Abstract
Background
Identification of the biological markers of anorexia nervosa (AN) is crucial for the development of new treatments. We aimed to determine whether AN is associated with disturbances in the nonconscious neural processing of innate signals of emotion and whether these disturbances persist after weight gain.
Methods
In a retest design, 28 adolescent females with AN were tested at first admission to hospital and again after they had gained weight. Matched healthy control participants were tested at the same times. We assessed emotion-elicited event-related potentials (ERPs) during overt and covert presentation of emotion expressions, scores on an emotion-identification behavioural task, and symptom measures. We performed between and within group analyses.
Results
Individuals with AN had a marked alteration in ERPs relative to healthy controls. Irrespective of the form of stimulus, early and late ERP components were significantly reduced in AN patients at baseline (when underweight) and on retest (after weight gain), especially in the temporo-occipital regions, suggesting a persistent disruption of the early automatic appraisal of salient emotional signals.
Limitations
This study could have been improved with a longer standardized retest interval.
Conclusion
There is likely a core, generic disturbance in AN in the early “automatic” neural processing of emotion irrespective of weight or nutritional status. New innovative emotion-based psychologic or pharmacologic treatments targeting these nonconscious processes may prove beneficial.
Introduction
Anorexia nervosa (AN) is an eating disorder characterized by low body weight, an intense fear of weight gain, body image distortion and a disruption of menstrual function.1 There is increasing recognition of the importance of understanding the neurobiology of AN. As summarized by Kaye,2 the stereotypical presentation of this illness, the evidence of heritability, the typical age of onset and the discrepancy between the high prevalence of societal pressure for thinness and the low prevalence of eating disorders all point to a biological basis of AN. A biological basis for AN is underscored by its poor prognosis3 and treatment-resistant nature.4 Identification of candidate biological markers, which reflect endophenotypes for AN, is a starting point for the development of effective treatments.5 For example, the well-replicated finding of a deficit in set shifting6,7 led to the development of targeted cognitive remediation treatments.8 Yet existing treatments have limited effectiveness for many individuals with AN.9 Identification of other candidate biological markers will provide a first step in developing complementary new treatments for AN.
In our integrative neuroscience model of AN, we propose that disturbances in emotion processing are candidate biological markers of AN.10 This model uses a definition of emotion that highlights early automatic reactions to significant emotion cues11 consistent with an evolutionary context and the concept of “primary emotion.”12,13 In this definition, alterations in emotion processing in AN may occur automatically and do not require conscious awareness, thereby producing a deficit that is difficult to access for treatment. In other words, “emotion” is distinct from “feeling”; the latter relies on the subjective experience of emotional reactions, which comes with brain–body feedback and awareness of this feedback.11
This definition of emotion is in agreement with the involvement of 2 parallel pathways in the brain for processing emotional information. Seminal fear conditioning work by Le Doux14 referred to these pathways as the “low road” and the “high road.” Subsequent lesion and functional neuroimaging studies have provided convergent evidence for low- and high-road pathways that process visual cues of emotion.15–17 The low road bypasses the visual cortex and relays low-level sensory cues directly and rapidly to the amygdala, allowing emotional reactions to occur automatically and without conscious awareness of these cues. The high road provides a slower, indirect pathway via the visual cortex that allows for conscious elaboration of these cues and feedback about them.
To our knowledge, no study to date has examined AN in terms of emotion processing using brain function recordings that have “real time” resolution to capture low- and high-road activity. Certainly, a broader construct of emotional function has been examined in AN using functional brain imaging techniques with symptom provocation stimuli such as food and body images,18,19 self-report measures of alexithymia20 and behavioural studies of emotion identification and attentional bias.21 However, functional magnetic resonance imaging captures changes in blood oxygenation that occur every couple of seconds, requiring inferences about the source of earlier activity. Moreover, food stimuli may be conditioned stimuli for AN and may engage neural systems other than the innate low- and high-road pathways. Similarly, self-report has a resolution of seconds, and behavioural studies typically have a resolution of greater than 500 milliseconds. Further, because self-report relies on the participant’s conscious awareness, these studies are likely capturing feelings as well as earlier emotional processing.
Event-related potentials (ERPs) provide a high-resolution measure of brain activity that captures direct neural activity on a real-time millisecond scale immediately following stimulus presentation.22 Facial expressions of emotion are pertinent, biologically important emotion cues that are universally perceived.23 Facial expressions of the primary emotions (anger, disgust, fear, surprise, happiness and sadness) are innate low-level cues that signal danger or reward (e.g., potential sources of threat in a given environment can be signaled by the fear facial expression of another person). The 6 basic facial expressions have evolved from behavioural responses associated with action tendencies to minimize danger or maximize reward.12 Empirical evidence suggests that certain features of facial expressions can trigger emotion (action tendencies) in the viewer as shown by early patterns of brain and body activation within 150 ms.16,24
Event-related potentials have previously been recorded in response to facial emotion cues using specially designed backward masking protocols to capture activity in parallel low- and high-road pathways.16,17 Low-road activity is isolated by use of a masking stimulus that interrupts sustained activity associated with the slower high road, while the absence of a mask allows this sustained activity to continue. These conditions have been referred to as “covert” versus “overt” and found to preferentially modulate neural activity within 200 ms of stimulus onset (low road) versus 200 ms and beyond (addition of high-road activity).17,25 The use of a masking protocol may also limit other influences of conscious elaboration, such as cognitive appraisal, that occur with repeated stimulus trials.
