Abstract
Hidradenitis suppurativa is a chronic disease that affects the apocrine gland-bearing regions of the body. The etiology of this disorder is poorly understood, but most likely is a complex process involving follicular apocrine occlusion with subsequent perifolliculitis. Many treatment options have been reported with varying degrees of success, including topical and oral therapy and surgical procedures. Recently, TNF-α antagonists have been reported as effective therapy in a few patients. We report here a patient who initially responded to infliximab but developed an infusion reaction to this medication. After subsequent treatment with adalimumab, the patient's disease improved dramatically and has been maintained under excellent control for over 15 months. We propose that TNF-α inhibitors, particularly monoclonal antibody based agents, are a viable treatment option in patients with severe, recalcitrant HS and that a patient may be safely and successfully treated with the fully human monoclonal antibody adalimumab in cases in which the chimeric monoclonal antibody infliximab therapy is not tolerated.
Key Words: Hidradenitis suppurativa, Adalimumab, Infliximab, Tumor necrosis factor-alpha, Biologics
Introduction
Hidradenitis suppurativa (HS) is a chronic skin disease characterized by abscess and sinus tract formation in areas of the body with apocrine sweat glands. The exact pathophysiology of HS is still not understood but it is likely that a multitude of factors, including genetics, bacterial infection, hormones, inflammation, mechanical occlusion, and possibly even smoking, play a role [1]. HS tends to start after puberty, persists for years and may worsen over time. The reported prevalence is between 0.25–5%. It is more common in females and African-Americans, and it has been associated with Crohn's disease and arthropathy. Furthermore, 38% of patients presenting with HS have a positive family history for the disease [2]. A multitude of treatment options are available, including topical and systemic agents, surgery, and recently TNF-α inhibitors.
Case Report
A 47-year-old white female with a 10-year history of HS was referred for the treatment of painful nodules and purulent draining sinuses in her axillary, inguinal, and mammary areas. Our patient is thin and otherwise healthy. Previous treatment for her HS included combinations of numerous topical antibiotics including clindamycin and chlorhexidine scrubs, oral antibiotics including doxycycline, intralesional triamcinolone, isotretinoin, and systemic prednisone. In addition, multiple surgical procedures, including excision and grafting of the affected skin, were performed, ultimately all with unsuccessful outcomes. After failure of these treatments, the patient began therapy with infliximab and had near complete clearing of her active disease. However, within 15 months of infliximab therapy, the medication's efficacy diminished to 10 days per infusion. At 18 months, the patient developed a severe infusion reaction with urticaria and the medication was discontinued.
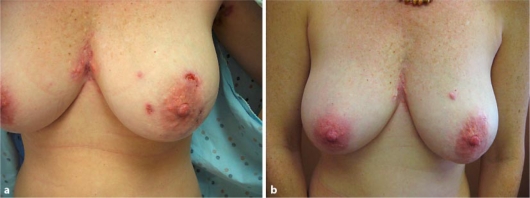
Several weeks after discontinuation of infliximab, physical exam revealed the return of her disease (fig. 1 a). We sought a treatment regimen that would effectively treat her disease and minimize adverse effects. Because of her previous successful treatment with a TNF-α inhibitor, we proposed a trial of another biologic agent, adalimumab.
Fig. 1.
a Pre-treatment. b After 15 months.
Before treatment with adalimumab, the patient had a complete blood count, liver function tests, a purified protein derivative test, chest radiography, and hepatitis B, hepatitis C, and human immunodeficiency virus antibody panel, which showed no abnormalities. She had no personal history of malignancy. The patient was begun on 40-mg injections of adalimumab every other week. The patient was re-evaluated 3 months into her treatment and her HS was significantly improved with a decrease in drainage from old lesions and a decrease in the formation of new lesions. Over the past 15 months of adalimumab therapy, the patient has remained clear except for the development of a few small nodules which were effectively managed with intralesional triamcinolone (fig. 1 b).
Discussion
HS represents a spectrum of disease ranging from tender papules with minimal pain to deep-seated nodules, which can serve as a nidus for recurrent systemic infection, fistula formation with internal organs such as the bladder, or even squamous cell carcinoma [3]. The condition was classically thought to occur when blocked apocrine glands forced perspiration and bacteria into surrounding tissue, causing subcutaneous inflammation and infection. More recent studies have indicated that HS is caused by follicular occlusion first, which then occludes the apocrine glands and causes perifolliculitis [4].
