Abstract
Background
We investigated the differential effects of serotonergic and noradrenergic antidepressants on brain activation in patients with major depressive disorder during a Stroop task. We predicted that pretreatment hyperactivity in the rostral anterior cingulate cortex would predict better treatment outcomes.
Methods
In total, 20 patients underwent naturalistic open-label clinical treatment with citalopram (n = 12) or reboxetine (n = 8). We performed functional magnetic resonance imaging at baseline and after 6 weeks of treatment.
Results
There were no significant group differences in clinical characteristics, treatment outcomes or baseline fMRI activations. The group by time interaction revealed significant voxels in the right amygdala–hippocampus complex (p < 0.05, family-wise error corrected by use of the bilateral amygdala and hippocampus mask image as a small volume), indicating a posttreatment blood oxygen level–dependent signal decrease in the citalopram group. Pretreatment hyperactivity in the rostral anterior cingulate cortex was not related to symptom improvement.
Limitations
Our study was a nonrandomized clinical trial.
Conclusion
These results indicate that serotonergic and noradrenergic antidepressants have a differential effect on brain activity, especially in the amygdala and hippocampus.
Introduction
Neuroimaging studies have provided an essential contribution to our understanding of the neurobiologic underpinnings of major depressive disorder (MDD). A network comprised mainly of the limbic, frontal and cingulate regions has been shown to be altered with regard to function and brain structure in this disorder.1 Although optimized treatment strategies, including numerous modern antidepressants, have recently been developed, fewer than 50% of patients with MDD show full remission.2,3 Motivated by these data, a growing number of neuroimaging studies have focused on the prediction of response to antidepressant treatment and thus on the identification of neurofunctional markers that may be used to discriminate responders from nonresponders.
The majority of previous neuroimaging studies have focused on resting-state cerebral glucose metabolism using positron emission tomography (PET) to identify brain areas that correlate with symptom normalization and using baseline brain activity to predict treatment outcome. Most studies have reported a reciprocal pattern of normalization of pre-frontal, parietal and cingulate pretreatment hypometabolism and of limbic–paralimbic decreases in glucose metabolism or blood flow (e.g., in the subgenual cingulate cortex, hippocampus and amygdala after antidepressant treatment with a selective serotonin reuptake inhibitor [SSRI]).4–6 In contrast, significant decreases in metabolic brain activity in the rostral anterior cingulate (rACC) and prefrontal brain areas after treatment with an SSRI or a serotonin–norepinephrine reuptake inhibitor (NRI) have also been reported.7,8
In addition to differences in sample composition and anti-depressant medication, one reason for these inconsistent results may be the acquisition of resting-state scans in which an individual’s depressed symptoms may interact with the uncontrolled scanning condition of “mind wandering” and thus contribute to heterogeneity. Obtaining brain responses to cognitive or affective challenges in the course of treatment has the advantage of a more standardized scanning condition and allows selective hypothesis testing targeted depressive core symptoms.
Applying a continuous performance test and 18F-deoxyglucose-PET scanning, Buchsbaum and colleagues9 observed normalization of glucose metabolism in terms of increased metabolic activity after 10 weeks of placebo-controlled treatment with sertraline; these changes were predominantly in the frontoparietal brain network. A functional magnetic resonance imaging (fMRI) study by Walsh and colleagues10 used the n-back task and reported a greater load-dependent response in the inferior frontal and parietal cortex in patients with MDD before treatment, with no differences in task accuracy between patients and healthy controls (although differences in reaction times were present). The authors detected activation normalization in these areas after 8 weeks of treatment with fluoxetine and reported a significant relation between load-response activity and clinical outcome in the frontotemporal network. Additionally, in a study involving 19 acutely depressed patients, Fu and colleagues11 observed reduced capacity for activation in the left amygdala and in the frontoparietal network in a facial affect recognition task after 8 weeks of fluoxetine treatment.
With regard to prediction of treatment response, the rACC (Brodmann area [BA] 24/32) and subgenual cingulate cortex (BA 25) have been shown to play a prominent role. These regions have been related to more successful antidepressant treatment outcomes, indicating a beneficial effect of higher pretreatment metabolic activity12 and theta activity13 as well as lower metabolism.7
In our previous study14 with the Stroop Color–Word test, an established neuropsychologic test of cognitive control processes, our main finding was that unmedicated depressed patients showed relative hyperactivity in the rACC despite normal behavioural performance. We interpreted these results as an inability of the depressed patients to inhibit affective interferences, which they probably compensate for with stronger cognitive control exerted by the dorsolateral pre-frontal cortex to produce normal behavioural performance.
In light of these findings, one of our main goals was to investigate the effect of antidepressant treatment on the inefficient neural cognitive control processing in depressed patients and to determine how these changes relate to responses to serotonergic or noradrenergic therapy.
Noradrenergic and serotonergic pathways show anatomic overlap.15 Despite potentially different side-effects and influences of different depressive symptoms, this overlap may lead to the suggestion that both SSRIs and NRIs exert a comparable antidepressant effect through the final common pathways targeting the same brain structures.16 Thus, we expected to find comparable activation normalization in the frontocingulate brain areas and amygdala–hippocampal regions in terms of activation decreases for both medication groups. In the same brain regions, we expected to find an association between improved depressive symptoms and decreased blood oxygen level–dependent (BOLD) signals. In addition, we hypothesized a predictive role of pretreatment hyperactivity in the rACC for treatment outcome in both groups.
Methods
Participants
We recruited 20 patients who met the DSM-IV criteria for MDD according to the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID)17 from the in- and outpatient service of the Department of Psychiatry and Psychotherapy of the Friedrich-Schiller University, Jena. Detailed demographic and clinical characteristics are summarized in Table 1. Twelve of the patients included in this study were part of a previously published study.14
Table 1.