In this study, we aimed to test whether there are early deficits in nonconscious neural processing on a millisecond time scale in AN in response to innately important emotion cues (as opposed to cues that are illness-specific such as food or body images). We hypothesized that individuals with AN would exhibit differential emotion processing as shown by ERPs, and we proposed that such a deficit would be a candidate marker of AN. Research into the biology of this psychiatric illness is complex because of the physical complications associated (i.e., malnourishment and low weight). We also aimed to test whether these deficits exist irrespective of starvation and low weight. We assessed the ERPs of AN and matched healthy controls elicited during covert and overt presentation of innately important stimuli. We then retested the AN individuals after refeeding and weight gain and examined changes within the clinical sample as a function of weight gain.
Methods
Participants
A total of 42 first-admission inpatient, female adolescents aged 12–18 years with AN were recruited from 2 specialist eating disorder programs for adolescents, based in large university teaching hospitals in Sydney, Australia. The diagnosis of AN was based on psychiatrist and pediatrician consensus (S.M., S.C., M.K.) according to DSM-IV criteria.1 The symptom severity of the eating disorder was measured by use of the Eating Disorders Inventory-326 at baseline and retest. We recruited 34 healthy control participants matched for age, sex and years of education.
Screening for inclusion and exclusion criteria has been described in detail.25 For both groups, we included participants with normal (or corrected to normal) hearing and vision, and those with a premorbid intelligence quotient (IQ) estimate within the normal range. We excluded participants with physical brain injury (causing loss of consciousness for > 10 min), neurologic disorder, serious medical or genetic condition (other than AN for the clinical group) and substance use. For the control group, we excluded participants with a DSM-IV Axis 1 disorder, determined by use of assessments from the Somatic and Psychological Health Report (SPHERE),27 the Patient Health Questionnaire (PHQ-9), which included items on eating difficulties,28 the 21-item version of the Depression Anxiety and Stress Scale (DASS21) (www2.psy.unsw.edu.au/groups/dass//Down_W6/dass21.doc; this version has been validated against the Beck Depression and Anxiety Inventories and includes Australian norms29) and items for family history of psychiatric disorder.
After a complete description of the study to participants and their families, written informed consent was obtained from both adolescents and their parents/guardians. The study was approved by the human research ethics committees at Sydney West Area Health Service and Children’s Hospital at Westmead.
Study design
A retest design was used in a naturalistic, treatment-as-usual setting. For AN participants, testing was performed before (baseline) and after weight restoration (retest). Baseline was considered to be admission to the inpatient program with testing performed between days 3 and 10 of the hospital stay, once the acute medical complications of starvation, including hypothermia, bradycardia, hypotension and dehydration had been addressed. We performed the retest after a refeeding intervention when weight was restored to, or very close to, a minimum healthy weight as determined by measures of body composition, hormonal levels and weight. The mean retest interval for AN participants was 65 days. Percentiles of the retest time represent the continuous distribution of this retest interval with the 25th percentile being 38 days, the 50th percentile being 50 days and the 75th percentile being 81 days.
Owing to funding limitations, only a small subset of control participants (n = 11) were retested. Because ERPs have been shown to possess adequate retest reliability (r = 0.75)30 and for the purposes of statistical power, we focused our analyses on the test–retest comparison of AN participants relative to baseline controls.
Facial emotion task
Behavioural data
We used a behavioural task for emotion identification to assess the accuracy and time taken for emotion identification.31 In this task, facial expressions of disgust, fear, anger, sadness, happiness and neutral (each depicted by 8 different individuals, totaling 48 stimuli) were presented in a pseudorandom sequence for 2 seconds each. Participants identified (via computer mouse click) the verbal label for each emotional expression from among the 6 expression options. Accuracy and reaction time (RT) for both correct and incorrect responses were recorded.
Event-related potential data acquisition
Using standardized LabNeuro protocols,32,33 we recorded electroencephalography (EEG) data during perception of the facial expressions of emotion stimuli, using the previously established Facial Expressions of Emotions for Brain Activation protocol.24,34 From the EEG recordings, we identified the ERPs elicited by these stimuli, presented under overt and covert conditions.
Participants were required to view a series of neutral facial expressions embedded among emotional facial stimuli (happy, sad, fear, disgust, anger expressions) selected from a set of faces developed by Gur and colleagues.35 The stimuli were equal in terms of size, grey-scale parameters and central alignment of the face within the image (with eyes as the midpoint reference).
In this active viewing task, participants were instructed to pay attention to the faces presented on the screen because they would be asked questions about them at the end of the task, but they were not required to make an overt response to the stimuli during the task. This active viewing method was selected because it has been previously found to elicit enhanced responses in comparison with similar tasks requiring overt responses36 and to minimize any influence of age-related cognitive ability.
The participants were presented with blocks of face stimuli under both overt (to elicit conscious controlled processing) and covert (nonconscious, to engage automatic processing) conditions. There were 32 presentations of each facial expression category (4 blocks of 8 stimuli), and stimulus blocks were presented in a pseudorandom order to avoid priming effects. Two or more blocks of the same emotional valence were not presented consecutively, and stimulus blocks within each expression category were not preceded by blocks of a given emotional valence more than once.
