Abstract
Mitochondria of eukaryotic organisms contain populations of DNA molecules that are packed into higher-order structures called mitochondrial nucleoids (mt-nucleoids). In Saccharomyces cerevisiae, the compaction of mitochondrial DNA (mtDNA) into mt-nucleoids is mediated primarily by the high-mobility group (HMG) box-containing protein Abf2, which is an important player in stabilization and metabolism of mtDNA. Although it is evident that analogous proteins must exist in other yeast species, an apparently fast divergence rate has precluded their identification, characterization and comparative analysis. Using in silico analysis of the complete genome sequence of the pathogenic yeast Candida albicans we predicted that the ORF 19.400/19.8030 assigned as GCF1 encodes a putative mitochondrial HMG box-containing protein. In contrast to Abf2p, which contains two HMG boxes, Gcf1p contains only one C-terminal HMG box. In addition, it contains one putative coiled-coil domain with a potential role in protein dimerization. Fluorescence microscopy analysis of a C-terminally tagged Gcf1p with green fluorescent protein (GFP) revealed its mitochondrial localization in both heterologous (S. cerevisiae) and native (C. albicans) hosts. Biochemical analyses of DNA-binding properties indicate that Gcf1p is, similarly to Abf2p, a non-specific DNA-binding protein. To analyse the role of Gcf1p in mtDNA metabolism, we constructed strains lacking one functional allele of the GCF1 gene and carrying one GCF1 allele under the control of the MET3 promoter. Under repressible conditions this strain exhibited a more than 3000-fold decrease in levels of GCF1 mRNA, which was correlated with a substantial decrease in the number of mtDNA copies as well as recombination intermediates. The dramatic effect of reduced levels of Gcf1p on mtDNA metabolism indicates that the protein is involved in essential molecular transactions that relate to the mitochondrial genome.
INTRODUCTION
Similarly to its nuclear counterpart, mitochondrial DNA (mtDNA) is bound by a variety of proteins with different roles in stabilization, maintenance and inheritance of the genome (Kuroiwa, 1982). It is now generally accepted that the resulting DNA–protein structures [mitochondrial nucleoids (mt-nucleoids)] are fundamental segregating units of mtDNA (Okamoto et al., 1998; Zelenaya-Troitskaya et al., 1998; MacAlpine et al., 2000), and investigations of their structure and dynamics are essential for understanding the principles that govern mitochondrial inheritance (Chen & Butow, 2005; Kucej & Butow, 2007).
In Saccharomyces cerevisiae, the major mt-nucleoid component is the non-histone protein Abf2p (Caron et al., 1979; Diffley & Stillman, 1991, 1992). Abf2-like proteins, found in several yeast species (Miyakawa et al., 1996, 2000; Umezaki & Miyakawa, 2002), are tightly bound to mt-nucleoids and exhibit a high affinity for dsDNA (Miyakawa et al., 1995). In vitro, Abf2p bends the DNA backbone, resulting in compaction of dsDNA molecules into 190 nm structures resembling the mt-nucleoids (Brewer et al., 2003). The Abf2 protein belongs to the high-mobility-group (HMG) box superfamily containing two tandem HMG boxes responsible for the non-sequence-specific binding of Abf2p to the minor groove of DNA. Similarly to other members of the HMG box-containing protein family, Abf2p exhibits affinity for distorted DNA (e.g. Holliday junctions, DNA cruciforms, DNA bulges and crossovers in supercoiled DNA) and induces looping of linear DNA molecules (Diffley & Stillman, 1991, 1992; Brewer et al., 2003; Friddle et al., 2004). In vivo, Abf2p is essential for stabilization of mtDNA in cells grown on a fermentable carbon source such as glucose (Diffley & Stillman, 1991). In addition, it has been shown that Abf2p influences the level of yeast mtDNA recombination intermediates (MacAlpine et al., 1998) and is involved in remodelling of mt-nucleoids in response to changes in mitochondrial metabolism (Kucej et al., 2008).
The involvement of Abf2p in stabilization of S. cerevisiae mtDNA indicates that this HMG box-containing protein is an important component of the budding yeast mt-nucleoid. However, extensive searches through databases have not led to identification of an Abf2p homologue, even in relatively closely related yeast species (Nosek et al., 2006). The ability of otherwise unrelated HMG box-containing proteins such as bacterial protein HU, human mitochondrial transcriptional activator mtTF1/TFAM and mitochondrially targeted non-histone protein NHP6A to functionally replace Abf2p both in vivo and in vitro (Parisi et al., 1993; Kao et al., 1993; Megraw & Chae, 1993) suggests that the presence of the HMG box is sufficient for stabilization of mtDNA. Therefore, it is evident that in different phylogenetic branches the roles of Abf2p have been adopted by unrelated HMG box-containing proteins.
In Podospora anserina, the mitochondrial HMG-like protein mthmg1 has been shown to protect mtDNA from deletions (Dequard-Chablat & Allandt, 2002). With the exception of two HMG boxes, mthmg1 does not display significant homology with any mitochondrial HMG box-containing protein (mtHMG). The repertoire of currently known mtDNA packaging proteins was recently expanded by a protein named Glom, purified from the mt-nucleoids of the slime mould Physarum polycephalum. Glom has a lysine-rich region with a proline-rich domain in the N-terminal half and two HMG boxes in the C-terminal half (Sasaki et al., 2003). Again, with the exception of HMG boxes, Glom does not exhibit homology with Abf2p.
Recently, we have performed a comparative analysis of homologues of the components of mt-nucleoids, especially proteins containing HMG boxes fulfilling the role(s) of Abf2p (Nosek et al., 2006). We were able to identify Abf2p homologues only in species closely related to Saccharomyces spp. Even in these cases, homology between the corresponding proteins is low (e.g. 28.5 % identity between S. cerevisiae and Kluyveromyces lactis Abf2p) compared with that of other mt-nucleoid proteins (Nosek et al., 2006). Analysis of available genome sequences does not reveal a clear sequence homologue of Abf2p in species below the Ashbya gossypii branch on the phylogenetic tree, indicating that species such as Candida albicans, Candida parapsilosis, Debaryomyces hansenii, Yarrowia lipolytica and Schizosaccharomyces pombe employ alternative modes of mtDNA packaging. This divergence might be caused by dramatic alterations in the Abf2p sequence without affecting its function. According to an alternative possibility, another mtHMG might have been recruited to ensure mtDNA packaging. Indeed, in C. albicans and D. hansenii we have found ORFs encoding proteins [EAK92709 in C. albicans (CaO19.400, CaO19.8030; GCF1); CAG90643 in D. hansenii (DEHA0G15059 g)] containing at least one HMG box, one coiled-coil domain and a putative mitochondrial N-terminal leader sequence (Nosek et al., 2006). There are two interesting features exhibited by both proteins: (i) their lengths (245 aa in CaGcf1p; 275 aa in DhGcf1) are similar to that of the human mtTF1 (246 aa), and (ii) they are relatively lysine-rich, analogously to the N-terminal domain of the mtDNA packaging protein (Glom) of the slime mould (Sasaki et al., 2003). SMART analysis revealed that, in contrast to Abf2p, mtTF1, mthmg1, Glom and DhGcf1p, CaGcf1p contains only one HMG box, indicating that in C. albicans a single HMG box is sufficient for mtDNA packaging. Gcf1 protein thus seems to represent a member of a novel class of mtDNA packaging proteins, and therefore we decided to pursue a study of its role in the maintenance of C. albicans mtDNA. Importantly, a homologue of Gcf1p has been subsequently identified biochemically as a component of the mt-nucleoid of C. parapsilosis (Miyakawa et al., 2009).
In this work we performed in silico analysis of mtHMGs and analysed biochemical properties and physiological functions of the Gcf1 protein in C. albicans. Using green fluorescent protein (GFP) fused to the C terminus of Gcf1p we demonstrated that it is targeted to mitochondria of both its native (C. albicans) and heterologous (S. cerevisiae) hosts. We have analysed DNA-binding properties of Gcf1p using its recombinant version expressed in Escherichia coli. We tested the ability of GCF1 to complement Δabf2 mutation in S. cerevisiae. Finally, we analysed the consequences of deletion of GCF1 in C. albicans on mtDNA metabolism in this pathogenic yeast.
METHODS
Yeast strains.
C. albicans CBS562NT was provided by Dr H. Fukuhara (Institut Curie, Orsay, France). C. albicans CAI4 (ura3 : : λimm434/ura3 : : λimm434) was provided by Dr A. J. Mitchell (Columbia University, New York, USA). S. cerevisiae strain GG595 (MATa/MATα ura3−/ura3−) was obtained from dr. H. Y. Steensma (Leiden University, The Netherlands), and strain YAM 101 (MATa/MATα Δabf2 : : TRP1/ABF2 ade2-1/ade2-1 ura3-1/ura3-1 his3-11,15/his3-11,15 trp1-1/trp1-1 leu2-3, 112/leu2-3,112 canl-100/canl-100) was provided by Dr D. A. Clayton (Howard Hughes Medical Institute, Chevy Chase, USA). The haploid strains used for complementation experiments YAM 101-1a (MATa ABF2 ade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 canl-100) and YAM 101-1b (MATa Δabf2 : : TRP1 ade2-1 ura3-1 his3-11,15 leu2-3,112 canl-100) were prepared by tetrad dissection of the strain YAM 101. Yeast cultures were grown in YPD medium [1 % (w/v) yeast extract (Difco), 2 % (w/v) Bacto Peptone (Difco), 2 % (w/v) glucose] at 28 °C. In the case of C. albicans, media were supplemented with 80 μg uridine ml−1. Synthetic medium was 0.17 % (w/v) Yeast Nitrogen Base without amino acids and ammonium sulfate (Difco), 0.5 % (w/v) ammonium sulfate (Lachema) containing 2 % (w/v) glucose (SD), 3 % (v/v) glycerol (SG) or 2 % (w/v) galactose (SGal). Transformation of S. cerevisiae was done as described by Gietz & Schiestl (1995). Electroporation of C. albicans was performed according to the protocol of De Backer et al. (1999).
