/impulsive type.
A certified neuroradiologist (A.F.-B.), who was unaware of the individual’s identity, evaluated all MRI scans. The control and ADHD individuals were recruited from the general community of the urban areas of Querétaro City and México City by means of an open invitation poster. The study took place from March 2006 to February 2008.
After having fully explained the study to all participants, written informed consent was obtained. Certified physicians obtained a full medical history and performed a physical examination of each participant. The Institutional Review Board of the General Hospital of Querétaro, México, approved the study protocol and the informed consent document.
Clinical measures
The diagnosis of ADHD was made using the MINI-plus (Spanish version) for all participants by 2 experienced, certified psychiatrists. The kappa agreement index for the diagnosis of adult ADHD between the 2 psychiatrists was 0.85 (p < 0.001). To quantify the severity of ADHD and to correlate severity with the volumes of the brain structures, we used both versions of the Friederichsen, Almeida, Serrano, Cortes Test (FASCT):22 the self-reported (FASCT-SR) and the observer (FASCT-O) scales. The FASCT scale was specifically developed to assess ADHD in adults.
The mother of each patient completed the FASCT-O. Although the FASCT was designed to screen and measure the severity of ADHD in adults, the agreement kappa indices for the diagnosis of ADHD between the semistructured interview (MINI-plus) and a FASCT-SR or a FASCT-O score of 23 points or greater were 0.82 and 0.88, respectively. The FASCT-SR has 3 factors: hyperactivity and deficits in memory; organization and functional impairment; and low frustration tolerance. The FASCT-O scale has 4 factors: hyperactivity and memory; organization and functional impairment; low frustration tolerance; and legal problems. More detailed information about the FASCT scale has been published elsewhere.22 All participants were tested on the Weschler Adult Intelligence Scale, 3rd edition (WAIS-III)29 by a certified neuropsychologist.
Data acquisition
A 1-T Philips new Intera MRI machine (release 10.3; Philips Medical Systems) was used for all scans. A fast-field echo T1 3-dimensional (3-D) volumetric sequence produced 190 continuous 1.0-mm thick coronal slices without any gaps between slices.
The acquisition parameters were echo time 6.9 ms, repetition time 25 ms, flip angle 30°, acquisition matrix 230 × 230 mm, field of view 256 mm and voxel size 1.0 mm3. The MRI scanner was located at the Instituto de Neurobiología Universidad Nacional Autónoma de México, Campus Juriquilla Querétaro, México. There were no variations in the acquisition, such as the use of different scanners, scanner upgrade or changes in pulse sequence. No equipment calibration was done during the MRI scanning phase of the study.
Data processing
Voxel-based morphometry
We used the Statistical Parametric Mapping software version 5 (SPM5; Wellcome Trust Centre of Neuroimaging, Institute of Neurology, University College of London) and Matlab R2007a (version 7.4.0) to analyze the MRI data. To improve the registration of the MRI images, we used the diffeomorphic anatomic registration through exponentiated Lie algebra algorithm (DARTEL).30–32
The DARTEL technique was developed to analyze deformations in terms of spatial transformations by applying concepts from differential geometry (e.g., diffeomorphism and Lie algebra), which were introduced to study infinitesimal transformations. Deformations are parameterized by a flow field or slope field and are considered a member of Lie algebra, which is exponentiated to produce a deformation field, defining an objective function that is optimized locally in terms of a nonlinear extended least squares technique known as the Levenberg–Marquardt strategy. This technique is an iterative procedure that locates the minimum of a function in terms of the combination of the square of nonlinear functions. Once the objective function is built, the parameters that describe a spatial transformation between the source and reference (template) images are optimized, considering the template as a deformable probability density.33 The DARTEL technique is superior to standard VBM because it improves registration using the Levenberg–Marquardt strategy. We used a constant Eulerian velocity framework, which allows a rapid scaling and squaring method to be used in the computations.
Because DARTEL produces a more accurate registration, it improves the sensitivity of finding differences and localizing differences between groups in the concentration of grey or white matter. This is because the images processed by DARTEL require less smoothing (full-width at half-maximum [FWHM] Gaussian kernel 8 mm instead of 12 mm, as is usually used in standard VBM), thus improving the interpretation of the results.30,31
We performed smoothing using a Gaussian kernel with a FWHM of 8 mm. We chose this parameter because the detection of the differences between groups would be greater, according to the study by Davatzikos and colleagues.34 The final step was to convert these images to the Montreal Neurologic Institute (MNI) space. This was achieved by matching the grey matter component of the template with a probability map of grey matter tissue in MNI space. Afterwards, the spatial transformation that maps from MNI space to the space of the DARTEL template was combined with the deformations estimated by DARTEL for each individual.