For the overt (conscious) perception condition, the stimulus presentation time was 500 ms, with an average inter-stimulus interval of 1 second (jittered by ± 0.05 s). The covert (nonconscious) perception task used a backward masking procedure, in which faces of each emotional valence, including neutral facial expressions, were presented for 10 ms and immediately followed by a neutral face mask for 150 ms. This method has been previously shown to prevent the detection of the initial 10 ms stimulus beyond chance levels.17 For the assessment of effects of nonconscious emotion perception, the masked emotion faces are compared with masked neutral faces.
Event-related potential data reduction
Event-related potential waveforms comprise a series of negative- and positive-going deflections in electrical brain activity, which define the components of interest. Component names (e.g., N120) indicated their direction (N, negative-going deflection; P, positive-going deflection) and the latency at which they typically peak (e.g., 120 ms). We calculated average ERPs for each emotion stimuli. Individual single-trial ERP epochs were filtered with a low-pass Tukey (cosine taper) filter function that attenuated frequencies above 25 Hz. We then averaged single trials to form conventional ERPs, and we identified peak components within defined latency windows, validated by visual inspection across individual subjects for each recording site.
At the temporal, occipital and midline regions of focal interest, we identified the following ERP components (and latency windows) for the temporal (T5, T6) and occipital (O1, O2) regions: P120 (80–180 ms poststimulus), N170 (120–220 ms) and P300 (300–400 ms). The corresponding medial (Fz) components identified were the N120 (80–150 ms, concomitant to the P120), vertex positive peak (standard terminology for a positive ERP component elicited by face stimuli, equivalent to a P200 elicited by other stimuli: 120–220 ms, concomitant to the N170) and early P300 (230–330 ms, concomitant to the N250). Note that whereas the latency windows for these components may overlap, this is a consequence of having to allow for temporal variations across electrode sites. At each site, there was a distinctive sequence of positive- and negative-going components. These ERP components have been shown to be modulated by facial emotion in both overt and covert conditions in previous studies with large samples.24,34
Each of these specific components was selected for analyses because of their interpretative relevance. The P120 (and N120) was selected because it is an early component modulated by threat expressions,24 the N170 (and VPP) because it represents a marker of experimental task and is modulated especially by face stimuli,37–39 and the P300 because it represents later, controlled, contextual evaluation of facial stimuli.39 We scored the ERP components using a baseline-to-peak method, such that peak amplitude and peak latency were determined. We defined outliers as values beyond 2.5 standard deviations from the relevant group mean, and we excluded outliers from the analyses.
Statistical analyses
For the analysis of behavioural data and clinical questionnaires, we used independent t tests to investigate the presence of between-group differences, and we used paired samples t tests to examine within-group differences for demographic (e.g., body mass index [BMI]) and symptom variables (e.g., mood). We used a 2-tailed α level of 0.05.
To analyze the ERP data, we performed repeated-measures multivariate analyses of variance (MANOVA) to analyze the amplitude and latency for each ERP component, with group (underweight AN v. controls) as the between-groups factor, and emotion (e.g., happy, fear) and laterality (left: T5, O1 v. right: T6, O2) as the within-subjects factors.
For baseline–retest change for AN participants, we used repeated-measures ANOVAs for each component with emotion and time (baseline v. retest) as within-subjects factors.
We performed MANOVAs and ANOVAs to test the amplitude and latency of each ERP component at each brain region (temporal, occipital, frontal) and electrode site (e.g., T5, T6, O1) within the overt and covert conditions. The focal effects of interest were group main effects (i.e., underweight AN v. controls, weight-gain AN v. controls). We applied the Greenhouse–Geisser correction if the covariance matrix did not meet the sphericity assumption. Because of multiple ERP comparisons, we used a conservative α level of 0.01 to determine significant ERP effects.
Results
Participants
Of the initial group of 42 AN patients, 5 had a BMI greater than 17.5 at assessment; we considered these patients ineligible for inclusion. Of the remaining 37, 9 had incomplete data owing to movement or related artifacts in the ERP recording. Thus, we included 28 AN participants in the baseline analysis. Of the original 37 participants, 7 did not complete the retest (18.9% drop-out rate) and 5 had incomplete retest data owing to artifacts. The final sample for retest was 25 AN participants. For paired sample analyses, we included only matched participants, thereby leaving 21 AN participants in the within-subject analyses.
Of the 28 AN participants at baseline, 24 were not taking medication, 2 were taking a selective serotonin reuptake inhibitor (SSRI) and 2 were taking both an SSRI and an atypical antipsychotic. Twenty-seven participants were of the restricting AN subtype and one participant was of the binge–purge subtype. The mean duration of illness (time from the commencement of first weight loss to baseline testing) was 9.7 months.
Participants with AN did not differ significantly from healthy controls on premorbid IQ (p = 0.74). Body mass index values on the day of testing were significantly higher for controls (t60 = −11.598, p < 0.001). For AN individuals, there was a significant increase in BMI (t20 = −11.571, p < 0.001) between testing occasions. There was a significantly higher level of depression, anxiety and stress among AN participants than among controls (depression t60 = 7.817, p < 0.001; anxiety t60 = 6.958, p < 0.001; stress t60 = 7.604, p < 0.001; Table 1).
Table 1.
