Abstract
Although much has been learned in recent decades about the deterioration of muscular strength, gait adaptations, and sensory degradation among older adults, little is known about how these intrinsic changes affect biomechanical parameters associated with slip-induced fall accidents. In general, the objective of this laboratory study was to investigate the process of initiation, detection, and recovery of inadvertent slips and falls. We examined the initiation of and recovery from foot slips among three age groups utilizing biomechanical parameters, muscle strength, and sensory measurements. Forty-two young, middle-age, and older participants walked around a walking track at a comfortable pace. Slippery floor surfaces were placed on the track over force platforms at random intervals without the participants’ awareness. Results indicated that younger participants slipped as often as the older participants, suggesting that the likelihood of slip initiation is similar across all age groups; however, older individuals’ recovery process was much slower and less effective. The ability to successfully recover from a slip (thus preventing a fall) is believed to be affected by lower extremity muscle strength and sensory degradation among older individuals. Results from this research can help pinpoint possible intervention strategies for improving dynamic equilibrium among older adults.
INTRODUCTION
Injuries associated with slip and fall accidents continue to pose a significant problem to older adults, in terms of both human suffering and economic losses. Many studies have indicated that with advancing age there is an increasing incidence of fatal slip and fall injuries (Agnew & Suruda, 1993; Campbell, Reinken, Allan, & Martinez, 1981; Donald & Bulpitt, 1999; Murphy, 2000; Rubenstein et al., 1988). Falls are the leading cause of death resulting from injury among older adults (over age 75) and the second highest cause of accidental death for 45- to 75-year-olds (National Safety Council, 2002). The National Safety Council reported that in 2001, 15,400 Americans met their death by falling, and of these deaths, the majority (over 80%) were people over 65 years of age (National Safety Council, 2002). In terms of the injuries, more than 25% of fall-related injuries in older adults result from slips, and 66% of fall-related hip fractures occur on wet or slippery floor surfaces (Norton, Campbell, Lee-Joe, Robinson, & Butler, 1997). Additionally, falls and hip fractures among older adults rank among the most serious public health problems in the United States, with costs expected to exceed $43.8 billion by the year 2020 (Englander, Hodson, & Terregrossa, 1996). Furthermore, fall accidents are the second leading cause of work-related fatalities, after motor vehicle accidents, and compared with victims of all disabling injuries, victims of disabling falls are more likely to be older than 55 years (Courtney, Sorock, Manning, Collins, & Holbein-Jenny, 2001).
A review of literature on slip and fall accidents indicates that multiple mechanisms are involved. Numerous studies have identified age-related risk factors for falling. Factors intrinsic and extrinsic to older adults and the hazards and demands of the environment contribute to most falls in varying degrees (for a comprehensive review, refer to American Geriatrics Society et al., 2001, and Tinetti, 2003). In general, the ability to walk safely and preserve balance (keeping the body’s center of mass [COM] over its base of support) in the event of a slip and fall is dependent on intact visual, vestibular, proprioceptive, and musculoskeletal systems. However, with advancing age, a variety of physiologic changes affecting these systems may interfere with gait and balance, placing older individuals at a higher risk for slip and fall accidents.
Major physiologic changes affecting the potential for slip and fall accidents begin to appear in the mid-20s. In general, isometric muscle strength peaks in the mid-20s and then decreases slowly until after 50 years of age, when there is an accelerated decline (Astrand & Rodahl, 1986; Larsson, 1982; Rice & Cunningham, 2001). These declines in strength development appear to stem from changes in muscle contraction mechanisms (Thelen, Schultz, Alexander, & Ashton-Miller, 1996), in mitochondrial enzyme activity (Houmard et al., 1998), and in the proportion of fast-twitch to slow-twitch muscle fibers (Lexell, 1995). Studies suggest that age-related changes in muscle strength have an important effect on the initiation of and recovery from slip and fall accidents (Bonder & Wagner, 1994; Larsson, Grimby, & Karlsson,1979; Whipple, Wolfson, & Amerman, 1987; Wolfson, Whipple, Amerman, Kaplan, & Kleinberg, 1985; Woolacott, 1986).
Evidence in support of this hypothesis comes from a number of investigations indicating an age-related decline in voluntary muscle strength and increased likelihood of slips and falls. Age-related decrease in leg strength has been associated with mobility and balance problems and considered a risk factor for falls among older adults (Judge, Lindsey, Underwood, & Winsemius, 1993; Nevitt, Cummings, Kidd, & Black, 1989; Stephen, John, Philippa, & Kaarin, 1994; Tinetti, Liu, & Claus, 1993; Tinetti, Speechley, & Ginter, 1988). For example, Wolfson et al. (1985) and Larsson et al. (1979) reported that ankle and quadriceps muscle strength was significantly lower for those who fall as compared with nonfallers. Additionally, reduced lower extremity muscle strength has been implicated as a factor contributing to nursing home placement (Hubert, Bloch, & Fries, 1993; Wolfson, Judge, Whipple, & King, 1995) and to increased risk of falling (Whipple et al., 1987).
A number of studies have documented the decline of postural control (keeping the body’s COM over its base of support during quiet stance and active movement) caused by sensory degradation among older adults (Sheldon, 1963; Woolacott, Shumway-Cook, & Nashner, 1982). This decline of postural control is believed to be an integrative process associated with a greater risk of falling (Brocklehurst, Robertson, & James-Groom, 1982; Isaacs, 1985; Maki, Holliday, & Topper, 1994; Overstall, Exton-Smith, Imms, & Johnson, 1977). Sensory inputs important for balance are vision, proprioception, and vestibular sensations (Lacour, Vidal, & Xerri, 1983; Nashner, 1982).
Vision plays a major role in maintaining stability, both at stance and while undergoing movement such as walking (Tinetti & Speechley,1989). Visual acuity, adaptation to the dark, peripheral vision, contrast sensitivity, and accommodation, all of which are related to stability, may be affected by age-related changes (Cohn & Lasley, 1985; Goldman, 1986; Kornzweig, 1977). For example, age-related decrements in peripheral vision may impair an older individual’s use of visual reference information. Narrowing the visual field may deprive the older person of that part of the visual field that is most sensitive to movement (Stelmach & Worringham, 1985), and as a result, postural control may be compromised. Furthermore, elderly individuals’ diminished sensitivity to low visual spatial frequencies may also lead to decreased postural stability (Lord, Clark, & Webster, 1991). Older adults tend to rely more heavily on visual cues (e.g., the location of stable surroundings) for the maintenance of balance, whereas younger adults rely more on proprioceptive and vestibular cues. Redfern and DiPasquale (1997) reported that the postural stability of older individuals, as compared with younger individuals, was affected more by moving visual environments. They also concluded that postural instability was most likely caused by a function of the sensitivity of older people to visual and proprioceptive inputs and of their difficulty in handling sensory conflicts to the postural control system. Visual deficits then may result in an increase in the time taken for the visual system to alert the central nervous system to a potential fall, thereby increasing the likelihood of fall accidents.
Studies indicate that proprioceptive deficits are also significantly higher in older individuals (Rabbitt & Rogers, 1965; Skinner, Barrack, & Cook, 1984; Wollacott et al., 1982). The pro-prioceptive system contributes to stability, particularly during changes of position. Woolacott et al. (1982) demonstrated the significance of ankle proprioception for balance retention in older adults by utilizing a moveable platform that permitted stabilization of either the visual field or the support surface. Comparing the postural sway, they found that older individuals had large increases in postural sway when ankle pro-prioception was eliminated (via a moveable platform). This large increase in postural sway was associated with a greater risk of falling (Isaacs, 1985). Proprioceptive information also plays a vital role in modification of internal models using feed-forward control mechanisms (Bard, Fleury, Teasdale, Paillard, & Nougier, 1995; Sainburg, Ghilardi, Poizner, & Ghez, 1995). During slip perturbation, motor programs have to be modified to maintain dynamic stability. Modification of the motor program is closely associated with visual input as well as proprioceptive input (plantar cutaneous receptors, muscle and joint mechanoreceptors). However, when visual cues conflict with environmental cues, proprioceptive input may be the quickest and most accurate modality associated with balance maintenance (Ghez & Sainburg, 1995). As a result, older adults’ proprioceptive deficits may hinder optimum balance recovery during slip-induced perturbations and increase the likelihood of fall accidents.
Studies on the vestibular system indicate a marked decline in the vestibular apparatus among older adults (i.e., reduction of hair cells of the cristae ampullares [semicircular canals] and of the macula of the utriculus and sacculus; Johnsson & Hawkins, 1972; Rosenhall & Rubin, 1975). The major contribution of the vestibular apparatus to posture is in maintaining the whole-body balance by perceiving the changes in direction as well as motion by adjusting the activity of the postural muscles (Inglis, Shupert, Hlavacka, & Horak, 1995; Keshner, Allum, & Pfaltz, 1987). In the normal state, vestibular receptors in each labyrinth generate resting activity; subsequent head movement produces equal and opposite alteration in the activity in each ear. This in turn leads to the appropriate compensatory eye and muscle response for maintenance of posture. As indicated, the vestibular system contributes to stabilizing the eyes and head in space but is also important during fast postural movements (Petersen, Magnusson, Fransson, & Johansson, 1994), such as in eliciting fall responses (Melvill & Watt, 1971a, 1971b) and in resolving conflicting visual and proprioceptive information (Nashner, 1982). As a result, older adults’ degraded vestibular system may hinder the optimum balance recovery response and may increase their likelihood of slips and falls (Kristinsdottir, Jarnlo, & Magnusson, 2000).
Increasing age can also have an effect on gait because of postural and balance changes. Older adults tend to have a shorter step length and a broader walking base, which results in an increase in stance time and double support time (Gillis, Gilroy, Lawley, Mott, & Wall, 1986; Imms & Edholm, 1979; Murray, Kory, & Clarkson, 1969; Winter, Patla, Frank, & Walt, 1990). Many researchers have observed that on slippery floor surfaces, participants tend to shorten their step length in order to reduce foot velocities, foot shear forces, and the likelihood of slipping (Cooper & Glassow, 1963, pp. 140–175; Ekkubus & Killey, 1973). As a result, the shorter step length and the broader walking base of older adults are thought to result in a more stable or safer gait pattern; however, these gait adaptations may have some important implications for initiation of slip-induced falls. Winter et al. (1990) and Lockhart (1997) reported that horizontal heel velocity during the heel contact phase of the gait cycle was significantly higher for older participants than for younger ones, even though the walking velocity of the older participants was slower. This increase in horizontal heel velocity during a critical time of weight transfer might increase the potential for slip-induced falls if the friction between the heel and the floor is reduced by contamination of the floor surface. Thus general gait instability among older individuals, and specifically higher horizontal heel velocity during the critical phase of the gait cycle, may increase the friction demand (i.e., required coefficient of friction, or RCOF) and thereby increase the likelihood of slip-induced fall accidents.