Demographic and clinical characteristics of patients with major depressive disorder and controls
| Group; mean (SD)*† |
|||
|---|---|---|---|
| Characteristic | Reboxetine, n = 8 | Citalopram, n = 12 | Controls, n = 20 |
| Age, yr, mean | 36.1 (10.8) | 42.4 (12.8) | 37.6 (10.8) |
| School education, yr | 10.5 (1.4) | 10.5 (1.2) | 10.8 (1.2) |
| Sex, female:male | 7:1 | 11:1 | 18:2 |
| Age at onset, yr | 34.3 (12.0) | 30.9 (12.2) | – |
| Duration of illness, yr | 8.3 (6.4) | 5.2 (7.7) | – |
| No. of previous episodes | 1.3 (0.8) | 1.0 (1.3) | – |
| Logical IQ score | 98.6 (10.4) | 104.8 (13.5) | |
| Trail Making Test20 Part A score | 34.8 (9.1) | 35.7 (16.5) | – |
| Trail Making Test20 Part B score | 80.6 (25.8) | 77.2 (31.6) | – |
| Before treatment | |||
| HRSD score | 24.4 (4.7) | 23.4 (4.4) | – |
| BDI score | 25.5 (8.1) | 25.5 (11.3) | 3.6 (8.3) |
| After treatment | |||
| HRSD score | 8.8 (5.8) | 8.3 (6.3) | – |
| BDI score | 16.3 (11.2) | 15.1 (12.1) | – |
| Treatment response, % change in HRSD score | 65.5 (16.5) | 66.2 (23.7) | – |
| No. of patients in remission after treatment (HRSD score < 7) | 4 | 6 | – |
We included patients with an acute episode of MDD, aged 18–55 years and a score of 18 or greater on the Hamilton Rating Scale for Depression (HRSD, 21-item version).18 We excluded patients with a current comorbid axis I disorder (according to SCID), a history of manic episodes, and past or current neurologic disorders. None of the patients in the present sample met the DSM-IV criteria for a personality disorder. To assess intellectual abilities, patients performed sub-test U3 from the Leistungs-Prüf-System (LPS)19 test of logical abstract reasoning. We further tested executive function with the Trail Making Test Part A and Part B.20
We recruited 20 healthy controls matched for sex, age and education (Table 1) through an advertisement in a local newspaper; the controls were screened for psychiatric or neurologic diseases. Controls with past or current neurologic or psychiatric diseases and/or first-degree relatives with axis I psychiatric disorders were excluded from the study. Two experienced clinical psychologists (C.S., G.W.) assessed the current presence of psychiatric disease by use of a checklist of DSM-IV criteria. To assess general psychopathology, all healthy controls performed self-ratings and evaluations using the Symptom Checklist-90-R21 and the Beck Depression Inventory (BDI)22 for assessing self-reported depressive symptoms.
All participants were right-handed, according to the modified version of the Annett handedness inventory,23 and all participants provided written informed consent before participating in the study. The study protocol was approved by the Ethics Committee of the University of Jena.
Treatment procedure
During pretreatment clinical evaluation and at the time of the baseline fMRI scan, all patients were free of psychotropic medication for at least double the half-life of any previously administered psychotropic drugs. For benzodiazepines, the wash-out period was 5 half-lives of the previously administered benzodiazepine. After the baseline fMRI scan, the depressed patients underwent prospective, naturalistic open-label, nonrandomized controlled clinical treatment with either the NRI reboxetine or the SSRI citalopram. The choice of antidepressant type and dose was determined by usual clinical practice routines. Patients were treated in the inpatient and outpatient service of the university hospital.
All psychopathological rating scales were administered by a rater (G.W., C.S.) who was unaware of the pharmacologic treatment.
At the end of the 6-week period, 8 patients were taking reboxetine and 12 were taking citalopram. Patients taking citalopram received an average daily dose of 27.9 (standard deviation [SD] 8.3) mg/day. Patients taking reboxetine received an average daily dose of 5.28 (SD 1.22) mg/day. After the first fMRI scan, 3 patients from the reboxetine group and 1 patient from the citalopram group took a benzodiazepine for an average of 1 week. None of the patients took a sleeping medication during treatment.
We defined treatment response as a minimum reduction of 50% in HRSD scores from baseline, and we defined remission as an HRSD end-point score of less than 7. Six weeks after the baseline fMRI scan, patients received a follow-up fMRI scan.
Cognitive paradigm
The Stroop Color–Word test was presented in an event-related design and consisted of 2 conditions (congruent and incongruent). In the congruent condition, colour words were presented in the colour denoted by the corresponding word; in the incongruent condition, colour words were displayed in 1 of 3 colors not denoted by the word. Two possible answers were presented below the word to minimize contextual memory demand. The participants were asked to indicate the colour by pressing 1 of 2 buttons, which corresponded spatially to both possible answers. Stimuli were presented for 1500 ms with an interstimulus interval of 10.5 seconds and were jittered over the repetition time. Further details of the paradigm can be found in our previous paper.14
Functional MRI parameters
We collected functional data using a 1.5-T Siemens Magnetom Vision whole-body system equipped with a circularly polarized transmit/receive head volume coil. Participants’ heads were immobilization with pads within the coil. We obtained a series of overall 440 T2*-weighted images using a single-shot echo planar sequence (repetition time 2000 ms, echo time 60 ms, flip angle 90°) with 19 contiguous transversal slices of 5-mm thickness in 2 separate sessions (220 scans in each session). Matrix size was 64 × 64 pixels with in-plane resolution of 3.75 × 3.75 mm and field of view of 240 mm.
Statistical analyses
We used SPM2 for image preprocessing and SPM5 software for statistical analyses (www.fil.ion.ucl.ac.uk/spm). We discarded the first 4 scans per session to obtain steady-state tissue magnetization.
We corrected the images of the remaining 216 scans from each session for differences in slice time acquisition, realigned to the first image of each session and normalized to the Montreal Neurological Institute reference brain. The data were smoothed with a Gaussian kernel (10-mm full-width at half-maximum), high-pass filtered with a cutoff period of 128 seconds and corrected for serial correlations choosing the first-order autoregressive model AR(1).
We then created individual images of parameter estimates in a fixed-effects model, which we then entered into the second-level analyses. During the second-level random-effects analyses, a 2-way analysis of variance (ANOVA) design matrix within SPM5 was set up with the between-subjects factor group (MDD patients and controls) and the within-subjects factor task (congruent and incongruent Stroop conditions) to test for differences in the baseline fMRI scans. Next, we set up within SPM5 a 2-way ANOVA design matrix with the between-subjects factor group (patients with citalopram or reboxetine) and the within-subjects factor treatment (before and after 6 weeks’ antidepressant treatment) to test for differential treatment effects.
Because we expected to find an aberrant activation pattern predominantly in the cognitive-demanding incongruent condition, we restricted our analyses to this dependent variable. To test the relation between symptomatic improvement and treatment-associated changes in brain activation patterns, we set up a fixed-effect model at a single-subject level with both fMRI scanning times in a single model and put the contrast images into a regression analysis with the percentage of symptomatic improvement as assessed by HRSD.