In 10 out of their 12 histologic samples of HS, Yu and Cook identified squamous epithelium-lined structures, which likely represent abnormally dilated hair follicles, in the form of cysts or sinuses within the dermis; inflammation of the apocrine glands was seen in only 4 out of 12 samples. In these samples the inflammation was also seen surrounding eccrine glands, hair follicles, and the epithelium-lined structures, suggesting that apocrine gland inflammation is a secondary phenomenon rather than an initiating event [1]. In 60 specimens studied by Jemec and Hansen, only 12% contained apocrine gland involvement [5]. In a 2005 review by Sellheyer and Krahl, serial histologic specimens demonstrated progression of HS from perifollicular hyperkeratosis and subsequent comedo development to rupture of the follicular infundibulum which caused local dermal inflammation [4]. Over time, it is believed that these granulomatous infiltrates cause the formation of the abscesses seen in HS.
The critical role of follicular occlusion was also highlighted by a comparison of specimens from affected individuals and controls that demonstrated follicular occlusion only in the axillary and inguinal skin of all affected individuals [6]. Further support for a primary folliculitis was found in another study that demonstrated an infundibulum centered T-cell rich infiltrate in early lesions. The T cells were chiefly HLA-DR positive with a low percentage of Leu-8 positive lymphocytes [7]. Therefore, HS is likely an inflammatory disease of the hair follicle that can be included among other diseases of follicular occlusion and should be considered a part of the follicular occlusion tetrad [8].
A number of treatment options are currently used to treat HS (table 1) [9, 10]). First-line measures include weight loss, smoking cessation, and hygiene measures. If these measures fail, subsequent therapy includes the use of long-term topical and systemic antibiotics, hormonal treatment, intralesional corticosteroids, systemic retinoids, and surgical procedures. For many patients, the disease is characterized by recurrences regardless of the treatment modality used. Therefore, it is critical that the benefits of these therapies must be weighed against potential adverse effects.
Table 1.
Treatment of hidradenitis suppurativa
| Treatment | Level of evidence∗ | Efficacy |
|---|---|---|
| Local therapy | ||
| Clindamycin | 1 | superior to placebo |
| Intralesional steroids | 3 | effective |
| Antimicrobial washes | 3 | unknown |
| Systemic therapy | ||
| Tetracycline# | 1 | equivalent to topical clindamycin |
| Cyproterone | 1;2 | no effect over estrogen therapy; possibly effective over antibiotics |
| Finasteride | 2 | partial efficacy |
| Isotretinoin | 2 | limited efficacy |
| Clindamycin + rifampicin | 2 | effective |
| Zinc salts | 2 | effective |
| Etretinate | 3 | effective |
| Acitretin | 3 | effective |
| Cyclosporine | 3 | effective |
| Dapsone | 2 | effective |
| Systemic steroids | 3 | possibly effective |
| Methotrexate | 3 | weak to none |
| TNF-α inhibitors | 2 | effective in some patients |
| Physical modalities | ||
| Incision/drainage | effective for pain only | |
| Surgical treatment | 2 | effective (for severe disease only) |
| Radiation treatment | possibly effective | |
| CO2 laser | 2 | effective (compared to surgery) |
| Botulinum toxin | 3 | effective |
| Photodynamic therapy | 2 | not effective |
1 = RCT; 2 = retrospective, case-control, small therapeutic trials, case series; 3 = case report, common practice.
When discernible
or related drug.
Given the important role of TNF-α in granuloma formation, we considered the possibility that adalimumab may be an effective treatment for HS. Our patient enjoyed marked improvement early in the treatment of her HS with infliximab, but this response was not sustained over months. Since 25% of the sequence of infliximab is mouse-derived, it has been associated with the development of antibodies against the murine portion of the antibody, which may lead to infusion reactions and loss of efficacy [11]. In studies of patients with Crohn's disease, adalimumab has been shown to be effective in patients who lost response to infliximab [12]. Adalimumab is a fully humanized anti-TNF-α antibody. It has a high specificity and affinity for TNF-α. Adalimumab was first tested in 1997 for rheumatoid arthritis and was developed to have low immunogenicity, avoiding the need for concomitant administration of immunosuppressants such as methotrexate.
Despite its benefit in the treatment of recalcitrant HS, adalimumab does have known associated risks. The drug carries warnings regarding reactivation of latent TB and invasive fungal infections, both of which have been reported in patients treated with adalimumab. Other adverse events reported following all TNF-α antagonist treatment include lupus-like syndromes [13], demyelinating disorders [14], and congestive heart failure [15]. Controversy exists as to the risk of lymphoma associated with adalimumab use [16, 17]. In addition, the cost and the lack of long-term efficacy data should be considered before using adalimumab to treat HS.