Demographic and clinical characteristics of the study participants
| Group; mean (SD) |
Control v. underweight AN |
Underweight AN v. weight-gain AN |
|||||
|---|---|---|---|---|---|---|---|
| Characteristic | Healthy controls, n = 34 | Underweight AN, n = 28 | Weight-gain AN, n = 21* | t test | p value | t test | p value |
| Age, yr | 14.98 (1.70) | 15.14 (1.67) | 15.73 (1.56) | t60 = 0.364 | 0.72 | t20 = −3.315 | 0.003 |
| Education, yr | 10.05 (1.72) | 10.32 (1.70) | t47 = 0.555 | 0.58 | — | ||
| Body mass index | 21.81 (2.64) | 16.25 (0.83) | 18.33 (0.79) | t60 = −11.598 | < 0.001‡ | t20 = −11.571 | < 0.001 |
| DASS21† | |||||||
| Depression | 2.06 (3.02) | 19.89 (11.76) | 12.9 (11.58) | t60 = 7.817 | < 0.001‡ | t19 = 1.906 | 0.07 |
| Anxiety | 2.09 (2.99) | 12.5 (7.44) | 6 (7.79) | t60 = 6.958 | < 0.001‡ | t19 = 3.999 | 0.001 |
| Stress | 2.88 (3.72) | 18.00 (9.96) | 12.8 (10.37) | t60 = 7.604 | < 0.001‡ | t19 = 1.777 | 0.09 |
| Eating Disorders Inventory-326 | |||||||
| Ineffectiveness composite | 94.14 (19.41) | 93.66 (18.85) | — | t19 = 0.125 | 0.90 | ||
| Interpersonal problems composite | 97.10 (16.88) | 101.05 (16.39) | — | t20 = −1.177 | 0.25 | ||
| Affective problems composite | 102.05 (19.42) | 93.24 (15.71) | — | t20 = 2.569 | 0.018 | ||
| Overcontrol composite | 95.10 (17.54) | 94.34 (19.89) | — | t20 = 0.320 | 0.08 | ||
| General psychological maladjustment composite | 440.48 (64.39) | 430.00 (64.01) | — | t20 = 0.993 | 0.033 | ||
AN = anorexia nervosa; DASS21 = Depression Anxiety and Stress Scale, 21-item version; SD = standard deviation.
Although there were 25 patients tested at retest, paired samples t tests only include cases in which there was the same patient ID on both occasions.
Higher scores indicate more severe mood or eating disorder symptoms, or composite scores.
Levine’s test for equality of variance indicated significantly different variances between the groups.
We compared the clinical characteristics of the AN participants whose data were excluded (owing to artifact or technical problems) with those whose data were included. No significant differences were observed in age, duration of illness, education or clinical symptoms with the exception of BMI being slightly lower in those who were excluded (p = 0.036).
Baseline: underweight AN versus controls
There were no significant differences between underweight AN patients and controls in emotion identification accuracy or the reaction time for correctly identified emotions or incorrectly identified emotions.
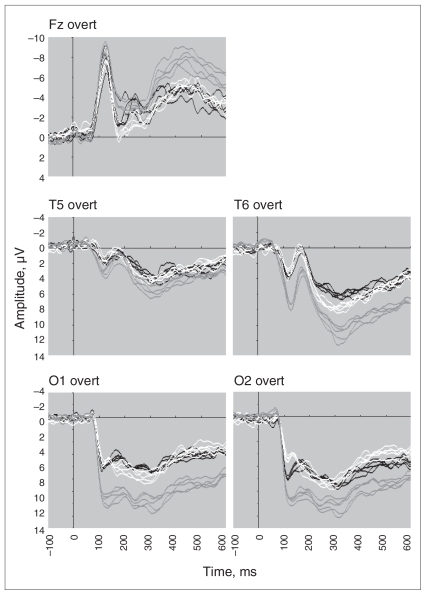
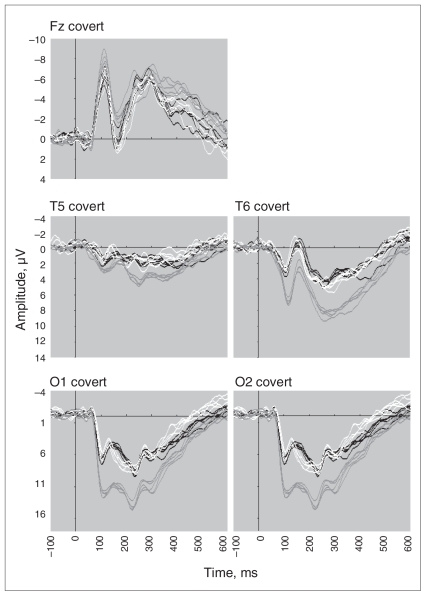
For both overt and covert conditions, visual inspection of emotion ERP components identified between-group effects across the entire waveform, which were confirmed by statistical analyses (Figs. 1 and 2). Overall, these effects reflected a shift in the direction of negativity irrespective of the individual ERP component.
Fig. 1.
Event-related potential waveforms for the frontal (Fz), temporal (left T5 and right T6 sites) and occipital (left O1 and right O2) brain regions elicited by overt facial expressions of happy, fear, sad, anger and disgust in participants with anorexia nervosa when underweight (black), after weight gain (white) and in matched healthy controls (grey).
Fig. 2.
Event-related potential waveforms for the frontal (Fz), temporal (left T5 and right T6 sites) and occipital (left O1 and right O2) brain regions elicited by covert facial expressions of happy, fear, sad, anger and disgust in participants with anorexia nervosa when underweight (black), after weight gain (white) and in matched healthy controls (grey).