Volume calculations
We performed a search for small volumes of interest in the global maxima of SPM5 using a sphere whose radius provided statistically significant p values at the cluster and voxel levels. With this sphere radius, a 3-D sphere mask image with a radius equal to the radius used in the small volume-of-interest search was constructed by use of the WFU PickAtlas tool (version 2.4). We used this mask to calculate the volume for each participant where SPM showed statistical differences in the concentration of grey matter between groups. The number of voxels that met the p value threshold produced a “bulb” whose size allowed us to perform a small volume search using a sphere of 10 mm for the whole sample and 5 mm for the analysis by sex. We chose these values because a greater radius exceeded the significance level and a lower one would not have covered the entire portion of the statistical difference between groups shown by the whole-sample SPM analysis.
When we compared healthy and ADHD women and healthy and ADHD men, the SPM analysis showed a bulb with a smaller size than the one obtained in the whole-sample analysis. Thus, the sphere radius was set at 10 mm to create the 3-D mask to calculate the volume for each participant when comparing all healthy and ADHD participants. We chose a sphere radius of 5 mm to create the 3-D mask to calculate the volume for each participant in the sex comparison. The 3-D mask was built on the MNI coordinates where SPM showed the maximum statistical difference between groups. We applied this mask to the SPM.mat file that contained the statistical information for the 40 individuals (or 20 in the case of the sex analysis). This mask was applied to each individual scan with the SPM5 extension known as “volumes toolbox,” which gives the volume of the portion of grey matter of each individual where SPM showed statistical differences between groups.
Statistical analyses
We used the χ2 test for nominal characteristic data. We used the Fisher exact test if any of the expected frequencies were less than 2 or if more than half of the expected frequencies were less than 5. For numerical data, we used a t test or the Mann–Whitney U test, according to the result of a test for normality (Kolmogorov–Smirnov, Lilliefor correction).
We calculated either Pearson (r) or Spearman (rho) coefficients (depending on the distribution of the data), and we used these coefficients to analyze the correlation between clinical measures and the volume of the portion of the caudate where SPM found statistical differences between the ADHD patients and healthy controls. All tests were 2-tailed.35 These analyses were carried out using the Statistical Package for the Social Sciences (version 17, SPSS Inc.).
Grey matter concentration
For the whole-brain analyses, we analyzed the data using a t test for 2 samples, which was included in the SPM5 software. We selected a p value threshold of 0.001 for 20 voxels. Our intention was to find a cluster of at least 20 voxels whose concentration of grey matter had a low probability of being equal between healthy controls and ADHD patients.
Once the SPM of the whole brain was obtained, we performed a small volume search at the MNI coordinates x = 18, y = 6, z = 22 and x = 18, y = 2, z = 44 (right caudate) using a sphere with a radius of 10 mm. At the voxel level, the p values were corrected for multiple comparisons, and a family-wise error (FWE) and false discovery rate (FDR) corrections were applied to the voxels that built the cluster that reached significance at the cluster-level.
To detect structural differences or similarities between healthy male or female controls and ADHD men or ADHD women, respectively, we performed a separate statistical analysis. The contrast parameters for both sexes were a p value threshold of 0.001 and a threshold of 20 contiguous voxels. For men, a small volume correction was used at MNI coordinates x = 21, y = 9, z = 18 (right caudate) using a sphere with a radius of 5 mm. For women, the small volume correction was used at MNI coordinates x = 21, y = 9, z = 18 (right caudate) using a sphere with a radius of 5 mm.
After obtaining the volume of the grey matter in each individual where SPM showed a statistical difference, we performed a 2-tailed t test for independent groups (previous normality test of Kolmogorov–Smirnov, Lilliefor correction) to compare the proportional grey-matter volume of the right caudate between healthy controls and ADHD patients. The same statistical analysis was used to compare the volume in healthy women and ADHD women and in healthy men and ADHD men. This test was 2-tailed and was done with SPSS version 17.
Results
There were no differences in demographic variables (e.g., age, sex, somatometric parameters, income level and IQ) between healthy controls and ADHD patients (Table 1). All participants were right-handed Hispanic people. The ADHD patients more frequently had a history of school problems than did the healthy adults (40% v. 5%, p = 0.02). The mean number of DSM-IV-TR criteria for inattention among adults with ADHD was 7.60 (standard deviation [SD] 1.18) and 0.40 (SD 0.68) for healthy controls (Mann-Whitney U = 0.00, Z = 5.73, p < 0.001).
Table 1.