We chose a significance level of p < 0.001 with a spatial extent of 25 voxels. In addition, we masked treatment-related statistics inclusively with the overall effect (p < 0.05 uncorrected) to only reveal changes above baseline. For the cluster-level statistics, we used nonstationary cluster extent correction, as implemented in the SPM toolbox.24
We used a small volume correction to perform multiple comparison correction using the family-wise-error (FWE) theory. The small volumes were created by means of the Wake Forest University pickatlas (www.fmri.wfubmc.edu/) and based on our initial hypotheses.
We applied the Kolmogorov–Smirnov test to examine the distribution of the performance data. The distribution of response accuracies but not of reaction times was significantly different from a normal distribution both in controls and patients. Therefore, reaction time was analyzed with SPSS 14.0.1 (www.spss.com) by use of ANOVA. We nonparametrically analyzed the response accuracy by use of the Mann–Whitney U test for between-group differences and the Wilcoxon test for within-group differences.
Results
Clinical data
In the 2-factors ANOVA for group (SSRI v. NRI) and time (before v. after treatment), there was a significant main effect of time (F1,18 = 211.4, p < 0.001) on the HRSD score, but there was no significant main effect of group or time by group interaction.
Post-hoc t tests revealed that the HRSD and BDI scores were not significantly different between patients in the SSRI and NRI groups at baseline (Table 1). In addition, there were no significant treatment effects on depression severity scores. In total, 16 of 20 depressed patients showed a reduction in symptoms of more than 50% after 6 weeks of antidepressant treatment, and 10 of 20 patients were in remission after 6 weeks.
Behavioural performance
When comparing the reaction times of depressed patients with those of healthy controls, there was no significant main effect of group (control v. depressed patients) and no interaction of group and task (congruent v. incongruent condition). There was a significant main effect of task (F1,37 = 154.67, p < 0.001), which indicated a reliable induction of the Stroop interference effect.
Repeated-measures ANOVA testing for treatment-related effects on performance of the Stroop task showed a significant main effect of task (F1,18 = 86.04, p < 0.001) and a significant task by time interaction (F1,18 = 4.13, p = 0.05), indicating a stronger reduction in response time after antidepressant treatment in the incongruent than in the congruent condition. No other main effects or interactions were statistically significant, indicating no significant differences in Stroop task performance between the medication groups before or after 6 weeks of antidepressant treatment. No significant differences were found for response accuracy.
Functional MRI results
Group comparison between healthy controls and patients
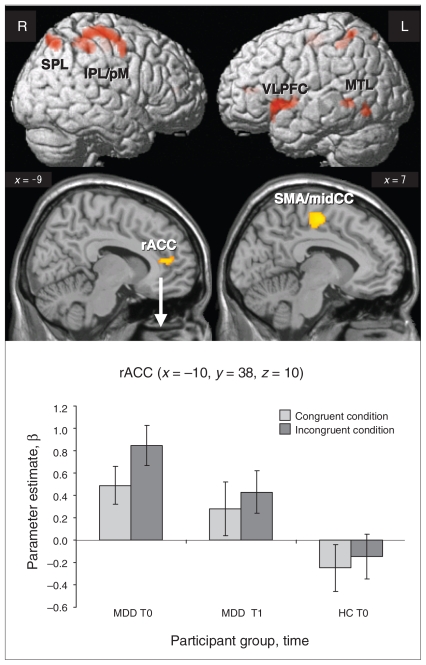
In a 2-way ANOVA with the factors group (controls v. patients) and task (congruent v. incongruent condition), we found a relative hyperactivity in patients in the fronto–parieto–temporal network and rACC in the incongruent condition (Fig. 1 and Table 2). The results of the opposite contrast were not significant. For the incongruent condition after 6 weeks of antidepressant treatment, there were no differences between patients and controls (baseline) in relative hyper- or hypoactivity.
Fig. 1.
Comparison of the brain activation during the incongruent task in 20 patients with major depressive disorder (MDD) and 20 controls (p < 0.001, cluster size > 24 voxels). Parameter estimates (β) and standard error of the mean extracted from the cluster in rostral anterior cingulate cortex for the congruent and incongruent conditions are shown. HC = healthy controls; IPL = inferior parietal lobe; midCC = midcingulate cortex; MTL = middle temporal lobe; pM = premotor cortex; rACC = rostral anterior cingulate cortex; SMA = supplementary motor cortex; SPL = superior parietal lobe; T0 = blood oxygen level–dependent (BOLD) signal before antidepressant therapy; T1 = BOLD signal after antidepressant therapy; VLPFC = ventrolateral prefrontal cortex.
Table 2.
Regions that showed significantly* increased blood oxygen level–dependent signal in depressed patients compared with healthy controls in the incongruent condition
| Talairach coordinate |
||||||
|---|---|---|---|---|---|---|
| Region of activation | Cluster size | x | y | z | t value | pFDR-corrected |
| Right inferior parietal cortex (BA 40) | 540† | 48 | −34 | 59 | 4.55 | 0.05 |
| Right premotor cortex (BA 6) | 46 | −16 | 60 | 4.43 | 0.05 | |
| Left inferior parietal cortex (BA 40) | 63 | −46 | −42 | 57 | 3.70 | 0.05 |
| Right superior parietal lobe (BA 7) | 157 | 36 | −63 | 55 | 5.04 | 0.05 |
| Right medial frontal gyrus (BA 6) | 249 | 6 | −11 | 50 | 4.35 | 0.04 |
| Left middle temporal gyrus (BA 22) | 48 | −61 | −47 | 2 | 3.60 | 0.07 |
| Left middle occipital gyrus (BA 37) | 81 | −48 | −62 | −5 | 3.99 | 0.05 |
| Left ventrolateral PFC (BA 44/45/47) | 387† | −48 | 17 | −3 | 4.59 | 0.05 |
| Left rostral ACC (BA 24) | 50 | −10 | 37 | 7 | 3.57 | 0.07 |
ACC = anterior cingulate; BA = Brodmann area; FDR = false discovery rate; PFC = prefrontal cortex.
Significant at p < 0.001 before FDR correction (cluster size > 24 voxels).
Significant after correction for multiple comparisons (p < 0.05).
