Abstract
Multiple myeloma is a haematologic malignancy caused by clonal expansion of malignant plasma cells and associated with bone disease and hypercalcaemia. Myeloma cells are in close proximity to sites of active bone resorption and the interactions between those cells, osteoblasts and osteoclasts, are crucial not only for the bone distraction but for the proliferation of bone marrow cells as well. Recent studies have revealed that numerous regulating factors of osteoblast and osteoclast activity interfere with the pathogenesis of multiple myeloma's bone disease and that the understanding of the pathophysiological pathways involved is the first step towards discovering novel potential therapeutic approaches.
Keywords: multiple myeloma, bone disease, hypercalcaemia, osteoblasts, osteoclasts
Multiple myeloma (MM) is a haematologic malignancy that is caused by clonal expansion of malignant plasma cells. It was first described in 1848 and today represents 10-15% of all malignant hematological diseases1. Clinical manifestations of multiple myeloma are variable and derive either directly from the neoplasmatic infiltration of bone marrow (osteoporosis, pathologic fracture, bone pain, anaemia) or indirectly from aberrant function of humoral immunity and from secretion of clonal protein1,2.
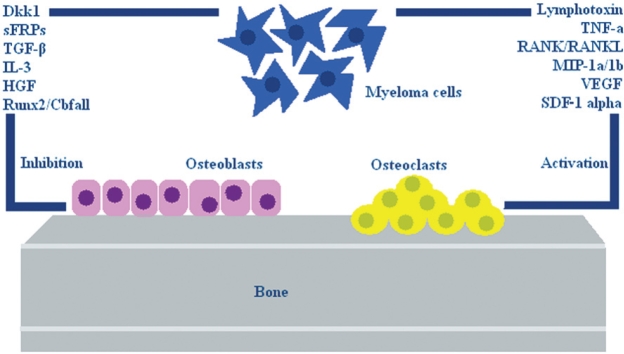
MM bone disease is associated with excessive tumorinduced, osteoclast-mediated bone destruction3. During the past decades our understanding of the pathogenesis of myeloma bone disease has significantly improved. In particular, key mediators of the osteoclastic bone resorption in myeloma have been identified, including receptor activator of nuclear factor- B ligand (RANKL), macrophage inflammatory protein-1a (MIP-1α), stromal derived factor-1 alpha (SDF-1a), transforming growth factor beta (TGF-β), dickkopf homolog 1 (Dkk1) and secreted frizzled-related proteins (sFRPs). Many of these factors seem to be disregulated in patients with MM, and probably participate in bone disease either alone or concomitantly (Figure 1).
Figure 1. Factors involved in the pathogenesis of myeloma bone disease. Myeloma bone disease is associated with an increased osteoclast-mediated bone destruction and osteoblast inhibition and numerous factors have been already identified.
Pathophysiology of increased osteoclast-mediated bone destruction in MM
Lymphotoxin, interleukins and tumor necrosis factoralpha (TNF-α), represent osteoclast activating factors, secreted by myeloma cells, although it remains unclear whether these factors are also produced by accessory cells involved in cell-cell interactions3,4. The involvement of these factors in the development of myeloma bone disease is well established in vitro; however their role in vivo remains obscure5. Nowadays, it is well known that the interactions between myeloma cells and bone marrow stromal cells play a crucial role in the progression of myeloma bone disease as well as in the disequilibrium and dysregulation of the factors involved in its aetiopathogenesis5.
The RANK/RANKL/OPG system
Receptor activator of nuclear factor κ B (RANK), receptor activator of nuclear factor κ B ligand (RANKL) and osteoprotegerin (OPG), represent members of the TNF receptor family that play an important role in bone metabolism affecting osteoclast formation and activity5.
RANKL is a polypeptide of 217 aminoacids which is expressed on the surface of both osteoblasts and bone marrow stromal cells6. The interaction between RANKL and RANK, a specific receptor present on osteoclast progenitors and mature osteoclasts, stimulates osteoclast proliferation and bone resorption7. On the other hand, OPG is a soluble receptor of 100-110 kD6, secreted by osteoblasts and bone marrow stromal cells, that exerts the exact opposite biological activity. Thus, through binding of RANKL, this molecule antagonizes the action of RANK resulting in the inhibition of osteoclast-mediated bone destruction8. The role of these molecules has been confirmed in various in vivo studies where, RANK and RANKL knockout mice presented extensive osteopetrosis, while in OPG knockout mouse models severe osteoporosis with fractures was reported5,6.