Sociodemographic, psychometric and clinimetric characteristics of the study participants
| Group; mean (SD)* |
||||
|---|---|---|---|---|
| Characteristic | Healthy control | ADHD | T38* | p value |
| Age, yr, mean (SD) [range] | 27.57 (2.6) [25–35] | 28.95 (4.01) [25–35] | −1.2 | 0.21 |
| Sex, male:female, no. (%) of participants | 10 (50) : 10 (50) | 10 (50) : 10 (50) | χ12 = 0.0 | 1.0 |
| Weight, kg | 66.52 (10.20) | 72.47 (12.32) | −1.6 | 0.10 |
| Height, m | 1.64 (0.08) | 1.67 (0.07) | −1.2 | 0.21 |
| Body mass index | 24.42 (2.35) | 25.64 (2.44) | −1.3 | 0.20 |
| Right-handed, no (%) of participants | 20 (100) | 20 (100) | — | |
| Hispanic, no. (%) of participants | 20 (100) | 20 (100) | — | |
| History of school problems, no. (%) of participants† | 1 (5) | 8 (40) | 0.02§ | |
| Education, no. (%) of participants‡ | ||||
| Junior high school | 0 (0) | 2 (10) | 0.49§ | |
| Senior high school | 2 (10) | 4 (20) | 0.66§ | |
| College or university | 11 (55) | 8 (40) | 0.34§ | |
| Postgraduate | 7 (35) | 6 (30) | 0.74§ | |
| Monthly income, no. (%) of participants | χ12 = 1.1 | 0.28 | ||
| US$2692–6461 | 16 (80) | 13 (72) | ||
| > US$5638 | 4 (20) | 7 (35) | ||
| WAIS-III score | ||||
| Verbal scale | 102.55 (9.61) | 103.95 (10.05) | −0.4 | 0.65 |
| Executive scale | 99.25 (11.46) | 103.55 (10.47) | −1.2 | 0.22 |
| Total | 100.15 (11.51) | 102.85 (10.17) | −0.7 | 0.43 |
| DSM-IV criteria | ||||
| Inattention | 0.40 (0.68) | 7.60 (1.18) |
U = 0.0 Z = 5.7 |
< 0.001 |
| Hyperactivity/impulsivity | 1.30 (1.83) | 6.65 (1.81) | − 9.6 | < 0.001 |
| FASCT | ||||
| Self-reported score | 10.85 (5.81) | 34.00 (6.67) | −11.8 | < 0.001 |
| Observer score | 11.45 (9.43) | 29.10 (8.44) | −6.2 | < 0.001 |
ADHD = attention deficit hyperactivity disorder; DSM-IV = Diagnostic and Statistical Manual of Mental Disorders, 4th edition; FASCT = Friederichsen, Almeida, Serrano, Cortes Test;22 SD = standard deviation; WAIS-III = Weschler Adult Intelligence Scale, 3rd edition.29
Unless otherwise indicated.
Including academic achievement and misconduct problems.
Maximum education degree obtained.
The Fisher exact test.
The mean number of hyperactivity/impulsivity criteria was 6.65 (SD 1.81) for ADHD individuals and 1.30 (SD 1.83) for healthy controls (t38 = −9.63, p < 0.001). The mean score on the FASCT-SR scale was 34.0 (SD 6.67) points for ADHD patients and 10.85 (SD 5.81) points for healthy controls (t38 = −11.8, p < 0.001). The mean FASCT-O score was 29.10 (SD 8.44) points for ADHD patients and 11.45 (SD 9.43) points for healthy participants (t38 = −6.23, p < 0.001; Table 1).
Grey matter concentration
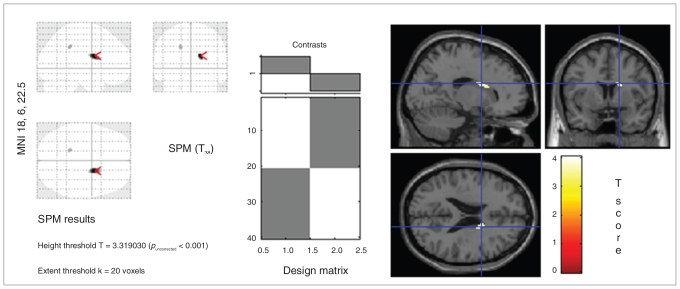
The results for the whole sample are shown in Figure 1. Note that there is a statistical difference in the right caudate between groups. In both sexes, there were significant differences between ADHD patients and healthy controls in the right caudate. For the whole sample the cluster-level results at MNI coordinates x = 18 mm, y = 6 mm, z = 22 mm (right caudate) were pcorr = 0.004 and expected voxels per cluster (Ke) = 147.
Fig. 1.
Portion of the right caudate where voxel-based morphometry showed statistical differences in the concentration of grey matter between 20 adults with attention deficit hyperactivity disorder and 20 healthy controls. The statistical parametric mapping (SPM), design matrix and SPM projection over the Montreal Neurological Institute T1 template are shown. The SPM contrast was set at a p value threshold of 0.001 and a voxel threshold of 20. The settings for small volume correction with a volume-of-interest search were a radius of 10 mm at global maxima 18, 6 and 22.
The voxel-level values were as follows: pFWE-corr = 0.014, pFDR-corr = 0.004, t = 4.04, and Z = 3.67. At MNI coordinates x = 18 mm, y = 2 mm, z = 44 mm (right caudate), the voxel-level values were pFWE-corr = 0.016, pFDR-corr = 0.004, t = 3.60, Z = 3.60; FWHMestimated = 10.6 mm, 11.7 mm and 10.7 mm; resel count = 3.2; and voxel size = 1.5 mm, 1.5 mm, 1.5 mm.