DNA manipulations.
The oligonucleotides listed in Table 1 were synthesized by Invitrogen or Sigma-Aldrich. PCRs (20–50 μl) were performed using 1 U Vent DNA polymerase (New England Biolabs) or Taq DNA polymerase (Invitrogen) and contained each dNTP at 200 μM, corresponding primers at 1 μM and 100 ng genomic DNA or 1 ng plasmid DNA. PCR fragments were purified from agarose gels using a Qiagen Gel Extraction kit. Southern blot analysis was performed as described by Sambrook & Russell (2001). DNA-modifying enzymes were used according to the instructions provided by the corresponding suppliers.
Table 1.
Oligonucleotides used in this study
| Name | Sequence | Application* |
|---|---|---|
| CA_HMG_F+START | ATGTTGAGATCATTTGTAACATC (23-mer) | 1, 3 |
| CA_HMG_R | AAAGTCATCCTCCACTTTGTAG (22-mer) | 1, 4 |
| CA_HMG_R+STOP | TCAAAAGTCATCCTCCACTTTG (22-mer) | 1 |
| 5BamORF | ATGGATCCATGTTGAGATCATTTGTAACA (29-mer) | 1, 2 |
| REsp3ORF | ATCGTCTCTACATAAAGTCATCCTCCACTTTGTA (34-mer) | 1 |
| 5GFPEsp3 | ATCGTCTCAATGTCTAAAGGTGAAGAATTA (30-mer) | 1, 3 |
| 3PstGFP | ATCTGCAGTTATTTGTACAATTCATCCAT (29-mer) | 1 |
| 3PstORF | ATCTGCAGGTCAGTAATTCACTTTTCCGG (29-mer) | 1, 3, 4 |
| R3OUT | ATGAGCTCTATTTCTCGAATCAGTTGATT (29-mer) | 1, 2, 3 |
| D3orf | ATTGCGGCCGCCTCAATCAACTAAAAGAGGTC (32-mer) | 1 |
| R5orf | ATACTCGAGAGATGAACAACTAAAATTGAC (30-mer) | 1 |
| D5OUT | ATGGGCCCACTAATATTTTACCTGCAGGG (29-mer) | 1 |
| pMET | ATGAAAAGACAAGAGAGC (18-mer) | 4 |
| GFP | AGATGGTGATGTTAATGG (18-mer) | 4 |
| pGEX5 | GGCAAGCCACGTTTGGTG (18-mer) | 4 |
| pGEX3 | GGAGCTGCATGTGTCAGAGG (20-mer) | 4 |
| 5ORFterm | ATCTGCAGAGAACTCAATCAACTAAAA (27-mer) | 3 |
| D3OUT | ATTGCGGCCGCCGATGGATGGATGACGAGAAC (32-mer) | 2 |
| IMH3OUT | AGAGGTATGGGTTCCATCGAT (21-mer) | 3 |
| 3PstKO | ATCTGCAGTGCCTTCTCTTCCTCGGGGAA (29-mer) | 2 |
| CaAbf2Rev | TCAATAGTCAAATAAAGTAGC (21-mer) | 3 |
| CaUra3in3 | TAATTGGCCAGTCTTTTTCAAATAAGC (27-mer) | 2 |
| CamtHMG1in5 | GTGGTACCATCACCGACTTCA (21-mer) | 3 |
| CamtHMG1in3 | ATCCGCAGCAACCGTCAATT (20-mer) | 3 |
| MPAout3 | AGATCCACTAGTTCTAGAGCGGCC (24-mer) | 3 |
| MPAout5 | CTAGAAAGTATAGGAACTTCCTCGAG (26-mer) | 3 |
| D5R5in | ATATTCTCCATTATACTTTGTATTGG (26-mer) | 2 |
| D3R3in | AACTATGGTCAAAAGGGTCTAAAAA (25-mer) | 2 |
| CanEco31 | ACGTGGTCTCATGGAATTAACACCCACACAG (31-mer) | 2 |
| Can3CBam | GCGGATCCTAAATTAGTCGACTAGT (25-mer) | 2 |
| 5RTcaAct1 | TGTCTTTGTACTCTTCTGGTAGAAC (25-mer) | 5 |
| 3RtcaAct1 | CTAGTAGTGAAACTGTAACCACGTT (25-mer) | 5 |
| 5RTcaGCF1 | CAAAAGCTATTTTACTCCTAAACCA (25-mer) | 5 |
| 3RTcaGCF1 | ATCTTCTTTACTGATTTGGTATTGC (25-mer) | 5 |
| 5RTcaNAD2 | TTCAAAATGGGATTAGCACCTT (22-mer) | 5 |
| 3RTcaNAD2 | TGTATATGCTGCCACGGCTA (20-mer) | 5 |
*1, PCR amplification for cloning; 2, PCR amplification to prepare probe for Southern blot hybridization; 3, PCR verification; 4, sequencing; 5, qRT-PCR.
Construction of plasmids.
For expression in E. coli, GCF1 amplified from genomic DNA using primers CA_HMG_F+START and CA_HMG_R+STOP was cloned into pGEX-2T (Amersham-Pharmacia) linearized with EcoRI and blunt-ended with Klenow fragment of DNA polymerase I. For in vivo localization in S. cerevisiae, the PCR fragment obtained by amplification of GCF1 using primers CA_HMG_F+START and CA_HMG_R was ligated into pUG35 (Niedenthal et al., 1996; provided by Dr J. H. Hegemann, Heinrich-Heine-Universität, Düsseldorf, Germany) linearized with SmaI. For in vivo localization in C. albicans, GCF1 was PCR-amplified using primers 5BamORF and REsp3ORF and digested with BamHI and Esp3I. The ORF encoding an enhanced version 3 of yeast GFP (yEGFP3) was PCR-amplified using the primers 5GFPEsp3 and 3PstGFP and digested with PstI and Esp3I. Both PCR products were ligated into the pCaExp vector (kindly provided Dr P. E. Sudbery, Sheffield University, UK) digested with BamHI and PstI. For complementation experiments in S. cerevisiae, GCF1 was PCR-amplified using primers CA_HMG_F+START and CA_HMG_R+STOP, and ligated into pYES2/CT (Invitrogen) linearized with EcoRI and blunt-ended with Klenow fragment of DNA polymerase I. All plasmid constructs were verified by restriction enzyme mapping as well as by DNA sequence analysis.
Complementation of the Δabf2 mutant of S. cerevisiae by GCF1.
Vectors pYES2/CT and pYES/2CT-GCF1 were transformed into S. cerevisiae strains ABF2 and Δabf2 and cells were plated on minimal glycerol medium. Cells from individual clones were inoculated into 2 ml minimal glycerol medium and cultivated for 24 h at 28 °C. Minimal media containing glucose, glycerol or galactose were inoculated from the pre-culture at a concentration of 1×105 cells ml−1. The cell suspensions were cultivated for 24 h at 28 °C, and cells (200 cells per plate) were spread on minimal glucose and glycerol medium and cultivated for 3 days at 28 °C. Frequency of standard (grande) and petite colonies was determined by triphenyl tetrazolium chloride (TTC) assay (Ogur et al., 1957).
Purification of glutathione S-transferase (GST) fusion protein.
Recombinant GST–Gcf1 protein was purified as either GST fusion or native protein. An overnight bacterial culture (20 ml) was inoculated into 1 l LBA medium [3.2 % (w/v) tryptone (FisherBiotech), 2 % (w/v) yeast extract (Difco), 0.5 % (w/v) NaCl, ampicillin (100 μg ml−1)] and grown at 37 °C to final OD600 0.7. The culture was then induced for 3 h at 30 °C with 1 mM IPTG (Sigma). Cells were washed once with ice-cold double-distilled water, resuspended in 20 ml buffer G [20 mM Tris/HCl, pH 7.4, 100 mM NaCl, 0.1 % Triton X-100, 10 mM 2-mercaptoethanol, protease inhibitor mixture (Complete, Boehringer Mannheim), 50 U DNase I (Promega)], sonicated, and centrifuged at 25 000 g for 30 min at 4 °C to remove insoluble material. The supernatant was mixed with 0.5 ml glutathione-agarose (Sigma) prewashed with buffer G, and incubated for 60 min on ice with occasional mixing. Beads were loaded onto a 5 ml column, and washed with 100 ml buffer G (without protease inhibitor mixture) followed by 50 ml ice-cold solution A (20 mM Tris/HCl, pH 7.4, 100 mM NaCl). The washed beads were resuspended in 1 ml solution A [supplemented with 2.5 mM CaCl2 and RNase A (75 μg ml−1)] containing thrombin (0.05 U μl−1, Amersham Biosciences) and incubated overnight at 4 °C. The cleaved Gcf1p was eluted five times in 1.0 ml solution A and stored at −80 °C. GST–Gcf1 fusion protein and GST were eluted from the column by 50 mM glutathione in solution A. Fractions were assayed for purity by SDS-PAGE (Laemmli, 1970) and stained with Coomassie Brilliant Blue.