Although much has been learned over the last few decades about the deterioration of muscular strength, gait adaptations, and sensory degradation among older adults, information regarding the relationships between intrinsic changes associated with aging and biomechanical responses during slip-induced falls is unclear. In general, the objective of this research was to investigate the process of initiation, detection, and recovery of inadvertent slips and falls.
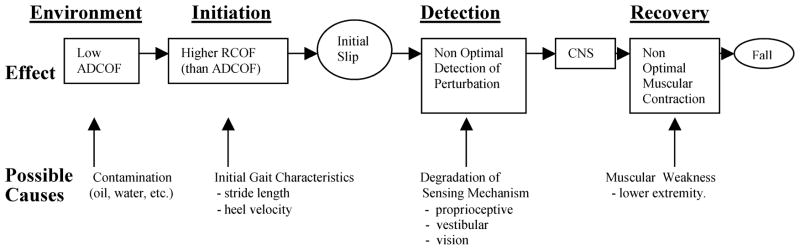
A hypothetical inadvertent slip and fall situation is illustrated in Figure 1, with possible causes and effects. The process is divided into four distinct phases (environment, initiation, detection, and recovery). The environmental phase considers the effects of contamination. As noted by Chaffin, Woldstad, and Trujillo (1992), “any fluid contaminant between two sliding surfaces will provide lubrication and thereby lower the dynamic COF values” (p. 285). Therefore, presence of contamination (oil, water, etc.) will reduce the available dynamic COF (ADCOF) of the floor surfaces. Consequently, slip is initiated by the combination of low DCOF and higher RCOF. As indicated earlier, initial gait characteristics such as stride length and heel velocity may affect RCOF because of the increase in horizontal foot force. If older individuals’ initial gait characteristics are altered (e.g., higher heel velocity) by intrinsic deficits associated with increased age, the potential for slip-induced falls may increase. Therefore, one objective of this study was to investigate the initiation phase of unexpected slips and falls by comparing the initial gait characteristics (e.g., step length and heel velocity) of older individuals and their younger counterparts to explore the effects of gait characteristics on severity of slip initiation (i.e., RCOF).
Figure 1.
The process of initiation, detection, and recovery of inadvertent slips and falls with possible causes and effects.
In terms of the detection and recovery phases of slip and fall accidents, the central nervous system must undertake certain processing stages (detection phase) if a fall is to be avoided or compensated for (recovery phase). First, during the detection phase, if a potential fall is imminent, sensory input must trigger or alert those centers responsible for response selection. This alerting process may be initiated by one or more of the following sensory inputs: proprioception, vision, and vestibular function. At the input stage, any age-related disruption in the quality of the signal from the periphery may increase the likelihood of slips and falls. In addition, diminished strength of lower extremity muscles of older individuals may make it difficult to rapidly adjust the whole-body COM to prevent falls. Thus the second objective of this study was to investigate the detection and recovery phases of unexpected slips and falls by comparing sensory organization scores, strength of lower extremity muscles, and slip performance measures (e.g., slip distances, sliding heel velocity, and sliding heel acceleration) of older individuals and their younger counterparts to further explore the effects of muscular and sensory degradation on slip performance measures.
From the work conducted thus far, it is hypothesized that gait adaptations, deterioration of muscular strength, and sensory degradation among older individuals would have a greater effect on the severity of their slips and falls as compared with that of their younger counterparts. In particular, it is expected that older adults’ gait adaptations (e.g., shorter step length and higher heel contact velocity) would affect the friction demand characteristic at the shoe-floor interface at the time of the heel contact and increase the distance slipped initially. It is also expected that older adults’ deterioration of muscular strength and sensory degradation would adversely affect the detection and recovery processes of slip and fall accidents, increasing the speed of slipping and distance slipped, ultimately leading to more falls.
Relevance
Although epidemiological studies on slip and fall accidents reveal a higher incidence of fatal injuries among older individuals, the relationship between age-related factors and slip and fall accidents is unclear. Particularly, the initiation, detection, and recovery processes associated with slip-induced falls, and age-related factors influencing these processes, are not well understood. As such the objective of this study was to investigate the process of initiation, detection, and recovery of inadvertent slips and falls. Understanding this information will help identify which of the processes associated with slip-induced falls are most critical for older adults and help pinpoint intervention strategies. Additionally, this study can provide knowledge concerning possible intervention strategies (muscle strengthening and balance exercises, etc.) for improving dynamic equilibrium.
METHOD
Participants
Fourteen young individuals (7 men, 7 women; age [mean, SD] 22.6 ± 2.1 years; height 169.7 ± 6.1 cm; weight 68.7 ± 9.6 kg), 14 middle-age individuals (7 men, 7 women, age 46.9 ± 13.6 years; height173.5 ± 6.3 cm; weight 75.5 ±16.1kg), and 14 older individuals (7 men, 7 women; age 75.5 ± 6.8 years; height 170.2 ± 6.4 cm; weight 76.8 ± 13.3 kg) participated in this experiment. The young participants were recruited from the general student population at Texas Tech University, and the two other groups were recruited from the local community. Most of the older participants were not engaged in regular exercise program (only 2 out of 14 individuals were involved in self-regulated exercise program). Prior to participating in the experiment, older participants were examined by a physician to ensure that they were in generally good physical health. Participants also received a peripheral neuropathy examination in the Neurology Department at St. Mary’s Hospital in Lubbock, Texas. Participants were excluded from the study based on these tests (below 50% of the norm) and the physician’s professional judgment. All participants signed a release form approved by the Texas Tech University Institutional Review Board. All participants were compensated for their time and effort.
Apparatus
Two commonly used floor materials were used in this experiment: outdoor carpet (Beau Lieu 0.25-inch [0.63 cm] Olefin) and vinyl tile (Arm-strong). The vinyl tile surface was covered with motor oil (10W40) to reduce the coefficient of friction (COF). The available dynamic COF (ADCOF) for each surface was measured using a standard 4.54-kg (10-pound) horizontal pull slip meter with a rubber sole material on the force platform and found to be 1.80 for the outdoor carpet and 0.08 for the oily vinyl tile. DCOF measurements were conducted at a constant velocity of 20 cm/s. Averages of 10 measurements on each of the two floor surfaces were used as final DCOF values. Standard shoes with rubber soles were supplied to all participants to maintain constant COF levels.
Walking trials were conducted on a rectangular track using an overhead fall-arresting rig as shown in Figure 2. The wood deck was approximately 6.7 × 6.7 m and permitted a straight walking path (participants were instructed to walk straight after turning). The entire deck was covered with carpet. The test surfaces (oily vinyl floor tiles) were mounted on a platform that was connected to the force plates (black box on the track; see Figure 2). A remote-controlled floor changer (RCFC) was used to change the test floor surfaces so as to provide unexpected slippery conditions. The RCFC unit was composed of a DC motor (LEESON Electric Corp.) and a gliding shaft (ACME Thread) attached to a platform. The DC motor was controlled by a remote control unit. Once triggered, the DC motor turned the ACME Thread (maximum 1750 revolutions/min), thereby moving the platform to a desired floor level. The test surfaces were mounted on a platform, which was connected to the force plates. The overall function of the system allowed a participant to walk under experimental conditions without being aware of the floor surface change.
Figure 2.
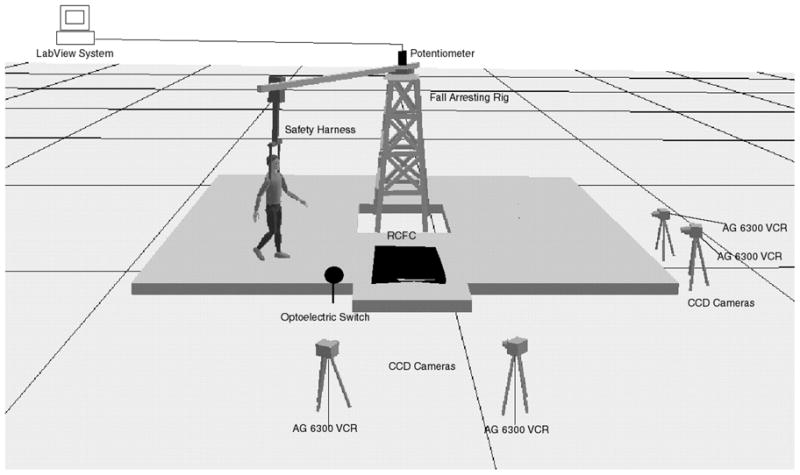
Field layout of the experiment, including fall-arresting rig, potentiometer interfaced with the LabView system, safety harness, force platforms, optoelectric switch, CCD cameras (4), AG 6300 VCR (4), and remote control floor changer (RCFC) units with baseline carpet floor material.
A fall-arresting rig was used to protect participants from falling during the experiment. The rig consists of a full-body parachute harness attached to an automated overhead suspension arm. A feedback control system allowed the arm to sense the position of the participant and increase or reduce velocity to stay overhead. The rig was designed to permit the participant to fall approximately 15 cm before arresting the fall and stopping the forward motion. An Ariel Performance Analysis System (APAS) and four Panasonic video recorders (charge-coupled device [CCD]) were used to collect the 3-D posture data of the participants as they walked over the test surface. Twenty-six reflective markers were used to define a whole-body model. Posture data were sampled and recorded at a rate of 60 Hz. The ground reaction forces of the participants walking over the test surfaces were measured using two Bertec force plates (Bertec Corp., Columbus, OH) sampled at a rate of 600 Hz. Participants were also supplied with a Walkman® (playing old comedy routines) during the walking experiment to conceal any sound of the floor changer’s motors. A Sensory Organization Test (balance assessment) and a Motor Control Test were performed utilizing the NeuroTest system (NeuroCom Inc., EquiTest platform without the moving visual scene). Strength measurements were collected using a Chatillon© force transducer (Itin Scale Co. Inc., Brooklyn, NY) for isometric strength tests and Cybex II (Computer Sports Medicine, Inc., Norwood, MA) for isokinetic strength tests.
Procedures
The participants were scheduled to take part in two testing sessions within 1 week’s time. The participants attended a familiarization session before the experiment. During the familiarization, the fall-arresting system and walking conditions were introduced. Whole-body isometric strength measurements for the arms, legs, and torso were collected as described by Chaffin, Herrin, and Keyserling (1978) and Chaffin and Andersson (1991). Additionally, isokinetic strength (knee flexion) measurements were collected utilizing Cybex II. For the isokinetic strength test procedure, participants were seated with the right leg in 90° flexion. Participants were instructed to push against the padded plate “as hard as you can” continuously for approximately 5 s after a spoken cue. Participants were allowed to practice at least one trial before data were collected. Participants were asked to apply two dynamic maximum exertions at each of three levels randomly (30°, 60°, and 90°/s). Rest periods were given after each exertion for 45 s. The highest knee flexion torque was recorded as the isokinetic strength.
