Abstract
Muscle hydatidosis is rare, accounting only for 3–5% of all cases. We present a case series of 9 patients (8 male, one female, mean age 59.3 years, range 48-75 years) with primary echinococcosis of skeletal muscles. The cysts presented as soft tissue masses in 8 patients, whereas in one, the cyst was an incidental finding on a CT scan performed for investigation of a lung problem. All hydatid cysts were confined into muscles, without affecting the bone. The location was the thigh region in 6 patients (quadriceps in 4, biceps in 2), the popliteal fossa (gastrocnemius) in one, the humerus (triceps branchii) in one and the shoulder (infraspinatus) in one patient. MRI showed multi-vesicular cysts in all patients. Indirect hemagglutination serological test was positive in 6 out of 9 cases. En block surgical excision of the cysts was undertaken in all patients. Two patients received antihelminthic chemotherapy preoperatively. Histopathologic findings confirmed the diagnosis. No recurrence occurred during the follow-up period (1-8 years). Skeletal muscle echinococcosis should be considered in the differential diagnosis of limb masses, especially in endemic countries. A meticulous history taking and MRI imaging are essential, while pericystectomy is an effective method of treatment.
Keywords: hydatid cyst, skeletal muscle, echinococcosis
Hydatid disease, or echinococcosis, is classified as an helminthic infection caused by the tapeworm Echinococcus granulosus. This tapeworm species is endemic in certain regions (eg Australia, Argenitina, Africa, Eastern Europe, Middle East, and the Mediterrenean region, particularly Lebanon and Greece)1. It is most prevalent in sheep- and cattle-breeding areas, where the first step in the chain of transmission of this infestation occurs. The causative agent is introduced to the sheep dog through the faeces of livestock. The minute larval form of E. granulosus lives in the small intestine of the dog species. The eggs are passed in the faeces of an infected dog and can be transferred to any mammal that ingests them. After ingestion, the embryos are released from the eggs, traverse the intestinal mucosa, and are disseminated systemically via venous and lymphatic channels. The embryos are then carried to various parts of the body, where they develop into hydatid cysts. The cysts contain numerous scoleces, each capable of producing a small tapeworm when ingested by a dog. The most common locations for development of a parasitic cyst are the liver (65–75%) and the lung (25–30%)1,2 mainly because of their role as capillary filters3.
Muscle hydatidosis is rare, accounting only for 3–5% of all hydatidosis cases2–4, possibly because the cyst uses oxygen for growth while muscles usually contain lactic acid1,4. A case-series of primary hydatid cysts of skeletal muscles (triceps brachii, infraspinatus, gastrocnemius and quadriceps/biceps femoris), mimicking soft tissue lumps, is presented, offering information regarding clinical presentation, diagnosis and management of skeletal muscle echinococcosis.
Patients – Methods
Nine patients, eight male and one female, with mean age of 59 years (range 48-75 years) were treated for primary muscle echinococcosis from 2001 to 2008 (Table 1). None of the patients had significant co-morbidities, nor suffered from immunodeficiency syndromes. All patients lived in areas endemic for hydatid disease, five were actively associated with livestock and one patient reported having eaten uncooked meat during a trip in Eastern Europe 3 weeks before the appearance of a soft tissue in his thigh. The location of the mass was the anterior thigh region in 4 patients, the posterior thigh in 2, the popliteal fossa in one, the posterior humerus in one and the scapular region in one patient (Figure 1,2,3A). Eight patients presented with enlarging soft tissue masses of the lower (seven patients) or upper extremity (one patient). In one patient a cystic lesion was identified in the suprascapular region, as an incidental finding, undergoing chest CT scan for investigation of pneumonia. The two patients with upper extremity (posterior humerus and shoulder) cysts were pain-free, whereas all patients with lower extremity hydatid cysts presented with pain. One of them presented urgently to the orthopaedic outpatient clinic with clinical manifestations of a pending compartment syndrome of the thigh (Table 1).
Table 1. Patients and cysts characteristics.