The ratio of RANKL and OPG in patients with MM is markedly disturbed, with an increase in the expression of osteoclastogenic RANKL and decrease in the production of osteoprotective factor OPG5. The increase in the RANKL/OPG ratio was well established in various studies where the co-culture of bone marrow stromal cells and myeloma cells induced the RANKL expression by both cell types, while the OPG expression was concomitantly downregulated by bone marrow stromal cells only3, illustrating the importance of cell-cell interactions in the pathogenesis of myeloma bone disease. In a recent study, the RANKL/OPG ratio has been correlated with markers of bone resorption, confirming its potential use as a prognostic factor of both the severity of myeloma bone disease and the survival of MM9.
Macrophage Inflammatory proteins (MIPs)
Macrophage Inflammatory protein-1alpha (MIP-1α) is a chemokine produced by myeloma cells, which induces the proliferation and differentiation of osteoclasts, thus affecting bone resorption6. The expression of MIP- 1α in the serum of patients with MM is upregulated and correlated with both the severity of bone destruction and the overall outcome of the disease itself10. MIP-1α is produced in response to several stimuli and through the interaction with several chemokine receptors, such as CCR1 and CCR5, plays its role as a mediator in myeloma bone disease3,5. Recent findings in RANK knockout animal models have shown that the osteoclastogenic effect of MIP-1α is inhibited, suggesting that its action is potentially mediated through RANK/RANKL system11.
Additionally there is growing evidence that MIP-1β, a chemokine of the Regulated on Activation Normal T cell Expressed and Secreted (RANTES) family, induces osteoclast proliferation and differentiation and represents a potent mediator of myeloma bone disease12.
Vascular endothelial growth factor (VEGF)
It is well established that VEGF plays a crucial role in solid tumors' neovascularization and that the microvessel density of bone marrow, in patients suffering from MM is correlated with both disease progression and overall outcome5. Recent research findings lead to the conclusion that the secretion of this factor from myeloma cells induces osteoclast differentiation and that the blockade of VEGF expression inhibits angiogenesis and bone resorption13. All these findings support the hypothesis that VEGF has a role in the pathophysiology of increased osteoclast- mediated bone destruction in MM.
Stromal derived factor-1 alpha/CXC chemokine receptor-4 (SDF-1 alpha/CXCR4)
SDF-1 alpha is a member of the CXC chemokine family and its receptor CXCR4 is expressed on various types of cells, such as malignant cells and osteoclast progenitors5. The SDF-1 alpha/CXCR4 complex plays a key role in migration and differentiation of myeloma cells, while there is growing evidence that these molecules increase osteoclast activity and bone resorption14. This was related to an overexpression of many genes encoding RANK, RANKL, TRAP, MMP-9, CA-II and Cathepsin-K, which are osteoclast activating factors5.
Cell-cell interactions
It is well documented that cell-cell interactions play an important role in the pathogenesis of myeloma bone disease, given the fact that myeloma cells are in close proximity to sites of bone resorption5. These interactions involve both bone marrow stromal cells and osteoclasts and they result in osteoclast activation, proliferation and differentiation, either directly or through the upregulation of osteoclastogenic factors such as IL-6 and RANKL5. The final outcome is the development and progression of myeloma bone disease.
Pathophysiology of osteoblast inhibition
Although increased osteoclast activity and bone resorption represent the primary pathophysiological pathway in myeloma bone disease, it is well recognized that osteoblasts play a crucial role in bone formation and repair of osteolytic lesions. Thus, a reduction in bone formation due to osteoblast inhibition might be a potential mechanism which results in bone destruction. Dickkopfs (Dkks), secreted frizzled related proteins (sFRPs), IL-3, runt-related transcription factor-2 (Runx-2), hepatocyte growth factor (HGF) and transforming growth factor beta (TGF-β) represent factors which are dysregulated in patients with MM and which may regulate osteoblast function through the inhibition of their formation and differentiation5.