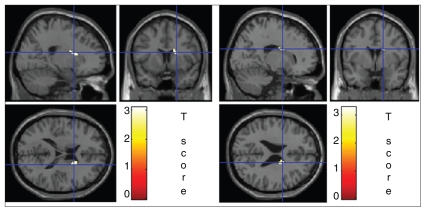
The comparison between healthy men and ADHD men gave the following results: cluster-level pcorr = 0.034, Ke = 77; voxel-level pFWE-corr = 0.047, pFDR-corr = 0.010, t = 3.10, Z = 2.74; FWHMestimated = 10.2 mm, 11.2 mm, 10.3 mm, and resel count = 1 (Fig. 2). The contrast between healthy women and ADHD women gave the following results: cluster-level pcorr = 0.049, Ke = 56; voxel-level pFWE-corr = 0.039, pFDR-corr = 0.017, t = 3.30; Z = 2.88, FWHMestimated = 10.4 mm, 11.5 mm, 10.4 mm, and resel count = 1.
Fig. 2.
Portion of the right caudate where voxel-based morphometry showed statistical differences between 10 controls and 10 adults with attention deficit hyperactivity disorder (left) in men and (right) women. The statistical parametric mapping (SPM) projection over the Montreal Neurological Institute T1 template is shown. The SPM contrast was set at a p value threshold of 0.01 and a voxel threshold of 20. The settings for small volume correction in men with a volume-of-interest search were a radius of 5 mm at global maxima of x = 21, y = 9, z = 18, and the settings in women were a radius sphere of 5 mm at global maxima of x = 17, y = 0, z = 25.
Grey matter volume
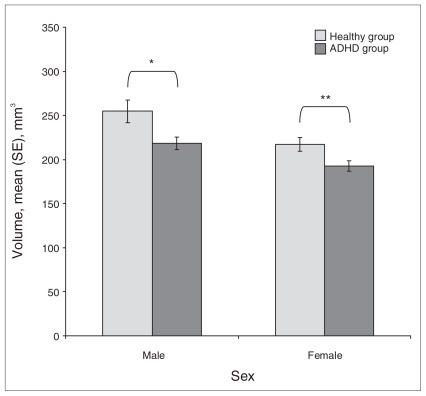
For the whole sample, the calculated volume of the portion of the right caudate that showed a low concentration of grey matter was 580 mm3 (SD 0.08) for healthy controls and 525 mm3 (SD 0.05) for ADHD patients (t38 = 2.59, p < 0.001). The results of the comparisons between healthy women and ADHD women are presented in Figure 3. The ADHD men and women both had a smaller volume of the portion of the right caudate with low-concentration grey matter than their respective healthy controls.
Fig. 3.
Comparison by sex of the volume of the portion of the right caudate where voxel-based morphometry (VBM) showed differences in the concentration of grey matter between healthy controls and patients with attention deficit hyperactivity disorder (ADHD). The bars represent the mean volume of the portion of the right caudate where VBM showed differences in concentration between healthy and ADHD individuals. *t18 = 2.36, p = 0.029, difference between means = 36 mm3, 95% confidence interval (CI) 4–68 mm3, Cohen d = 1.11. **t18 = 2.32, p = 0.032; difference between means = 20 mm3, 95% CI 2–48 mm3, Cohen d = 1.09. SE = standard error.
Correlations within the whole sample population
There were significant correlations between the volume of the low-concentration grey matter portion of the right caudate and the DSM-IV-TR criteria for inattention, impulsivity and hyperactivity. The greatest value was the correlation of volume with the number of hyperactivity criteria (rho38 = −0.51, p < 0.001). For the FASCT-SR and FASCT-O subscales, the strongest correlation was observed with the “organization problems factor”(r38 = −0.48, p < 0.001 and r38 = −0.38, p < 0.001; Table 2).
Table 2.
Correlation between the volume of the portion of the right caudate where the voxel-based morphometry showed statistical difference in the concentration of grey matter and the clinical measures
| Clinical measure | Coefficient correlation* | p value |
|---|---|---|
| DSM-IV-TR | ||
| Inattention | rho38 = −0.35 | 0.02 |
| Hyperactivity | rho38 = −0.51 | < 0.001 |
| Impulsivity | rho38 = −0.37 | < 0.001 |
| FASCT, self-reported version | ||
| Hyperactivity | r38 = −0.32 | 0.04 |
| Organization problems | r38 = −0.48 | < 0.001 |
| Low tolerance to frustration | r38 = −0.47 | < 0.001 |
| Total score | r38 = −0.49 | < 0.001 |
| FASCT, observer version | ||
| Hyperactivity | r38 = −0.31 | 0.05 |
| Organization problems | r38 = −0.38 | 0.01 |
| Low tolerance to frustration | r38 = −0.23 | 0.14 |
| Legal problems | rho38 = −0.19 | 0.21 |
| Total score | r38 = −0.39 | 0.01 |
DSM-IV-TR = Diagnostic and Statistical Manual of Mental Disorders, 4th edition, text revised; FASCT = Friederichsen, Almeida, Serrano, Cortes Test.22
Calculated with the Spearman correlation coefficient (rho) and the Pearson correlation coefficient (r).