DNA-binding assays in vitro.
Gel retardation assays were performed as described by Sasaki et al. (2003). In brief, purified GST–Gcf1 or Gcf1 protein (20–200 ng) was mixed with NdeI-digested C. albicans mtDNA (purified as described by Defontaine et al., 1991). The samples were incubated for 1 h in DNA-binding buffer (20 mM Tris/HCl, pH 7.4, 1 mM EDTA, 50 mM glutathione) at room temperature and then loaded onto a 0.8 % (w/v) agarose gel. DNA fragments and DNA–protein complexes were separated at 5–10 V cm−1 for 45–60 min in 45 mM Tris-borate,1 mM EDTA (0.5× TBE) buffer. After electrophoresis, the agarose gels were stained for 20 min with ethidium bromide (0.5 μg ml−1) followed by 10 min destaining in distilled water. For radioactive electrophoretic mobility shift assays (EMSAs) (Fig. 3d), C. albicans mtDNA was digested with a combination of BamHI and PvuII and dephoshorylated with shrimp alkaline phosphatase, and the DNA fragments were labelled by T4 polynucleotide kinase using [γ-32P]ATP as a substrate. DNA-binding assays were performed as described above. Nonlabelled mtDNA digested with a combination of BamHI and PvuII was used as a DNA competitor.
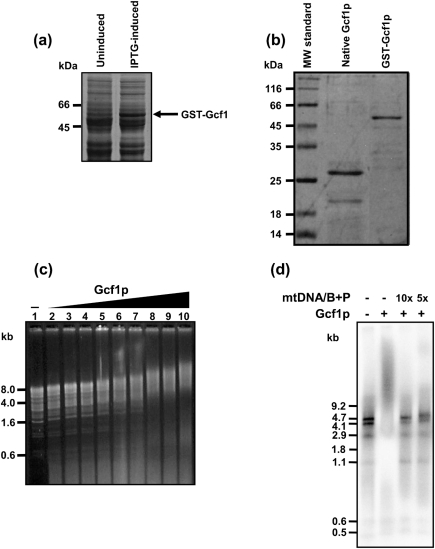
Fig. 3.
Gcf1 is a DNA-binding protein. GCF1 was expressed in E. coli as native or GST-fusion protein. (a) In contrast to uninduced cultures, crude protein extracts obtained from IPTG-induced cells contain a polypeptide (arrow) with a molecular mass corresponding to that of the GST–Gcf1 fusion protein. (b) Native (thrombin-cleaved) Gcf1p and GST–Gcf1p after purification on glutathione-agarose. (c) Gcf1 (40, 80, 120, 160, 200, 240, 280, 320 and 360 ng; lanes 2–10) protein was added to 300 ng NdeI-digested mtDNA of C. albicans. Lane 1, NdeI-digested mtDNA of C. albicans without addition of Gcf1p. Purified GST was used as a negative control (data not shown). (d) GST–Gcf1p (50 ng) was incubated with 40 ng terminally labelled fragments of mtDNA of C. albicans obtained by restriction with a combination of BamHI and PvuII in the absence or presence of nonlabelled mtDNA, as indicated. The numbers above the lanes indicate the molar excess of the competitor DNA. B, BamHI; P, PvuII.
Construction of a strain with a conditional allele of GCF1.
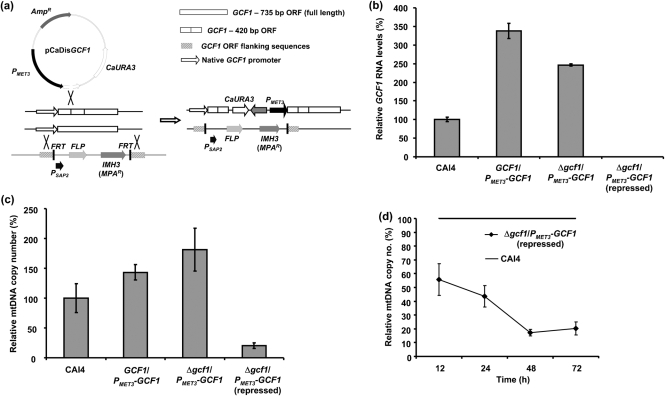
For the disruption of one allele, GCF1, 400 bp from the gene sequence was PCR-amplified using primers 5BamORF and 3PstORF, digested with BamHI and PstI, and ligated into the plasmid pCaDis (Care et al., 1999; the plasmid was kindly provided by Dr P. E. Sudbery, Sheffield University, UK) digested with the same restriction enzymes. The ligation mixture was transformed into E. coli DH5α and the plasmids were verified by restriction enzyme analysis. The resulting plasmid pCaDisGCF1 was digested with BstBI approximately in the middle of the inserted 400 bp and transformed into C. albicans strain CAI4 by electroporation. Homologous recombination yielded the GCF1 gene under control of the MET3 promoter (Fig. 4a). Transformants were verified by both PCR and Southern blot analyses, and the resulting strain was designated GCF1/PMET3–GCF1.
Fig. 4.
Schematic illustration of preparation of the strain carrying one conditional allele of GCF1, quantification of mRNA levels and mtDNA copy numbers. (a) One allele of GCF1 was disrupted by integration of plasmid pCaDisGCF1 into the GCF1 ORF by homologous recombination of a short coding region (400 bp). The second allele was replaced by the MPAR-FLP cassette containing sequences homologous to flanking sequences of the GCF1 ORF at both sides of the cassette. The arrow is pointing at a schematic structure of a double mutant of GCF1 lacking one allele and containing the MET3-driven second allele of GCF1. FRT, FLP recombination target; FLP, flip-flop recombinase; P, promoter. (b) Relative GCF1 mRNA levels of strains CAI4, GCF1/PMET3-GCF1 and Δgcf1/PMET3-GCF1 grown under derepressed and repressed conditions as determined by qRT-PCR, normalized using CaACT1 as a standard. GCF1/PMET3-GCF1 and Δgcf1/PMET3-GCF1 grown under derepressed conditions show two- to threefold increases in GCF1 mRNA compared with the wild-type, while repression of the MET3 promoter-controlled GCF1 allele resulted in an approximately 3200-fold decrease in GCF1 mRNA compared with the wild-type. Error bars, sd. (c) Relative mtDNA copy numbers of strains CAI4, GCF1/PMET3-GCF1 and Δgcf1/PMET3-GCF1 grown under derepressed and repressed conditions as determined by qRT-PCR; normalization was carried out using CaACT1 as a standard. Error bars, sd. In strains GCF1/PMET3-GCF1 and Δgcf1/PMET3-GCF1 grown under derepressed conditions, average mtDNA copy numbers are elevated by 25–50 %, whereas the strain Δgcf1/PMET3-GCF1 grown under repressed conditions for 48 h exhibits an approximately 80 % reduction in the number of mtDNA copies compared with the wild-type. (d) Relative mtDNA copy numbers of strain Δgcf1/PMET3-GCF1 grown under repressed conditions, and passaged every 12 h for 3 days, ensuring linear growth to OD600 1.0 at each sampling time point. Twelve hours of growth under repressed conditions already decreases mtDNA copy numbers by 40 %, and further passaging reveals a reduction to 20 % of wild-type level after 48 h. Error bars, sd. In (b), (c) and (d), results for the CAI4 wild-type strain were defined as 100 %.
For disruption of the second allele of GCF1, the sequences (1000 bp) flanking the GCF1 ORF were PCR-amplified using primers R5orf and D5OUT (5′ end) and R3OUT and D3orf (3′ end), and after digestion with XhoI and ApaI (5′ end) and SacI and NotI (3′ end) were ligated into the vector pSFI (provided by Dr J. Morschhäuser, Universität Würzburg, Germany) containing an MPAR-FLP cassette (Wirsching et al., 2000). The cassette enables selection of transformants based on their resistance to mycophenolic acid (MPA; final concentration 10 μg ml−1 in synthetic media) and removal of MPAR by expression of flip-flop (FLP) recombinase driven from the SAP2 promoter (Wirsching et al., 2000; Fig. 4a). The ligation mixture was transformed into E. coli DH5α and the resulting plasmids were verified by restriction enzyme analysis. The MPAR-FLP cassette containing flanking sequences was cut out of the plasmid with ApaI and SacI and used for electroporation of C. albicans strain GCF1/PMET3–GCF1 (see above). The transformants were verified by PCR and Southern blot analyses, and the strain carrying one conditional allele of GCF1 and lacking the second allele of GCF1 was named Δgcf1/PMET3–GCF1.
mtDNA purification.