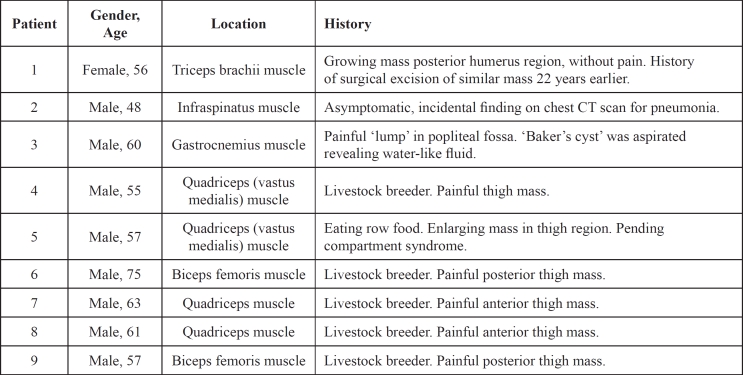
Figure 1. T1 weighted MRI depicted a mass of the infraspinatus muscle.
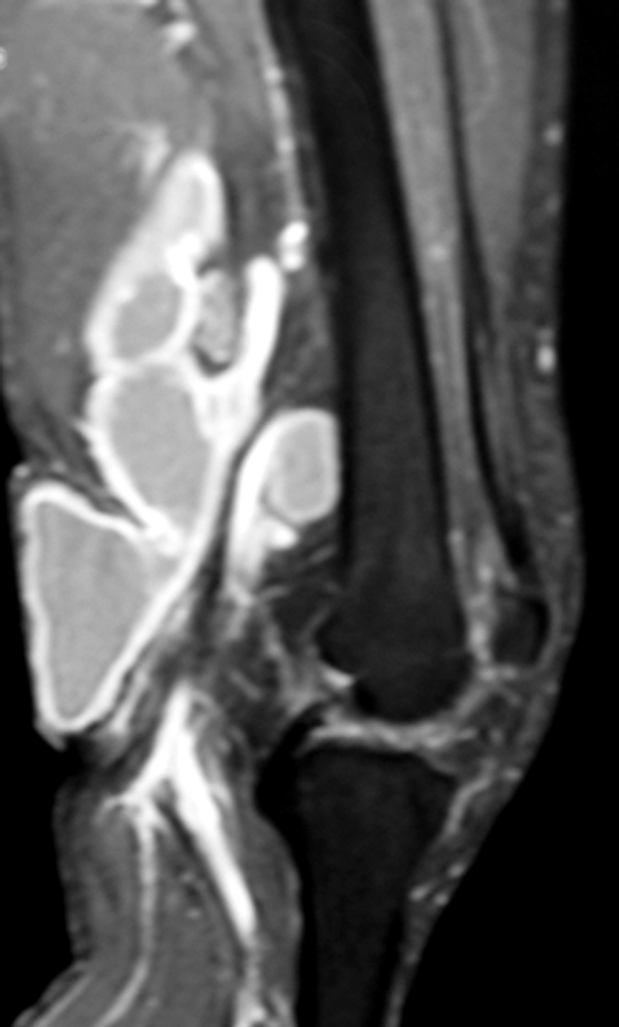
Figure 2. T2 weighted MRI of a cystic mass arising from the origin of the right gastrocnemius muscle.
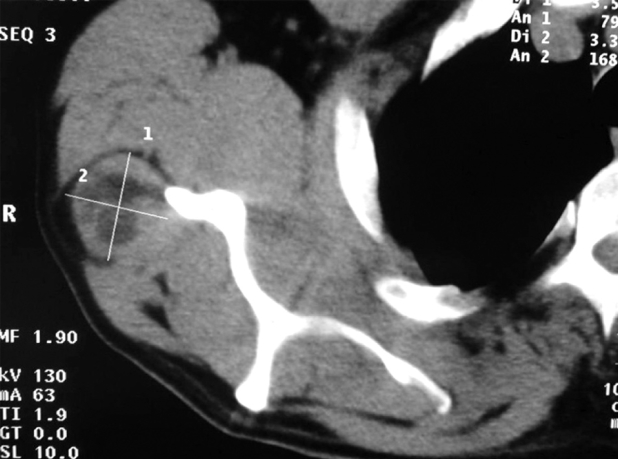
Figure 3. A:T2 weighted MRI of an anterior thigh multivesicular cyst, affecting the quadriceps muscle, extending from above the knee to the groin. B: Postoperative MR imaging 12 months after surgical excision. No evidence of recurrence.