Afterward, the NeuroCom Sensory Organization Test (SOT) and Motor Control Test (MCT) were performed at the Neurology Department at St. Mary’s Hospital in Lubbock, Texas, by the principal author to assess the balance profile and muscle latency (perturbation reaction time) of the participants (Cohen, Heaton, Congdon, & Jenkins, 1996; Ford-Smith, Wyman, Elswick, & Fernandez, 1995). Participants were asked to stand on the NeuroCom dual-force platforms with their feet in the required position on the platforms while wearing a safety harness. During the test, participants were instructed to close and open their eyes while the force plates moved forward and backward (translation) and also up and down (rotation). Individual measurements lasted 20 s, and the total testing period lasted 20 min. For the Sensory Organization Test, there was a total of three trials for each of four conditions: Condition 1 = eyes open, stable platform; Condition 2 = eyes closed, stable platform; and Condition 3 = eyes open, sway-referenced platform (i.e., elimination of proprioceptive feedback via calibrated support surface movement associated with the participant’s anteroposterior body sway); Condition 4 = eyes closed, sway-referenced platform. For the Motor Control Tests, there were two trials for each of six conditions: Condition 1 = small forward translation of the force plates; Condition 2 = medium forward translation of the force plates; Condition 3 = large forward translation of the force plates; Condition 4 = small backward translation of the force plates; Condition 5 = medium backward translation of the force plates; Condition 6 = large backward translation of the force plates.
During the walking experiment, the participants walked (natural cadence) across each floor condition for 10 min. A baseline measurement was collected on the carpeted floor surface before the slippery floor surface was introduced. While walking, participants were instructed to focus their eyes on an LED located approximately 2 m above and 3 m away from the testing area. A secondary task, which required them to call out when the light was on and when it was off, was used to ensure that they attended to the LED and not the floor surface. During the 10-min session, two slippery conditions were randomly introduced by the system, and measurements of participant’s posture and ground reaction forces were recorded. (For the data analysis, the second trial was used only if the first trial was not acceptable – e.g., if the participant stepped on the edge of the force platform.) Locations of the slippery surfaces were also randomly distributed by the two floor changers (e.g., two force plate locations) to provide a situation of inadvertency.
Dependent Measures
The converted coordinate data from the motion analysis system and ground reaction forces were observed during a typical slip behavior to provide robust definitions of the gait parameters. (For a comprehensive review of the dependent measures, refer to Grönqvist et al., 2001, and Lockhart, Woldstad, & Smith, 2003.) The converted coordinate data for each of the 26 body markers (defining a14-segment whole-body model; see Figure 3) and the ground reaction forces were digitally smoothed using a fourth-order, zerolag, low-pass Butterworth filter (Winter, 1990) with a cutoff frequency of 6 Hz.
Figure 3.
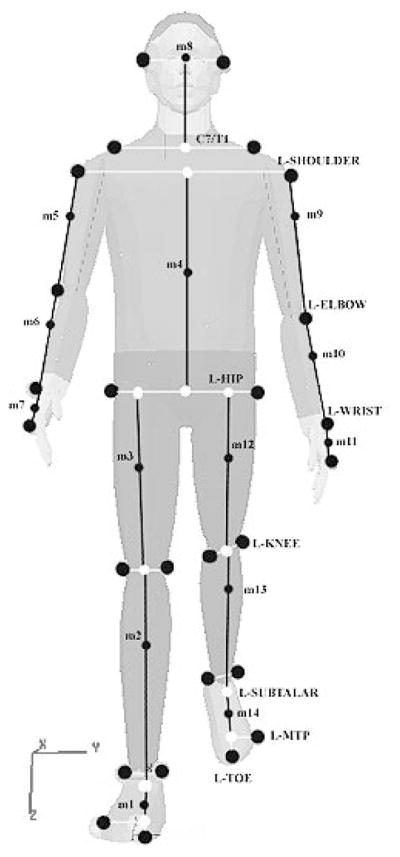
Locations of marker placements and internal landmarks used to generate whole-body center of mass and calculations of slip parameters.
Gait Parameters
Step length (SL)
The linear distance in the direction of progression between successive points of foot-to-floor contact of the first foot (X1, Y1) and other foot (X2, Y2) was measured on both floor surfaces. The step length was calculated from the difference between consecutive positions of the heel contacting the floor (resultant) using the general distance formula.
Step length index (SLI)
Normalized step length index was calculated by dividing the participant’s step length by his or her height.
Heel contact velocity (Vhc)
The instantaneous horizontal (plane of progression) heel velocity (Vhc) at heel contact was calculated utilizing heel velocities in the horizontal direction at the foot displacement of 1/60 s (Δt) before and after the heel contact phase of the gait cycle on both floor surfaces.
Velocity of the whole-body COM (VCOM)
Sagittal and frontal link (14) segment models (Lockhart et al., 2003) were used to calculate position and velocities of the whole-body COM. The sagittal and frontal models utilized the 14-component link-segment system defined by Mackinnon and Winter (1993) and the anthropometric model (Winter, 1990). The relative horizontal COM velocity of the whole body was calculated by averaging displacement over three time intervals (50 ms) of the instantaneous COM velocity before the heel contact phase of the gait cycle on both floor surfaces.
Friction demand (RCOF)
The required coefficient of friction (RCOF) was obtained by dividing the horizontal ground reaction force by the vertical ground reaction force (Fh/Pv) after heel contact (Peak 3 as defined by Perkins, 1978) on the carpeted floor surface to obtain the initial friction demand.
Slip Parameters
Definitions of the slip parameters were based on the observed typical slip behavior as illustrated by Figure 4 (adapted from Lockhart, Woldstad, & Smith, 2002). Heel contact was defined as the time when the vertical ground reaction force exceeded 10 N.
Figure 4.
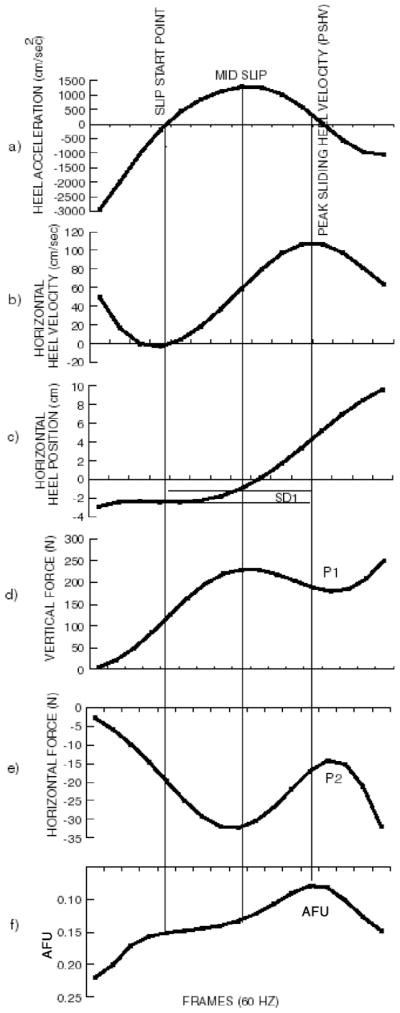
Composite view of the slip parameters. Adapted with permission from STP 1424 Metrology of Pedestrian Locomotion and Slip Resistance, copyright ASTM International, 100 Barr Harbor Dr., West Conshohocken, PA 19428 (Lockhart et al., 2002).
Initially, as indicated by the horizontal heel position (Figure 4c), the heel does not slip forward. Horizontal heel velocity decreases (Figure 4b) as the heel quickly decelerates (Figure 4a) during this time period. This (no slip) is believed to be the result of the position of the whole-body COM (closer to the other stance foot; Mackinnon & Winter, 1993) during the heel contact phase of the gait cycle. Shortly after heel contact (approximately 60 ms, as the forefoot comes down and the COM shifts toward the sliding heel), the heel begins to slip forward (Figure 4c). Afterward, the sliding heel reaches peak sliding heel velocity (Figure 4b). During this slipping period the heel accelerates, reaching the maximum (Figure 4a) near the midpoint of the sliding heel velocity profile (Figure 4b). After reaching maximum heel velocity, the sliding heel velocity decreases to minimum, halting further slipping (not complete on Figure 4).
Lockhart et al. (2003) indicated that the fall-arresting harness may influence slip parameters beyond certain point of slipping time. For example, Point 1 (P1) of the vertical force profile illustrates that there is a big decrease in vertical force (Figure 4d) as the participant slips (after reaching peak sliding heel velocity). This decrease in vertical force may have resulted when the participant tried to compensate for a slip by utilizing the fall-arresting harness and, in the process, may also may have affected the horizontal force profile (P2 in Figure 4e). Thus, beyond the peak heel velocity point, the fall-arresting harness may affect the biomechanical parameters of slip severity (slip distance, slipping velocity, etc). Utilizing this concept, in order to represent all slip-recovery and slip-fall trials uniformly, we did not calculate slip parameters beyond the peak heel velocity point.
Adjusted friction utilization (AFU)
The ratio between the horizontal and vertical ground reaction force was calculated during slip-grip responses on the oily vinyl tile floors (Figure 4f). AFU represents the ability to adjust dynamic frictional requirements during slipping (Grönqvist et al., 2001). The significance of this ratio is that it indicates when the gait compensation for a slip is most likely to occur.
Initial slip distance (SDI)
The initial distance traveled by the foot after the heel contact phase of the gait cycle was measured to provide information concerning the severity of slip initiation. The slip start point was defined as the point where nonrearward positive acceleration of the foot after heel contact occurred (where the first minimum of the vertical heel velocity after the heel contact occurred), and the slip stop point for SDI was defined as the point at which peak heel acceleration occurred after the slip start point (Lockhart et al., 2003; Grönqvist et al., 2001). SDI was calculated utilizing a general distance formula.
Slip Distance II (SDII)
Slip Distance II (SDII) was developed to provide information concerning the slip behavior after the initiation of slips. The start point for the SDII was defined as an SDI slip stop point and the end of slip (for the purpose of calculation) as the time when the first maximum of horizontal heel velocity occurred after the slip start point occurred (Lockhart et al., 2003; Grönqvist et al., 2001). SDII was also calculated utilizing a general distance formula.
Sliding heel velocity (Vs)
The relative sliding heel velocity (Vs) of the heel after heel contact was calculated (to assess severity of slips and falls) by averaging the instantaneous sliding heel velocity during the slip start and peak sliding heel velocity points on the oily vinyl floor surface.
Sliding heel acceleration (Hacc)
The relative heel acceleration of the heel after heel contact was calculated by averaging the acceleration of the heel during the slip start and slip stop points on the oily vinyl floor surface.