C. albicans strains were grown to OD600=1.5 as described above. Mitochondria were purified according to Daum et al. (1982) with slight modifications. In brief, cells were harvested, washed once with H2O and once with 1.2 M sorbitol, and resuspended in Zymolase-buffer (1.2 M sorbitol, 20 mM KPi buffer, pH 7.4, to 1 ml per 0.15 g wet weight cells). Zymolase 20T was added to 8 mg per g of cells (wet weight) and incubated at 30 °C for 10–15 min until conversion of cells to spheroplasts. After centrifugation, spheroplasts were resuspended in homogenization buffer [10 mM Tris/HCl, pH 7.4, 1 mM EDTA, 0.6 M sorbitol, 0.2 % (w/v) BSA, 1 mM PMSF] and lysed by 20 strokes in a tight fitting Dounce homogenizer. Lysates were centrifuged twice at 3500 r.p.m. in an SS34 rotor (Sorvall) for 5 min at 4 °C, and the resulting supernatant once at 9000 r.p.m. in an SS34 rotor for 15 min. The crude mitochondrial pellet was resuspended in lysis buffer (50 mM EDTA, 10 mM Tris/HCl, pH 7.4, 75 mM NaCl). SDS was added to a final concentration of 1 % (w/v) and the mixture was kept on ice for 10 min. Subsequently, proteinase K was added to a final concentration of 0.5 mg ml−1, and the samples were incubated at 37 °C for 30 min. Lysates were extracted with phenol : chloroform (1 : 1), DNA was ethanol-precipitated, washed and dried, and finally resuspended in T10E1 buffer (10 mM Tris/HCl, pH 7.4, 1 mM EDTA), aliquoted and stored at −70 °C until use. Samples (10 μg) of mtDNA were subjected to restriction enzyme digestions according to the manufacturer's protocols (Fermentas) and separated by 2D agarose gel electrophoresis (2D-AGE).
2D-AGE, hybridization and detection of mtDNA recombination and replication intermediates.
Neutral 2D-AGE was carried out as described elsewhere (Brewer & Fangman, 1987). Fragments between 5.5 and 11 kb were separated on 0.3 % (w/v) 1× TBE-agarose gels at 0.9 V cm−1 and room temperature for 24 h in the first dimension, followed by separation on 0.8 % (w/v) 1× TBE-agarose gels containing ethidium bromide (300 ng ml−1) at 3 V cm−1 and 4 °C for 15 h in the second dimension. Southern blots of 2D gels were hybridized with specific PCR probes of C. albicans mtDNA as indicated (Table 1) for 3–4 h at 65 °C in 7 % (w/v) SDS, 250 mM NaHPO4 (pH 7.2), 1 mM EDTA and 0.1 % (w/v) BSA (Church & Gilbert, 1984). Blots were then washed twice for 15 min at 65 °C with wash buffer [5 % (w/v) SDS, 40 mM NaHPO4 (pH 7.2), 1 mM EDTA]. Filters were exposed to X-ray films for 1–14 days or to Storage Phosphor Screens (GE Healthcare) for 12–72 h. Signal detection of screens was performed with a Typhoon Trio Phosphor Imager (GE Healthcare).
Purification of total cellular DNA and RNA and quantitative real-time RT-PCR (qRT-PCR).
C. albicans strains were grown as described above and total DNA was isolated according to our standard procedure (Sedman et al., 2005). Total RNA was purified from C. albicans strains as follows. Cultures (3 ml) were grown to mid-exponential phase as described above, harvested and resuspended in 100 μl ice-cold RNA-buffer (50 mM Tris/HCl, pH 7.4, 100 mM NaCl, 10 mM EDTA). Glass beads (50 μl) were added and the samples were vortexed at maximum speed for 3 min at 4 °C. RNA-buffer [450 μl containing 1 % (w/v) SDS] and 450 ml equilibrated phenol were added, and the samples were vortexed as described above followed by centrifugation for 10 min at 4 °C, precipitation of RNA with an equal volume of 2-propanol for 60 min at room temperature, sedimentation of RNA by centrifugation and resuspension of the resulting RNA in RNase-free water. Total RNA samples (5 μg) were treated with DNase I (Fermentas), and 1 μg of the resulting RNA was used in reverse transcriptase reactions according to the supplier's protocol (Fermentas). cDNA (50 ng) or total cellular DNA (50 ng), were analysed by qRT-PCR using the AB 7500 Real-Time PCR System (Applied Biosystems; conditions: 40 cycles; 95 °C 20 s, 60 °C 30 s, 72 °C 33 s). Maxima SYBR Green qPCR Master Mix (Fermentas) was used to amplify a 170 bp portion of GCF1, CaACT1 or CaNAD2 using RT-PCR primers according to Table 1. For all independent RNA preparations, DNase I-treated RNA without reverse transcriptase was included as a control in qRT-PCR experiments to exclude false-positive results due to DNA contamination (Maxima standard protocol, Fermentas). All experiments were performed on four biological replicates for each strain and were analysed in triplicates.
Fluorescence microscopy.
S. cerevisiae strain GG595 was transformed with pUG35-GCF1 or pUG35, and cells of C. albicans CAI4 were transformed with pGCF1-GFP digested with StuI (in the RP10 gene), allowing its insertion into the genome. Cells were cultivated in synthetic glucose medium for 24 h at 28 °C. The cells were centrifuged at 3000 g for 5 min, and washed three times with sterile distilled water. 4′,6-diamidino-2-phenylindole (DAPI) was added to a final concentration of 1 μg ml−1 and the cell suspension was incubated for 1 h at room temperature. The cells were visualized by an Olympus BX50 microscope equipped with a DP70 camera (Olympus Optical) and an appropriate filter set.
In silico analyses.
Homologues of CaGcf1 protein (orf19.400/orf19.8030) were identified in the Candida database of the Broad Institute (http://www.broad.mit.edu/annotation/genome/candida_group/MultiHome.html) using the blastp algorithm at default settings (i.e. E value <10−3, BLOSUM62 matrix). The query revealed a single hit per genome in C. albicans, Candida guilliermondii, Candida lusitaniae, C. parapsilosis, Candida tropicalis, D. hansenii and Lodderomyces elongisporus. Similarly, TBlastN searches identified single candidates in assembled genome contigs of Candida dubliniensis, C. parapsilosis (The Wellcome Trust Sanger Institute; http://www.sanger.ac.uk/Projects/Fungi/), and Pichia stipitis (DOE Joint Genome Institute; http://genome.jgi-psf.org/Picst3/Picst3.home.html). The sequence alignments and comparisons were done using the AlignX utility of the Vector NTI 10.1.1 package (Invitrogen) and the GeneDoc program (Nicholas et al., 1997). Mitochondrial import sequences were detected using MitoProt II (Claros & Vincens, 1996; http://ihg2.helmholtz-muenchen.de/ihg/mitoprot.html). The potential to form coiled-coil domains based on the analysis of heptad repeats in three windows of 14, 21 and 28 amino acids was calculated with PCOILS (http://toolkit.tuebingen.mpg.de/pcoils/) using the MTIDK matrix. The HMG boxes were predicted using SMART (http://dylan.embl-heidelberg.de/; Letunic et al., 2006) and InterProScan (http://www.ebi.ac.uk/Tools/InterProScan/). Phylogenetic analyses were performed using the mega 4 software package (Tamura et al., 2007).
RESULTS AND DISCUSSION
In silico analysis of mtHMGs
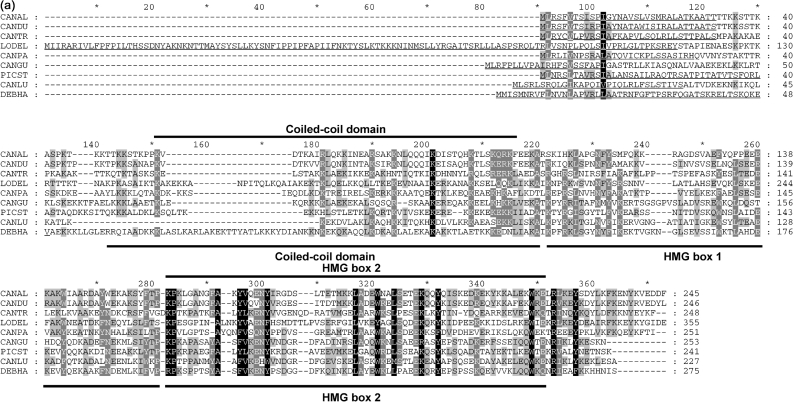
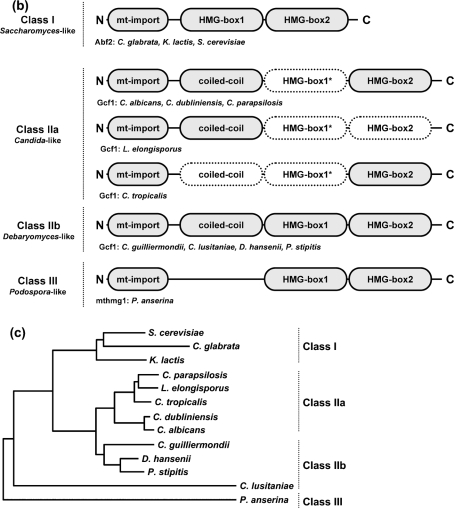
With the aim of identifying putative mitochondrial functional homologues of S. cerevisiae Abf2 protein in non-Saccharomyces species, we first performed an in silico analysis of available genomic sequences. We identified a putative mitochondrial HMG box-containing protein encoded by the genome of D. hansenii (DhGcf1p) and subsequently its homologue in C. albicans (CaGcf1p) using the sequence of the HMG box of the Abf2 protein of K. lactis as a query (Nosek et al., 2006). Following inspection of available genomic sequences, we identified homologous sequences in the genomes of C. dubliniensis, C. guilliermondii, C. parapsilosis, C. tropicalis, L. elongisporus and P. stipitis (Fig. 1a). Importantly, a Gcf1 homologue was also identified by proteomic analysis of mt-nucleoids in C. parapsilosis (Miyakawa et al., 2009). In contrast to their D. hansenii counterpart, which contains two putative HMG boxes, Gcf1p of C. albicans contains only one region with clear HMG sequence motifs. Comparison of amino acid sequences of several yeast mitochondrial HMG box-containing proteins indicates a relatively high rate of evolutionary diversification, and suggests that they can be separated into several families based on the number of putative HMG boxes as well as the presence/absence of a coiled-coil domain supposedly involved in protein dimerization (Fig. 1b). Thus, acquisition of the second HMG box may have been a prerequisite for loss of the coiled-coil domain in the S. cerevisiae group. Based on these data, we suggest naming the group of all mtHMGs the mtHMG superfamily and, based on their modular structure (Fig. 1b), dividing it into four groups, namely class I (Saccharomyces Abf2-like), class IIa (Candida Gcf1-like), class IIb (Debaryomyces Gcf1-like) and class III (Podospora mthmg1-like). These subfamilies are consistent with the distribution of the corresponding species on the phylogenetic tree (Fig. 1c). The division of class II into two subclasses is based on the fact that all members of class IIb mtHMGs contain all three modules found in HMG box proteins, while the HMG box 1 was detected neither by SMART nor by the InterProScan utility in any of the class IIa proteins, indicating that this domain rapidly accumulates changes within this region. We suggest that the class IIb members represent an ancestral type of mtHMG.