Clinical presentation and history taking raised the suspicion of hydatid disease, to be included in the differential diagnosis. Routine blood tests (full blood count, biochemical markers, liver function tests, clotting profile), CRP and ESR were within normal limits in all patients. Indirect hemagglutination serological test was positive in 6 out of 9 cases (higher than 1/8000 titer). Plain radiographs of the affected region showed diffuse soft tissue swelling without bone erosion or soft tissue calcification, whereas magnetic resonance (MR) images depicted multivesicular cysts, confined to the soft tissues, not affecting the bone, in all patients (Figure 1,2,3A). CT scan of chest and abdomen did not reveal any other organ involvement in any of the patients.
Elective surgery for cyst excision was planned for all patients. Routine antihelminthic chemotherapy was not administered. However, two of the patients were considered as high for pre- and intraoperative dissemination and were treated with albendazole and praziquantel for 28 days, prior to surgery. One of them had previously undergone a cyst aspiration and the other patient presented with a rapidly enlarging cystic mass in his thigh, and severe pain (pending compartment syndrome). It was decided, after consultation of the Infectious Diseases specialist, to avoid surgical excision in this acute setting. Antihelminthic therapy (albendazole and praziquantel for 28 days) were initiated in order to slow the progression and reduce the risk of cyst rupture that could result in intra- and post- operative complications. These two patients were treated with albendazole and praziquantel postoperatively for 3 months.
Results
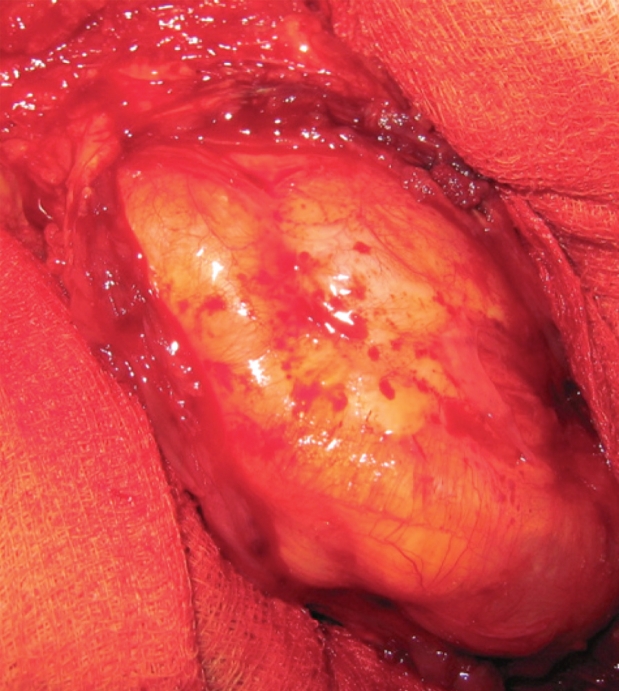
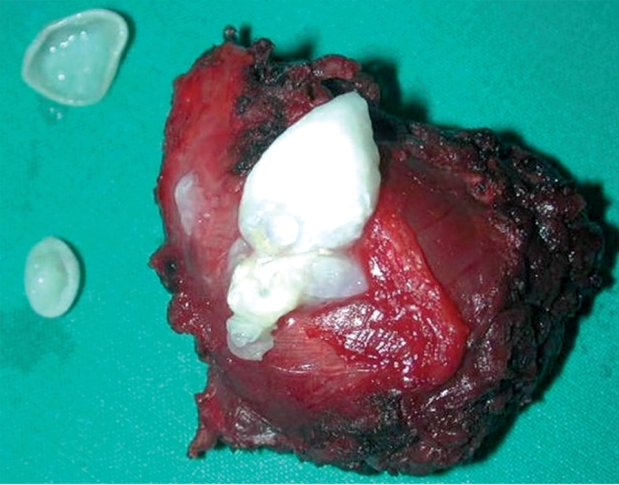
En block surgical excision of the masses under general anesthesia was undertaken, by experienced orthopaedic surgeons (Figure 4). The proximity of the cysts to vessels was taken into account and care was taken to remove the masses en block without perforating the cyst wall, along with surrounding muscle fibers. After cyst excision extensive wash-out of the surgical field was carried out and the wounds were closed in layers, with drains remaining in situ for 24 hours. The cysts were multivesicular containing daughter cysts and were filled with clear water-like fluid with scoleces, typical for hydatid disease (Figure 5).