Pretreatment differences between the SSRI and NRI groups
Before treatment, the NRI group showed significantly higher activity only in the superior temporal gyrus (BA 39, x = –51, y = –55, z = 21, cluster size = 55, t = 3.84, p < 0.001, pFDR-corrected = 0.78). In the opposite contrast, we only detected relative hyperactivity in the fusiform gyrus (BA 19, x = –30, y = –71, z = –13, cluster size = 40, t = 3.97, p < 0.001, pFDR-corrected = 0.85) in the SSRI group.
There were no significant group differences at baseline with regard to the frontocingulate brain network and amygdala–hippocampus activation.
Effects of antidepressant treatment
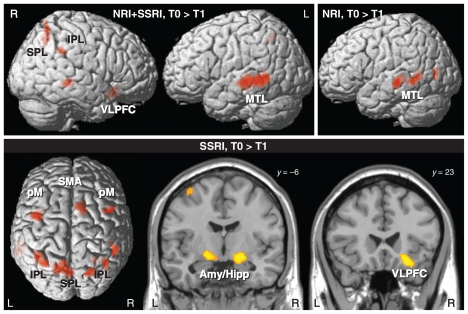
In the main effect of time for all patients, there was a significant decrease in BOLD signal after treatment predominantly in the left middle temporal lobe, right inferior parietal lobule, right ventrolateral prefrontal cortex and bilaterally in the superior parietal lobe (Fig. 2 and Table 3). No significant voxels were detected in the opposite contrast.
Fig. 2.
Main effect of time for both medication groups together (noradrenalin reuptake inhibitors [NRI] and selective serotonin reuptake inhibitors [SSRI]) as well as separately for the citalopram (SSRI) and reboxetine (NRI) groups. There were significant reductions in the blood oxygen level–dependent (BOLD) signal during the incongruent condition after antidepressant treatment (p < 0.001, cluster size > 24 voxels). No significant BOLD signal increases were observed. Amy/Hipp = amygdala–hippocampus complex; IPL = inferior parietal lobe; MTL = middle temporal lobe; pM = premotor cortex; SMA = supplementary motor cortex; SPL = superior parietal lobe; VLPFC = ventrolateral prefrontal cortex.
Table 3.
Regions that showed significant* decreases in blood oxygen level–dependent signal after 6 weeks of antidepressant treatment
| Group; before > after treatment for the incongruent condition | Cluster size | Talairach coordinate |
t value | pFDR-corrected | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| All patients | ||||||
| Left middle temporal gyrus (BA 21/22) | 1152† | −48 | −24 | −4 | 5.64 | 0.02 |
| Right middle temporal gyrus (BA 22) | 52 | 65 | −33 | 0 | 3.95 | 0.04 |
| Right insula (BA 13) | 36 | 44 | −27 | 0 | 3.84 | 0.04 |
| Right inferior frontal gyrus (BA 47) | 116 | 24 | 19 | −16 | 4.55 | 0.02 |
| Right superior parietal lobule (BA 7) | 92 | 38 | −55 | 58 | 3.62 | 0.06 |
| Left superior parietal lobule (BA 7) | 51 | −12 | −54 | 47 | 4.15 | 0.03 |
| Left superior parietal lobule (BA 7) | 28 | −16 | −61 | 64 | 3.92 | 0.04 |
| Right inferior parietal lobule (BA 40) | 78 | 48 | −39 | 33 | 4.75 | 0.02 |
| Right inferior parietal lobule (BA 40) | 26 | 51 | −42 | 54 | 3.92 | 0.04 |
| Left cerebellum | 45 | −4 | −47 | −9 | 4.27 | 0.03 |
| Citalopram | ||||||
| Right amygdala/Hippocampus | 583† | 18 | −12 | −9 | 4.85 | 0.07 |
| Left amygdala/Hippocampus | −18 | −8 | −8 | 4.6 | 0.07 | |
| Right inferior frontal gyrus (BA 47) | 154 | 24 | 19 | −16 | 4.64 | 0.07 |
| Right middle frontal gyrus (BA 6) | 85 | 46 | 8 | 44 | 3.71 | 0.07 |
| Left middle frontal gyrus (BA 6) | 141† | −42 | 2 | 48 | 4.41 | 0.07 |
| Right superior frontal gyrus (BA 6) | 51 | 10 | 15 | 64 | 4.47 | 0.07 |
| Right medial frontal gyrus (BA 6) | 34 | 14 | 6 | 49 | 3.88 | 0.07 |
| Left middle temporal gyrus (BA 22) | 134 | −59 | −37 | −3 | 4.09 | 0.07 |
| Right superior temporal gyrus (BA 21) | 37 | 48 | −20 | −7 | 4.00 | 0.07 |
| Left superior temporal gyrus (BA 21) | 110 | −46 | −23 | −2 | 4.07 | 0.07 |
| Right superior parietal lobule (BA 7) | 85 | 22 | −67 | 57 | 4.08 | 0.07 |
| Right superior parietal lobule (BA 7) | 73 | 14 | 6 | 49 | 3.77 | 0.07 |
| Right superior parietal lobule (BA 7) | 25 | 26 | −52 | 50 | 3.65 | 0.07 |
| Left superior parietal lobule (BA 7) | 261† | −16 | −61 | 64 | 4.29 | 0.07 |
| Right inferior parietal lobule (BA 40) | 72 | 51 | −40 | 54 | 4.33 | 0.07 |
| Left inferior parietal lobule (BA 40) | 188† | −38 | −48 | 54 | 4.09 | 0.07 |
| Left cerebellum | 39 | −4 | −47 | −9 | 3.76 | 0.07 |
| Reboxetine | ||||||
| Left middle temporal gyrus (BA 21) | 151† | −38 | −52 | 6 | 4.66 | 0.19 |
| Left superior temporal gyrus (BA 22) | 136 | −51 | −25 | −2 | 4.39 | 0.19 |
| Left parahippocampal gyrus (BA 30) | 75 | −22 | −37 | 0 | 3.94 | 0.19 |
BA = Brodmann area; FDR = false discovery rate.
Significant at p < 0.001 before FDR correction (cluster size > 24 voxels).
Significant after correction for multiple comparisons (p < 0.05).
When we tested the effects of antidepressant treatment for both medication groups separately, the SSRI group had a significant reduction in BOLD signal after treatment bilaterally in the amygdala–hippocampus complex (largest cluster), right ventrolateral prefrontal cortex, supplementary motor cortex extending to the midcingulate cortex (BA 24), bilaterally in the premotor cortex, and in the superior parietal lobe and inferior parietal lobule (Fig. 2).