Inhibitors of Wnt signaling pathway
The Wnt (the name comes from Wg/wingless and Int genes) signaling pathway has a crucial role in the regulation of skeletal biology and seems to be involved in the pathogenesis of diseases of altered bone mass, such as osteoporosis and MM15. This pathway exerts its biological action through the activation or inhibition of various factors, such as glycogen synthase kinase- 3 beta, axin, catenin-β and activated protein C and thus controls the formation, differentiation and survival of both precursors and mature osteoblasts16. A great number of molecules which antagonize the functions of Wnt signaling in the extracellular matrix have been identified, such as Dkks, sFRPs and Wnt inhibitory factor 1 (Wif-1), resulting in the suppression of osteoblast function and the progression of myeloma bone disease15,16.
Dkk1 is a member of the Dickkopf family and is expressed by bone marrow stromal cells, osteoblasts and myeloma cells5. In vitro studies have demonstrated that this soluble antagonist of the Wnt signaling pathway inhibits bone formation5. Additionally, the role of this molecule was confirmed in vivo, in studies with transgenic mice overexpressing Dkk1 which developed severe osteopenia, while mutations of low density lipoprotein receptor- related protein 5 (LRP5), that reduce its affinity for Dkk1, were related to increased bone mass. Although the precise role of this molecule in humans remains to be determined, Dkk1 levels were increased in both bone marrow plasma and peripheral blood of patients suffering from MM in relation to the healthy population5. In addition, Dkk1 was demonstrated to have an inhibitory effect on osteoblast differentiation5.
Apart from Dkk1, myeloma cells secrete various Wnt signalling antagonists, such as sFRPs (sFRPs 1-4)16. It was demonstrated that sFRP3 is overexpressed by myeloma cells in patients with MM and along with Dkk1 contributes to the development of osteolytic lesions which occur in myeloma bone disease16. Furthermore, sFRP2 is also secreted by certain MM cell lines and there is evidence that this factor induces bone resorption and inhibits osteoblast differentiation17.
TGF-β
TGF-β holds a key role in pathophysiological mechanisms in malignant diseases and is secreted by bone matrix during osteoclast mediated bone destruction5. It represents a multifunctional growth factor which, and in the case of MM, exerts its biological action through the inhibition of osteoblast differentiation5. In addition, in vitro studies have shown that the inhibition of TGF-β signaling results in the blockade of the inhibitory action of myeloma cells on osteoblast differentiation18.
Runx2/Cbfal1
Runx2 represents a transcription factor, which controls skeletogenesis through the differentiation of mesenchymal stem cells to osteoblasts16. This was demonstrated with the use of animal models, where Runx2 knockout mice showed a complete lack of osteoblasts and ossification, establishing the importance of this factor in osteoblastogenesis5. In humans, the co-culture of myeloma cells with osteoblast progenitors, inhibited the formation and differentiation of osteoblasts as well as the expression of osteocalcin, alkaline phosphatase and collagen I genes, which represent biological markers of osteoblast differentiation. The effect of Runx2 on osteoblasts is mediated by interkeukin-7 and various cell-cell interactions5.
Interleukin-3 (IL-3)
IL-3 represents a cytokine with a potential inhibitory effect on osteoblast formation and differentiation in patients with MM5. It was demonstrated, both in human and animal models, that IL-3 has an indirect effect on the differentiation of primary stromal cells to osteoblasts, while it was found that the expression of this factor in the bone marrow of patients with MM is upregulated in relation to healthy controls4,19. In addition it was found that TNF- enhances the inhibitory effect of IL-319. Furthermore, Lee et al, have found that this cytokine is also involved in osteoclast activation and enhances osteoclast formation in vitro4. These data suggest that IL-3 exerts a dual function, affecting both osteoclast mediated bone resorption and osteoblast bone formation.
Hepatocyte growth factor (HGF)
Myeloma cells secrete HGF and thus its levels are increased in the serum of patients with MM5. This increase was positively correlated with a poor outcome and negatively correlated with the level of alkaline phosphatase in the serum of the patients5. These data, in combination with recent findings which showed that HGF inhibits bone morphogenetic protein (BMP) osteoblastogenesis in vitro and suppresses Runx2 gene expression, demonstrate that this growth factor may hold a key role in the pathogenesis of myeloma bone disease as well as in the discovery of targeted pharmacotherapy in the future.
The ubiquitin-proteasome pathway
The ubiquitin-proteasome pathway represents a cellular system which degrades various proteins involved in the proliferation and survival processes of myeloma cells20. Current researches, both in vivo and in vitro, with the use of animal models, suggest that this pathway regulates osteoblast differentiation and osteosynthesis20. This regulation is based on the modification of the expression of Wnt signalling and transcription factor Runx28.