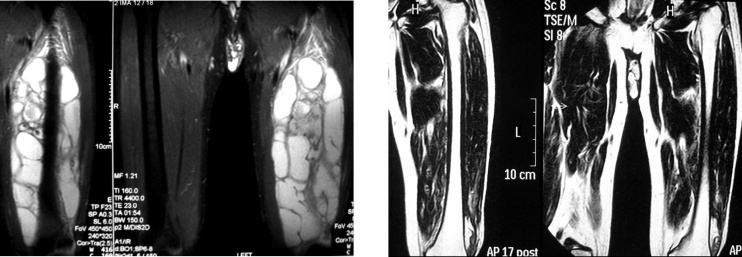
Figure 4. After incision of the triceps brachii muscle, a soft, non-tender, cystic mass, surrounded by a capsule, was exposed.
Figure 5. Hydatid cyst containing daughter cysts, excised en block with part of the surrounding infraspinatus muscle.
In all patients the cysts were confined into the muscles, not affecting neurovascular structures or bones and the diagnosis of muscle echinococcosis was confirmed by histopathologic examination. A cyst wall with an outer chitinous (fibrous laminar) layer, surrounded by granulation tissue and inner germinal layer, led to the diagnosis of skeletal muscle echinococcosis.
In a follow-up period ranging from 12 months to 8 years, recovery was uneventful, without local or systemic complications, and without clinical signs of recurrence in all cases (Figure 3B). The patient with a popliteal fossa hydatid cyst, had an extension deficit of 10 degress. All other patients regained full range of motion of their limbs. All returned to their normal activities.
Discussion
Among patients treated for hydatidosis, unusual sites are observed in about 15% of cases3, with highest rates in endemic areas1,3. In an article reviewing 1275 patients treated for hydatidosis from 1949 to 1993, the authors reported 66 (5.2%) hydatid cysts developed in unusual sites5. The most common skeletal muscle sites include the hip and thigh3–10 and the shoulder and humerus2–4 regions. Bone, including spine can also be affected3,11,12. Six of the nine cases presented in the current study were located in the thigh anatomical region, (Figure 3A) and none of the nine cases affected the bone.
The clinical examination usually reveals a painless enlarging soft tissue mass and additional evaluation is necessary for the diagnostic process. Muscle echinococcosis should be included in the differential diagnosis of limb masses, especially in endemic areas3–5. Six patients in our series were associated with livestock, living in agricultural areas of an endemic country for echinococcosis. The remarkable point regarding one of our patients, with the 'aggressive' enlargement of the mass over a 3 months period, is the importance of careful history taking: the patient recalled having eaten non-cooked meat 3 weeks before the mass in his thigh started to develop.
Serology may not always be helpful in diagnosing primary muscle hydatidosis. A negative test does not rule out the diagnosis of echinococcosis1. Cysts in the liver elicit positive antibody responses in 90% of cases, whereas up to 50% of patients with lung hydatid disease are seronegative1. Arazi et al3 found that 27% (4 of 15) in their case series of musculoskeletal echinococcosis had a positive indirect hemagglutination test. In our series, indirect hemagglutination testing for echinococcosis was positive in 6 of 9 patients. Imaging evaluation may be not be specific and accurate and can also indicate other pathological processes, such as malignancy, including sarcoma, or infection. Endovesicular daughter cysts that are commonly seen in hepatic hydatid disease imaging, are not usually seen on ultrasound or CT of skeletal muscle cysts, and calcification is rare13,14. MRI is the examination of choice in case of suspicion of hydatid disease due to its ability to demonstrate adequately most features of hydatid disease, with the exception of calcifications. The multiplanar imaging and the excellent soft tissue contrast provide valuable information on the extent of the disease. The classic MRI findings include a multivesicular cyst, a low-intensity rim ("rim sign") on T2-weighted images or a detached membrane3,13,14. The most pathognomonic sign is that of daughter cysts within larger cysts1. In our cases, MRI was able to demonstrate a multivesicular cyst containing multiple daughter cysts (Figure 1,2,3A).
