Abstract
Schwannomas occurring in the gastrointestinal tract are rare, and among them, schwannomas of the large intestine are extremely rare. In this paper, we report a case of a macroscopically atypical schwannoma of the transverse colon. The case is a female aged 67. Stool occult blood test was positive, and colonoscopy revealed a protruded lesion resembling a type 1 carcinoma measuring 4 cm with a reddish and uneven surface on the transverse colon. The surface was smooth and lobulated in observation with indigo carmine spray, and granulation tissue was revealed by biopsies. CT of the abdomen showed an irregular mass, and clinical examinations could not rule out malignancy. Therefore, partial transverse colectomy with peripheral lymph node dissection was performed. Histologically, proliferation of spindle cells was observed originating from the muscularis propria, and most of the upper part of the lesion was replaced by granulation tissue. In immunohistochemical staining, S-100 protein and NSE were positive while KIT, CD34, desmin and smooth muscle actin were negative, and the tumor was therefore diagnosed to be a schwannoma. In addition, since the MIB-1 labeling index was low and virtually no mitosis was observed, it was diagnosed as benign tumor.
Key Words: Schwannoma, Transverse colon, Granulation
Introduction
Although there has been an increase in reports of mesenchymal tumors in recent years, schwannomas of the large intestine are extremely rare. We report a case of schwannoma of the transverse colon accompanied by granulation tissue in whom malignancy could not be ruled out in preoperative diagnosis. Therefore, we obtained a definite diagnosis based on pathological findings from surgically resected specimens. We discuss our case along with a literature review.
Case Report
A 67-year-old female patient was introduced to our hospital for a complete examination in May 2007 because stool occult blood test was positive in her annual medical checkup. Her medical history included appendectomy at age 22, mastectomy for left breast cancer at age 50, and hypertension and hyperlipidemia, for which she is currently being treated, since age 47. No significant abnormal findings were observed including tumor markers (CEA, CA19-9). Barium enema examination showed a steep edged mass shadow measuring 3 cm with clearly defined boundaries in the left side of the transverse colon.
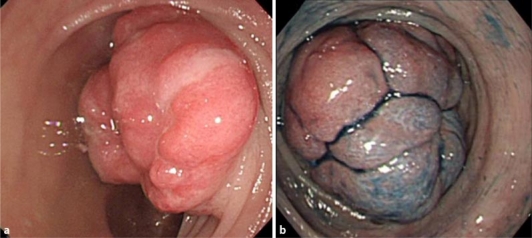
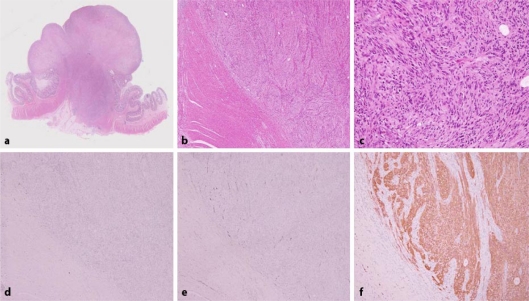
Colonoscopy revealed a protruded lesion measuring 4 cm occupying in the transverse colon (fig. 1). The surface was reddish and uneven, and its lobulated form appeared when examined by indigo carmine spraying. Pit pattern was unclear. However, mobility between the mucosa and the tumor was poor. Biopsies revealed that the tumor consisted of granulated tissue indicating blood vessel proliferation, inflammatory cell infiltration and fibrin deposition. The epithelium was almost entirely gone, and malignant cells were not observed. Endoscopic ultrasonography showed a low-echoic mass with clearly defined boundaries in the 2nd and lower layers, and the 4th low echoic layer was stretched and disrupted by pressure from the tumor. Internal echoes were comparatively uniform. CT scan revealed an irregular mass measuring 3 cm near the transverse colonic splenic flexure. There were several paracolic lymph nodes 5 mm or less in size. Since the possibility of malignant tumors could not be ruled out, the tumor was treated by transverse colectomy with D2 lymph node dissection. The tumor mass measured 33 × 23 × 17 mm and was a solid tumor of yellowish color, proliferating primarily in the muscularis propria. Microscopically, spindle cells proliferated primarily in the muscularis propria in a fascicular or storiform pattern, and nuclear palisading was observed in part. However, the majority of the upper portion of the lesion was replaced by granulation tissue and exhibited fibrin attachment and neutrophil infiltration. Furthermore, virtually no mitosis was observed. In immunohistochemical studies, S-100 protein and NSE were positive. All other markers including KIT, CD34, desmin and smooth muscle actin were negative (fig. 2). A final diagnosis of a schwannoma was made. It was diagnosed to be benign because nuclear atypicality was not observed and the MIB-1 marker was low. Metastasis to lymph nodes was not observed. The patient was discharged from the hospital on the 12th day after surgery, and she is presently followed at an outpatient clinic.