Current therapeutic approaches in myeloma bone disease
The optimal therapy for bone disease and hypercalcaemia associated with cancer is the effective primary tumor reduction, making direct antitumor treatment necessary. Since MM is a disease without radical treatment, antihypercalcaemic therapy should be considered as an intermediate measure with no ultimate effect on patients survival3,21.
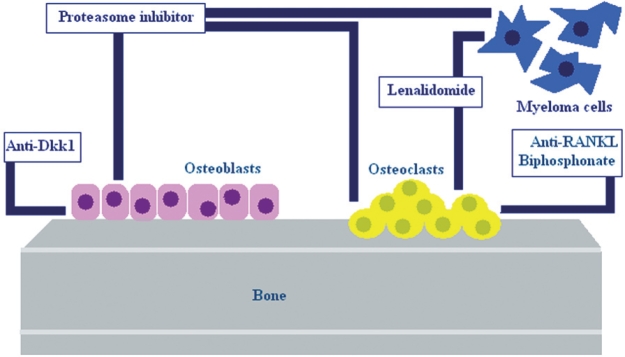
Except for saline hydration and calciuresis, there are measures that affect bone metabolism, such as bisphosphonates. Bisphosphonates are inhibitors of osteoclastic bone resorption and are considered to be effective agents in the treatment of hypercalcemia associated with cancer, osteoporosis and other situations associated with increased bone resorption21. Glucocorticoids and calcitonin can be used also in combination with bisphosphonates3,21. Although many pharmacologic agents can be used in the management of myeloma bone disease and associated hypercalcaemia, recent advances in our understanding of factors involved in pathogenesis of MM bone disease have identified novel therapeutic targets (Figure 2).
Figure 2. Therapeutic approaches in myeloma bone disease. These agents regulate bone metabolism either by increasing osteoblastic bone formation or decreasing osteoclast activity and differentiation.
Lenalidomide
Thalidomide, was initially introduced for the treatment of MM because of its anti-angiogenic properties22. Due to its potential teratogenicity, several agents referred as "immunomodulatory drugs" (IMiDs) were developed. IMiDs are thalidomide analogues and have many of thalidomides biological properties, but they have less nonhaematologic toxicity22,23. Lenalidomide is one of IMiDs and has been recently used in the management of patients with relapsed/ refractory MM22. IMiDs reduce angiogenesis through the inhibition of VEGF and as a result this leads to alteration of bone marrow environment and inhibition of myeloma cell growth and proliferation23. These agents also reduce the secretion of growth and survival factors, induce direct apoptosis of myeloma cells, promote the cytotoxic activity of natural killer and T cells against myeloma cells by stimulating their proliferation and the secretion of interleukin 2 and interferon gamma, and downregulate the activity of nuclear factor -kappa beta23. Except for their anti-myeloma effect, recent studies have demonstrated that these agents have effect on bone metabolism of patients with MM, reduce osteoclast formation and function in vitro and biochemical markers of bone resorption24.
Proteasome inhibitors
Proteasome inhibition is increasingly being employed as an antitumor strategy in several cancers including MM25. The ubiquitin-proteasome pathway (UPP) regulates normal protein degradation processes essential for cell cycle, inflammation, transcription, DNA replication and apoptosis26. The 26S proteasome consists of a 20S catalytic complex and a 19S regulatory complex, where the ubiquitin tags bind. Thus, proteins are transported to the catalytic complex for hydrolysis into small polypeptides26.
Blockade of UPP using proteasome inhibitor Bortezomib, is an effective therapy for relapsed/refractory MM, when administrated alone or in combination with dexamethasone and/or chemotherapeutic agents5,26. Bortezomib is the only agent to date proved to have a survival benefit in the settings of relapsed MM27.
As mentioned above, preclinical studies showed that the ubiquitin-proteasome machinery regulates osteoblast differentiation and bone formation. Several clinical studies suggest that the response to Bortezomib observed in patients with MM was associated with a significant increase in alkaline phosphatase (ALP) and osteocalcin and support the hypothesis that a direct stimulatory effect on bone formation process could occur during Bortezomib treatment28. Additionally, Bortezomib has been shown to inhibit Dkk1 expression in bone and bone-derived cells, suggesting a novel mechanism by which the proteasome inhibitor may modulate bone formation in the bone microenvironment5,25. Thus, proteasome inhibitor Bortezomib may be effective both for the treatment of MM and the associated bone disease.