The NRI group showed decreased BOLD signal after reboxetine treatment in the left superior (BA 22) and middle temporal lobe (BA 21) as well as in the left parahippocampal gyrus (BA 30).
Medication group by time interaction
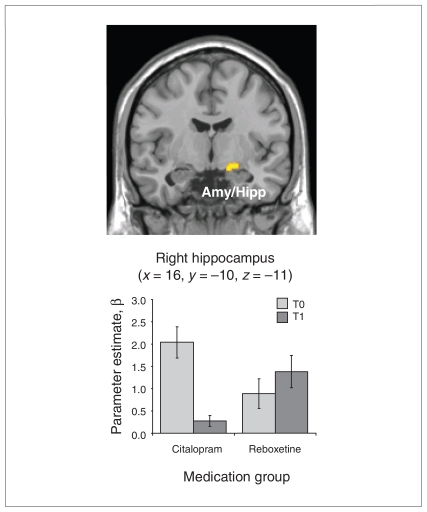
The group by time interaction contrast revealed significant voxels in the right amygdala–hippocampus area (x = 16, y = –10, z = –11, cluster size = 25, t = 3.72, p < 0.001), indicating a BOLD signal decrease in the SSRI group after treatment and a slight increase in the NRI group (Fig. 3). This finding was still significant at the FWE-corrected threshold of p < 0.05, when a small volume correction based on the bilateral amygdala–hippocampus mask image was applied. The opposite contrast was not significant.
Fig. 3.
Medication group by time interaction (p <0.001, cluster size > 24 voxels; p < 0.05 family-wise error–corrected based on small volume correction). Parameter estimates (β) and standard error of the mean were extracted from the right amygdala–hippocampus complex. Patients who received citalopram showed a noticeable decrease in blood oxygen level–dependent signal after 6 weeks of antidepressant treatment, whereas patients who received reboxetine (noradrenalin reuptake inhibitor) showed a slight increase in activity. Amy/Hipp = amygdala–hippocampus complex; T0 = before antidepressant treatment; T1 = after antidepressant treatment.
Prediction of treatment response
There was a nonsignificant relation between pretreatment BOLD signal in the rACC and relative symptom improvement in the whole-brain analysis and when a small volume correction was applied. When we tested this association separately for the SSRI and NRI groups, we found no significant correlations, despite a close to significant p value for the correlation coefficient between rACC activity and BDI (p = 0.07). We observed that in the SSRI group, the direction of this correlation was negative for BDI (r = –0.42, p = 0.19), whereas in the NRI group this association was positive for BDI (r = 0.57, p = 0.18).
After we divided the depressed patients into 2 groups on the basis of the remission criteria (10 in remission v. 10 not in remission), we did not find a significant difference in the initial or the posttreatment BOLD signal in the rACC. There was no significant reduction of the observed initial hyperactivity in the rACC after treatment, although a slight decrease was observed (Fig. 1).
Effect of remission status
There were no significant differences in the frontoparietal or amygdala–hippocampal regions before or after antidepressant treatment. Patients in remission were not significantly different from those not in remission with regard to brain activation during the incongruent condition before treatment. Patients not in remission showed a significantly higher BOLD signal relative to those in remission in the left and right occipital lobe. After treatment, patients not in remission showed a higher BOLD signal in the posterior cingulate, whereas those in remission showed a significantly higher BOLD signal bilaterally in the occipital lobe and insula, as well as in the left cerebellum and left thalamus (Table 4).
Table 4.
Regions that showed significant* differences in blood oxygen level–dependent signal between patients in remission (HRSD score below 7) and those not in remission for the incongruent condition
| Patient group comparison; region of activation | Cluster size | Talairach coordinate |
t value | pFDR–corrected | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| No remission > remission, before AD treatment | ||||||
| Left occipital lobe (BA 18) | 229† | −10 | −82 | 23 | 5.26 | 0.28 |
| Right occipital lobe (BA 18) | 188† | 26 | −71 | 28 | 5.87 | 0.28 |
| No remission > remission, after AD treatment | ||||||
| Right posterior cingulate (BA 31) | 28 | −14 | −35 | 35 | 5.1 | 0.99 |
| Remission > no remission, after AD treatment | ||||||
| Left/right occipital lobe (BA 17/18) | 1755† | 6 | −89 | 10 | 7.17 | 0.02 |
| Left insula (BA 13) | 286† | −40 | −2 | 4 | 6.17 | 0.02 |
| Right insula (BA 13) | 60 | 44 | −7 | 13 | 4.88 | 0.03 |
| Left cerebellum | 435† | −10 | −55 | −7 | 5.15 | 0.03 |
| Left thalamus | 43 | −24 | −25 | 0 | 4.8 | 0.04 |
AD = antidepressant; BA = Brodmann area; FDR = false discovery rate; HRDS = Hamilton Rating Scale for Depression.18
Significant at p < 0.001 before FDR correction (cluster size > 24 voxels).
Significant after correction for multiple comparisons (p < 0.05).
Correlation with symptom reduction
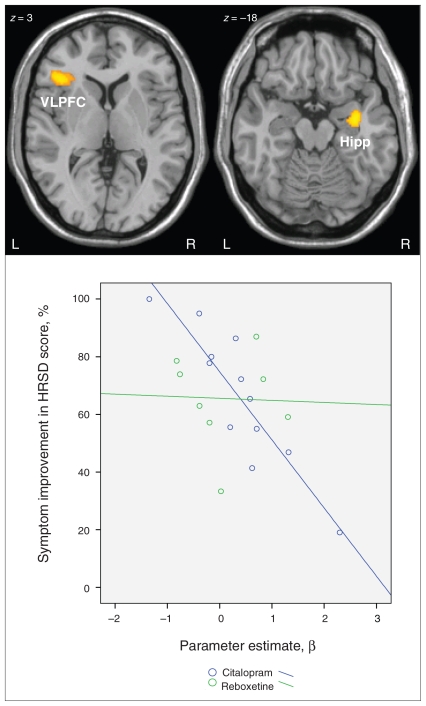
There was a significant negative correlation in the SSRI group between symptom improvement and changes in BOLD signal between baseline and after antidepressant treatment in the right hippocampus, left ventrolateral prefrontal cortex and supplementary motor cortex (Fig. 4 and Table 5). We did not find any significant positive correlations in the SSRI group. In the NRI group, there was a nonsignificant negative and positive relation between BOLD signal decrease and relative symptom improvement.
Fig. 4.