Correlations within the sexes
For men, there were significant correlations between the volume of the portion of the right caudate with a low concentration of grey matter and the DSM-IV-TR criteria for inattention, impulsivity and hyperactivity. The strongest correlation was with the number of hyperactivity criteria (r18 = −0.72, p < 0.001). The highest correlation was observed with organization and functional impairment on the FASCT-SR and FASCT–O scales (r18 = −0.636, p < 0.001 and r18 = −0.452, p = 0.04, respectively; Table 3).
Table 3.
Correlation by sex between the volume of the portion of the right caudate where the voxel-based morphometry showed statistical difference in the concentration of grey matter and the clinical measures
| Men |
Women |
|||
|---|---|---|---|---|
| Clinical measure | Coefficient correlation, r18 | p value | Coefficient correlation, r18 | p value |
| DSM-IV-TR | ||||
| Inattention | −0.57 | < 0.001 | −0.51 | 0.02 |
| Hyperactivity | −0.72 | < 0.001 | −0.45 | 0.04 |
| Impulsivity | −0.68 | < 0.001 | −0.51 | 0.02 |
| FASCT, self-reported version | ||||
| Hyperactivity | −0.40 | 0.07 | −0.45 | 0.04 |
| Organization problems | −0.63 | < 0.001 | −0.49 | 0.02 |
| Low tolerance to frustration | −0.57 | 0.01 | −0.63 | < 0.001 |
| Total score | −0.72 | < 0.001 | −0.54 | 0.01 |
| FASCT, observer version | ||||
| Hyperactivity | −0.43 | 0.04 | −0.45 | 0.04 |
| Organization problems | −0.45 | 0.04 | −0.45 | 0.04 |
| Low tolerance to frustration | −0.35 | 0.13 | −0.31 | 0.17 |
| Legal problems | −0.28 | 0.21 | −0.11 | 0.64 |
| Total score | −0.52 | 0.01 | −0.44 | 0.04 |
DSM-IV-TR Diagnostic and Statistical Manual of Mental Disorders, 4th edition, text revised; FASCT = Friederichsen, Almeida, Serrano, Cortes Test.22
For women, the strongest correlation between the volume and the number of DSM-IV-TR criteria was for impulsivity (r18 = −0.51, p = 0.02). The strongest FASCT scale correlations were observed for the “low tolerance to frustration factor” of FASCT-SR (r18 = −0.63, p < 0.001) and the “hyperactivity factor” of FASCT-O (r18 = −0.45, p = 0.04) (Table 3).
Discussion
Our results show that the portion of the right caudate with a statistically lower concentration of grey matter than the surrounding tissue has a smaller volume and lower concentration of grey matter in adults with ADHD compared with healthy controls. The same results were obtained in a separate analysis by sex. The volume of this portion of the right caudate was significantly correlated with the number of criteria of the 3 ADHD clusters of the DSM-IV-TR. This volume was also associated with most of the individual factors and with total scores on the FASCT-SR and FASCT-O scales.
Studies that have compared the morphometric characteristics of the caudate in adults with ADHD26,27 have found no differences between healthy controls and ADHD patients. In contrast, we found significant differences. We hypothesize that these contradictory results are because of differences in the sample characteristics and methods, particularly in the measurement of the caudate nucleus. The most notable differences were in the image analysis methods used. We used VBM with DARTEL, whereas others have used a semiautomated technique.
The studies by Biederman and colleagues27 and Siedman and colleagues26 used the same image analysis method developed by Filipek and Kennedy.36–38 This approach includes the selection of region of interest by the user and may produce somewhat imprecise anatomic validity.39 However, the reliability of VBM is uncertain. Nonetheless, in a study published by Segall and colleagues,40 the authors evaluated the consistency of the results obtained in different studies and samples. This study included 327 patients with schizophrenia and 266 healthy individuals across 13 study sites using SPM–VBM with a Gaussian kernel of 10 mm. The samples came from 2 multisite, independent studies: the Functional Biomedical Informatics Research Network and the Mind Clinical Imaging Consortium. Different makes and models of scanners were used, as were different strengths of magnetic fields (e.g., 1.5, 3 and 4 T) and different sequences and slice thicknesses (1.2, 1.5 and 1.6 mm). Despite these methodological variations, similar results were obtained across studies. The strongest similarity was observed in the rostral section of the superior temporal lobe, where the schizophrenia patients showed a smaller concentration of grey matter.40 The extrapolation of the results from this study must be handled with caution because Segall and colleagues40 included patients with schizophrenia, and the DARTEL algorithm was not used.