Anti-RANKL therapy
The increase in our understanding of the mechanisms involved in the development of destructive osteolytic bone disease and the following hypercalcaemia observed in MM leads to the discovery of novel potential therapeutic approaches. As mentioned above, the dysregulation of the RANKL/RANK/OPG system is a crucial mechanism in the development of myeloma bone disease5. This knowledge has led to the development of RANKL antagonists, such as OPG-Fc and RANKL-Fc, that inhibit the action of RANKL29. These inhibitors are specific and effective and have been used in several published preclinical studies30.
A phase 1 trial evaluated the use of AMGN-0007 as a potential therapeutic agent in the treatment of bone disease in patients with MM or breast carcinoma with confirmed lytic bone lesions. AMGN-0007 is a recombinant OPG construct that blocks differentiation and activation of osteoclasts through binding to RANKL31.
A recent study in postmenopausal women showed that AMG162 is more effective in decreasing bone turnover markers at even lower doses32. Also two potential concerns with Fc-OPG therapy are cited. The first is the generation of anti-Fc-OPG antibodies, which might react with endogenous Fc-OPG and neutralize its activity. The second concern is that the binding of Fc-OPG to TNF could inhibit the role of this factor in tumor growth32. Thus, AMG162 has been proposed as safer and more efficacious therapeutic inhibitor of RANKL.
Denosumab, a novel agent which is fully human monoclonal antibody against RANKL is under investigation in clinical trials to date. Initially, this monoclonal antibody was IgG1 type, known as AMG161. Because of the fact that AMG161 was shown to have a cytotoxic effect on target cells and induced the apoptosis of RANKLproducing cells (osteoblasts, T-cells, stromal cells), it was converted into a noncytotoxic IgG2 antibody, known as AMG16229. Studies in postmenopausal women with low bone mineral density (BMD) have shown that Denosumab compared with bisphosphonates could decrease bone resorption and increase bone mineral density30. The duration of suppression of bone turnover is dose-depended32. A phase 1 trial concerning patients with MM and lytic bone lesions showed that Denosumab was effective in rapid dose-depended suppression of bone resorption, lasting at least three months and has no direct effect on osteoblast activity33.
It should be noted that bisphosphonates and anti- RANKL therapy suppress osteoclastic bone resorption, while the proteasome inhibitors increase osteoblastic bone formation. Denosumab takes precedence over bisphosphonates at certain points. Concerning the adverse events from the use of bisphosphonates, renal toxicity is a potential complication33. A potential advantage of Denosumab is that this factor requires a short period of time to clear over, whereas bisphosphonates half-life time is at least five years29. Due to this long-term bone turnover inhibition, bones are fragile and microfractures are accumulated. Another disadvantage of bisphosphonates is the inability to use them in combination with anabolic agents29.
Apart from the anti-RANKL therapy, several preclinical studies investigate numerous agents involved in bone metabolism, such as MIP-1a, CCR1, CCR5 and Dkk14,34.
Conclusions
Multiple myeloma is a heamatologic malignancy caused by clonal expansion of malignant plasma cells, associated with bone disease and hypercalcaemia as its primary metabolic complication. Although the pathogenetic mechanisms of disease have been clarified to a large extent, MM remains an incurable disease with low life expectancy. While there are many therapeutic approaches in myeloma bone disease and subsequent hypercalcaemia, none is entirely satisfactory and effective, and the identification of novel therapeutic targets seems to be a definite and imperative need.