There was a significant negative correlation (p < 0.001, cluster size > 24 voxels) in the selective serotonin reuptake inhibitor group between symptom improvement as assessed by the Hamilton Ration Scale for Depression (HRSD)18 score and changes in blood oxygen level–dependent signal in the incongruent condition before and after antidepressant treatment. The scatter plot shows the correlation of the parameter estimates (β) extracted from the right hippocampal cluster and symptom improvement in HRSD for both medication groups. Hipp = hippocampus; VLPFC = ventrolateral prefrontal cortex.
Table 5.
Regions that showed a significant* negative correlation between symptom improvement and changes in blood oxygen level–dependent signal before and after antidepressant treatment among patients in the selective serotonin reuptake inhibitor group
| Talairach coordinate |
||||||
|---|---|---|---|---|---|---|
| Region of activation | Cluster size | x | y | z | t value | pFDR–corrected |
| Right hippocampus | 91† | 34 | −6 | −20 | 6.90 | 0.35 |
| Left inferior frontal gyrus (BA 45) | 141† | −36 | 26 | 3 | 6.44 | 0.35 |
| Left supplementary motor area (BA 6) | 58 | −13 | −23 | 62 | 5.30 | 0.35 |
BA = Brodmann area; FDR = false discovery rate.
Significant at p < 0.001 before FDR correction (cluster size > 24 voxels).
Significant after correction for multiple comparisons (p < 0.05).
Discussion
Before antidepressant treatment, the patient group exhibited distinct hyperactivation in a predominantly frontocingulate network including the rostral ACC, despite normal behavioural performance on the Stroop Color–Word Test, which corroborates our earlier results.14 After 6 weeks of antidepressant treatment, no significant hyperactivation in patients with MDD relative to healthy controls could be detected, which identifies these activation abnormalities as a potential marker of psychopathology. A systematic investigation of the differential treatment effects revealed that the activation normalization in the patient group as a whole was mainly attributable to activation decreases in the citalopram group, although both medication groups benefited with regard to depressive symptom improvement and were comparable with regard to brain activation before treatment. The predominant reduction in the activity of the amygdala–hippocampus complex may constitute a specific neurophysiologic effect of the SSRI medication, which was statistically distinguishable from the effect of reboxetine.
The treatment response (independent of pharmacologic effects) was not sufficient to explain the observed difference in brain activation because there were no significant differences in the frontoparietal or amygdala–hippocampus regions between patients in remission and those not in remission in terms of pre- and posttreatment changes.
Hence, although our findings support the efficacy of citalopram and reboxetine to normalize activation abnormalities in patients with MDD to a certain degree, they suggest that only citalopram treatment leads to effective activation decreases in a psychopathologically relevant corticolimbic network. This result is remarkable because we used a cognitive task but not an affective paradigm, such as processing of emotional faces11 or affective pictures.25 Furthermore, a nonsignificant difference in pretreatment activity of this brain area was detected between patients and controls, implying that citalopram effects specifically limbic activity despite a normal level of activation.
Previous studies that investigated the effect of SSRIs on brain activity in patients with MDD reported, in agreement with our data, a reduction in resting state metabolism4,5 or a reduced capacity for activation in terms of BOLD signal in the hippocampus and amygdala during affective processing.11,26 The independence of the type of task used strengthens the notion of the specific impact of SSRIs on hippocampus–amygdala complex activity. Furthermore, studies in which citalopram27,28 or escitalopram29 were placebo-controlled and repeatedly administered to healthy controls concordantly reported a reduction in BOLD signal in the amygdala–hippocampus complex during execution of affective tasks after treatment.
It seems that the primary mechanism of chronic SSRI action is to reduce limbic activity and, thus, to potentially influence the hyperactive frontoparietal network,30 whereas reboxetine has only a limited impact on limbic activity. A normalization of the frontoparietal brain network was consistently reported by previous studies using SSRI medication independent of the paradigms used.5,10,11
In the reboxetine group, only a reduction in the middle temporal gyrus could be observed, which potentially reflects a phasic effect of increased availability of noradrenaline on the ventral pathways of visual processing in the Stroop task, as reported in previous studies.15
The noradrenergic and serotonergic pathways show close anatomic overlap and are highly intertwined in the cortical and subcortical regions. However, recent studies have reported differences in the local distribution of norepinephrine transporters. The highest levels of binding to the norepinephrine transporters have been reported in the locus coeruleus complex, raphe nuclei and thalamus, and low levels were reported in the basolateral amygdala, hippocampal and striatal regions.31,32 In contrast, using the 5-HT transporter radioligand 3H-citalopram, it has been shown that there is a markedly higher concentration of the serotonin transporter in the limbic lobe regions such as the anterior cingulate, subgenual cortex, hippocampus, entorhinal and insular cortices, and the temporal pole.33 Additionally, the 5-HT1A receptor was found to be abundantly present in the hippocampus, and its binding was negatively related to performance in an explicit memory task.34 This anatomic divergence in the local distribution of serotonergic and noradrenergic transporters and receptors may provide a basis for the observed differential effects citalopram and reboxetine on brain activation.
It should be noted that both medication groups benefited comparably from 6 weeks of antidepressant treatment with regard to depressive symptom improvement despite clear neurofunctional differences.
In a recent review of 112 randomized controlled trials, Cipriani and colleagues35 compared the efficacy and acceptability of 12 new-generation antidepressants. They reported that reboxetine was significantly less efficacious than the other antidepressants, including citalopram. We therefore speculate that the impact of citalopram on brain activation might be a criterion for long-term clinical effects (i.e., it may differentiate both medication groups with regard to further symptom improvement and the probability of relapse). Otherwise, we have to consider that we used a cognitive task during fMRI, which only indirectly characterizes depressive symptoms.
The negative correlation between treatment-related symptom improvement and activation changes in the right hippocampus, left ventrolateral prefrontal cortex and supplementary motor cortex, which we only found in the SSRI patient group, is surprising because we expected to detect symptom improvement related to BOLD signal reduction. This negative correlation suggests that patients with better SSRI treatment outcomes demonstrated a relative increase in BOLD signal in the frontolimbic network after 6 weeks of AD therapy, whereas patients with poor treatment outcomes had a relative reduction in BOLD signal.