Postural Control
Sensory Organization Test (SOT) scores
SOT scores were based on the assumption that a normal individual can exhibit anterior to posterior sway over a total range of 12.5° (6.25° anterior, 6.25° posterior) without losing balance. An overall equilibrium score was calculated by comparing the angular difference between the participant’s calculated maximum anterior to posterior COM displacements to this theoretical maximum displacement (i.e., SOT scores). The result was expressed as a percentage between 0% and 100%, with 0% indicating sway exceeding the limits of stability and 100% indicating perfect stability. Subcomponent scores of vision, vestibular, and proprioceptive systems were calculated via the NeuroCom system utilizing SOT conditions (see the Procedures section). Conditions 1 and 2 were used to test a participant’s ability to use input from the proprioceptive system to maintain balance. Conditions 1 and 3 were used to test a participant’s ability to use input from visual system to maintain balance. Conditions 2 and 4 were used to test a participant’s ability to use input from the vestibular system to maintain balance (El-Kashlan et al., 1998; Ruckenstein & Shepard, 2000).
Motor Control Test (MCT) time
The MCT time was defined as the delay (in milliseconds) between the onset of a translation and the onset of the participant’s active response to the support surface movement. The muscle latency time was based on the MCT protocols utilizing the six conditions described in the Procedures section. The composite muscle latency time was determined as the average of the latency scores following medium and large translations (Black, 2001; Nashner, Black, & Wall, 1982).
Strength
Isometric muscle strength
Whole-body isometric strength measurements were collected for the arms, legs, and torso (Chaffin & Andersson, 1991; Chaffin et al., 1978).
Isokinetic knee flexion strength
Three isokinetic knee flexion strength measurements were collected, and the highest knee flexion torque was recorded as the isokinetic strength.
Treatment of Data
The dependent measures – step length (SL), step length index (SLI), horizontal heel contact velocity (Vhc), and velocity of the whole-body COM (VCOM) – were analyzed using 3 ×2 (Age × Floor) two-way repeated measures analyses of variance (ANOVAs). RCOF was analyzed using a one-way ANOVA on the carpeted floor surface. Slip distances (SDI and SDII), sliding heel velocity (Vs), sliding heel acceleration (Hacc), and adjusted friction utilization (AFU) were analyzed using one-way ANOVAS on the oily vinyl tile floor surface. Additionally, SOT scores, MCT time, and isometric/isokinetic muscle strengths were analyzed using one-way ANOVAs. Results were considered significant at α ≤ .05. For these analyses, floor (where applicable) was treated as a within-subjects effect, whereas age was a between-subjects effect.
Bivariate correlation analyses were also performed between the slip parameter (SDII) and physical parameters (SOT, MCT, and maximal isometric leg strength) in order to elaborate onthe effects of sensory and musculoskeletal changes associated with aging and outcome of slips.
RESULTS
Strength
Isometric muscle strength (arms, legs, and torso)
The results of a one-way ANOVA indicated no statistically significant (p > .05) arm, F(2, 39) = 0.987, p ≈ .38, or torso, F(2, 39) = 2.25, p ≈ .12, strength differences among the age groups. However, older participants’ leg strength was significantly (p ≤ .05) lower than that of their younger counterparts, F(2, 39) = 4.224, p ≈ .02. Post hoc comparison of means (Tukey-Kramer honestly significant difference [HSD]) indicated that the older age group’s leg strength was significantly less than that of both the young and middle age groups, and the middle age group’s leg strength was significantly less than that of the younger age group.
Table 1 summarizes the mean values and standard deviations for each of the dependent measures as a function of age group.
TABLE 1.
Summary of the Dependent Variables
| Young (18–29 years) | Middle (35–59 years) | Old (>65 years) | |
|---|---|---|---|
| Variables | Mean (SD) | Mean (SD) | Mean (SD) |
| Maximal Isometric Strength | |||
| Arms (N) | 327.93 (116) | 324.85 (114) | 278.28 (85) |
| Torso (N) | 730.46 (294) | 699.71 (287) | 530.35 (139) |
| Leg (N)* | 923.00 (333) | 800.78 (321) | 577.92 (164) |
| Maximal Isokinetic Knee Flexion Strength | |||
| 30°/s (Nm)* | 58.14 (17.5) | 55.28 (18.7) | 41.92 (12.5) |
| 60°/s (Nm)* | 52.85 (16.3) | 48.64 (16.8) | 34.64 (11.3) |
| 90°/s (Nm)* | 45.71 (15.0) | 40.21 (14.5) | 25.57 (8.94) |
| Sensory Organization | |||
| MCT (ms)* | 123.78 (10.8) | 131.40 (7.50) | 139.70 (5.20) |
| SOT (%)* | 83.85 (3.75) | 77.60 (3.65) | 67.13 (7.20) |
| Somatosensory* | 94.50 (1.95) | 92.64 (1.96) | 90.35 (5.65) |
| Vision* | 91.92 (3.17) | 87.92 (3.75) | 82.21 (6.38) |
| Vestibular* | 73.07 (5.37) | 64.71 (7.47) | 50.00 (11.1) |
| Gait Parameters | |||
| Step length (cm)* | 65.35 (7.34) | 67.63 (9.05) | 59.12 (7.67) |
| SL index (SLI)* | 0.38 (0.04) | 0.39 (0.04) | 0.34 (0.03) |
| Heel contact velocity (cm/s) | 31.03 (14.5) | 32.11 (13.5) | 42.31 (17.9) |
| COM velocity (cm/s)* | 109.99 (14.4) | 115.53 (18.2) | 99.87 (16.5) |
| RCOFa | 0.176 (0.01) | 0.188 (0.02) | 0.192 (0.02) |
| AFUb* | 0.074 (0.01) | 0.010 (0.01) | 0.120 (0.01) |
| Sliding heel velocity (cm/s)b* | 44.05 (35.1) | 63.95 (31.7) | 75.84 (39.7) |
| Sliding heel acceleration (cm/s2)b* | 609.50 (79.2) | 907.86 (73.5) | 912.11 (66.5) |
| Initial slip distance (SDI; cm)b | 1.09 (0.39) | 2.30 (0.39) | 2.17 (0.36) |
| Total slip distance (SDII; cm)b* | 4.98 (4.81) | 7.65 (4.92) | 11.80 (9.43) |
Measured on carpeted floor surface only.
Measured on oily vinyl floor surface only.
Significant (p > .05) across age groups.
Isokinetic knee flexion strength
The results of a one-way ANOVA indicated that older participants’ knee flexion strengths at each level (30°/s, 60°/s, and 90°/s) were all significantly (p ≤ .05) lower than those of their younger counterparts: 30°/s, F(2, 39) = 3.87, p ≈ .03; 60°/s, F(2, 39) = 5.63, p ≈ .007; and 90°/s, F(2, 39) = 8.85, p ≈ .0007. Post hoc comparison of means (Tukey-Kramer HSD) indicated that the older age group’s knee flexion strengths at all levels were significantly less than those of both the young and middle age groups, and the middle age group’s knee flexion strength was significantly less than that of the younger group.
Postural Control
Motor Control Test (MCT) time
The results of a one-way ANOVA indicated that older participants’ muscle latency times were significantly (p ≤ .05) longer than the those of the middle-age and younger participants, F(2, 39) = 13.77, p < .0001. Post hoc comparison of means indicated that the older age group’s MCT time was significantly longer than that of both the young and middle age groups, and the middle age group’s MCT time was significantly longer than that of the younger age group.
Sensory Organization Test (SOT) scores
Older participants’ SOT scores were significantly (p ≤ .05) lower than those of their younger counterparts, F(2, 39) = 33.27, p < .0001. Post hoc comparison of means indicated that the older age group’s SOT score was significantly less than that of both the young and middle age groups, and the middle age group’s SOT score was significantly less than that of the younger age group. Older adults’ itemized SOT scores (somatosensory, vision, vestibular) were also significantly (p ≤ .05) lower than those of their younger counterparts – somatosensory, Conditions 1 and 2, F(2, 39) = 4.61, p < .01; vision, Conditions 1 and 3, F(2, 39) = 15.41, p < .001; vestibular, Conditions 2 and 4, F(2, 39) = 27.55, p < .001 – with post hoc comparisons similar to those discussed previously.
Gait Parameters
Step length (SL) and step length index (SLI)
The result of a two-way ANOVA indicated that older participants’ SL was significantly (p ≤ .05) shorter than that of their younger counterparts, F(2, 39) = 4.735, p ≈ 0.01. Similarly, older participants’ SLI was significantly (p ≤ .05) lower than that of their younger counterparts, F(2, 39) = 6.676, p ≈ .003. Additionally, there were no statistically significant (p > .05) floor effects on SL, F(2, 39) = 3.166, p ≈ .05, or SLI, F(2, 39) = 3.975, p ≈ .06. Post hoc comparison of means indicated that the middle age group’s SL and SLI were significantly longer and higher than those of the younger and older participants, and the older age group’s SL and SLI were significantly shorter and lower than those of their younger counterparts.
Heel contact velocity (Vhc)
The result of a two-way ANOVA indicated no statistically significant (p > .05) horizontal heel contact velocity (Vhc) differences among the age groups, F(2, 39) = 2.0889, p ≈ .06. Additionally, there were no statistically significant (p > .05) floor effects on Vhc, F(2, 39) = 0.846, p ≈ .43.
Velocity of the whole-body COM (VCOM)
The result of a two-way ANOVA indicated that older participants’ VCOM was significantly (p ≤ .05) slower than that of their younger counterparts, F(2, 39) = 4.404, p ≈ .04. Additionally, there were no statistically significant (p > .05) floor effects on VCOM, F(2, 39) = 0.846, p ≈ .43. Post hoc comparison of means indicated that the middle age group’s VCOM was significantly faster than that of their younger and older counterparts, and the older age group’s VCOM was significantly slower than that of the younger age group.
Friction demand (RCOF)
The one-way ANOVA of RCOF indicated no statistically significant (p > .05) differences among the age groups, F(2, 39) = 2.392, p < .10.
Slip Parameters
Adjusted friction utilization (AFU)
The result of the one-way ANOVA indicated that the older participants’ AFU was significantly (p ≤ .05) higher than that of their younger counterparts, F(2, 39) = 13.197, p < .0001. Post hoc comparison of means indicated that the younger group’s AFU was significantly lower than that of the middle and older age groups and that there were no significant differences between the middle and older age groups.