The frontal P300 was later during overt processing in the underweight AN group than in the control group (F1,44 = 8.58, p = 0.005). Relative to controls, the amplitudes for the temporal P120 (F1,51 = 15.86, p < 0.001) and P300 (F1,46 = 14.04, p < 0.001) were significantly lower in the underweight AN group. Relative to controls, the amplitude for the occipital P120 (F1,54 = 22.08, p < 0.002), N170 (F1,45 = 10.59, p < 0.001) and P300 (F1,49 = 10.50, p = 0.002) were significantly lower in the underweight AN group.
The frontal P300 was later during covert processing in the AN underweight group relative to controls (F1,45 = 20.00, p < 0.001). Relative to controls, the amplitude for the temporal P120 (F1,39 = 18.91, p < 0.001) and P300 (F1,36 = 14.52, p = 0.001) were significantly lower in the underweight AN group. Relative to controls, the amplitude for the occipital P120 (F1,50 = 29.49, p < 0.001), N170 (F1,52 = 12.31, p < 0.001) and P300 (F1,48 = 10.85, p = 0.002) were significantly lower in the underweight AN group. The occipital P300 was later in the AN underweight group (F1,47 = 13.06, p = 0.001) than in the control group.
Baseline: underweight AN versus retest weight-gain AN
Compared with underweight baseline, after weight gain the AN participants had significantly lower anxiety on the DASS (t19 = 3.999, p = 0.001) and a corresponding decrease in affective problems on the EDI-3 (t20 = 2.569, p = 0.018) (Table 1).
After weight gain, the AN participants exhibited no significant change in the accuracy of identifying emotions, but they had faster reactions times for the correct identification of happy facial expressions (t16 = 2.656, p = 0.036). There was no significant change in reaction time for incorrect responses.
For both overt and covert conditions, ERP components showed a general but slight shift in the direction of reduced negativity after weight gain (Figs. 1 and 2). However, these effects were not significant. The only result close to the correct significance level was observed for P300 for temporal sites for the covert condition (p = 0.013).
Retest: weight-gain AN versus controls
As an additional confirmation of the stability of the ERPs within the AN sample, AN patients who had gained weight were compared with control participants. These analyses confirmed a very similar pattern of results as for the underweight AN patients and controls.
The frontal N120 amplitude was lower during overt processing in the AN weight-gain group than in the control group (F1,52 = 9.98, p = 0.003). Relative to controls, the amplitude for the temporal P120 was significantly lower in the weight-gain AN group (F1,49 = 9.26, p < 0.004). Relative to controls, the amplitude for the occipital P120 (F1,45 = 20.47, p < 0.001), N170 (F1,39 = 9.30, p < 0.004) and P300 (F1,42 = 9.34, p = 0.004) were significantly lower in the weight-gain AN group.
The frontal P300 was later during covert processing in the AN weight-gain group than in the control group (F1,41 = 8.33, p = 0.006). Relative to controls, the amplitude for the temporal P120 (F1,33 = 13.19, p = 0.001) and P300 (F1,36 = 20.49, p < 0.001) were significantly lower in the weight-gain AN group. The temporal N170 was earlier in the AN weight-gain group than in the control group (F1,40 = 7.56, p = 0.009). Relative to controls, the amplitude for the occipital P120 (F1,40 = 28.44, p < 0.001), N170 (F1,44 = 13.88, p = 0.001) and P300 (F1,38 = 19.46, p < 0.001) were significantly lower in the weight-gain AN group.
Discussion
Through the measurement of ERPs and biologically salient signals of emotion, our findings provide new evidence that AN is associated with disturbances in the automatic, nonconscious neural activity in response to innately important stimuli, which exist irrespective of overt or covert stimuli presentation and irrespective of weight status.
Consistent with our predictions, individuals with AN showed alterations in neural processing as early as 100 ms after stimulation; these alterations persisted until after 400 ms. That is, ERP disturbances were present in both the automatic and more elaborative phases of processing. Group differences were found across all brain regions, although the effects were particularly apparent in the temporo-occipital regions. Inspection of the ERP waveforms revealed a shift in the direction of negativity in AN patients to all emotion expressions, suggesting reduced processing of all emotionally laden content regardless of valence. This deficit in global emotion processing may manifest in the clinical features measured through self-report and behavioural tasks. For example, if there is a neural hyporesponsiveness to emotion cues, it is feasible that this may have an effect on difficulties in the identification and description of feelings as reported in behavioural and alexithymia self-report studies. Relations among neural responses, self-report and behavioural tasks would be an interesting avenue for future studies.
In our integrative conceptualization of AN,10 we hypothesized that nonconscious early neural alterations would affect disturbances in “feeling” (conscious experience of emotion) in AN patients (e.g., high levels of alexithymia20 and depression and anxiety40).
It is notable given the highly significant ERP findings (typically at the p < 0.001 level) that there were no corresponding differences between groups for the behavioural measure of emotion identification. Previous behavioural studies have also reported no effects in AN patients for emotion recognition.41 Event-related potentials may provide a more sensitive and direct measure of the neural basis of emotion deficits than measures that capture behavioural output. It is possible that the less neural reactivity seen in the ERPs is associated with the high levels of self-reported mood symptoms in AN patients. Previous research has found similar ERP waveforms in individuals with high levels of anxiety and depression,34 although there is some specificity of the current findings to AN because these non-AN anxiety and depression studies reported significant ERP latency effects, as opposed to the amplitude effects seen in this study. A longitudinal design is required to adequately investigate the possibility that anxiety or mood may be producing these emotion-related ERPs found in AN patients.