References
- 1.Bataille R, Harousseau JL. Multiple myeloma. N Engl J Med. 1997;336:1657–1664. doi: 10.1056/NEJM199706053362307. [DOI] [PubMed] [Google Scholar]
- 2.Blad J, Kyle RA. Multiple myeloma in young patients: clinical presentation and treatment approach. Leuk Lymphoma. 1998;30:493–501. doi: 10.3109/10428199809057562. [DOI] [PubMed] [Google Scholar]
- 3.Oyajobi BO. Multiple myeloma/hypercalcemia. Arthritis Res. Ther. 2007;9:S4. doi: 10.1186/ar2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JW, Chung HY, Ehrlich LA, Jelinek DF, Callander NS, Roodman GD. IL-3 expression by myeloma cells increases both osteoclast formation and growth of myeloma cells. Blood. 2004;103:2308–2315. doi: 10.1182/blood-2003-06-1992. [DOI] [PubMed] [Google Scholar]
- 5.Edwards CM, Zhuang J, Mundy GR. The pathogenesis of the bone disease of multiple myeloma. Bone. 2008;42:1007–1013. doi: 10.1016/j.bone.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giuliani N, Colla S, Rizzoli V. Update on the pathogenesis of osteolysis in multiple myeloma patients. Acta Bio Medica Ateneo Parmenese. 2004;75:143–152. [PubMed] [Google Scholar]
- 7.Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175–179. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- 8.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lthy R. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 9.Terpos E, Szydlo R, Apperley JF, Hatjiharissi E, Politou M, Meletis J. Soluble receptor activator of nuclear factor kappaB ligand-osteoprotegerin ratio predicts survival in multiple myeloma: proposal for a novel prognostic index. Blood. 2003;102:1064–1069. doi: 10.1182/blood-2003-02-0380. [DOI] [PubMed] [Google Scholar]
- 10.Terpos E, Politou M, Szydlo R, Goldman JM, Apperley JF, Rahemtulla A. Serum levels of macrophage inflammatory protein-1 alpha (MIP-1alpha) correlate with the extent of bone diseaseand survival in patients with multiple myeloma. Br J Haematol. 2003;123:106–109. doi: 10.1046/j.1365-2141.2003.04561.x. [DOI] [PubMed] [Google Scholar]
- 11.Oyajobi BO, Franchin G, Williams PJ, Pulkrabek D, Gupta A, Munoz S. Dual effects of macrophage inflammatory protein-1 alpha on osteolysis and tumor burden in the murine 5TGM1 model of myeloma bone disease. Blood. 2003;102:311–319. doi: 10.1182/blood-2002-12-3905. [DOI] [PubMed] [Google Scholar]
- 12.Barill-Nion S, Bataille R. New insights in myeloma-induced osteolysis. Leuk Lymphoma. 2003;44:1463–1467. doi: 10.3109/10428190309178765. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka Y, Abe M, Hiasa M, Oda A, Amou H, Nakano A. Myeloma cell-osteoclast interaction enhances angiogenesis together with bone resorption: a role for vascular endothelial cell growth factor and osteopontin. Clin Cancer Res. 2007;13:816–823. doi: 10.1158/1078-0432.CCR-06-2258. [DOI] [PubMed] [Google Scholar]
- 14.Zannettino AC, Farrugia AN, Kortesidis A, Manavis J, To LB, Martin SK. Elevated serum levels of stromal-derived factor 1-alpha are associated with increased osteoclast activity and osteolytic bone disease in multiple myeloma patients. Cancer Res. 2005;65:1700–1709. doi: 10.1158/0008-5472.CAN-04-1687. [DOI] [PubMed] [Google Scholar]
- 15.Westendorf JJ, Kahler RA, Schroeder TM. Wnt signaling in osteoblasts and bone diseases. Gene. 2004;341:19–39. doi: 10.1016/j.gene.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 16.Giuliani N, Rizzoli V, Roodman D. Multiple myeloma bone disease: pathophysiology of osteoblast inhibition. Blood. 2006;108:3992–3996. doi: 10.1182/blood-2006-05-026112. [DOI] [PubMed] [Google Scholar]
- 17.Oshima T, Abe M, Asano J, Hara T, Kitazoe K, Sekimoto E. Myeloma cells suppress bone formation by secreting a soluble Wnt inhibitor, sFRP-2. Blood. 2005;106:3160–3165. doi: 10.1182/blood-2004-12-4940. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi T, Kronenberg H. Minireview: transcriptional regulation in development of bone. Endocrinology. 2005;146:1012–1017. doi: 10.1210/en.2004-1343. [DOI] [PubMed] [Google Scholar]