Sliding heel velocity (Vs)
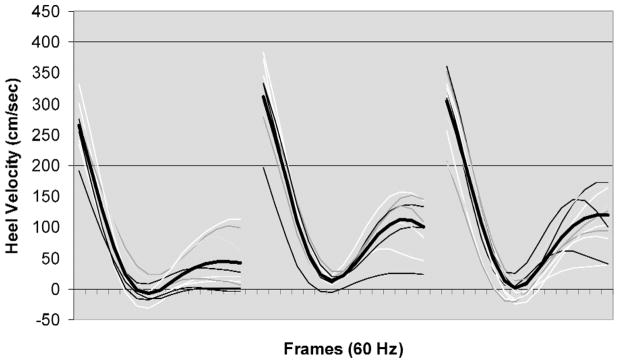
The result of the one-way ANOVA indicated that older participants’ Vs was significantly (p ≤ .05) faster than that of their younger counterparts, F(2, 39) = 5.536, p < .007. Post hoc comparison of means indicated that the older age group’s Vs was significantly faster than that of the younger participants and that there were no significant differences between the middle and older age groups. Figure 5 illustrates the results of individual trials and means (darker line) of heel velocity before heel contact (Vhc) and after heel contact (Vs) on the oily vinyl floor surface among the three age groups.
Figure 5.
Heel velocity profiles of young (left panel), middle (middle panel), and older (right panel) age groups (116 ms before and 116 ms after heel contact). Each tick marker (frame) represents 1/60 s. Heel contact was defined as the time when the vertical ground reaction force exceeded 10 N. The darker lines represent averages of heel contact velocities in each of the age groups.
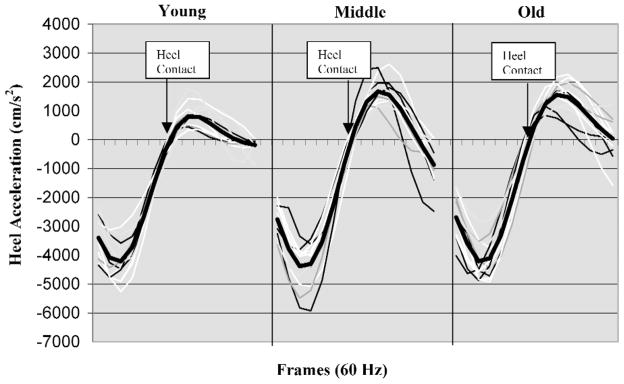
Sliding heel acceleration (Hacc)
The result of the one-way ANOVA indicated that older participants’ Hacc was significantly (p ≤ .05) faster than that of their younger counterparts, F(2, 39) = 5.448, p < .008. Post hoc comparison of means indicated that the older age group’s Hacc was significantly faster than that of the younger age group and that there were no significant differences between the middle and older age groups. Figure 6 illustrates the results of individual trials and means (darker line) of heel acceleration before heel contact (Vhc) and after heel contact (Vs) on the oily vinyl floor surface among young and older individuals.
Figure 6.
Sliding heel velocity profiles of three age groups (116 ms before and 116 ms after heel contact). Each tick marker (frame) represents 1/60 s. Heel contact was defined as the time when the vertical ground reaction force exceeded 10 N. The darker lines represent averages of sliding heel velocities in each of the age groups.
Slip distances (SDI and SDII)
The results of a one-way ANOVA on SDI indicated no statistically significant differences among the age groups, F(2, 39) = 2.989, p ≈ .06. The results of a one-way ANOVA on SDII indicated significant differences with respect to age group, F(2, 39) = 3.69, p ≈ .03. Post hoc comparison of means indicated that the older age group’s SDII was significantly longer than that of both the young and middle age groups and that there were significant differences between the middle and older age groups.
Fall Characteristics
Frequency of slips and falls
Slip and fall classification was obtained by comparing horizontal velocity of the whole-body COM (VCOM) with sliding heel velocity (Vs) of the individuals on theoily vinyl floor surface (during the period of slipping). In order to objectively assess an actual fall, we identified a fall as whenever two conditions were met simultaneously: (a) when the Vs was greater than the VCOM during the slipping period and (b) when the participant’s body dropped toward the floor after slipping and was arrested by the harness before impact (assessed by visual inspections of video recordings of the actual fall trials). The results indicated that younger individuals experienced 4 falls, middle-age individuals experienced 8 falls, and older individuals experienced 12 falls.
Fall recovery threshold (FRT)
FRT analysis was performed utilizing fallers’ slip parameters (Table 2) to provide information regarding the relationship among slip parameters (initial slip distance, sliding heel velocity, and sliding heel acceleration) and fall accidents in each age groups. In other words, we investigated whether or not different age groups exhibited different threshold values (i.e., slip parameters) during a fall event. In general, when the participants in each age group exceeded the FRT limits, a fall resulted. The results of FRT analysis indicated that the younger individuals’ recovery threshold was higher on the average and suggests that the recovery threshold is not all the same for the entire population. Thus, in a given situation, older individuals are at a higher risk for fall accidents (i.e., younger individuals can slip longer and faster and not fall).
TABLE 2.
Slip Recovery Index Across Three Age Groups
| Age Group | Sliding Heel Velocity (cm/s) | SDI (cm) | Hacc (cm/s2) |
|---|---|---|---|
| Young | 144.45 | 3.91 | 1580 |
| Middle | 145.26 | 3.80 | 1310 |
| Old | 107.63 | 3.12 | 1220 |
Bivariate correlation analyses
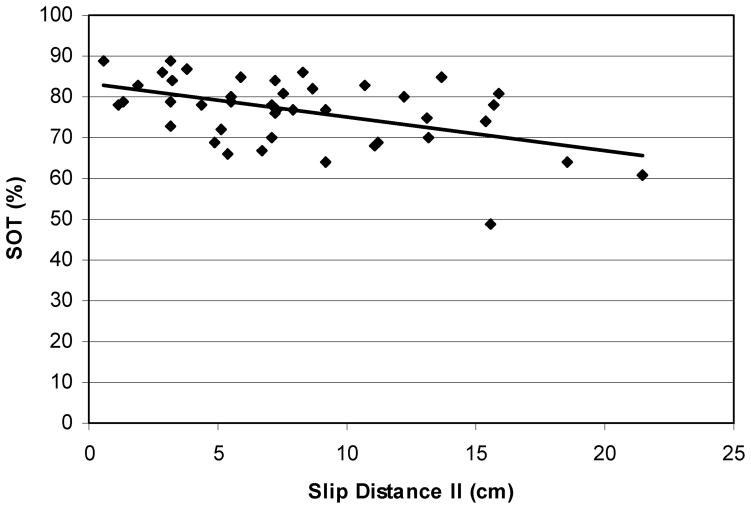
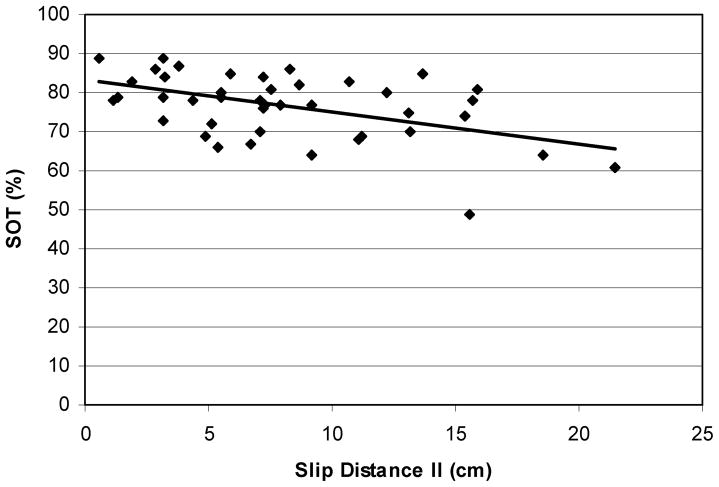
Bivariate correlation analyses were performed between the slip parameter (SDII) and physical parameters (SOT, MCT, and maximal isometric leg strength) in order to elaborate on the effects of sensory and musculoskeletal changes associated with aging and outcome of slips. In general, individuals with lower SOT scores slipped longer (Figure 7, r = −.49, p ≈ .005). The relationship between MCT and SDII indicated that individuals with higher MCT slipped longer (Figure 8, r = .20, p ≈ .03).
Figure 7.
Relationship between distance slipped and sensory organization scores of each participants (r = −.49). In general, individuals with lower SOT scores slipped farther.
Figure 8.
The relationship between muscle latency times and distances slipped (r = .2). In general, individuals with longer muscle latency times slipped farther.
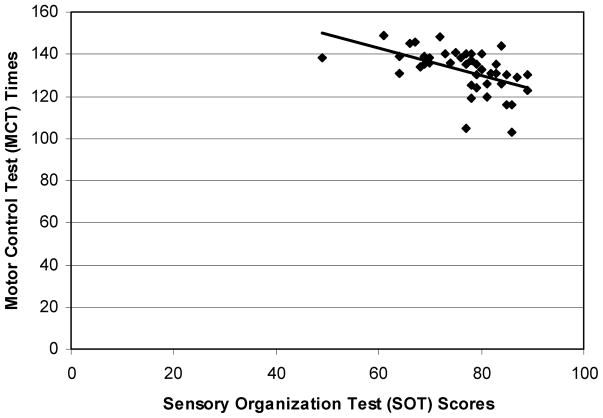
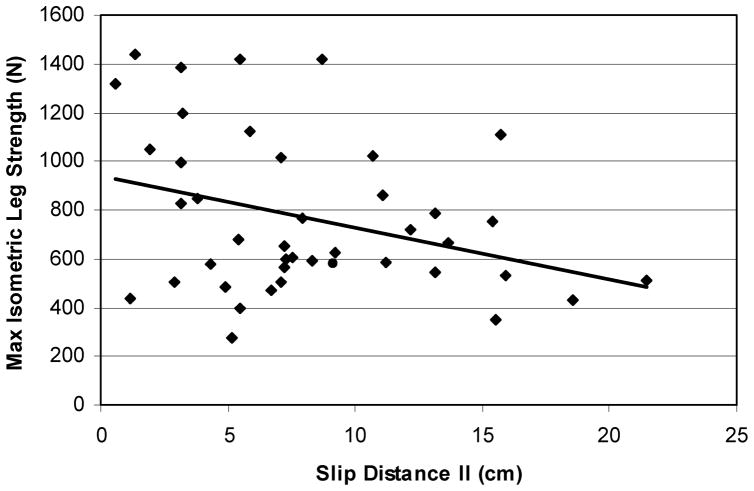
Furthermore, a relationship between SOT and MCT suggests that individuals with higher SOT scores took less time to actively respond to the support surface movements (Figure 9, r = −0.51, p ≈ .0002). The relationship between maximal isometric leg strength and SDII indicated that individuals with stronger lower extremities slipped less (Figure 10, r = −0.57, p ≈ .002). Additionally, older adults’ lower extremity strength was lower than that of their younger counterparts, and they slipped longer than their younger counterparts did.
Figure 9.
The relationship between SOT scores and MCT times (r = −.51). In general, individuals with higher SOT scores took less time to actively respond to the support surface movements.
Figure 10.