In this study, we assessed parallel neural pathways of non-conscious processing through the inclusion of covert and overt presentations. The ERP differences in AN were apparent across both conditions and showed a similar pattern within the first few hundred milliseconds of neural activity. These findings point to a core, early biological disturbance in the neural systems for nonconscious processing of emotion that may be compounded by subsequent conscious evaluation. Given that ERP disturbances were present even when overt attention was controlled, it is unlikely that disturbances could be accounted for solely by higher-order cognitive appraisal processes. The presence of higher-order executive functioning deficits in AN6,7 might reflect (or be compounded by) the effects of earlier alterations in emotion processing.
Our ERP findings extend and are consistent with previous AN neuroimaging19 and autonomic arousal studies42 that have reported neural disturbances in response to stimuli that are relevant to the illness and are therefore emotionally provocative for AN individuals. It is likely that the effects observed on high spatial resolution techniques, which capture activity about 2 seconds after stimulus presentation, are capturing the deficits that occur at the millisecond level, as was seen in the ERPs.
Although visual inspection of the ERP waveforms among the AN participants across testing occasion revealed some changes with weight gain toward to the control level, there were no statistically significant differences within the sample. The stability of the ERP disturbances in AN was further confirmed when individuals who had gained weight were compared with controls; the majority of the effects observed remained after weight gain. However, individuals with AN were retested only after weight restoration and not when they had “recovered.” In fact, despite weight gain, the patients continued to exhibit high levels of depression and symptoms of eating disorders.
Limitations
Because the study was conducted in a naturalistic, treatment-as-usual setting, individuals were retested at various points (although most were retested at hospital discharge). The present study could have been improved by standardizing the retest interval. It also would have been advantageous to retest the same individuals after a longer period to allow for both physiologic and psychologic recovery. The benefit of the current retest design is that we can be confident that the deficits were not entirely an effect of malnourishment, low weight or the medical instability that necessitated inpatient admission.
A further limitation related to the naturalistic design was that a small percentage of participants were taking medication. There is evidence to suggest that psychotropic medications influence EEGs and ERPs.43,44 Although less than 15% of the sample was taking medication, the influence of medication on the current findings cannot be ruled out.
Given the evidence of structural brain abnormalities in AN,45 we cannot rule out the possibility that these structural disturbances did not influence the ERP profile. Ideally, future research would examine magnetic resonance images and ERPs in the same participant sample. It would also be valuable to examine the ERPs in individuals with AN in response to food stimuli. We expect that overactivation would be observed. If this overactivation occurs nonconsciously and as early as 100 milliseconds after viewing a food stimulus, it is conceivable that this emotion abnormality could predominate over controlled, higher-order cognitive appraisals (e.g., “I need to eat to survive”), which emerge later during information processing.
Lastly, future studies could examine differences according to emotional valence through the computation of difference scores for positive- and negative-valenced emotions.
Conclusion
Given our findings, it is possible that treatments targeting the conscious appraisal of situations/stimuli will not be able to modify these nonconscious, reflexive responses occurring on a millisecond scale. Although limited studies have been conducted, cognitive-based treatments that presuppose conscious awareness have been found to be less effective than nonspecific clinical management in acutely ill AN patients.46 New, innovative emotion-based psychologic or pharmacologic treatments that target these nonconscious processes may prove beneficial. There may be some utility in treatments that emphasize repetition of new responses to counter these maladaptive automatic responses.
To our knowledge, this is the first study to examine the timing of neural processing in response to emotional stimuli in AN and the first to use face emotional stimuli, allowing us to conclude that there are indeed difficulties in innate emotional processing in this illness. This study provides preliminary evidence of a candidate neural marker of AN, which needs further examination in controlled studies. Our findings suggest that the deficits found in emotion-related neuroimaging and arousal studies are not restricted to food or body cues. A core, generic emotion vulnerability may exist in vulnerable individuals that generalizes to food at the onset of the illness.
Acknowledgements
We acknowledge the support of the Brain Resource International Database (under the auspices of Brain Resource; www.brainresource.com) for support in data acquisition and quantification. Brain Resource had no role in the design or implementation of the project. All scientific decisions are made independently of Brain Resource operations via the scientific division, BRAINnet (www.brainnet.net). Dr. Williams holds a Pfizer Senior Research Fellowship. Ms. Hatch was supported by a University Postgraduate Award (UPA) (University of Sydney) and The Millennium Foundation Research Scholarship Stipend Enhancement.
Footnotes
Preliminary data for a subset of patients included in this article were presented at the 6th International Congress of Neuropsychiatry, Sydney, Sept. 10–14, 2006 (oral presentation), and at the 14th Annual Meeting of the Organization for Human Brain Mapping in Melbourne, Australia, June 15–19, 2008 (poster).
Previously published at www.jpn.ca
Competing interests: None declared for Drs. Madden, Kohn, Clarke and Touyz and Ms. Hatch. Dr. Gordon is CEO of Brain Resource, with significant financial interest in the company. Dr. Williams is a minor shareholder (< 1%) in Brain Resource and has received fees from them for work unrelated to this study.