Fig. 1.
Colonoscopy. a The reddish and uneven lesion resembling type 1 tumor was observed in the transverse colon. b The surface was smooth and lobulated in observation with indigo carmine spraying.
Fig. 2.
Histopathological examination findings. The majority of the upper portion of the lesion was replaced by granulation tissue (a). Spindle cells proliferated in a fascicular pattern (b). Nuclear palisading was observed in part (c). KIT (d), CD34 (e), desmin and smooth muscle actin were negative and S-100 (f) protein and NSE were positive in immunohistochemical staining.
Discussion
Schwannomas are tumors that originate in Schwann cells and commonly appear along the peripheral nerves of the head, cervix, extremities and other parts of the body. In the digestive tract, they most commonly originate in Auerbach's nerve plexus in the intestinal wall, and at times, they originate in Meissner's nerve plexus. In macroscopic morphology, those originating in Auerbach's nerve plexus are hemispherical or broad-based protrusions while those originating in Meissner's nerve plexus are considered to often display morphology similar to spherical pedunculated polyps [1]. Schwannomas that originate in the digestive tract are rare. Most of them occur in the stomach or small intestine, and those occurring in the large intestine are extremely rare.
Schwannomas in the large intestine occur most commonly at middle to advanced age, and there are no differences between the sexes. Tumors measure 4 cm in diameter on average, and the possibility of malignancy is thought to increase if the size is 5 cm or larger. Although they are clinically asymptomatic for the largest part, if the tumor is large in diameter, it can exhibit bowel movement disorders, pain, hemorrhaging or palpable tumor masses. Definite preoperative diagnosis is difficult, and procedures such as needle biopsy using endoscopic ultrasonography are recommended [2].
The diagnosis is considered to be definite if the proliferation of spindle cells is histopathologically observed through HE staining and if both KIT (CD117) and CD34 are stained negative and S-100 protein is stained positive in immunohistochemical staining [3]. Spiral-like forms consisting of densely arrayed spindle-shaped tumor cells, palisade arrangements and loose reticular networks of tumor cells can be cited as histological features and Verocay corpuscles and lymphoid cuff are characteristic [4, 5, 6]. In addition, blood vessels in tumors show hyalinization of the wall and clot formation. These changes in the blood vessels cause lack of blood flow which then leads to tissue necrosis. In our case, it is conjectured that replacement of the submucosal layer with thick granulation tissue occurred to restore these tissues. CT showed uneven tumor unlike normal schwannomas, which substantiates this conjecture. We believe that this also added to the difficulty of making a diagnosis based on preoperative colonoscopic biopsies.
In regard to the treatment method, a tumor size of 5 cm or more is considered to be a criterion for surgery because recurrence and prognosis vary greatly if the tumor is more than 5 cm in diameter. If a definite preoperative diagnosis is possible or if infiltration or metastasis is not observed in diagnostic imaging, the standard treatment is surgical partial colectomy, including laparoscopic surgery, where lymph node dissection is not performed [7]. Moreover there are recent reports of endoscopic removal of schwannomas originated in Meissner's nerve plexus[8, 9]. In Japan, however, there are reports of 16 cases of malignant schwannomas of the large intestine, including the report of Suda et al. [10, 11, 12, 13]. In our case, lymph node dissection was performed because the tumor did not appear to be a typical submucosal tumor, malignant epithelial tumor could not be rules out preoperatively and lymph node metastasis was also suspected in findings during surgery.