In a recent study by Peelle and colleagues,41 MRI scans of 330 participants (18–78 yr) were analyzed using SPM5 software. Images of the grey matter were normalized to either an MNI template (the “standard” approach) or to an average template using DARTEL. Modulation and smoothing at 8 mm FWHM were done in the standard approach and the DARTEL algorithm. The images obtained with DARTEL were not as blurred as the images obtained with the standard approach. Further, registration errors were noted in the standard approach but not in the DARTEL approach. An analysis of age-related decreases in grey matter volume showed more focal results using DARTEL than using the standard approach, where registration errors were observed on the edges of the images.41
It is not surprising that we found a reduced volume of a portion the caudate, given that studies using diverse functional imaging techniques (e.g., positron emission tomography) have shown a malfunction in the striatum of adults with ADHD.42–44
Considering our findings and the fact that few studies involving adult ADHD patients have been published, there is a need for additional research into the volume of the caudate nucleus in adults with ADHD.
Limitations
Given the cross-sectional design of this work, our results can only be applied to patients aged 25 to 35 years. The results obtained with the image analysis method used here must be considered with caution because any significant differences found using VBM could be explained by a number of causes, such as a real increase or decrease in the concentration of brain tissue, errors in the classification of the brain tissues or the complex folding of the brain cortex.
A critique of VBM is that it is sensitive to systematic shape differences attributable to misregistration from the spatial normalization step;45 nevertheless, this does not imply that VBM is invalid.46 Indeed, VBM is a completely automated method and is free of user bias. It is sensitive to differences in the local composition of brain tissue types, by means of discounting positional and other large-scale volumetric differences of the gross anatomy. Additionally, it uses probabilistic maps to achieve better segmentation.47
Although some authors33,34 have examined the effect of the size of the Gaussian kernel (FWHM) on t scores when comparing 2 groups using VBM or other techniques, those results have limited applicability to this study because the parameters of registration used in VBM by Davatzikos and colleagues,34 for example, are referred to as VBM without the use of the DARTEL algorithm. The VBM technique has some flaws and some advantages, and some issues remain unclear (e.g., the optimal kernel size and the reliability indices for the measures of VBM with the DARTEL algorithm).
Conclusion
Given the limited data about the possible anatomic abnormalities of the right caudate in adults with ADHD and our results, we suggest that additional studies with the following features should be performed: longitudinal, multimodal (e.g., structural and functional techniques) studies with a larger sample size and a broader age range, and using homogenized image analysis methods whose validity and reliability are well-known and robust.
The results of this study suggest a reduction of a portion of the right caudate in adults with ADHD; this reduction is correlated with the severity of illness.
Acknowledgments
Secretaria de Salud del Estado de Querétaro, México, and Consej Nacional de Ciencia y Tecnología provided grants for this work. Programa de Doctorado en Ciencias Biomédicas de la Universidad Nacional Autónoma de México provided academic and research guidance.
Footnotes
Competing interests: None declared.
Contributors: Drs. Almeida Montes, Ricardo-Garcell, Prado Alcántara, Martínez García and Fernández-Bouzas designed the study. Drs. Almeida Montes, Prado Alcántara and Ávila Acosta acquired the data, which Drs. Almeida Montes, Ricardo-Garcell, Barajas De La Torre, Prado Alcántara, Martínez García and Fernández-Bouzas analzyed. Drs. Almeida Montes, Ricardo-Garcell, Prado Alcántara and Fernández-Bouzas wrote the article. All authors reviewed the article and approved its publication.