One interpretation might be that the hyperactivity at baseline, which normalizes with SSRI treatment, characterizes the patients who are strongly affected by the illness (i.e., patients with high ratings on the HRSD). This indirectly confirms the notion that the hyperactivation exhibited by the patients before treatment are psychopathologically meaningful and constitute some kind of treatment-sensitive state marker of the illness. Even at a weak significance threshold, initial hyperactivity in the right hippocampus (r = –0.67, p = 0.02) and the left ventrolateral prefrontal cortex (r = –0.68, p = 0.01) were related to poorer treatment response, supporting this notion. These patients seemed to possess a small potential for symptom improvement or they may need a longer time to reach remission. However, these patients were not characterized by significant differences in cognitive performance in comparison to treatment responders, such as in the Trail Making Test, Stroop test or logical IQ.
In contrast, less affected patients with comparatively smaller relative hyperactivation before (and after) treatment seemed to possess a strong potential for treatment-related symptom improvement. It is important to note, however, that this normalization of activation in association with comparatively small symptom improvement was only detectable in the SSRI group, which provides further evidence for the clear effect of citalopram on frontolimbic activity. In contrast to our results, Drevets and colleagues4 found a positive correlation between subcortical metabolic normalization and symptom improvement. An overall greater treatment related improvement (HRSD score of 24.0 before treatment v. 4.7 after treatment) in this study might be one explanation for the different finding.
Predictive role of rACC in treatment outcome
When testing our hypothesis of rACC hyperactivity being a predictor of the outcome of antidepressant treatment, we failed to detect any significant relations. Moreover, after 6 weeks of antidepressant treatment, we detected only a slight and nonsignificant decrease in BOLD signal, which means that the patients still exhibited an inability to deactivate this brain area activity during the incongruent Stroop condition.
These results are in contrast with the results of some previous studies, which reported a predictive role of rACC hyperactivity in antidepressant treatment outcome.12,13 However, other studies have also detected a negative relation7 or a non-significant relation5 during paroxetine treatment. Differences in the methods among the previous studies and our study may explain the divergent findings.
The often-used resting state condition may potentially contribute to variance heterogeneity because of a little-standardized scanning condition. Further sources of variance relate to the definition of response (e.g., the use of different questionnaires), the length and kind of antidepressant treatment (e.g., variation from 6 weeks to 4–6 months of treatment) and antidepressants, which varied from tricycle medication to SSRIs and bupropion.5,12,13,25,36 Furthermore, we predominantly investigated female patients in contrast to predominantly or solely male patients as in other studies.5,12 Because male and female depressed patients clearly differ with regard to their illness characteristics, illness severity and concurrent symptoms,37 there is reason to assume that there may also be differences at the cerebral level.
We suppose that the rACC hyperactivity observed in the present study represents a task-specific activation, which reflects the effect of depressive psychopathology on cognitive control processes. Walsh and colleagues10 reported a negative correlation of a more dorsal part of the ACC with treatment response in an n-back task only at a reduced statistical threshold, which may represent a spurious finding and is thus in agreement with our findings.
Furthermore, we previously observed that higher rACC activity was related to larger grey matter reductions in the medial orbitofrontal cortex.38 Thus, our nonsignificant finding fits well with this association, which we would not expect to change with 6 weeks of antidepressant treatment. In this vein, we still detected an inability to deactivate the rACC in depressed patients after treatment, even if only half of our patient attained the remission criteria.
Limitations
We performed this study as a naturalistic open-label, nonrandomized trial, which may introduce potential selection bias. However, the medication groups did not differ in initial depression severity as assessed by HRSD and BDI. In addition, the observed differences in BOLD signal at baseline included the fusiform gyrus and superior temporal lobe but did not include areas concerned in our primary hypothesis, including the amygdala–hippocampus and the frontocingulate regions. Moreover, both medication groups were not significantly different with regard to behavioural performance on the Stroop task before or after antidepressant treatment. Furthermore, we could clearly demonstrate a distinct effect of SSRI therapy on amygdala–hippocampal activity despite comparable clinical symptom improvement in both medication groups. Our main goal was to investigate the differential effects of citalopram and reboxetine on brain activation patterns. Thus, because patients given reboxetine and citalopram had comparable depression severity, cognitive performance and brain activation patterns at baseline, a potential selection bias was regarded as negligible.
Moreover, patients given citalopram and reboxetine did not differ in performance on the Stroop task before or after antidepressant treatment. Thus, the significant group by time interaction in the amygdala–hippocampus complex cannot be explained by potential differences in task performance or practice effects, which are regarded as negligible.
The wash-out period was at least 2 half-lives of the previous antidepressant medication. Therefore, an ongoing effect of antidepressants even after discontinuation could not be entirely ruled out, particularly in light of the long-term downstream adaptive processes on pre- and postsynaptic receptors and of changes in the intracellular signaling pathways.39 To exclude any potential long-term effects of antidepressant treatment a much longer washout period would be required, but this was not ethically justifiable for the group of patients with clinically manifest depression.
Acknowledgments
This work was supported by the German Federal Ministry of Education and Research (grants AQ4 FKZ01ZZ0405 and 01GW0740), the Interdisciplinary Center for Clinical Research of the University of Jena and Thuringian Ministry of Science, Research, and Art (grant B307-04004).
Footnotes
Competing interests: None declared.
Contributors: Drs. Wagner, Sobanski, Sauer and Schlösser designed the study. Drs. Wagner and Reichenbach and Ms. Schachtzabel acquired the data, which Drs. Wagner, Koch and Schlösser analyzed. Drs. Wagner and Koch wrote the article, which Drs. Koch, Sobanski, Reichenbach, Sauer and Schlösser and Ms. Schachtzabel reviewed. All authors approved the final version submitted for publication.