Histopathologically, the diagnosis of whether a tumor is malignant or not is based on the disparities in cell size, cell density, degree of mitosis and so forth. The labeling index of Ki-67 antibodies (MIB-1) with highly assessable reproducibility is recommended as an indicator of malignancy, and that more than 10% is diagnosed to be malignant. In our case, we were able to diagnose the tumor essentially as a benign schwannoma because the labeling index of MIB-1 was extremely low and mitosis was not observed either. Presence of hemorrhaging, necrosis and ulceration are the main characteristics of malignant schwannomas and are often used to macroscopically differentiate malignancy from benignity [14]. However, there have been 18 reports of benign schwannomas that have appeared in the Japana Centra Revuo Medicina during the past ten years. Among them, necrotic or granulation tissue was observed in biopsies of 5 cases, or 25%, including our own cases. In addition, in a report by Miettinen et al., ulceration was observed in 8 of 15 cases, or about half, of spindle cell type schwannomas [15]. As in our own case, there have also been cases that were difficult to macroscopically differentiate as cancer [16]. Thus, based on literature review, schwannomas that are macroscopically accompanied by necrotic tissue may often be benign. Therefore, it is necessary to be careful when treating schwannoma with granulation.
References
- 1.Otsuka T, Ando M, Kurahasi M, et al. A case report of intussusception caused by a schwannoma of the caecum. Jpn J Gastroenterol Surg. 2006;39:614–619. [Google Scholar]
- 2.Fukami Y, Terasaki M, Sakaguchi K, et al. A case of a preoperatively diagnosed schwannoma of the rectum. J Jpn Surg Assoc. 2006;67:834–837. [Google Scholar]
- 3.Miettinen M, Lasota J. Gastrointestinal stromal tumors – definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1–12. doi: 10.1007/s004280000338. [DOI] [PubMed] [Google Scholar]
- 4.Hasegawa T, Tashiro T, Sekine S, et al. Pathology and classification of gastrointestinal submucosal tumors including GIST. Stomach Intestine. 2004;39:396–404. [Google Scholar]
- 5.Kitajima M, Takita N, Yamaguchi H, et al. A case of neurilemoma of sigmoid colon. J Jpn Soc Coloproctol. 2005;66:1972–1975. [Google Scholar]
- 6.Emanuel P, Pertsemlidis DS, Gordon R, Xu R. Benign hybrid perineurioma-schwannoma in the colon. A case report. Ann Diagn Pathol. 2006;10:367–370. doi: 10.1016/j.anndiagpath.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Jacobson BC, Hirsch MS, Lee JH, et al. Multiple asymptomatic plexiform schwannomas of the sigmoid colon. Gastrointest Endosc. 2001;53:801–804. doi: 10.1067/mge.2001.115317. [DOI] [PubMed] [Google Scholar]
- 8.Otake Y, Abe T, Ohta A, et al. A case of endoscopically resected schwannoma in the sigmoid colon. Prog Dig Endosc. 2001;59:118–119. [Google Scholar]
- 9.Inagawa S, Hori M, Shimazaki J, et al. Solitary schwannoma of the colon: report of two cases. Surg Today. 2001;31:833–838. doi: 10.1007/s005950170060. [DOI] [PubMed] [Google Scholar]
- 10.Fujimoto Y, Oya M, Kuroyanagi H, et al. A case of transverse colon schwannoma. J Jpn Soc Coloproctol. 2007;60:286–291. [Google Scholar]
- 11.Fujii M, Tanaka Y, Saito T. A case of malignant peripheral nerve sheath tumor of the rectum. J Jpn Soc Coloproctol. 2006;59:395–398. [Google Scholar]
- 12.Fotiadis CI, Kouerinis IA, Papandreou I, et al. Sigmoid schwannoma: a rare case. World J Gastroenterol. 2005;11:5079–5081. doi: 10.3748/wjg.v11.i32.5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suda K, Adati M, Kajiyama Y, et al. A case of laparoscopically resected schwannoma in the transverse colon. Shujutsu. 1997;51:1567–1571. [Google Scholar]
- 14.Shimizu T, Nakata M, Inaba S, et al. A case report of metachronous quartet malignant tumors including primary malignant schwannoma of the small intestine. Jpn J Gastroenterol Surg. 2002;35:1433–1437. [Google Scholar]
- 15.Miettinen M, Shekitka KM, Sobin LH. Schwannomas in the colon and rectum. Am J Surg Pathol. 2001;25:846–855. doi: 10.1097/00000478-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Braumann C, Guenther N, Menenakos C, Junghans T. Schwannoma of the colon mimicking carcinoma: a case report and literature review. Int J Colorectal Dis. 2007;22:1547–1548. doi: 10.1007/s00384-006-0264-9. [DOI] [PubMed] [Google Scholar]