Contributors: Ms. Hatch and Drs. Kohn, Clarke, Touyz, Gordon and Williams designed the study. Ms. Hatch and Dr. Kohn acquired the data. Ms. Hatch and Drs. Madden, Kohn and Touyz analyzed the data. Ms. Hatch and Drs. Madden, Kohn, Clarke, Gordon and Williams wrote the article, which Ms. Hatch and Drs. Kohn, Touyz and Gordon reviewed. All authors approved publication.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed., text revised. Washington (DC): The Association; 2000. [Google Scholar]
- 2.Kaye W. Neurobiology of anorexia and bulimia nervosa. Physiol Behav. 2008;94:121–35. doi: 10.1016/j.physbeh.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinhausen HC. The outcome of anorexia nervosa in the 20th century. Am J Psychiatry. 2002;159:1284–93. doi: 10.1176/appi.ajp.159.8.1284. [DOI] [PubMed] [Google Scholar]
- 4.Fairburn CG. Evidence-based treatment of anorexia nervosa. Int J Eat Disord. 2005;37:S26–30. doi: 10.1002/eat.20112. [DOI] [PubMed] [Google Scholar]
- 5.Bulik CM, Hebebrand J, Keski-Rahkonen A, et al. Genetic epidemiology, endophenotypes, and eating disorder classification. Int J Eat Disord. 2007;40:S52–60. doi: 10.1002/eat.20398. [DOI] [PubMed] [Google Scholar]
- 6.Tchanturia K, Morris RG, Brecelj Anderluh M, et al. Set shifting in anorexia nervosa: an examination before and after weight gain, in full recovery and relationship to childhood and adult OCPD traits. J Psychiatr Res. 2004;38:545–52. doi: 10.1016/j.jpsychires.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Holliday J, Tchanturia K, Landau S. Is impaired set-shifting an endophenotype of anorexia nervosa? Am J Psychiatry. 2005;162:2269–75. doi: 10.1176/appi.ajp.162.12.2269. [DOI] [PubMed] [Google Scholar]
- 8.Tchanturia K, Davies H, Campbell IC. Cognitive remediation therapy for patients with anorexia nervosa: preliminary findings. Ann Gen Psychiatry. 2007;6:14. doi: 10.1186/1744-859X-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bulik CM, Berkman ND, Brownley KA. Anorexia nervosa treatment: a systematic review of randomized controlled trials. Int J Eat Disord. 2007;40:310–20. doi: 10.1002/eat.20367. [DOI] [PubMed] [Google Scholar]
- 10.Hatch A, Madden S, Kohn M, et al. Anorexia nervosa: towards and integrative neuroscience model. Eur Eat Disord Rev. doi: 10.1002/erv.974. In press. [DOI] [PubMed] [Google Scholar]
- 11.Williams LM, Gatt JM, Hatch A, et al. The integrate model of emotion, thinking and self-regulation: an application to the ‘Paradox of aging’. J Integr Neurosci. 2008;7:367–404. doi: 10.1142/s0219635208001939. [DOI] [PubMed] [Google Scholar]
- 12.Darwin C. The expression of the emotions in man and animals. 3rd ed. New York (NY): Oxford University Press; 1998. [Google Scholar]
- 13.Damasio AR. The feeling of what happens. London (UK): Vintage, Random House; 1999. [Google Scholar]
- 14.Le Doux JE. The emotional brain: the mysterious underpinnings of emotional life. New York (NY): Touchstone; 1996. [Google Scholar]
- 15.Whalen PJ, Rauch SL, Etcoff NL. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci. 1998;18:411–8. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liddell BJ, Williams LM, Rathjen J, et al. A temporal dissociation of subliminal versus supraliminal fear perception: an event-related potential study. J Cogn Neurosci. 2004;16:479–86. doi: 10.1162/089892904322926809. [DOI] [PubMed] [Google Scholar]
- 17.Williams LM, Liddell BJ, Rathjen J, et al. Mapping the time course of nonconscious and conscious perception of fear: an integration of central and peripheral measures. Hum Brain Mapp. 2004;21:64–74. doi: 10.1002/hbm.10154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seeger G, Braus DF, Ruf M, et al. Body image distortion reveals amygdala activation in patients with anorexia nervosa — a functional magnetic imaging study. Neurosci Lett. 2002;326:25–8. doi: 10.1016/s0304-3940(02)00312-9. [DOI] [PubMed] [Google Scholar]
- 19.Uher R, Murphy T, Brammer MJ, et al. Medial prefrontal cortex activity associated with symptom provocation in eating disorders. Am J Psychiatry. 2004;161:1238–46. doi: 10.1176/appi.ajp.161.7.1238. [DOI] [PubMed] [Google Scholar]
- 20.Bourke M P, Taylor GJ, Parker JD, et al. Alexithymia in women with anorexia nervosa: a preliminary investigation. Br J Psychiatry. 1992;161:240–3. doi: 10.1192/bjp.161.2.240. [DOI] [PubMed] [Google Scholar]
- 21.Zonnevijlle-Bender MJ, van Goozen SH, Cohen-Kettenis PT, et al. Do adolescent anorexia nervosa patients have deficits in emotional functioning? Eur Child Adolesc Psychiatry. 2002;11:38–42. doi: 10.1007/s007870200006. [DOI] [PubMed] [Google Scholar]
- 22.Gordon E, Rennie C, Toga A, et al. Human brain imaging technologies. In: London GE, editor. Integrative neuroscience: bringing together biological psychological and clinical models of the human brain. London (UK): Harwood Academic Press; 2000. pp. 233–44. [Google Scholar]