- 19.Ehrlich LA, Chung HY, Ghobrial I, Choi SJ, Morandi F, Colla S. IL-3 is a potential inhibitor of osteoblast differentiation in multiple myeloma. Blood. 2005;106:1407–1414. doi: 10.1182/blood-2005-03-1080. [DOI] [PubMed] [Google Scholar]
- 20.Kropff M, Bisping G, Wenning D, Berdel WE, Kienast J. Proteasome inhibition in multiple myeloma. Eur J Cancer. 2006;42:1623–1639. doi: 10.1016/j.ejca.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 21.Stewart AF. Clinical practice. Hypercalcemia associated with cancer. N Engl J Med. 2005;352:373–379. doi: 10.1056/NEJMcp042806. [DOI] [PubMed] [Google Scholar]
- 22.Bartlett JB, Tozer A, Stirling D, Zeldis JB. Recent clinical studies of the immunomodulatory drug (IMiD) lenalidomide. Br J Cancer. 2005;93:613–619. doi: 10.1038/sj.bjc.6602774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar S, Rajkumar SV. Thalidomide and lenalidomide in the treatment of multiple myeloma. Eur J Cancer. 2006;42:1612–1622. doi: 10.1016/j.ejca.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Terpos E, Dimopoulos MA, Sezer O. The effect of novel antimyeloma agents on bone metabolism of patients with multiple myeloma. Leukemia. 2007;21:1875–1884. doi: 10.1038/sj.leu.2404843. [DOI] [PubMed] [Google Scholar]
- 25.Oyajobi BO, Garrett IR, Gupta A, Flores A, Esparza J, Muoz S. Stimulation of new bone formation by the proteasome inhibitor, bortezomib: implications for myeloma bone disease. Br J Haematol. 2007;139:434–438. doi: 10.1111/j.1365-2141.2007.06829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chauhan D, Hideshima T, Anderson KC. Targeting proteasomes as therapy in multiple myeloma. Adv Exp Med Biol. 2008;615:251–260. doi: 10.1007/978-1-4020-6554-5_12. [DOI] [PubMed] [Google Scholar]
- 27.Richardson PG, Mitsiades C, Schlossman R, Munshi N, Anderson K. New drugs for myeloma. Oncologist. 2007;12:664–689. doi: 10.1634/theoncologist.12-6-664. [DOI] [PubMed] [Google Scholar]
- 28.Giuliani N, Morandi F, Tagliaferri S, Lazzaretti M, Bonomini S, Crugnola M. The proteasome inhibitor bortezomib affects osteoblast differentiation in vitro and in vivo in multiple myeloma patients. Blood. 2007;110:334–338. doi: 10.1182/blood-2006-11-059188. [DOI] [PubMed] [Google Scholar]
- 29.Schwarz EM, Ritchlin CT. Clinical development of anti-RANKL therapy. Arthritis Res Ther. 2007;9:S7. doi: 10.1186/ar2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamdy NA. Denosumab: RANKL inhibition in the management of bone loss. Drugs Today (Barc) 2008;44:7–21. doi: 10.1358/dot.2008.44.1.1178467. [DOI] [PubMed] [Google Scholar]
- 31.Body JJ, Greipp P, Coleman RE, Facon T, Geurs F, Fermand JP. A phase I study of AMGN-0007, a recombinant osteoprotegerin construct, in patients with multiple myeloma or breast carcinoma related bone metastases. Cancer. 2003;97:887–892. doi: 10.1002/cncr.11138. [DOI] [PubMed] [Google Scholar]
- 32.Bekker PJ, Holloway DL, Rasmussen AS, Murphy R, Martin SW, Leese PT. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL in postmenopausal women. J Bone Miner Res. 2004;19:1059–1066. doi: 10.1359/JBMR.040305. [DOI] [PubMed] [Google Scholar]
- 33.Body JJ, Facon T, Coleman RE, Lipton A, Geurs F, Fan M. A study of the biological receptor activator of nuclear factorkappaB ligand inhibitor, denosumab, in patients with multiple myeloma or bone metastases from breast cancer. Clin Cancer Res. 2006;12:1221–1228. doi: 10.1158/1078-0432.CCR-05-1933. [DOI] [PubMed] [Google Scholar]
- 34.Yaccoby S, Ling W, Zhan F, Walker R, Barlogie B, Shaughnessy JDJr. Antibody-based inhibition of DKK1 suppresses tumor-induced bone resorption and multiple myeloma growth in vivo. Blood. 2007;109:2106–2111. doi: 10.1182/blood-2006-09-047712. [DOI] [PMC free article] [PubMed] [Google Scholar]