Abstract
Because of its basal position on the phylogenetic tree of vertebrates, the lamprey embryo would be expected to exhibit segmental head mesoderm. Recent observations, however, show that the lamprey does not have any somite-like segments in the head. Coelomic head cavities that are most conspicuous in elasmobranch embryos, do not appear to represent universal vertebrate traits. From the perspective of generative constraint, segmental structures in the vertebrate body can be classified into primary segments, which arise as segmental embryonic primordia such as somites and pharyngeal pouches, and secondary segments whose patterns are determined by the presence of primary segments. Secondary segments include neural crest derivatives and epibranchial placodes that are not initially segmented. The head mesoderm of vertebrates is secondarily regionalized into several domains that do not impose any secondary segmental patterns on other structures. Thus, the vertebrate head is characterized by a lack of segmental generative constraint in its mesoderm. Classical segmental theories are now refuted because they attempted to equate the vertebrate head with that of the amphioxus, whose rostral somites are considered primary segments, which are absent from vertebrates.
Introduction
Many studies on head segments of vertebrates have been conducted by comparative zoologists. Interest in vertebrate head segments first arose because of a transcendental concept of morphology [Goethe 1790 (cited in Gaupp 1898), 1820] and was subsequently revived in the search for a common embryonic design reflecting the morphology of a common ancestor (Goodrich 1930). Current studies of vertebrate head segments involve the discipline of evolutionary developmental biology, in which regulatory gene networks and their functions are compared between vertebrates and nonvertebrate chordates to elucidate evolutionary changes in developmental programs (gene regulatory networks) that result in the vertebrate body plan (see Olsson et al. 2005, and S. Kuratani and T. Schilling in this issue).
Several types of segments are recognized or assumed present in the vertebrate head (see Jefferies 1986 for a classification of segmental theories). For example, pharyngeal arches and cranial (branchiomeric) nerves innervating the arches are iterated in the ventral part of the head. In the vertebrate embryo, these nerves arise on even-numbered rhombomeres (r2, r4, and r6), the segmental bulges in the hindbrain (Lumsden and Keynes 1989). Similar neuromeric segments are also present in the forebrain. Consequently, the neuromeres in the neuraxis were thought by some morphologists to reflect the segmental design of the vertebrate axis (reviewed by Jarvik 1980). However, the most intriguing issue in the history of comparative embryology has concerned the hypothesis that the mesodermal segments in the head are equivalent (serially homologous) to the somites in the trunk. In a general sense, Goethe's vertebral theory of the skull (Goethe 1790, 1820) is based on this theory, as are many of the concepts that are based on mesodermal segmentation (Fig. 1C). Although Goodrich's scheme (Fig. 1A) includes the peripheral nervous system and a differentiated skeletal system, if these anatomical structures are removed, it is apparent that the scheme assumes that the paraxial mesoderm is segmented along the entire axis as “somites” (Fig. 1B).
Fig. 1.
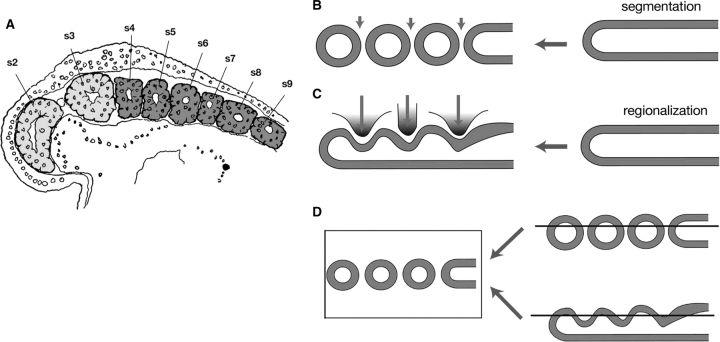
Segmental views of the vertebrate head. (A) Goodrich's (1930) formulation of the vertebrate head is based on the morphology of the mid-pharyngula of elasmobranchs. (B) When the skeletal and peripheral nervous systems are removed from the scheme, it is evident that it was based on the segmentation of the paraxial mesoderm. Note that each of the head somites (pm, mm, hm, s0–3) is associated ventrally with the pharyngeal arch mesoderm. (A) and (B) were modified from Kuratani et al. (1999). (C) Typical segmentalist perspective. The vertebrate head is assumed to contain only one type of segmentation that involves metamerism of paraxial mesodermal segments and pharyngeal arches. (D) An example of a nonsegmentalist theory that assumes independent patterns of metamerisms for somites and pharynegal arches but questions the presence of segments in head mesoderm. Abbreviations: hm, hyoid somite; mm, mandibular somite; ot, otic vesicle; pm, premandibular somite; pv, Platt's vesicle; s0–7, somites. (C) and (D) were modified from Kuratani (2003).