Considerable interest exists in the use of biologic agents for HS. Their use in HS was pioneered with intravenous infliximab in a female patient treated for Crohn's disease who had concomitant HS. The patient had significant reduction in her HS within a few treatments with infliximab [18]. Our patient shared this similar success with infliximab initially, before its efficacy diminished. In contrast, a recent open-label study of etanercept in 15 patients failed to show a statistically significant improvement in disease control [19].
The use of adalimumab in HS is limited thus far to a few case reports and small case series in patients with refractory disease (table 2) [20, 21, 22, 23, 24, 25]). Due to the role of inflammation in HS, biologic therapy will likely play a role in the future treatment of refractory HS. The seemingly disparate efficacy of the TNF-α receptor fusion protein etanercept and the monoclonal antibodies infliximab and adalimumab in treating HS suggest that there may be a difference in mechanism due to the structural differences in these biologic agents. One study showed that in a mouse model of pulmonary tuberculosis, a monoclonal anti-TNF-α antibody was better able to penetrate into granulomas than a TNF-α receptor fusion protein [26]. Given the granulomatous nature of HS, this finding may explain the possible superiority of the monoclonal antibodies in treating this condition. However, more rigorous prospective trials are necessary to fully determine the efficacy of these biologics in treating HS.
Table 2.
Reports of treatment of hidradenitis suppurativa with adalimumab
| Authors | Type of study | Number of patients | Treatment | Duration of disease | Duration of response∗ | Adverse events |
|---|---|---|---|---|---|---|
| Harde and Mrowietz | case report | 1 | 80 mg SC × l, then 40 mg QW | 12 years | 6 months | none |
| Yamauchi and Mau | case series | 3 | range: 40 mg EOW to 40 mg QW | range: 3 months to 5 years | 1 year | none |
| Moul and Korman | case report | 1 | 40 mg EOW | 20 years | 4 months | none |
| Scheinfeld | case report | 1 | 40 mg EOW, then QW | 15 years | not reported | none reported |
| Blanco etal. | case series | 6 | 40 mg EOW, up to QW prn | 22.5 years (mean) | 21.5 months (mean); range: 13-29 | mild injection site pain; facial cellulitis in 1 patient |
| Sotiriou etal. | case series | 3 | 40 mg EOW | 4.3 (mean) | discontinued treatment at 3 months in all patients | none reported |
At time of publication.
References
- 1.Yu CC, Cook MG. Hidradenitis suppurativa: a disease of follicular epithelium, rather than apocrine glands. Br J Dermatol. 1990;122:763–769. doi: 10.1111/j.1365-2133.1990.tb06264.x. [DOI] [PubMed] [Google Scholar]
- 2.Jemec GB, Heidenheim M, Nielsen NH. The prevalence of hidradenitis suppurativa and its potential precursor lesions. J Am Acad Dermatol. 1996;35:191–194. doi: 10.1016/s0190-9622(96)90321-7. [DOI] [PubMed] [Google Scholar]
- 3.Constantinou C, Widom K, Desantis J, Obmann M. Hidradenitis suppurativa complicated by squamous cell carcinoma. Am Surg. 2008;74:1177–1181. [PubMed] [Google Scholar]
- 4.Sellheyer K, Krahl D. 'Hidradenitis suppurativa' is acne inversa! An appeal to (finally) abandon a misnomer. Int J Dermatol. 2005;44:535–540. doi: 10.1111/j.1365-4632.2004.02536.x. [DOI] [PubMed] [Google Scholar]
- 5.Jemec GB, Hansen U. Histology of hidradenitis suppurativa. J Am Acad Dermatol. 1996;34:994–999. doi: 10.1016/s0190-9622(96)90277-7. [DOI] [PubMed] [Google Scholar]
- 6.Attanoos RL, Appleton MA, Douglas-Jones AG. The pathogenesis of hidradenitis suppurativa: a closer look at apocrine and apoeccrine glands. Br J Dermatol. 1995;133:254–258. doi: 10.1111/j.1365-2133.1995.tb02624.x. [DOI] [PubMed] [Google Scholar]
- 7.Boer J, Weltevreden EF. Hidradenitis suppurativa or acne inversa. A clinicopathological study of early lesions. Br J Dermatol. 1996;135:721–725. [PubMed] [Google Scholar]
- 8.Lam J, Krakowski AC, Friedlander SF. Hidradenitis suppurativa (acne inversa): management of a recalcitrant disease. Pediatr Dermatol. 2007;24:465–473. doi: 10.1111/j.1525-1470.2007.00544.x. [DOI] [PubMed] [Google Scholar]