Fig. 1.
In silico analysis of mtHMGs. (a) Amino acid sequence alignment of yeast mtHMGs (class II members). The sequences were aligned using the AlignX utility of the Vector NTI 10.1.1 program package (Invitrogen) and the GeneDoc utility (Nicholas et al., 1997). CANAL, C. albicans; CANDU, C. dubliniensis; CANGU, C. guilliermondii; CANLU, C. lusitaniae; CANPA, C. parapsilosis; CANTR, C. tropicalis; DEBHA, D. hansenii; LODEL, L. elongisporus; PICST, P. stipitis. Positions of coiled-coil domain and HMG boxes in CaGcf1 (CANAL; subclass IIa) and DhGcf1 (DEBHA; subclass IIb) predicted by SMART are indicated above and below the alignment, respectively. Mitochondrial import presequences identified using MitoProtII are underlined. The probabilities of protein import into mitochondria were 0.9939 (CANAL), 0.9976 (CANDU), 0.9378 (CANGU), 0.8854 (CANLU), 0.9673 (CANPA), 0.6683 (CANTR), 0.9763 (DEBHA), 0.9986 (LODEL) and 0.9446 (PICST). (b) Modular structures of fungal mtHMGs. Domains predicted with low confidence are shown with dotted borders. The coiled-coil domain in the N-terminal region of CANAL, CANDU, CANGU, CANLU, CANPA, DEBHA, LODEL and PICST proteins was predicted by PCOILS with probabilities of 0.9–1.0 in at least two heptad repeat frames (i.e. 14, 21 and 28 amino acids). In the C. tropicalis homologue the predicted PCOILS probabilities were significantly lower (<0.6, 0.7 and 0.8 for frames 14, 21 and 28, respectively). The HMG boxes (Pfam and SMART accession numbers are PF00505 and SM00398, respectively) were predicted using SMART (normal mode) and InterProScan at default settings. The region corresponding to HMG box 1 is weakly conserved among the members of class II. This domain has not been detected by SMART and InterProScan in any of the subclass IIa proteins. Note that in the case of L. elongisporus the prediction of HMG box 2 was below the threshold, but its presence was inferred from the sequence homology. The three classes of fungal mtHMGs were defined using the protein length and the presence of protein domains. (c) Phylogenetic trees of fungal species with known mtDNA packaging proteins. The neighbour-joining tree was calculated from the D1/D2 sequences of the large subunit rRNA gene. The two groups within class II correspond to the split on the phylogenetic tree, which also supports the idea that subclass IIb architecture is ancestral to the subclass I and IIa proteins.
Gcf1 protein is localized into mitochondria of both C. albicans and S. cerevisiae
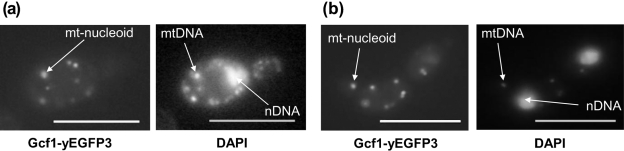
In silico analysis of Gcf1 amino acid sequences indicated the presence of an N-terminal mitochondrial targeting sequence (psort II and MITOPROT indicated 69.6 and 99.39 % probability of mitochondrial localization, respectively). To verify that the protein is targeted to mitochondria, we constructed two plasmids carrying Gcf1 with C-terminally fused GFP. The first plasmid, pUG35-GCF1, was designed for expression of the fusion proteins in S. cerevisiae. Fig. 2(a) shows that when expressed in S. cerevisiae, Gcf1p targets GFP into mitochondria, while the GFP without Gcf1 at the N terminus is localized in the cytoplasm (data not shown). Similarly, when the Gcf1–GFP fusion protein was expressed in C. albicans using the plasmid pGCF1-GFP as expression vector, most of the fluorescence was concentrated into the cellular compartments whose co-staining with DAPI indicates that they represent mitochondria (Fig. 2b).
Fig. 2.
Gcf1 co-localizes with mtDNA. Co-localization of fusion protein Gcf1–GFP with mtDNA in the cells of S. cerevisiae (a) and C. albicans (b) visualized by fluorescence microscopy. The cells expressing Gcf1–GFP were stained with DAPI as described in Methods. Bars, 10 μm.
After our in silico identification (Nosek et al., 2006), the Gcf1 protein was implicated in regulation of promoter activity of HWP1, a gene encoding a hyphae-specific adhesin in C. albicans (Kim et al., 2007). There are two possible explanations for this observation. Gcf1p may exhibit dual (mitochondrial and nuclear) localization and thus fulfil two distinct roles in the cell. The nuclear isoform may participate in gene regulation and the mitochondrial isoform in packaging of mtDNA and/or mtDNA metabolism. However, because Kim et al. (2007) used crude protein extracts as a source for purification of proteins binding to the promoter region, the binding of Gcf1p to the nuclear sequence might have been artefactual. For example, Gcf1p as a non-specific DNA-binding protein could have been released from mitochondria upon preparation of protein extracts, resulting in its binding to the Hwp1 promoter in vitro. In either case, our experiments clearly demonstrate mitochondrial localization of Gcf1p and thus indicate its role in mtDNA packaging and/or metabolism.
Mitochondrially targeted HMG box proteins seem to be the fastest evolving component of mt-nucleoids (Miyakawa et al., 2009). Therefore, it is difficult to reconstruct the phylogenetic relationships among the various members of the mtHMG family. To circumvent this problem we compared the HMG boxes of selected members of the mtHMG superfamily, including Gcf1, with the HMG boxes of various HMG1-containing proteins, in a similar manner to that reported by Baxevanis & Landsman (1995). The phylogenetic analysis revealed that the CaGcf1 HMG box clusters together with the CpGcf1 HMG1 box and one of the two HMG boxes of the DhGcf1 protein (DEBHA_Gcf1.2) (Supplementary Fig. S1). Although some branches of the phylogenetic tree must be taken with caution due to the weak homology, the presence of the second DhGcf1 HMG box (DEBHA_Gcf1.1) on a different branch and the relatively distant position of the Saccharomyces Abf2-like HMG boxes underline the relatively fast evolution, apparently due to modification of the HMG boxes, in proteins involved in mtDNA packaging.
Recombinant Gcf1 protein binds DNA in vitro
To demonstrate that Gcf1 is a DNA-binding protein, the entire ORF was expressed in E. coli in fusion with N-terminally located GST (Fig. 3a). The crude protein fraction was loaded onto glutathione agarose, and Gcf1 was eluted either with glutathione (GST–Gcf1) or by thrombin digestion (native Gcf1) (Fig. 3b). To assess the DNA-binding properties of Gcf1p we employed an assay used by Sasaki et al. (2003), which demonstrates preferential binding of Glom protein of P. polycephalum to certain regions of mtDNA. When we used purified mtDNA of C. albicans as a substrate, both GST–Gcf1 and native Gcf1 proteins exhibited DNA-binding activity in vitro when assayed by EMSA in agarose gels (Fig. 3c). GST alone at the same protein concentrations and under the same reaction conditions did not bind to DNA (data not shown). Using titrations of the Gcf1 protein in DNA-binding reactions we did not observe preferential binding to any of the restriction fragments of C. albicans mtDNA. Similar results were obtained when we used digested plasmid DNA instead of mtDNA as a substrate (data not shown). Furthermore, we used radioactively labelled mtDNA fragments in the EMSA assay. When Gcf1p was incubated with digested and labelled mtDNA, most of the fragments dramatically reduced their mobility in agarose gels (Fig. 3d, lanes 1 and 2). Shorter fragments were shifted less efficiently, possibly indicating a cooperative binding of Gcf1p. Ten- and fivefold molar excess of unlabelled mtDNA fragments competed quantitatively with the labelled sample for complex formation (Fig. 3d, lanes 3 and 4)
There are two limitations of the interpretations of these in vitro data. First, Gcf1p expressed in E. coli may lack appropriate post-translational modifications, which may affect its biochemical properties in C. albicans. Second, the CUG codon in C. albicans encodes serine instead of leucine. In the case of GCF1 there is a single CUG codon at position 21. However, substitution of serine for leucine at this position should not affect the DNA-binding properties of Gcf1p, since it lies within the N-terminal mitochondrial-targeting peptide and relatively far from the putative coiled-coil domain. In addition, note that the Ser21→Leu21 substitution does not affect mitochondrial targeting in S. cerevisiae (Fig. 2a). Taken together, our results indicate that Gcf1p, similarly to Abf2p, is a non-specific DNA-binding protein potentially involved in packaging of C. albicans mtDNA.