The relationship between maximal isometric leg strength and distance slipped (r = −0.57). In general, individuals with stronger lower extremities slipped less.
Multiple regression analysis
Multiple regression analysis was performed to describe and predict the relationship between the independent variables – age, height, weight, vision, proprioception, vestibular responses, MCT, and strength variables (strength variables were divided by the weight of the each participant) – and the dependent variables (SD: total slip distances [SDI + SDII]). The data were analyzed by utilizing techniques available for evaluating probabilistic process, functional specification of mean response, constant variance, and normality assumptions. The best subset regression method was applied to selecting the predictor variables utilizing cp (underspecified) and backward elimination procedure for dependent variable SD. The final model of SD is
in which vision = scores collected from Conditions 1 and 3 of computerized dynamic posturography, MCT = muscle latency response times associated with medium and large support surface movements, and LLI (lower leg index) = lower leg strength/body weight.
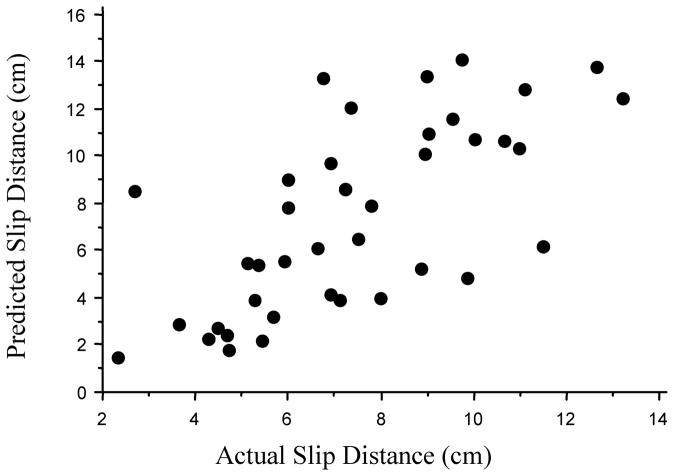
The prediction model further suggests that vision, reaction time, and muscle strength (lower extremity) were important for determining slip and fall severity (Figure 11).
Figure 11.
Illustration of actual slip distances and predicted slip distances. The prediction model further suggests that the vision, reaction time, and muscle strength (lower extremity) were important for determining slip distances.
DISCUSSION
Our purpose in this study was to investigate the process of initiation, detection, and recovery of inadvertent slips and falls – more specifically, how age-related gait adaptation, deterioration of muscular strength and sensory degradation among older individuals would affect biomechanical parameters of slip and fall accidents under slippery and nonslippery conditions. From the work conducted thus far, we hypothesized that gait adaptations, deterioration of muscular strength, and sensory degradation among older individuals would affect the severity of their slips and falls more than those of their younger counterparts. In particular, it was expected that older adults’ gait adaptations would adversely affect the slip initiation process (i.e., would increase the friction demand characteristic at the shoe-floor interface at the time of the heel contact, which would increase the distance slipped initially), leading to more falls. It was also expected that older adults’ deterioration of muscular strength and sensory degradation would adversely affect the detection and recovery processes of slip and fall accidents, increasing the speed of slipping and the distance slipped, ultimately leading to more falls.
Slip Initiation Process
The outcome of a slip-induced fall accident is highly influenced by friction demand characteristics. The works of Perkins and Wilson (1983), Redfern and Andres (1984), and Hanson, Redfern, and Mazumdar (1999) have suggested that the number of slip and fall events will increase as the differences between the friction demand characteristic (i.e., RCOF) and measured dynamic COF increases. Most slips that lead to falls occur when the frictional force opposing the movement of the foot is less than the shear force of the foot immediately after the heel contacts the floor (Perkins, 1978). Furthermore, as indicated by many researchers, initial gait characteristics such as step length, heel contact velocity, and walking velocity may influence the friction demand characteristic because of alterations of the horizontal foot shear force.
In close agreement with previous findings (Mills & Barrett, 2001; Winter, 1991, pp. 87–94), in this experiment older individuals’ step length was significantly shorter and walking velocity (i.e., COM velocity) was significantly slower, as compared with those of their younger counterparts. However, in contrast to previous studies (Lockhart, 1997; Mills & Barrett, 2001; Winter, 1991), we found no statistically significant differences in heel contact velocity. That no differences were found between young and older adults’ heel contact velocity in the present study may be reflective of the selection criterion (which aimed to include only healthy older individuals with no gait abnormalities) or a factor of walking velocity. Regardless of the influencing factors, shorter step length, slower walking velocity, and insignificant differences of heel contact velocity between the young and old groups in this test population suggest that the likelihood of slip initiations is similar across all age groups. This statement is further supported by the finding of no significant differences in initial friction demand characteristics (i.e., RCOF) and initial slip distance (SDI) among the three age groups. Given these results, it seems that older adults in the present study effectively adopted a strategy of stable gait pattern (i.e., shorter step length and slower walking velocity) to reduce the initiation of slipping. Why, then, did older adults in the present study fall more often than their younger counterparts did? The answer may lie hidden in the processes associated with detection of slips and recovery from falls.
Detection and Recovery
Nashner (1982) suggested that at the time of a potential slip-induced fall, the central nervous system must undertake certain processing stages if a fall is to be avoided or recovered from. First, during the detection of slip perturbation, sensory input must trigger or alert those centers responsible for response selection. This alerting process may be initiated by one or more of the following sensory inputs: proprioception, vision, and vestibular function (Nashner, 1982). Thus any age-related disruption in the quality of the signal from sensory inputs may increase the likelihood of slip-induced falls because of delayed response selection. Furthermore, after the response selection, muscular strength may play an important role in the recovery process (attenuation of lower extremity motions during slip perturbation).
In order to investigate the effects of sensory organization on the outcome of slips and falls, we collected equilibrium scores utilizing a computerized dynamic posturography. Similar to previous studies, in our study we found significant equilibrium score differences among the three age groups. The results of the Sensory Organization Test (SOT) and associated modalities (somatosensory, vision, and vestibular) indicated that equilibrium scores were lower for older individuals than for their younger counterparts. In order to provide the relationship between postural control during quiet stance and dynamic slip responses when walking over a slippery floor surface, bivariate correlation analysis between SOT scores and slip distance was performed. The results suggested that individuals with lower SOT scores (sensory degradation) slipped longer. Furthermore, older adults exhibited lower SOT scores along with longer slip distances. Slip behavior utilizing slip distance measures has been investigated by several researchers (Brady, Pavol, Ownings, & Grabiner, 2000; Perkins, 1978; Strandberg & Lanshammar, 1981). These investigators found that the severity of a slip (whether the slip resulted in a fall) was dependent on the distance that participants slipped (e.g., any slip distance more than 10 cm resulted in a fall). This relationship between equilibrium score and slip distance (SDII) suggests that older adults are more likely to fall as a result of a delayed response selection process.
This statement is further supported by the bivariate correlation analysis between the Motor Control Test (MCT) and slip distance (SDII). The relationship between MCT and SDII indicated that individuals with longer motor control times slipped longer, and older adults’ motor control time was significantly longer than that of their younger counterparts. Furthermore, the relationships between SOT and MCT suggest that individuals with higher SOT scores took less time to actively respond to the support surface movements. The effect of the sensory degradation and delayed response selection is further supported by higher adjusted friction utilization (AFU). AFU represents the ability to adjust dynamic frictional requirements during slipping (Grönqvist et al., 2001). The significance of this ratio is that it indicates when the gait compensation for a slip is most likely to occur. On average, the younger individuals’ AFU (.074) was adjusted within the dynamic friction requirements (.08). However, on average, the middle-age (AFU .10) and older individuals (AFU .12) could not. Consequently, the result was longer slip distance (SDII) and higher frequency of falls. Thus it seems that sensory degradation among older adults may result in increased frequency of slips and falls because of delayed response selection.
In this study, it is important to note that MCT and SOT scores are confounded with age because they were observed as opposed to being manipulated. Additional research that compares younger participants with poor MCT and SOT scores versus older participants with good scores would provide greater insight as to the nature of this effect. As such, although the mechanisms associated with these relationships are implicated, further studies investigating these mechanisms are needed to elucidate this possibility.
Furthermore, muscular strength may have also contributed to older adults’ fall frequencies. Do, Breniere, and Brenguier (1982) and Cham and Redfern (2001) suggested that strength generation and the ability to attenuate lower extremity motions are critical in determining whether or not a person can respond appropriately to balance perturbation (i.e., recovery from slips). Evidence in support of this hypothesis comes from a number of investigations indicating an age-related decline in voluntary muscle strength and rate of muscle force production and an increased likelihood of slips and falls. For example, Wolfson et al. (1985) and Larsson et al. (1979) reported that ankle and quadriceps muscle strength was significantly lower for those who fall as compared with nonfallers. Additionally, reduced lower extremity strength has been implicated as a factor contributing to nursing home placement (Hubert et al., 1993; Wolfson et al., 1995) and an increased risk of falling (Whipple et al., 1987). In this study, older adults’ lower extremities were significantly weaker than those of their younger counterparts (both isometric and isokinetic). A relationship between leg strength and SDII further indicates that individuals with stronger lower extremities slipped less, suggesting that recovery from a slip is associated with lower extremity muscle strength. Further studies investigating the mechanisms associated with muscle force productions and balance recoveries from slip perturbation (i.e., autonomic reflex-like postural responses independent of sensory organizations) are needed to reveal this possibility.
In conclusion, all participants slipped under slippery conditions. However, the older individuals could not control their slips, leading to more falls. Thus, in a given situation, older individuals are at a higher risk for fall accidents (i.e., younger individuals can slip longer and faster and not fall). The prediction model further supports the idea that vision, reaction time, and muscle strength (lower extremity) are important for determining slip and fall severity. Inability to control slipping responses may be a result of sensory degradation and muscle weakness. As such, possible intervention strategies (muscle strengthening and balance exercises) can be important for improving dynamic equilibrium for older adults. Furthermore, most of the current research on slips and falls concentrates predominantly on initiation of slips (i.e., RCOF); however, this study indicates that how slips result in a fall is important as well. Therefore, future research should focus not only on the dynamics of slips but also on the dynamics of falls.
A principal limitation in the current study may have arisen from the situation of inadvertency. Unexpected slips and falls were introduced utilizing the methods described. However, as in all laboratory experiments, a tendency to anticipate “complete unexpectedness” will be limited by equipment and laboratory settings. In order to avoid such anticipation, we had participants walk at a natural cadence for approximately 15 min prior to introducing the slippery surface. A comparison of the gait parameters (e.g., step length, heel contact velocity, and walking velocity) between the nonslippery and slippery floor surfaces suggest, although speculatively, that the participants’ walking characteristics were consistent throughout the experiment. Previous studies investigating gait pattern modification suggested that when participants knew they were walking onto a slippery floor surface, they tended to shorten their step length and reduce walking velocity (Ekkubus & Killey, 1973; Lockhart, 1997). The lack of significant differences in step length, heel contact velocity, and walking velocity between the slippery and nonslippery floor surfaces suggests that our participants were unaware of the floor surface change and thus did not alter their gait characteristics when stepping onto the slippery floor surface.