References
- 1.Biederman J, Faraone S, Spencer T, et al. Patterns of psychiatric comorbility, cognition, and psychosocial functioning in adults with attention deficit hyperactivity disorder. Am J Psychiatry. 1993;150:1792–8. doi: 10.1176/ajp.150.12.1792. [DOI] [PubMed] [Google Scholar]
- 2.Biederman J. Attention-deficit/hyperactivity disorder: a selective overview. Biol Psychiatry. 2005;57:1215–20. doi: 10.1016/j.biopsych.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 3.Hynd GW, Semrud-Clikeman M, Lorys A, et al. Brain morphology in developmental dyslexia and attention deficit/hyperactivity. Arch Neurol. 1990;47:919–26. doi: 10.1001/archneur.1990.00530080107018. [DOI] [PubMed] [Google Scholar]
- 4.Valera EM, Faraone SV, Murray KE, et al. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61:1361–9. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Qiu A, Crocetti D, Adler M, et al. Basal ganglia volume and shape in children with attention deficit hyperactivity disorder. Am J Psychiatry. 2009;166:74–82. doi: 10.1176/appi.ajp.2008.08030426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seidman LJ, Valera EM, Makris N. Structural brain imaging of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1263–72. doi: 10.1016/j.biopsych.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 7.Ellison-Wright I, Ellison-Wright Z, Bullmore E. Structural brain change in attention deficit hyperactivity disorder identified by meta-analysis. BMC Psychiatry. 2008;8:51–8. doi: 10.1186/1471-244X-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giedd JN, Blumenthal J, Molloy E, et al. Brain imaging of attention-deficit/hyperactivity disorder. Ann N Y Acad Sci. 2001;931:33–49. doi: 10.1111/j.1749-6632.2001.tb05772.x. [DOI] [PubMed] [Google Scholar]
- 9.Crinion J, Turner R, Grogan A, et al. Language control in the bilingual brain. Science. 2006;312:1537–40. doi: 10.1126/science.1127761. [DOI] [PubMed] [Google Scholar]
- 10.Packard MG, Knowlton BJ. Learning and memory functions of the basal ganglia. Annu Rev Neurosci. 2002;25:563–93. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- 11.Graybiel AM. The basal ganglia: learning new tricks and loving it. Curr Opin Neurobiol. 2005;15:638–44. doi: 10.1016/j.conb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Fairhall SL, Indovina I, Driver J, et al. The brain network underlying serial visual search. Comparing overt and covert spatial orienting, for activations and for effective connectivity. Cereb Cortex. 2009;19:2946–58. doi: 10.1093/cercor/bhp064. [DOI] [PubMed] [Google Scholar]
- 13.Heilman KM, Voeller KK, Nadeau SE. A possible pathophysiology substrate of attention deficit hyperactivity disorder. J Child Neurol. 1991;6(Suppl):S76–81. doi: 10.1177/0883073891006001s09. [DOI] [PubMed] [Google Scholar]
- 14.Halperin JM, Schulz KP. Revisiting the role of the prefrontal cortex in the pathophysiology of attention-deficit/hyperactivity disorder. Psychol Bull. 2006;132:560–81. doi: 10.1037/0033-2909.132.4.560. [DOI] [PubMed] [Google Scholar]
- 15.Castellanos FX, Giedd JN, Marsh WL, et al. Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Arch Gen Psychiatry. 1996;53:607–16. doi: 10.1001/archpsyc.1996.01830070053009. [DOI] [PubMed] [Google Scholar]
- 16.Filipek PA, Semrud-Clikeman M, Steingard RJ, et al. Volumetric MRI analysis. Comparing individuals having attention-deficit hyperactivity disorder with normal controls. Neurology. 1997;48:589–601. doi: 10.1212/wnl.48.3.589. [DOI] [PubMed] [Google Scholar]
- 17.Tremols V, Bielsa A, Soliva J, et al. Differential abnormalities of the head and body of the caudate nucleus in attention deficit hyperactivity disorder. Psychiatr Res. 2008;163:270–8. doi: 10.1016/j.pscychresns.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 18.McAlonan GM, Cheung V, Cheung C, et al. Mapping brain structure in attention deficit hyperactivity disorder: a voxel based MRI study of regional grey and white matter volume. Psychiatr Res. 2007;154:171–80. doi: 10.1016/j.pscychresns.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Castellanos FX, Lee PP, Sharp W, et al. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA. 2002;288:1740–8. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- 20.Kessler RC, Adler R, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States. Results of national co-morbility survey replication. Am J Psychiatry. 2006;163:716–23. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biederman J, Mick E, Faraone S. Age-dependent decline of symptoms of attention deficit hyperactivity disorder. Impact of remission definition and symptom type. Am J Psychiatry. 2000;157:816–8. doi: 10.1176/appi.ajp.157.5.816. [DOI] [PubMed] [Google Scholar]
- 22.Almeida Montes L, Friederichsen Alonso A, Olivia Hernández A, et al. [Construction, validity and reliability, of the screening scale “F.A.S.C.T.” for attention deficit hyperactivity disorder in adults (self-reported and observer versions)] [Article in Spanish] Actas Esp Psiquiatr. 2006;34:231–8. [PubMed] [Google Scholar]
- 23.Almeida Montes LG, Hernández García AO, Ricardo-Garcell J. ADHD prevalence in adult outpatients with nonpsychotic psychiatric illnesses. J Atten Disord. 2007;11:150–6. doi: 10.1177/1087054707304428. [DOI] [PubMed] [Google Scholar]