Complementation of S. cerevisiae Δabf2 mutant by GCF1
To analyse the functional similarity of the Gcf1 protein to S. cerevisiae Abf2p, we transformed wild-type as well as Δabf2-mutant S. cerevisiae strains with the expression plasmid pYES/2CT carrying GCF1 under the control of the GAL1 promoter. We took advantage of the fact that whereas cells lacking Abf2p rapidly lose mtDNA when grown on fermentable carbon sources (e.g. glucose or galactose), they remain respiratory-competent on a non-fermentable carbon source (e.g., glycerol) due to the activity of Aco1 protein (Chen et al., 2005). We observed that expression of GCF1 almost completely prevented loss of mtDNA when the transformants were grown on synthetic media containing galactose (Table 2). Similar results were obtained on media containing glycerol, which is consistent with the derepression of the GAL1 promoter under these conditions. In contrast, propagation of the transformants on glucose media resulted in a massive loss of mtDNA. These results indicate that Gcf1p function is able to partially substitute the function of the Abf2 protein in S. cerevisiae. Interestingly, galactose-induced expression of Gcf1 in a S. cerevisiae strain containing a wild-type ABF2 allele substantially increased the frequency of petite colonies (Table 2). Similar results were obtained when we performed the complementation experiment with GCF1 from C. parapsilosis (Supplementary Table S1). This suggests that co-expression of ABF2 and GCF1 leads to competition of these proteins for mtDNA substrate and a subsequent destabilization of the mitochondrial genome.
Table 2.
Complementation of S. cerevisiae Δabf2 mutant by GCF1
| Strain | ABF2 allele | Plasmid | Medium* | Number of colonies (%) | |
|---|---|---|---|---|---|
| Grande | Petite | ||||
| YAM 101-1a | ABF2 | pYES2/CT | SD | 99.7±0.3 | 0.3±0.3 |
| YAM 101-1a | ABF2 | pYES2/CT | SG | 98.2±1.6 | 1.8±1.6 |
| YAM 101-1a | ABF2 | pYES2/CT | SGal | 94.0±6.1 | 6.1±6.1 |
| YAM 101-1a | ABF2 | pYES2/CT-GCF1 | SD | 99.2±0.9 | 0.9±0.9 |
| YAM 101-1a | ABF2 | pYES2/CT-GCF1 | SG | 99.4±0.6 | 0.6±0.6 |
| YAM 101-1a | ABF2 | pYES2/CT-GCF1 | SGal | 48.3±2.4 | 51.7±2.4 |
| YAM 101-1b | Δabf2 | pYES2/CT | SD | 2.7±0.9 | 97.3±0.9 |
| YAM 101-1b | Δabf2 | pYES2/CT | SG | 22.2±20.7 | 77.8±20.7 |
| YAM 101-1b | Δabf2 | pYES2/CT | SGal | 9.0±4.5 | 91.0±4.5 |
| YAM 101-1b | Δabf2 | pYES2/CT-GCF1 | SD | 12.2±3.3 | 87.8±3.3 |
| YAM 101-1b | Δabf2 | pYES2/CT-GCF1 | SG | 77.2±6.9 | 22.8±6.9 |
| YAM 101-1b | Δabf2 | pYES2/CT-GCF1 | SGal | 52.6±17.6 | 47.4±17.6 |
*Synthetic medium containing glucose (SD), glycerol (SG) or galactose (SGal) as carbon source.
Construction of a C. albicans strain with a conditional allele of GCF1 and its phenotypic analysis
To analyse the role of GCF1 in mtDNA metabolism in C. albicans, we constructed a strain which lacks one allele of GCF1 and in which the second allele is replaced by a locus enabling conditional expression of GCF1 from the MET3 promoter repressible by 2.5 mM methionine/cysteine in the medium (Care et al., 1999; Fig. 4a). The correct insertion of both constructs into the genome was verified by both PCR and Southern blot analyses (data not shown). The resulting strain Δgcf1/PMET3-GCF1 was able to grow under both repressed and derepressed conditions. GCF1 mRNA levels in the wild-type (CAI4), GCF1/PMET3-GCF1 and Δgcf1/PMET3-GCF1 grown under repressed and derepressed conditions were analysed by qRT-PCR. CaACT1 mRNA encoding actin was used as a standard for normalization. GCF1 mRNA levels in the repressed strain Δgcf1/PMET3GCF1 were 3200-fold (±14 %) lower than in the wild-type strain after 12 h of growth in repression medium containing Met/Cys (Fig. 4b). Interestingly, both GCF1/PMET3-GCF1 and Δgcf1/PMET3-GCF1 strains grown under derepressed conditions exhibited two- to threefold elevated levels of GCF1 mRNA compared with the wild-type strain (Fig. 4b). Relative copy numbers of mtDNA in strains CAI4, GCF1/PMET3-GCF1 and Δgcf1/PMET3GCF1 were determined by qRT-PCR. As indicated in Fig. 4(c), the copy number of mtDNA in the repressed strain Δgcf1/PMET3-GCF1 was reduced by 80 % compared with the wild-type strain, whereas both the GCF1/PMET3-GCF1 and the Δgcf1/PMET3-GCF1 strains grown under derepressed conditions contained up to 50 % more mtDNA. Clearly, the elevated levels of GCF1-mRNA in the cells correlate with the observed higher copy numbers of mtDNA. Similarly, it has been demonstrated that moderate overexpression of S. cerevisiae ABF2 increases the amount of mtDNA by 50–100 % (Zelenaya-Troitskaya et al., 1998). On the other hand, S. cerevisiae cells lacking ABF2 exhibit rapid loss of mtDNA on fermentable carbon sources (e.g. glucose). Only when grown on non-fermentable carbon sources (e.g. glycerol) can Δabf2 strains of S. cerevisiae maintain their mtDNA (Diffley & Stillman, 1991). C. albicans is a petite-negative yeast, which cannot tolerate alteration or loss of its mtDNA. We were unable to knockout both alleles of GCF1. Although this could be due to technical reasons, it is possible and more likely that a strain lacking both functional alleles of GCF1 is not viable. Moreover, growing Δgcf1/PMET3-GCF1 under repressed conditions in synthetic minimal medium affected growth and slowed the cell cycle down three- to fivefold. Considering these observations and our data on GCF1 mRNA levels and mtDNA copy numbers it is plausible to propose that (i) GCF1 is an essential gene for mtDNA maintenance in C. albicans, and (ii) very low levels of GCF1 mRNA (and thus Gcf1 protein) could be sufficient to maintain levels of mtDNA that keep GCF1-shut-off mutants viable. This hypothesis is supported by our data shown in Fig. 4(d). Strain Δgcf1/PMET3GCF1, grown under repressed conditions, was passaged every 12 h for 3 days keeping the cultures in linear growth phase up to OD600 1.0. Analyses of samples taken at different time points show that during the first 24 h of passaging the cells, mtDNA copy numbers, as analysed by qRT-PCR, in this strain dropped down to 40 % of wild-type level. During the following 24 h, copy numbers dropped further, reaching a plateau at 20 % of wild-type level. This can be interpreted as follows: low GCF1 mRNA levels ensure a critical minimum level of Gcf1 protein, which in return supports maintenance of a vital amount of mtDNA in C. albicans. However, we cannot rule out the possibility that the vitality of the Δgcf1/PMET3-GCF1 strain under repressed conditions is caused by alternative protein factors (e.g. Aco1p) that could stabilize mtDNA in the absence of GCF1, as has been described for S. cerevisiae (Chen et al., 2005). In any case, a regulated expression of GCF1 enabled us to study the consequences of decreased levels of this protein on metabolism of mtDNA in C. albicans.