- 9.Jemec GB. Medical treatment of hidradenitis suppurativa. Expert Opin Pharmacother. 2004;5:1767–1770. doi: 10.1517/14656566.5.8.1767. DOI: 10.1517/14656566.5.8.1767. [DOI] [PubMed] [Google Scholar]
- 10.Revuz J. Hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2009;23:985–998. doi: 10.1111/j.1468-3083.2009.03356.x. DOI: 10.HH/j.1468-3083.2009.03356.x. [DOI] [PubMed] [Google Scholar]
- 11.Hanauer SB. Immunogenicity of infliximab in Crohn's disease. N Engl J Med. 2003;348:2155–2156. doi: 10.1056/NEJM200305223482121. author reply 2155-2156. [DOI] [PubMed] [Google Scholar]
- 12.Papadakis KA, Shaye OA, Vasiliauskas EA, et al. Safety and efficacy of adalimumab (D2E7) in Crohn's disease patients with an attenuated response to infliximab. Am J Gastroenterol. 2005;100:75–79. doi: 10.1111/j.1572-0241.2005.40647.x. [DOI] [PubMed] [Google Scholar]
- 13.Remicade (infliximab) prescribing information [package insert] Malvern, PA: Centocor, Inc.; 2007. [Google Scholar]
- 14.Mohan N, Edwards ET, Cupps TR, et al. Demyelination occurring during anti-tumor necrosis factor alpha therapy for inflammatory arthritides. Arthritis Rheum. 2001;44:2862–2869. doi: 10.1002/1529-0131(200112)44:12<2862::aid-art474>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 15.Wolfe F, Michaud K. Heart failure in rheumatoid arthritis: rates, predictors, and the effect of anti-tumor necrosis factor therapy. Am J Med. 2004;116:305–311. doi: 10.1016/j.amjmed.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 16.Humira (adalimumab) prescribing information [package insert]. 2008.
- 17.Burmester GR, Mease PJ, Dijkmans BA, et al: Adalimumab safety and mortality rates from global clinical trials of six immune-mediated inflammatory diseases. Ann Rheum Dis 2009 (Epub ahead of print). [DOI] [PMC free article] [PubMed]
- 18.Martinez F, Nos P, Benlloch S, Ponce J. Hidradenitis suppurativa and Crohn's disease: response to treatment with infliximab. Inilamm Bowel Dis. 2001;7:323–326. doi: 10.1097/00054725-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Lee RA, Dommasch E, Treat J, et al. A prospective clinical trial of open-label etanercept for the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 2009;60:565–573. doi: 10.1016/j.jaad.2008.11.898. DOI: 10.1016/j.jaad.2008.11.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamauchi PS, Mau N. Hidradenitis suppurativa managed with adalimumab. J Drugs Dermatol. 2009;8:181–183. [PubMed] [Google Scholar]
- 21.Blanco R, Martinez-Taboada VM, Villa I, et al. Long-term successful adalimumab therapy in severe hidradenitis suppurativa. Arch Dermatol. 2009;145:580–584. doi: 10.1001/archdermatol.2009.49. DOI: 10.1001/archdermatol.2009.49. [DOI] [PubMed] [Google Scholar]
- 22.Harde V, Mrowietz U. Treatment of severe recalcitrant hidradenitis suppurativa with adalimumab. J Dtsch Dermatol Ges. 2009;7:139–141. doi: 10.1111/j.1610-0387.2008.06918.x. DOI: 10.111 l/j.1610-0387.2008.06918.x. [DOI] [PubMed] [Google Scholar]
- 23.Moul DK, Korman NJ. The cutting edge. Severe hidradenitis suppurativa treated with adalimumab. Arch Dermatol. 2006;142:1110–1112. doi: 10.1001/archderm.142.9.1110. DOI: 10.1001/archderm.l42.9.1110. [DOI] [PubMed] [Google Scholar]
- 24.Scheinfeld N. Treatment of coincident seronegative arthritis and hidradentis supprativa with adalimumab. J Am Acad Dermatol. 2006;55:163–164. doi: 10.1016/j.jaad.2006.01.024. DOI: 10.1016/j.jaad.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 25.Sotiriou E, Apalla Z, Vakirlis E, Ioannides D. Efficacy of adalimumab in recalcitrant hidradenitis suppurativa. Eur J Dermatol. 2009;19:180–181. doi: 10.1684/ejd.2008.0599. DOI: 10.1684/ejd.2008.0599. [DOI] [PubMed] [Google Scholar]
- 26.Plessner HL, Lin PL, Kohno T, et al. Neutralization of tumor necrosis factor (TNF) by antibody but not TNF receptor fusion molecule exacerbates chronic murine tuberculosis. J Infect Dis. 2007;195:1643–1650. doi: 10.1086/517519. [DOI] [PubMed] [Google Scholar]