Incisional biopsy and marginal excision are contraindicated if hydatid disease is suspected, because of the likelihood of disease dissemination and possibility of allergic reaction1,2. It is critical to establish, or at least suspect, the diagnosis of hydatid disease preoperatively in order to limit the risk of anaphylactic shock or dissemination in the event of puncture or accidental opening of the cyst during resection2. The fluid of E. granulosus cysts contains significant amounts of foreign protein and is extremely toxic to the host. Subsequently, it is important to excise the cyst en block, as its rupture can cause anaphylactic shock or may release a large number of viable scoleces that will be implanted elsewhere and produce secondary cysts4. The treatment of choice in musculoskeletal hydatid disease is surgical excision (pericystectomy), potentially combined with antihelminthic medication3,4,9,15. Percutaneous aspiration, infusion of scolicidal agents and reaspiration (PAIR), under imaging (ultrasound or CT) guidance can be used as alternative to surgery in inoperable cases1. Percutaneous puncture with concomitant injection of ethanol and polidocanol in the cyst cavity has been recently reported to have treated muscle hydatosis in selected cases, without recurrence6.
Supplementary chemotherapy with antihelminthics for skeletal muscle hydatid disease is controversial and no evidence based recommendations can be made. Perioperative therapy with antihelminthic agents was evaluated by studies done on liver hydatid cysts16. A study reviewing comparative studies and randomized controlled trials, showed that there is a role for adjuvant treatment with albendazole16. Whether the combination of albendazole with praziquantel is superior to albendazole alone remains unclear16. The likely beneficial role for praziquantel in human hydatidosis may be in preventing encystment of protoscoleces following perioperative spillage17. In humans, the use of combination therapy has been reported in the treatment of inoperable hydatidosis17.
In cases affecting skeletal muscles - without other organ involvement - where surgical excision is possible, the rationale of adjuvant chemotherapy is to reduce the risk of dissemination during surgery and to prevent recurrence3,4. Bone involvement makes recurrence more likely after surgical excision, compared to muscle echinococcosis alone3. Although adjuvant administration of antihelminthic agents has been successful in treating musculoskeletal echinococcosis3,7,9,11 recurrence rates up to 50% have been reported in cases of bone involvement3, where resection of the diseased area is probably incomplete. Bonifacino et al10 proposed for the treatment of hydatid disease of bone, one cycle (28 days) of albendazole preoperatively, and six or more courses of 28 days postoperatively, with an interval of 14 days between them.
Antihelminthic chemotherapy was not routinely used. However, in one of our patients, the history of previous aspiration that could have caused dissemination of echinococcosis to the surrounding soft tissues or even the vessels, indicated the need for chemotherapy. In another patient, antihelminthic chemotherapy was initiated because of the acutely 'aggressive characteristics' of the hydatid cysts enlargement in this patients thigh. A combination of antihelminthic agents was used pre- and postoperatively.
Complete en block surgical resection without antihelminthic chemotherapy in the remaining seven patients, was successful without recurrence in a follow-up period ranging from 1 to 8 years. However, the previous history of a 'similar' mass excision 22 years earlier, in one of the patients of the present series, alerts for the likelihood for late recurrences.
The importance of inclusion of skeletal muscle echinococcosis in the differential diagnosis of soft tissue limb masses is underlined in the present report. A detailed medical history is essential, and although blood tests and imaging studies may not always be diagnostic, a meticulous surgical excision of the mass with surrounding tissues (pericystectomy) is considered an effective method of treatment.