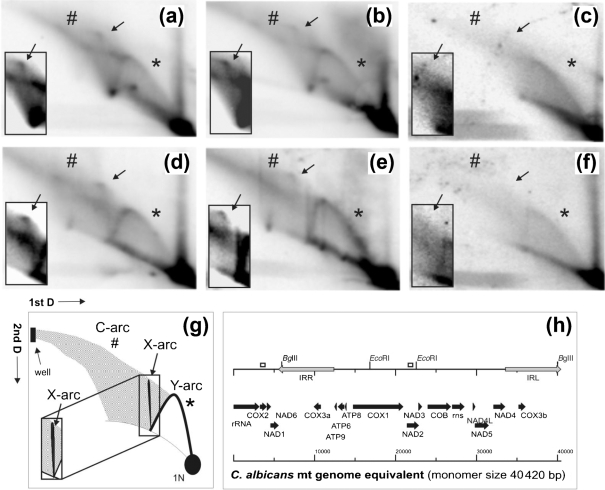
In order to demonstrate a direct effect of GCF1 on mtDNA metabolism in C. albicans we used 2D-AGE to analyse metabolic intermediates of mtDNA. In S. cerevisiae, the Abf2 protein binds directly to mtDNA, leading to its bending and compaction, and it has been indicated to play a role in mtDNA recombination (Stigter, 2004; Friddle et al., 2004; Zelenaya-Troitskaya et al., 1998). 2D-AGE is a powerful tool to investigate the topology of DNA molecules by separating them in the first dimension according to their mass under low voltage and in the absence of intercalating chemicals, and in the second dimension under higher voltage and using ethidium bromide to separate molecules according to their mass and shape. If DNA to be analysed is digested with restriction endonucleases prior to 2D-AGE, replication and recombination intermediates can be discriminated on the basis of a standard Y-arc formed by replication forks passing the restriction fragment analysed, an X-arc formed by Holliday junctions within the fragment, and an arc extending from larger linear double-stranded molecules to the well (C-arc), believed to be made up of highly branched complex recombination intermediates (Manchekar et al., 2006; J. M. Gerhold and others, unpublished results). 2D-AGE analyses of mtDNA from strain Δgcf1/PMET3-GCF1 grown under repressed conditions revealed a significant decrease in high-molecular-mass recombination structures and the absence of Holliday junctions (Fig. 5c, f) compared to the wild-type and Δgcf1/PMET3-GCF1 grown under derepressed conditions (Fig. 5a, b, d, e). The severe reduction of Gcf1p levels in C. albicans mitochondria under repressed conditions apparently leads to the observed decrease in recombination structures that could be used for initiation of mtDNA replication. Recently, it has been demonstrated that in yeast recombination is linked to the initiation of mtDNA replication (Ling et al., 2007; J. M. Gerhold and others, unpublished results). While the mechanistic details of this process are as yet unclear, it is most likely to be similar to the recombination-based replication observed in bacteriophage T4 (Mosig, 1998; Kreuzer et al., 1995). It is possible that the Gcf1 protein facilitates recombination by bending mtDNA and bringing together homologous DNA regions and proteins required for initiation of the process. Due to the reduced frequency of mtDNA recombination events in the repressed strain Δgcf1/PMET3-GCF1, the rate of mtDNA synthesis drops and the accumulation of vital levels of mtDNA takes longer than usually assumed for the wild-type cells. Therefore, cell cycle progression is slowed down.
Fig. 5.
2D electrophoresis. Purified mtDNA of CAI4 (a, d), Δgcf1/PMET3-GCF1 grown under derepressed conditions (b, e) and Δgcf1/PMET3-GCF1 grown under repressed conditions (c, f) was digested with BglII (a, b, c; fragment size 6328 bp, spanning base pairs 40 026 to 5934 in vivo) probed for a portion of cox2 (probe spanning base pairs 3285 to 3654), and EcoRI (d, e, f; fragment size 5726 bp, spanning base pairs 16 828 to 22 554) probed for nad2 (probe spanning base pairs 21 500 to 21 850). Positions of probes for cox2 and nad2 are indicated as open boxes in (h). (g) Graphical interpretation of (a–f). (h) An overview of a one-genome equivalent (monomer; 40 420 bp) of the C. albicans mitochondrial genome. Radiographs reveal a standard Y-arc [indicated by an asterisk; interpreted in (g)] of strand-coupled replication intermediates on all panels, albeit at reduced strength in (c) and (f). Each panel contains an overexposed section displaying the region of the descending Y-arc to the 2N spot (an almost fully replicated restriction fragment) and X-arc (indicated by an arrow) of Holliday junctions. In strain Δgcf1/PMET3-GCF1 grown under repressed conditions (c, f), X-arcs are reduced to a level below detection, and the C-arcs (indicated by #), which consist of complex high-molecular-mass recombination structures and branched molecules, are significantly reduced in comparison to CAI4 (a, d) and Δgcf1/PMET3-GCF1 grown under derepressed conditions (b, e).
As an alternative to a role in mtDNA replication initiation, a possible compaction function of Gcf1p together with its DNA-bending properties might influence mtDNA segregation and thereby slow down the cell cycle in the repressed Δgcf1/PMET3-GCF1 strain. However, DAPI staining of the repressed Δgcf1/PMET3-GCF1 strain did not indicate substantial changes in mt-nucleoid morphology (data not shown), and suggests therefore that the Gcf1 protein is dispensable for segregation of mtDNA in C. albicans.
Conclusions
We have characterized Gcf1p of C. albicans, a member of a novel subfamily of mtHMGs. Our results indicate that similarly to S. cerevisiae Abf2p, Gcf1p is a non-specific DNA-binding protein involved in mtDNA replication and recombination, and thus in the fine-tuning of mtDNA metabolism. It remains to be determined if and how other components of C. albicans mt-nucleoid participate in its function(s). One obvious candidate could be aconitase, which substitutes for a lack of Abf2p on non-fermentable carbon sources in S. cerevisiae (Chen et al., 2005). Indeed, Aco1p has been detected in preparations of mt-nucleoids in C. parapsilosis (Miyakawa et al., 2009). In any case, our results clearly demonstrate the importance of Gcf1p for optimal growth yields, and the data on mtDNA copy number, such as topological studies of replication and recombination intermediates, underline the effect of this protein on mtDNA metabolism.
Our paper underlines the relatively high rates of divergence of yeast mtHMGs (Nosek et al., 2006). The degree of sequence variability between the members of the four subfamilies of mtHMGs is so high that it is difficult to reconstruct their evolutionary history. One possibility is that the HMG boxes are shuffled between the proteins involved in mtDNA packaging. According to another scenario, the class IIb mtHMGs, containing two HMG boxes and one coiled-coil domain, may represent an ancestral type of mtHMG. Comparative analysis of Gcf1p with other members of the novel family of mtHMGs (CpGcf1, DhGcf1) as well as with more distantly related mtDNA packaging proteins, including Abf2, may lead to a better understanding of the means of stabilization and transmission of mtDNA in yeasts.
Acknowledgments
We wish to thank the members of our laboratories for discussions, and L. Kovac (Comenius University, Bratislava, Slovakia) for helpful comments and continuous support. In addition, we thank Dr D. A. Clayton (Howard Hughes Medical Institute, Chevy Chase, USA), Dr H. Fukuhara (Institut Curie, Orsay, France), Dr J. Hegemann (Heinrich-Heine-Universität, Düsseldorf, Germany), Dr A. J. Mitchell (Columbia University, New York, USA), Dr J. Morschhäuser (Universität Würzburg, Germany), Dr H. Y. Steensma (Leiden University, The Netherlands) and Dr P. E. Sudbery (Sheffield University, UK) for providing yeast strains and plasmids. Our work is supported by grants from the Fogarty International Research Collaboration Award [2-R03-TW005654-04A1 (L. T.)], Howard Hughes Medical Institute [55005622 (J. N.)], the Slovak grant agencies APVT [20-001604; VVCE-0064-07 (L. T.) and 0024-07; LPP-0164-06 (J. N.)], VEGA [1/3247/06; 1/0132/09 (L. T.) and 1/0219/08 (J. N.)], and Comenius University [UK/247/2008 (K. V.)], and J. S. and J. M. G. are supported by Estonian Science Foundation grant 7013 and Targeted Finance Program SF0180164. K. V. has been partly supported by a travel grant from the Estonian Ministry of Science and Education. J. M. G. wishes to thank Arnold Kristjuhan and Signe Värv for technical advice and discussion of qRT-PCR matters.
Abbreviations
AGE, agarose gel electrophoresis
DAPI, 4′,6-diamidino-2-phenylindole
EMSA, electrophoretic mobility shift assay
GST, glutathione S-transferase
HMG, high-mobility group
mtDNA, mitochondrial DNA
mtHMG, mitochondrial HMG box-containing protein
mt-nucleoid, mitochondrial nucleoid
qRT-PCR, quantitative real-time RT-PCR
Footnotes
A supplementary figure, showing phylogenetic trees of mitochondrial HMG box-containing proteins and the superfamily of HMG box-containing proteins encoded by yeast genomes, and a supplementary table of complementation of the S. cerevisiae Δabf2 mutant by Candida parapsilosis GCF1, with associated supplementary methods, are available with the online version of this paper.