- 24.Hesslinger B, Tebartz van Elst L, Thiel T, et al. Frontoorbital volume reductions in adult patients with attention deficit hyperactivity disorder. Neurosci Lett. 2002;328:319–21. doi: 10.1016/s0304-3940(02)00554-2. [DOI] [PubMed] [Google Scholar]
- 25.Makris N, Biederman J, Valera E, et al. Cortical thinning of the attention and executive function networks in adults with attention-deficit/hyperactivity disorder. Cereb Cortex. 2007;17:1364–75. doi: 10.1093/cercor/bhl047. [DOI] [PubMed] [Google Scholar]
- 26.Seidman LJ, Valera EM, Makris N, et al. Dorsolateral prefrontal and anterior cingulated cortex volumetric abnormalities in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2006;60:1071–80. doi: 10.1016/j.biopsych.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 27.Biederman J, Makris N, Valera E, et al. Towards further understanding of the co-morbidity between attention-deficit hyperactivity disorder and bipolar disorder: a MRI study of brain volumes. Psychol Med. 2008;38:1045–56. doi: 10.1017/S0033291707001791. [DOI] [PubMed] [Google Scholar]
- 28.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- 29.Weschler D. Weschsler adult intelligence scale. 3rd ed. San Antonio (TX): The Psychological Corporation; 1997. [Google Scholar]
- 30.Ashburner J. A fast diffemorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Klein A, Andersson J, Ardekani B, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46:786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ashburner J, Friston K. Computing average shaped tissue probability templates. Neuroimage. 2009;45:333–41. doi: 10.1016/j.neuroimage.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 33.Goldszal AF, Davatzikos C, Pham DL. An image-processing system for qualitative and quantitative volumetric analysis of brain images. J Comput Assist Tomogr. 1998;22:827–37. doi: 10.1097/00004728-199809000-00030. [DOI] [PubMed] [Google Scholar]
- 34.Davatzikos C, Genc A, Xu D, et al. Voxel based morphometry using RAVENS maps: methods and validation using simulated longitudinal atrophy. Neuroimage. 2001;14:1361–9. doi: 10.1006/nimg.2001.0937. [DOI] [PubMed] [Google Scholar]
- 35.Feinstein A. Principles of medical statistics. Boca Raton (FL): Chapman & Hall/CRC Press; 2002. Part II. Comparing two groups of data; pp. 163–323. [Google Scholar]
- 36.Filipek PA, Kennedy DN, Caviness VS, Jr, et al. Magnetic resonance imaging-based morphometry: development and applications to normal controls. Ann Neurol. 1989;25:61–7. doi: 10.1002/ana.410250110. [DOI] [PubMed] [Google Scholar]
- 37.Kennedy DN, Filipek PA, Caviness VS., Jr Anatomic segmentation and volumetric calculations in nuclear magnetic resonance imaging. IEEE Trans Med Imaging. 1989;8:1–7. doi: 10.1109/42.20356. [DOI] [PubMed] [Google Scholar]
- 38.Caviness VS, Jr, Kennedy DN, Richelme C, et al. The human brain age 7–11 years: a volumetric analysis based on magnetic resonance images. Cereb Cortex. 1996;6:726–36. doi: 10.1093/cercor/6.5.726. [DOI] [PubMed] [Google Scholar]
- 39.Friston K. A short history of SPM. In: Friston K, Ashburner J, Kiebel S, et al., editors. Statistical parametric mapping. The analysis of functional brain images. United Kingdom: Elsevier; 2007. pp. 3–9. [Google Scholar]
- 40.Segall JM, Turner JA, van Erp TG, et al. Voxel-based morphometric multisite collaborative study on schizophrenia. Schizophr Bull. 2009;35:82–95. doi: 10.1093/schbul/sbn150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peelle J, Cusack R, Henson R. Effects of normalization approach and global covariates on voxel-based morphometry: comparing DARTEL and standard SPM approaches using age-related cortical change [poster]. 15th Annual Meeting of the Organization for Human Brain Mapping; 2009 June 18–23; San Francisco, Calif. [Google Scholar]
- 42.Volkow ND, Wang GJ, Newcorn J, et al. Depressed dopamine activity in caudate and preliminary evidence of limbic involvement in adults with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2007;64:932–40. doi: 10.1001/archpsyc.64.8.932. [DOI] [PubMed] [Google Scholar]
- 43.Dougherty D, Bonab A, Spencer T. Dopamine transporter density in patients with attention deficit hyperactivity disorder. Lancet. 1999;354:2132–3. doi: 10.1016/S0140-6736(99)04030-1. [DOI] [PubMed] [Google Scholar]
- 44.Krause KH, Dresel SH, Krause J. Increased stratial dopamine transporter in adult patients with attention deficit hyperactivity disorder: effects of methylphenidate as measured by single photon emission tomography. Neurosci Lett. 2000;285:107–10. doi: 10.1016/s0304-3940(00)01040-5. [DOI] [PubMed] [Google Scholar]
- 45.Bookstein FL. “Voxel based morphometry” should not be used with imperfectly registered images. Neuroimage. 2001;14:1454–62. doi: 10.1006/nimg.2001.0770. [DOI] [PubMed] [Google Scholar]
- 46.Ashburner J, Friston K. Why voxel based morphometry should be used. Neuroimage. 2001;14:1238–43. doi: 10.1006/nimg.2001.0961. [DOI] [PubMed] [Google Scholar]
- 47.Davatzikos C. Why voxel-based morphometric analysis should be used with great caution when characterizing group differences. Neuroimage. 2004;23:17–20. doi: 10.1016/j.neuroimage.2004.05.010. [DOI] [PubMed] [Google Scholar]