Previously published at www.jpn.ca
References
- 1.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci. 2006;7:137–51. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- 3.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–17. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 4.Drevets WC, Bogers W, Raichle ME. Functional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolism. Eur Neuropsychopharmacol. 2002;12:527–44. doi: 10.1016/s0924-977x(02)00102-5. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy SH, Evans KR, Kruger S, et al. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am J Psychiatry. 2001;158:899–905. doi: 10.1176/appi.ajp.158.6.899. [DOI] [PubMed] [Google Scholar]
- 6.Mayberg HS, Brannan SK, Tekell JL, et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry. 2000;48:830–43. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- 7.Brody AL, Saxena S, Silverman DH, et al. Brain metabolic changes in major depressive disorder from pre- to post-treatment with paroxetine. Psychiatry Res. 1999;91:127–39. doi: 10.1016/s0925-4927(99)00034-7. [DOI] [PubMed] [Google Scholar]
- 8.Holthoff VA, Beuthien-Baumann B, Zundorf G, et al. Changes in brain metabolism associated with remission in unipolar major depression. Acta Psychiatr Scand. 2004;110:184–94. doi: 10.1111/j.1600-0447.2004.00351.x. [DOI] [PubMed] [Google Scholar]
- 9.Buchsbaum MS, Wu J, Siegel BV, et al. Effect of sertraline on regional metabolic rate in patients with affective disorder. Biol Psychiatry. 1997;41:15–22. doi: 10.1016/s0006-3223(96)00097-2. [DOI] [PubMed] [Google Scholar]
- 10.Walsh ND, Williams SC, Brammer MJ, et al. A longitudinal functional magnetic resonance imaging study of verbal working memory in depression after antidepressant therapy. Biol Psychiatry. 2007;62:1236–43. doi: 10.1016/j.biopsych.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 11.Fu CH, Williams SC, Cleare AJ, et al. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry. 2004;61:877–89. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- 12.Mayberg HS, Brannan SK, Mahurin RK, et al. Cingulate function in depression: a potential predictor of treatment response. Neuroreport. 1997;8:1057–61. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- 13.Pizzagalli D, Pascual-Marqui RD, Nitschke JB, et al. Anterior cingulate activity as a predictor of degree of treatment response in major depression: evidence from brain electrical tomography analysis. Am J Psychiatry. 2001;158:405–15. doi: 10.1176/appi.ajp.158.3.405. [DOI] [PubMed] [Google Scholar]
- 14.Wagner G, Sinsel E, Sobanski T, et al. Cortical inefficiency in patients with unipolar depression: an event-related fMRI study with the Stroop task. Biol Psychiatry. 2006;59:958–65. doi: 10.1016/j.biopsych.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 15.Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 2009;10:211–23. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- 16.Eriksson E. Antidepressant drugs: Does it matter if they inhibit the reuptake of noradrenaline or serotonin? Acta Psychiatr Scand Suppl. 2000;402:12–7. doi: 10.1034/j.1600-0447.2000.00003.x. [DOI] [PubMed] [Google Scholar]
- 17.Wittchen HU, Zaudig M, Fydrich T. SKID — Strukturiertes Klinisches Interview für DSM IV. Achse I und II. Göttingen (Germany): Hogrefe; 1997. [Google Scholar]
- 18.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horn W. Leistungsprüfsystem (LPS) Göttingen (Germany): Hogrefe; 1983. [Google Scholar]
- 20.Reitan RM. The relation of the trail making test to organic brain damage. J Consult Psychol. 1955;19:393–4. doi: 10.1037/h0044509. [DOI] [PubMed] [Google Scholar]
- 21.Franke G. Die Symptomcheckliste von Derogatis — Deutsche Version. Göttingen (Germany): Beltz Test; 1995. [Google Scholar]
- 22.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 23.Briggs GG, Nebes RD. Patterns of hand preference in a student population. Cortex. 1975;11:230–8. doi: 10.1016/s0010-9452(75)80005-0. [DOI] [PubMed] [Google Scholar]
- 24.Hayasaka S, Phan KL, Liberzon I, et al. Nonstationary cluster-size inference with random field and permutation methods. Neuroimage. 2004;22:676–87. doi: 10.1016/j.neuroimage.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 25.Davidson RJ, Irwin W, Anderle MJ, et al. The neural substrates of affective processing in depressed patients treated with venlafaxine. Am J Psychiatry. 2003;160:64–75. doi: 10.1176/appi.ajp.160.1.64. [DOI] [PubMed] [Google Scholar]
- 26.Sheline YI, Barch DM, Donnelly JM, et al. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50:651–8. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- 27.Harmer CJ. Serotonin and emotional processing: Does it help explain antidepressant drug action? Neuropharmacology. 2008;55:1023–8. doi: 10.1016/j.neuropharm.2008.06.036. [DOI] [PubMed] [Google Scholar]
- 28.Harmer CJ, Mackay CE, Reid CB, et al. Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol Psychiatry. 2006;59:816–20. doi: 10.1016/j.biopsych.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 29.Arce E, Simmons AN, Lovero KL, et al. Escitalopram effects on insula and amygdala BOLD activation during emotional processing. Psychopharmacology (Berl) 2008;196:661–72. doi: 10.1007/s00213-007-1004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeRubeis RJ, Siegle GJ, Hollon SD. Cognitive therapy versus medication for depression: treatment outcomes and neural mechanisms. Nat Rev Neurosci. 2008;9:788–96. doi: 10.1038/nrn2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Logan J, Wang GJ, Telang F, et al. Imaging the norepinephrine transporter in humans with (S,S)-[11C]O-methyl reboxetine and PET: problems and progress. Nucl Med Biol. 2007;34:667–79. doi: 10.1016/j.nucmedbio.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 32.Smith HR, Beveridge TJ, Porrino LJ. Distribution of norepinephrine transporters in the non-human primate brain. Neuroscience. 2006;138:703–14. doi: 10.1016/j.neuroscience.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 33.Varnas K, Halldin C, Hall H. Autoradiographic distribution of serotonin transporters and receptor subtypes in human brain. Hum Brain Mapp. 2004;22:246–60. doi: 10.1002/hbm.20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yasuno F, Suhara T, Nakayama T, et al. Inhibitory effect of hippocampal 5-HT1A receptors on human explicit memory. Am J Psychiatry. 2003;160:334–40. doi: 10.1176/appi.ajp.160.2.334. [DOI] [PubMed] [Google Scholar]
- 35.Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373:746–58. doi: 10.1016/S0140-6736(09)60046-5. [DOI] [PubMed] [Google Scholar]
- 36.Little JT, Ketter TA, Kimbrell TA, et al. Bupropion and venlafaxine responders differ in pretreatment regional cerebral metabolism in unipolar depression. Biol Psychiatry. 2005;57:220–8. doi: 10.1016/j.biopsych.2004.10.033. [DOI] [PubMed] [Google Scholar]
- 37.Marcus SM, Young EA, Kerber KB, et al. Gender differences in depression: findings from the STAR*D study. J Affect Disord. 2005;87:141–50. doi: 10.1016/j.jad.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Wagner G, Koch K, Schachtzabel C, et al. Enhanced rostral anterior cingulate cortex activation during cognitive control is related to orbitofrontal volume reduction in unipolar depression. J Psychiatry Neurosci. 2008;33:199–208. [PMC free article] [PubMed] [Google Scholar]
- 39.Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med. 2001;7:541–7. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]