References
- Baxevanis, A. D. & Landsman, D. (1995). The HMG-1 box protein family: classification and functional relationships. Nucleic Acids Res 23, 1604–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer, B. J. & Fangman, W. L. (1987). The localization of replication origins on ARS plasmids in S. cerevisiae. Cell 51, 463–471. [DOI] [PubMed] [Google Scholar]
- Brewer, L. R., Friddle, R., Noy, A., Baldwin, E., Martin, S. S., Corzett, M., Balhorn, R. & Baskin, R. J. (2003). Packaging of single DNA molecules by the yeast mitochondrial protein Abf2p. Biophys J 85, 2519–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Care, R. S., Trevethick, J., Binley, K. M. & Sudbery, P. E. (1999). The MET3 promoter: a new tool for Candida albicans molecular genetics. Mol Microbiol 34, 792–798. [DOI] [PubMed] [Google Scholar]
- Caron, F., Jacq, C. & Rouviere-Yaniv, J. (1979). Characterization of a histone-like protein extracted from yeast mitochondria. Proc Natl Acad Sci U S A 76, 4265–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X. J. & Butow, R. A. (2005). The organization and inheritance of the mitochondrial genome. Nat Rev Genet 6, 815–825. [DOI] [PubMed] [Google Scholar]
- Chen, X. J., Wang, X., Kaufman, B. A. & Butow, R. A. (2005). Aconitase couples metabolic regulation to mitochondrial DNA maintenance. Science 307, 714–717. [DOI] [PubMed] [Google Scholar]
- Church, G. M. & Gilbert, W. (1984). Genomic sequencing. Proc Natl Acad Sci U S A 81, 1991–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claros, M. G. & Vincens, P. (1996). Computational method to predict mitochondrially imported proteins and their targeting sequences. Eur J Biochem 241, 779–786. [DOI] [PubMed] [Google Scholar]
- Daum, G., Böhni, P. C. & Schatz, G. (1982). Import of proteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J Biol Chem 257, 13028–13033. [PubMed] [Google Scholar]
- De Backer, M. D., Maes, D., Vandoninck, S., Logghe, M., Contreras, R. & Luyten, W. H. M. L. (1999). Transformation of Candida albicans by electroporation. Yeast 15, 1609–1618. [DOI] [PubMed] [Google Scholar]
- Defontaine, A., Lecocq, F. M. & Hallet, J. N. (1991). A rapid miniprep method for the preparation of yeast mitochondrial DNA. Nucleic Acids Res 19, 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dequard-Chablat, M. & Allandt, C. (2002). Two copies of mthmg1, encoding a novel mitochondrial HMG-like protein, delay accumulation of mitochondrial DNA deletions in Podospora anserina. Eukaryot Cell 1, 503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diffley, J. F. & Stillman, B. (1991). A close relative of the nuclear, chromosomal high-mobility group protein HMG1 in yeast mitochondria. Proc Natl Acad Sci U S A 88, 7864–7868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diffley, J. F. & Stillman, B. (1992). DNA binding properties of an HMG1-related protein from yeast mitochondria. J Biol Chem 267, 3368–3374. [PubMed] [Google Scholar]
- Friddle, R. W., Klare, J. E., Martin, S. S., Corzett, M., Balhorn, R., Baldwin, E. P., Baskin, R. J. & Noy, A. (2004). Mechanism of DNA compaction by yeast mitochondrial protein Abf2p. Biophys J 86, 1632–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz, R. D. & Schiestl, R. H. (1995). Transforming yeast with DNA. Methods Mol Cell Biol 5, 255–269. [Google Scholar]
- Kao, L. R., Megraw, T. L. & Chae, C. B. (1993). Essential role of the HMG domain in the function of yeast mitochondrial histone HM: functional complementation of HM by the nuclear nonhistone protein NHP6A. Proc Natl Acad Sci U S A 90, 5598–5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S., Wolyniak, M. J., Staab, J. F. & Sundstrom, P. (2007). A 368-base-pair cis-acting HWP1 promoter region, HCR, of Candida albicans confers hypha-specific gene regulation and binds architectural transcription factors Nhp6 and Gcf1p. Eukaryot Cell 6, 693–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzer, K. N., Saunders, M., Weislo, L. J. & Kreuzer, H. W. E. (1995). Recombination-dependent DNA replication stimulated by double-strand breaks in bacteriophage T4. J Bacteriol 177, 6844–6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucej, M. & Butow, R. A. (2007). Evolutionary tinkering with mitochondrial nucleoids. Trends Cell Biol 17, 586–592. [DOI] [PubMed] [Google Scholar]
- Kucej, M., Kucejova, B., Subramanian, R., Chen, X. J. & Butow, R. A. (2008). Mitochondrial nucleoids undergo remodeling in response to metabolic cues. J Cell Sci 121, 1861–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroiwa, T. (1982). Mitochondrial nuclei. Int Rev Cytol 75, 1–59. [DOI] [PubMed] [Google Scholar]
- Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Letunic, I., Copley, R. R., Pils, B., Pinkert, S., Schultz, J. & Bork, P. (2006). SMART 5: domains in the context of genomes and networks. Nucleic Acids Res 34, D257–D260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling, F., Hori, A. & Shibata, T. (2007). DNA recombination-initiation plays a role in the extremely biased inheritance of yeast [rho−] mitochondrial DNA that contains the replication origin ori5. Mol Cell Biol 27, 1133–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAlpine, D. M., Perlman, P. S. & Butow, R. A. (1998). The high mobility group protein Abf2p influences the level of yeast mitochondrial DNA recombination intermediates in vivo. Proc Natl Acad Sci U S A 95, 6739–6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAlpine, D. M., Perlman, P. S. & Butow, R. A. (2000). The numbers of individual mitochondrial DNA molecules and mitochondrial DNA nucleoids in yeast are co-regulated by the general amino acid control pathway. EMBO J 19, 767–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchekar, M., Scissum-Gunn, K., Song, D., Khazi, F., McLean, S. L. & Nielsen, B. L. (2006). DNA recombination activity in soybean mitochondria. J Mol Biol 356, 288–299. [DOI] [PubMed] [Google Scholar]
- Megraw, T. L. & Chae, C. B. (1993). Functional complementarity between the HMG1-like yeast mitochondrial histone HM and the bacterial histone-like protein HU. J Biol Chem 268, 12758–12763. [PubMed] [Google Scholar]
- Miyakawa, I., Fumoto, S., Kuroiwa, T. & Sando, N. (1995). Characterization of DNA-binding proteins involved in the assembly of mitochondrial mt-nucleoids in the yeast Saccharomyces cerevisiae. Plant Cell Physiol 36, 1179–1188. [PubMed] [Google Scholar]
- Miyakawa, I., Okazaki-Higashi, C., Higashi, T., Furutani, Y. & Sando, N. (1996). Isolation and characterization of mitochondrial mt-nucleoids from the yeast Pichia jadinii. Plant Cell Physiol 37, 816–824. [DOI] [PubMed] [Google Scholar]
- Miyakawa, I., Kitamura, Y., Jyozaki, T., Sato, H. & Umezaki, T. (2000). Simple detection of a yeast mitochondrial DNA-binding protein, Abf2p, on SDS-DNA gels. J Gen Appl Microbiol 46, 311–316. [DOI] [PubMed] [Google Scholar]
- Miyakawa, I., Okamuro, A., Kinsky, S., Visacka, K., Tomaska, L. & Nosek, J. (2009). Mitochondrial nucleoids from the yeast Candida parapsilosis: expansion of the repertoire of proteins associated with mitochondrial DNA. Microbiology 155, (in press). [DOI] [PubMed]
- Mosig, G. (1998). Recombination and recombination-dependent DNA replication in bacteriophage T4. Annu Rev Genet 32, 379–413. [DOI] [PubMed] [Google Scholar]
- Nicholas, K. B., Nicholas, H. B., Jr & Deerfield, D. W., II (1997). GeneDoc: analysis and visualization of genetic variation. EMBnet News 4, 14––17. [Google Scholar]
- Niedenthal, R. K., Riles, L., Johnston, M. & Hegemann, J. H. (1996). Green fluorescent protein as a marker for gene expression and subcellular localization in budding yeast. Yeast 12, 773–786. [DOI] [PubMed] [Google Scholar]
- Nosek, J., Tomaska, L., Bolotin-Fukuhara, M. & Miyakawa, I. (2006). Structure and dynamics of the mitochondrial chromosome: an insight from complete yeast genomes analysis. FEMS Yeast Res 6, 356–370. [DOI] [PubMed] [Google Scholar]
- Ogur, M., St. John, R. & Nagai, S. (1957). Tetrazolium overlay technique for population studies of respiration deficiency in yeast. Science 125, 928–929. [DOI] [PubMed] [Google Scholar]
- Okamoto, K., Perlman, P. S. & Butow, R. A. (1998). The sorting of mitochondrial DNA and mitochondrial proteins in zygotes: preferential transmission of mitochondrial DNA to the medial bud. J Cell Biol 142, 613–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi, M. A., Xu, B. & Clayton, D. A. (1993). A human mitochondrial transcriptional activator can functionally replace a yeast mitochondrial HMG-box protein both in vivo and in vitro. Mol Cell Biol 13, 1951–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J. & Russell, D. W. (2001). Molecular Cloning: a Laboratory Manual, 3rd edn. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory.
- Sasaki, N., Kuroiwa, H., Nishitani, C., Takano, H., Higashiyama, T., Kobayashi, T., Shirai, Y., Sakai, A., Kawano, S. & other authors (2003). Glom is a novel mitochondrial DNA packaging protein in Physarum polycephalum and causes intense chromatin condensation without suppressing DNA functions. Mol Biol Cell 14, 4758–4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedman, T., Jõers, P., Kuusk, S. & Sedman, J. (2005). Helicase Hmi1 stimulates the synthesis of concatemeric mitochondrial DNA molecules in yeast Saccharomyces cerevisiae. Curr Genet 47, 213–222. [DOI] [PubMed] [Google Scholar]
- Stigter, D. (2004). Packaging of single DNA molecules by the yeast mitochondrial protein Abf2p: reinterpretation of recent single molecule experiments. Biophys Chem 110, 171–178. [DOI] [PubMed] [Google Scholar]
- Tamura, K., Dudley, J., Nei, M. & Kumar, S. (2007). MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24, 1596–1599. [DOI] [PubMed] [Google Scholar]
- Umezaki, T. & Miyakawa, I. (2002). Use of SDS-DNA PAGE for detection of mitochondrial Abf2p-like proteins and mitochondrial nuclease in Saccharomyces yeasts and Arxiozyma telluris. Cytologia (Tokyo) 67, 423–428. [Google Scholar]
- Wirsching, S., Michel, S. & Morschhäuser, J. (2000). Targeted gene disruption in Candida albicans wild-type strains: the role of the MDR1 gene in fluconazole resistance of clinical Candida albicans isolates. Mol Microbiol 36, 856–865. [DOI] [PubMed] [Google Scholar]
- Zelenaya-Troitskaya, O., Newman, S. M., Okamoto, K., Perlman, P. S. & Butow, R. A. (1998). Functions of the high mobility group protein, Abf2p, in mitochondrial DNA segregation, recombination and copy number in Saccharomyces cerevisiae. Genetics 148, 1763–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]