- 23.Ekman P, Friesen WV, Ellsworth P. Emotion in the human face. New York (NY): Pergamon; 1972. [Google Scholar]
- 24.Williams LM, Palmer D, Liddell BJ, et al. The when and where of perceiving signals of threat versus non-threat. Neuroimage. 2006;31:458–67. doi: 10.1016/j.neuroimage.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Williams LM, Hermens DF, Palmer D, et al. Misinterpreting emotional expressions in attention-deficit/hyperactivity disorder: evidence for a neural marker and stimulant effects. Biol Psychiatry. 2008;63:917–26. doi: 10.1016/j.biopsych.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 26.Garner DM. EDI-3 Eating Disorder Inventory-3: professional manual. Odessa (FL): Psychological Assessment Resources Inc; 2004. [Google Scholar]
- 27.Hickie I, Hadzi-Pavlovic D, Scott E, et al. SPHERE: A national depression project. Australas Psychiatry. 1998;6:248–50. [Google Scholar]
- 28.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9 — Validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav Res Ther. 1995;33:335–43. doi: 10.1016/0005-7967(94)00075-u. [DOI] [PubMed] [Google Scholar]
- 30.Williams LM, Hermens DF, Thein T, et al. Using brain-based cognitive measures to support clinical decisions in ADHD. Ped Neurology. 2010;42:118–26. doi: 10.1016/j.pediatrneurol.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 31.Williams LM, Mathersul D, Palmer DM, et al. Explicit identification and implicit recognition of facial emotions: I. Age effects in males and females across 10 decades. J Clin Exp Neuropsychol. 2009;31:257–77. doi: 10.1080/13803390802255635. [DOI] [PubMed] [Google Scholar]
- 32.Gordon E, Cooper N, Rennie C, et al. Integrative neuroscience: the role of a standardized database. Clin EEG Neurosci. 2005;36:64–75. doi: 10.1177/155005940503600205. [DOI] [PubMed] [Google Scholar]
- 33.Gordon E, Barnett KJ, Cooper NJ, et al. An “integrative neuroscience” platform: application to profiles of negativity and positivity bias (special issue volume) J Integr Neurosci. 2008;7:345–66. [PubMed] [Google Scholar]
- 34.Williams LM, Kemp AH, Felmingham K, et al. Neural biases to covert and overt signals of fear: dissociation by trait anxiety and depression. J Cogn Neurosci. 2007;19:1595–608. doi: 10.1162/jocn.2007.19.10.1595. [DOI] [PubMed] [Google Scholar]
- 35.Gur RC, Sara R, Hagendoorn M, et al. A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. J Neurosci Methods. 2002;115:137–43. doi: 10.1016/s0165-0270(02)00006-7. [DOI] [PubMed] [Google Scholar]
- 36.Lange K, Williams LM, Young AW, et al. Task instructions modulate neural responses to fearful facial expressions. Biol Psychiatry. 2003;53:226–32. doi: 10.1016/s0006-3223(02)01455-5. [DOI] [PubMed] [Google Scholar]
- 37.Bentin S, Allison T, Puce A, et al. Electrophysiological studies of face perception in humans. J Cogn Neurosci. 1996;8:551–65. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeffreys DA. A face-responsive potential recorded from the human scalp. Exp Brain Res. 1989;78:193–202. doi: 10.1007/BF00230699. [DOI] [PubMed] [Google Scholar]
- 39.Ashley V, Vuilleumier P, Swick D. Time course and specificity of event-related potentials to emotional expressions. Neuroreport. 2004;15:211–6. doi: 10.1097/00001756-200401190-00041. [DOI] [PubMed] [Google Scholar]
- 40.Kaye WH, Bulik CM, Thornton L, et al. Comorbidity of anxiety disorders with anorexia and bulimia nervosa. Am J Psychiatry. 2004;161:2215–21. doi: 10.1176/appi.ajp.161.12.2215. [DOI] [PubMed] [Google Scholar]
- 41.Kessler H, Schwarze M, Filipic S, et al. Alexithymia and facial emotion recognition in patients with eating disorders. Int J Eat Disord. 2006;39:245–51. doi: 10.1002/eat.20228. [DOI] [PubMed] [Google Scholar]
- 42.Friederich HC, Kumari V, Uher R, et al. Differential motivational responses to food and pleasurable cues in anorexia and bulimia nervosa: a startle reflex paradigm. Psychol Med. 2006;36:1327–35. doi: 10.1017/S0033291706008129. [DOI] [PubMed] [Google Scholar]
- 43.Saletu B, Anderer P, Saletu-Zyhlarz GM, et al. Classification and evaluation of the pharmacodynamics of psychotropic drugs by single-lead pharmaco-EEG, EEG mapping and tomography (LORETA) Methods Find Exp Clin Pharmacol. 2002;24(Suppl C):97–120. [PubMed] [Google Scholar]
- 44.Saletu B, Anderer P, Saletu-Zyhlarz GM, et al. EEG mapping and low-resolution brain electromagnetic tomography (LORETA) in diagnosis and therapy of psychiatric disorders: evidence for a key-lock principle. Clin EEG Neurosci. 2005;36:108–15. doi: 10.1177/155005940503600210. [DOI] [PubMed] [Google Scholar]
- 45.Frank GK, Bailer UF, Henry S, et al. Neuroimaging studies in eating disorders. CNS Spectr. 2004;9:539–48. doi: 10.1017/s1092852900009639. [DOI] [PubMed] [Google Scholar]
- 46.McIntosh W, Jordan J, Carter F, et al. Three psychotherapies for anorexia nervosa: a randomized controlled trial. Am J Psychiatry. 2005;162:741–7. doi: 10.1176/appi.ajp.162.4.741. [DOI] [PubMed] [Google Scholar]