Furthermore, the model of slips and falls in this study does not include the information-processing capabilities of the individuals. As such, our results may be confounded by an individual’s ability to process critical information in a timely manner. Future studies should link a human information-processing paradigm and biomechanics of reactive recovery during slip perturbation to refine the model of slips and falls.
Acknowledgments
This research was supported by the National Institutes of Health (National Institute for Occupational Safety and Health) Grant R03/Small Grant 1-R03-0803917-01. The paper’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Biographies
Thurmon E. Lockhart is an assistant professor of industrial and systems engineering and the director of the Locomotion Research Laboratory at Virginia Polytechnic Institute and State University, Blacksburg, Virginia. He received his Ph.D. in industrial engineering at Texas Tech University in 2000.
James L. Smith is a professor of industrial engineering at Texas Tech University, Lubbock, Texas. He received his Ph.D. in industrial engineering at the University of Auburn in 1980.
Jeffrey C. Woldstad is an associate professor of industrial and manufacturing engineering at Oregon State University, Corvallis, Oregon. He received his Ph.D. in industrial engineering at the University of Michigan in 1989.
Contributor Information
Thurmon E. Lockhart, Virginia Polytechnic Institute and State University, Blacksburg, Virginia
James L. Smith, Texas Tech University, Lubbock, Texas
Jeffrey C. Woldstad, Oregon State University, Corvallis, Oregon
References
- Agnew J, Suruda AJ. Age and fatal work-related falls. Human Factors. 1993;35:731–736. doi: 10.1177/001872089303500411. [DOI] [PubMed] [Google Scholar]
- American Geriatrics Society, British Geriatrics Society, & American Academy of Orthopaedic Surgeons Panel on Falls Prevention. Guideline for the prevention of falls in older persons. Journal of the American Geriatrics Society. 2001;49:664–672. [PubMed] [Google Scholar]
- Astrand PO, Rodahl K. Textbook of work physiology. New York: McGrawHill; 1986. [Google Scholar]
- Bard C, Fleury M, Teasdale N, Paillard J, Nougier V. Contribution of proprioception for calibrating and updating the motor space. Canadian Journal of Physiology and Pharmacology. 1995;73:246–254. doi: 10.1139/y95-035. [DOI] [PubMed] [Google Scholar]
- Black FO. Clinical status of computerized dynamic posturography in neurotology. Current Opinion in Otolarynogology –Head and Neck Surgery. 2001;9:314–318. [Google Scholar]
- Bonder BR, Wagner MB. Functional performance in older adults. Philadelphia: F. A. Davis; 1994. [Google Scholar]
- Brady RA, Pavol MJ, Owings TM, Grabiner MD. Foot displacement but not velocity predicts the outcome of a slip induced in young subjects while walking. Journal of Biomechanics. 2000;33:803–808. doi: 10.1016/s0021-9290(00)00037-3. [DOI] [PubMed] [Google Scholar]
- Brocklehurst JC, Robertson D, James-Groom P. Clinical correlates of sway in old age – Sensory modalities. Age and Ageing. 1982;11:1–10. doi: 10.1093/ageing/11.1.1. [DOI] [PubMed] [Google Scholar]
- Campbell AJ, Reinken J, Allan BC, Martinez GS. Falls in old age: A study of frequency and related factors. Age and Ageing. 1981;10:264–270. doi: 10.1093/ageing/10.4.264. [DOI] [PubMed] [Google Scholar]
- Chaffin DB, Andersson GBJ. Occupational biomechanics. New York: Wiley; 1991. [Google Scholar]
- Chaffin DB, Herrin GD, Keyserling WM. Pre-employment strength testing. Journal of Occupational Medicine. 1978;20:403–408. [PubMed] [Google Scholar]
- Chaffin DB, Woldstad JC, Trujillo A. Floor/shoe slip resistance measurement. American Industrial Hygiene Association Journal. 1992;53:283–289. doi: 10.1080/15298669291359672. [DOI] [PubMed] [Google Scholar]
- Cham R, Redfern MS. Lower extremity corrective reactions to slip events. Journal of Biomechanics. 2001;34:1439–1445. doi: 10.1016/s0021-9290(01)00116-6. [DOI] [PubMed] [Google Scholar]
- Cohen H, Heaton LG, Congdon SL, Jenkins HA. Changes in sensory organization test scores with age. Age and Ageing. 1996;25:39–44. doi: 10.1093/ageing/25.1.39. [DOI] [PubMed] [Google Scholar]
- Cohn TE, Lasley DJ. Visual depth illusion and falls in the elderly. Clinics in Geriatric Medicine. 1985;1:601–610. [PubMed] [Google Scholar]
- Cooper JM, Glassow RB. Kinesiology. New York: Mosby; 1963. [Google Scholar]
- Courtney TK, Sorock GS, Manning DP, Collins JW, Holbein-Jenny MA. Occupational slip, trip, and fall-related injuries – Can the contribution of slipperiness be isolated. Ergonomics. 2001;44:1118–1137. doi: 10.1080/00140130110085538. [DOI] [PubMed] [Google Scholar]
- Do MC, Breniere Y, Brenguier P. A biomechanical study of balance recovery during the fall forward. Journal of Biomechanics. 1982;15:933–939. doi: 10.1016/0021-9290(82)90011-2. [DOI] [PubMed] [Google Scholar]
- Donald IP, Bulpitt CJ. The prognosis of falls in elderly people living at home. Age and Ageing. 1999;28:121–125. doi: 10.1093/ageing/28.2.121. [DOI] [PubMed] [Google Scholar]
- Ekkubus CF, Killey W. Validity of 0.5 static coefficient of friction (James Machine) as a measure of safe walkway surfaces. Soap/Cosmetics/Chemical Specialties. 1973;49(2):40–45. [Google Scholar]
- El-Kashlan NI, Hussam K, Shaepard NT, Asher AM, Smith-Wheelock M, Telian SA. Evaluation of clinical measures of equilibrium. Laryngoscope. 1998;108:311–319. doi: 10.1097/00005537-199803000-00002. [DOI] [PubMed] [Google Scholar]
- Englander F, Hodson TJ, Terregrossa RA. Economic dimensions of slip and fall injuries. Journal of Forensic Science. 1996;41:733–746. [PubMed] [Google Scholar]
- Ford-Smith C, Wyman J, Elswick RK, Fernandez T. Test-retest reliability of the sensory organization test in nonin-stitutionalized older adults. Archives of Physical Medicine and Rehabilitation. 1995;76:77–81. doi: 10.1016/s0003-9993(95)80047-6. [DOI] [PubMed] [Google Scholar]
- Ghez C, Sainburg RL. Proprioceptive control of inter-joint coordination. Canadian Journal of Physiology and Pharmacology. 1995;73:273–284. doi: 10.1139/y95-038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis B, Gilroy K, Lawley H, Mott L, Wall JC. Slow walking speeds in healthy young and elderly females. Physiotherapy Canada. 1986;38:350–352. [Google Scholar]
- Goldman R. Decline in organ function with aging. In: Rossman I, editor. Clinical geriatrics. Philadelphia: Lippincott; 1986. pp. 23–59. [Google Scholar]
- Grönqvist R, Abeysekera J, Gard G, Hsiang SM, Leamon TB, Newman DJ, et al. Human-centered approaches in slipperiness measurement. Ergonomics. 2001;44:1167–1199. doi: 10.1080/00140130110085556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JP, Redfern MS, Mazumdar M. Predicting slips and falls considering required and available friction. Ergonomics. 1999;42:1619–1633. doi: 10.1080/001401399184712. [DOI] [PubMed] [Google Scholar]
- Houmard JA, Weidner ML, Gavigan KE, Tyndall GL, Hickey MS, Alshami A. Fiber type and citrate synthase activity in the human gastrocnemius and vastus lateralis with aging. Journal of Applied Physiology. 1998;85:1337–1341. doi: 10.1152/jappl.1998.85.4.1337. [DOI] [PubMed] [Google Scholar]
- Hubert HB, Bloch DA, Fries JF. Risk factors for physical disability in an aging cohort: The NHANES I epidemiologic follow-up study. Journal of Rheumatology. 1993;20:480–488. [PubMed] [Google Scholar]
- Imms FJ, Edholm OG. The assessment of gait and mobility in the elderly. Age and Ageing. 1979;8:261–267. doi: 10.1093/ageing/8.4.261. [DOI] [PubMed] [Google Scholar]
- Inglis JT, Shupert CL, Hlavacka F, Horak FB. Effect of galvanic vestibular stimulation on human postural responses during support surface translations. Journal of Neurophysiology. 1995;73:896–901. doi: 10.1152/jn.1995.73.2.896. [DOI] [PubMed] [Google Scholar]
- Isaacs B. Clinical and laboratory studies of falls in old people. Clinics in Geriatric Medicine. 1985;1:513–524. [PubMed] [Google Scholar]
- Johnsson LG, Hawkins JE., Jr Sensory and neural degeneration with aging, as seen in microdissections of the inner ear. Annals of Otology, Rhinology and Laryngology. 1972;81:179–193. doi: 10.1177/000348947208100203. [DOI] [PubMed] [Google Scholar]
- Judge JO, Lindsey C, Underwood M, Winsemius D. Balance improvements in older women: Effects of exercise training. Physical Therapy. 1993;73:254–265. doi: 10.1093/ptj/73.4.254. [DOI] [PubMed] [Google Scholar]
- Keshner EA, Allum JHJ, Pfaltz CR. Postural activation and adaptation in the sway stabilizing responses of normals and patients with bilateral deficit. Experimental Brain Research. 1987;69:77–92. doi: 10.1007/BF00247031. [DOI] [PubMed] [Google Scholar]
- Kornzweig AL. Visual loss in the elderly. Hospital Practice. 1977;12:51–59. doi: 10.1080/21548331.1977.11707163. [DOI] [PubMed] [Google Scholar]
- Kristinsdottir KE, Jarnlo GB, Magnusson M. Asymmetric vestibular function in the elderly might be a significant contributor to hip fractures. Scandinavian Journal of Rehabilitation Medicine. 2000;32:56–60. doi: 10.1080/003655000750045550. [DOI] [PubMed] [Google Scholar]
- Lacour M, Vidal PP, Xerri C. Dynamic characteristics of vestibular and visual control of rapid postural adjustments. In: Desmedt J, editor. Motor control mechanisms in health and disease (np) New York: Raven Press; 1983. [PubMed] [Google Scholar]
- Larsson L. Aging in mammalian skeletal muscle. In: Mortimer JA, Pirazzolo PJ, Meletta GJ, editors. The aging motor system (np) New York: Praeger; 1982. [Google Scholar]