References
- 1.White CJr, Weller PF. Echinococcosis. In: Braunwald E, Fauci AS, Kasper DL, Longo DL, Jameson JL, editors. Harrison's Principles of Internal Medicine. 15th edition. McGraw Hill; 2001. 1250 pp. [Google Scholar]
- 2.Tatari H, Baran O, Anlidag T, et al. Primary intramuscular hydatidosis of supraspinatus muscle. Arch Orthop Trauma Surg. 2001;121:93–94. doi: 10.1007/pl00013775. [DOI] [PubMed] [Google Scholar]
- 3.Arazi M, Ericoglou M, Odev K, Memik R, Ozdemir M. Primary echinococcus infestation of the bone and muscles. Clin Orthop Rel Res. 2005;432:234–241. doi: 10.1097/01.blo.0000149816.86222.2d. [DOI] [PubMed] [Google Scholar]
- 4.Duncan GJ, Tooke SM. Echinococcus infestation of the biceps brachii. A case report. Clin Orthop Rel Res. 1990;261:247–250. [PubMed] [Google Scholar]
- 5.Lucandri G, D'Elia G, Chiavellati L, et al. [Unusual location of hydatid cysts: clinical and therapeutic aspects] G Chir. 1994;15:529–537. [PubMed] [Google Scholar]
- 6.Ormeci N, Idilman R, Akyar S, et al. Hydatid cysts in muscle: a modified percutaneous treatment approach. Int J Infect Dis. 2007;11:204–208. doi: 10.1016/j.ijid.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 7.Thursky K, Torresi J. Primary muscle hydatidosis of the thigh: management of a complicated case with combination adjunctive albendazole and praziquantel chemotherapy. Clin Infect Dis. 2001;32:65–68. doi: 10.1086/318521. [DOI] [PubMed] [Google Scholar]
- 8.Kazakos CJ, Galanis VG, Verettas DA, Polychronidis A, Simopoulos C. Primary hydatid disease in femoral muscles. J Int Med Res. 2005;33:703–706. doi: 10.1177/147323000503300613. [DOI] [PubMed] [Google Scholar]
- 9.Tarhan NC, Tuncay IC, Barutcu O, Demirors H, Agildere AM. Unusual presentation of an infected primary hydatid cyst of biceps femoris muscle. Skeletal Radiol. 2002;31:608–611. doi: 10.1007/s00256-002-0524-x. [DOI] [PubMed] [Google Scholar]
- 10.Bonifacino R, Dogliani E, Craig PS. Albendazole treatment and serological follow-up in hydatid disease of bone. Int Orthop. 1997;21:127–132. doi: 10.1007/s002640050135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sapkas GS, Stathakopoulos DP, Babis GC, Tsarouchas JK. Hydatid disease of bone and joints. 8 cases followed for 4 – 16 years. Acta Orthop Scand. 1998;69:89–94. doi: 10.3109/17453679809002364. [DOI] [PubMed] [Google Scholar]
- 12.Schneppenheim M, Jerosch J. Echinococcosis granulosus / cysticus of the tibia. Arch Orthop Trauma Surg. 2003;123:107–111. doi: 10.1007/s00402-002-0461-0. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Diez AI, Ros Mendoza LH, Villacampa VM, Cozar M, Fuertes MI. MRI evaluation of soft tissue hydatid disease. Eur Radiol. 2000;10:462–466. doi: 10.1007/s003300050077. [DOI] [PubMed] [Google Scholar]
- 14.Von Sinner W, Strake L, Clark D, Sharif H. MR imaging in hydatid disease. Am J Roentgenology. 1991;157:741–745. doi: 10.2214/ajr.157.4.1892028. [DOI] [PubMed] [Google Scholar]
- 15.Franchi C, Di Vico B, Teggi A. Long-term evaluation of patients with hydatidosis treated with benzimidazole carbamates. Clin Infect Dis. 1999;29:3049. doi: 10.1086/520205. [DOI] [PubMed] [Google Scholar]
- 16.Falagas ME, Bliziotis IA. Albendazole for the treatment of human echinococcosis: a review of comparative clinical trials. Am J Med Sci. 2007;334:171–179. doi: 10.1097/MAJ.0b013e31814252f8. [DOI] [PubMed] [Google Scholar]
- 17.Mohamed AE, Yasawy MI, Al Karawi MA. Combined albendazole and praziquantel versus albendazole alone in the treatment of hydatid disease. Hepatogastroenterology. 1998;45:1690–1694. [PubMed] [Google Scholar]