- Larsson L, Grimby G, Karlsson J. Muscle strength and speed of movement in relation to age and muscle morphology. Journal of Applied Physiology. 1979;46:451–456. doi: 10.1152/jappl.1979.46.3.451. [DOI] [PubMed] [Google Scholar]
- Lexell J. Human aging, muscle mass, and fiber type composition. Journal of Gerontology, Series A. 1995;50A(Suppl):11–16. doi: 10.1093/gerona/50a.special_issue.11. [DOI] [PubMed] [Google Scholar]
- Lockhart TE. The ability of elderly people to traverse slippery walking surfaces. Proceedings of the Human Factors and Ergonomics Society 41st Annual Meeting; Santa Monica, CA: Human Factors and Ergonomics Society; 1997. pp. 125–129. [Google Scholar]
- Lockhart TE, Woldstad JC, Smith JL. Assessment of slip severity among different age groups: Metrology of pedestrian locomotion and slip resistance. ASTM STP. 2002;1424:17–32. [Google Scholar]
- Lockhart TE, Woldstad JC, Smith JL. Effects of age-related gait changes on biomechanics of slips and falls. Ergonomics. 2003;46:1136–1160. doi: 10.1080/0014013031000139491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord SR, Clark RD, Webster IW. Visual acuity and contrast to sensitivity in relation to falls in an elderly population. Age and Ageing. 1991;20:175–181. doi: 10.1093/ageing/20.3.175. [DOI] [PubMed] [Google Scholar]
- Mackinnon CD, Winter DA. Control of whole body balance in the frontal plane during human walking. Journal of Biomechanics. 1993;26:633–644. doi: 10.1016/0021-9290(93)90027-c. [DOI] [PubMed] [Google Scholar]
- Maki BE, Holliday PJ, Topper AK. A prospective study of postural balance and risk of falling in an ambulatory and independent elderly population. Journal of Gerontology. 1994;104:480–492. doi: 10.1093/geronj/49.2.m72. [DOI] [PubMed] [Google Scholar]
- Melvill JG, Watt DGD. Muscular control of landing from unexpected falls in man. Journal of Physiology (London) 1971a;219:729–737. doi: 10.1113/jphysiol.1971.sp009685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melvill JG, Watt DGD. Observations on the control of stepping and hopping movements in man. Journal of Physiology (London) 1971b;219:709–727. doi: 10.1113/jphysiol.1971.sp009684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills P, Barrett R. Swing phase mechanics of healthy young and elderly men. Human Movement Science. 2001;20:427–446. doi: 10.1016/s0167-9457(01)00061-6. [DOI] [PubMed] [Google Scholar]
- Murphy SL. National Vital Statistics Reports. 11. Vol. 48. Hyattsville, MD: National Center for Health Statistics; 2000. Deaths: Final data for 1998. [PubMed] [Google Scholar]
- Murray MP, Kory RC, Clarkson BH. Walking patterns in healthy old men. Journal of Gerontology. 1969;24:169–178. doi: 10.1093/geronj/24.2.169. [DOI] [PubMed] [Google Scholar]
- Nashner LM. Analysis of movement control in man using the movable platform. In: Desmedt J, editor. Motor control mechanisms in health and disease (np) New York: Raven Press; 1982. [Google Scholar]
- Nashner LM, Black FO, Wall C. Adaptation to altered support and visual conditions during stance: Patient with vestibular deficits. Journal of Neuroscience. 1982;5:117–124. doi: 10.1523/JNEUROSCI.02-05-00536.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Safety Council. Accidents facts. Itasca, IL: Author; 2002. [Google Scholar]
- Nevitt MC, Cummings SR, Kidd S, Black D. Risk factors for recurrent nonsyncopal falls: A prospective study. Journal of the American Medical Association. 1989;261:2663–2668. [PubMed] [Google Scholar]
- Norton R, Campbell J, Lee-Joe T, Robinson E, Butler M. Circumstances of falls resulting in hip fractures among older people. Journal of the American Geriatrics Society. 1997;45:1108–1112. doi: 10.1111/j.1532-5415.1997.tb05975.x. [DOI] [PubMed] [Google Scholar]
- Overstall PW, Exton-Smith AN, Imms FJ, Johnson AL. Falls in the elderly related to postural imbalance. British Medical Journal. 1977;1:261–264. doi: 10.1136/bmj.1.6056.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins PJ. Measurement of slip between the shoe and ground during walking. ASTM STP. 1978;649:71–87. [Google Scholar]
- Perkins PJ, Wilson MP. Slip resistance testing of shoes – New developments. Ergonomics. 1983;26:73–82. [Google Scholar]
- Petersen H, Magnusson M, Fransson PA, Johansson R. Vestibular disturbances at frequencies above 1 Hz affect human postural control. Acta OtoLaryngologica (Stockholm) 1994;114:225–230. doi: 10.3109/00016489409126048. [DOI] [PubMed] [Google Scholar]
- Rabbitt P, Rogers M. Age and choice between responses in a self-paced repetitive task. Ergonomics. 1965;8:435–444. doi: 10.1080/00140136508930824. [DOI] [PubMed] [Google Scholar]
- Redfern MS, Andres RO. The analysis of dynamic pushing and pulling: Required coefficients of friction. Proceedings of the 1984 International Conference on Occupational Ergonomics; Ontario. Mississauga, Canada: Human Factors Association of Canada; 1984. pp. 569–571. [Google Scholar]
- Redfern MS, DiPasquale JD. Biomechanics of descending ramps. Gait and Posture. 1997;6:119–125. [Google Scholar]
- Rice CL, Cunningham DA. Aging of the neuromuscular system: Influences of gender and physical activity. In: Shephard RJ, editor. Gender, physical activity, and aging. Boca Raton, FL: CRC Press; 2001. pp. 212–250. [Google Scholar]
- Rosenhall U, Rubin W. Degenerative changes in the human vestibular sensory epithelia. Acta Oto-Laryngologica (Stockholm) 1975;79:67–80. doi: 10.3109/00016487509124657. [DOI] [PubMed] [Google Scholar]
- Rubenstein LZ, Robbins AS, Schulman BL, Rosado J, Osterweil D, Josephson KR. Falls and instability in the elderly. Journal of the American Geriatrics Society. 1988;36:266–278. doi: 10.1111/j.1532-5415.1988.tb01811.x. [DOI] [PubMed] [Google Scholar]
- Ruckenstein MJ, Shepard NT. Balance function testing: A rational approach. Otolaryngologic Clinics of North America. 2000;33:507–518. doi: 10.1016/s0030-6665(05)70224-3. [DOI] [PubMed] [Google Scholar]
- Sainburg RL, Ghilardi MF, Poizner H, Ghez C. The control of limb dynamics in normal subjects and patients without proprioception. Journal of Neurophysiology. 1995;73:820–835. doi: 10.1152/jn.1995.73.2.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon JH. The effect of age on the control of sway. Gerontologia Clinica. 1963;5:129–138. doi: 10.1159/000244784. [DOI] [PubMed] [Google Scholar]
- Skinner HB, Barrack RL, Cook SD. Age-related decline in proprioception. Clinical Orthopaedics. 1984;184:208–211. [PubMed] [Google Scholar]
- Stelmach GE, Worringham CJ. Sensimotor deficits related to postural stability: Implications for falling in the elderly. Clinics in Geriatric Medicine. 1985;1:679–694. [PubMed] [Google Scholar]
- Stephen R, John A, Philippa W, Kaarin J. Physiological factors associated with falls in older community-dwelling women. Journal of the American Geriatrics Society. 1994;42:1110–1117. doi: 10.1111/j.1532-5415.1994.tb06218.x. [DOI] [PubMed] [Google Scholar]
- Strandberg L, Lanshammar H. The dynamics of slipping accidents. Journal of Occupational Accidents. 1981;3:153–162. [Google Scholar]
- Thelen DG, Schultz AB, Alexander NB, Ashton-Miller JA. Effects of age on rapid ankle torque development. Journal of Gerontology: Medical Sciences. 1996;51A:M226–M232. doi: 10.1093/gerona/51a.5.m226. [DOI] [PubMed] [Google Scholar]
- Tinetti ME. Clinical practice: Preventing falls in elderly persons. New England Journal of Medicine. 2003;348:42–49. doi: 10.1056/NEJMcp020719. [DOI] [PubMed] [Google Scholar]
- Tinetti ME, Liu WL, Claus E. Predictors and prognosis of inability to get up after falls among elderly persons. Journal of the American Medical Association. 1993;269:65–70. [PubMed] [Google Scholar]
- Tinetti ME, Speechley M. Prevention of falls among the elderly. Medical Intelligence. 1989;320:1055–1059. doi: 10.1056/NEJM198904203201606. [DOI] [PubMed] [Google Scholar]
- Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. New England Journal of Medicine. 1988;319:1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- Whipple RH, Wolfson LI, Amerman PM. The relationship of knee and ankle weakness to falls in nursing home residents: An isokinetic study. Journal of the American Geriatrics Society. 1987;35:13–20. doi: 10.1111/j.1532-5415.1987.tb01313.x. [DOI] [PubMed] [Google Scholar]
- Winter DA. Biomechanics and motor control of human movement. 2. New York: Wiley; 1990. [Google Scholar]
- Winter DA. The biomechanics and motor control of human gait: Normal, elderly and pathological. 2. Waterloo, Canada: University of Waterloo Press; 1991. [Google Scholar]
- Winter DA, Patla AE, Frank JS, Walt SE. Biomechanical walking pattern changes in the fit and healthy elderly. Physical Therapy. 1990;70:340–347. doi: 10.1093/ptj/70.6.340. [DOI] [PubMed] [Google Scholar]
- Wolfson L, Judge J, Whipple R, King M. Strength is a major factor in balance, gait, and the occurrence of falls. Journal of Gerontology. 1995;50A:64–67. doi: 10.1093/gerona/50a.special_issue.64. [DOI] [PubMed] [Google Scholar]
- Wolfson L, Whipple R, Amerman P, Kaplan J, Kleinberg A. Gait and balance in the elderly. Clinics in Geriatric Medicine. 1985;1:649–659. [PubMed] [Google Scholar]
- Woolacott MH. Aging and postural control: Changes in sensory organization and muscular coordination. International Journal of Aging and Human Development. 1986;23:97–101. doi: 10.2190/VXN3-N3RT-54JB-X16X. [DOI] [PubMed] [Google Scholar]
- Woolacott MH, Shumway-Cook A, Nashner L. Postural reflexes and aging. In: Mortiner JA, Pirozzolo FJ, Maletta GJ, editors. The aging motor system (np) New York: Praeger; 1982. [Google Scholar]