Classical segmental theories assumed that the iterating intervals of pharyngeal arches are identical to those of head segments. Therefore, the typical segmentalist theory assumed that each head somite was associated with a single pharyngeal arch, as is evident in the theories of Balfour (1878), van Wijhe (1882), Goodrich (1930), Bjerring (1977), and Jarvik (1980) (Fig. 1C). The dual segmental theory of Romer (1972), in which the segmental pattern of somites is considered independent of that of the gills (Fig. 1D), is typical of theories that arose in opposition to the aforementioned segmentalist theories. The series of reports by Froriep and Kuratani (Froriep 1882, 1885, 1902; Kuratani and Eichele 1993; Kuratani 1997, 2003) make assumptions similar to those of Romer (1972). In addition, “moderate segmentalists” recognized head mesodermal segments but denied (or questioned) their association with the pharyngeal arches (de Beer 1922, 1937; Damas 1944). In the modern context of evolutionary developmental biology, the primary areas of interest involve the absence or presence of paraxial mesodermal segmentation in the vertebrate embryonic head and whether the segments are related to branchiomeric elements. Thus, the issues in this field are similar to those considered by the “moderate” segmentalists.
Unlike comparative embryology that attempted to formulate an archetype of vertebrate head until the beginning of the 20th century, evolutionary developmental biology has attempted to identify an “ancestral” pattern based upon evolutionary history and concomitant changes in developmental mechanisms. The issue of head segmentation can now be rephrased as “Did the embryonic head of the vertebrate ancestor have somitomeric segmentation?” as is evident from the search for plesiomorphic features of the patterning program of the vertebrate head. Developmental biology, on the other hand, searches for generalized developmental programs of the vertebrate head (reviewed by Hunt et al. 1991; Graham 2001), which may or may not be equivalent to the ancestral pattern because such a generalized scheme may include vertebrate synapomorphies that are absent from the ancestor. The aims of this review are to refute the theory of head mesodermal segments, to examine the validity of Goodrich's scheme (and, indirectly, most segmentalist theories published prior to Goodrich's scheme), and to consider how to deal with this classical problem in the context of evolutionary development.
Development of the head mesoderm in cyclostomes
Unlike the clearly segmented paraxial mesoderm in the trunk, there are no clear segments in the embryonic head mesoderm of amniotes. However, Meier and his colleagues (Meier 1979, 1981; Anderson and Meier 1981; Meier and Tam 1982; Jacobson and Meier 1984; Meier and Packard 1984; reviewed by Jacobson 1988, 1993) claim to have observed incomplete segments called “somitomeres” in various gnathostome embryos by using scanning electron microscopy (SEM). The presence of these segments has never been confirmed by other scientists (see Freund et al. 1996; Jouve et al. 2002; also see S. Kuratani and T. Schilling in this issue). As the somitomeres in gnathostomes were thought to reflect their ancestral morphology, the embryos of basal species were expected to exhibit segments with even greater clarity. Two studies on cyclostomes showed clear segments in the head mesoderm.
Koltzoff (1901) and Damas (1944) illustrated the location of segmented head mesoderm in lamprey embryos and larvae based on histological observations of embryonic Petromyzon marinus and Lampetra fluviatilis (Fig. 2A). These segments are depicted as a rostral continuation of somites and are delineated by clear boundaries. However, Veit (1947), who described Petromyzon planeri embryos, and Kuratani et al. (1999), who conducted an SEM study of Lethenteron japonicum, did not observe segmental boundaries in the head mesoderm other than the posterior boundary of the premandibular mesoderm (Kuratani et al. 1999).
Fig. 2.
Is the head mesoderm of the lamprey segmented? (A) A parasagittal section of a young lamprey embryo published by Koltzoff (1901) (modified from de Beer 1937). The head mesoderm is colored light gray and real somites in the trunk are colored dark gray. In this section, numbering of the mesodermal “segments” starts from the yet undeveloped premandibular mesoderm (labeled “s1”) followed by the mandibular mesoderm (labeled “s2”). Thus, the first (real postotic) somite in the generic terminology is labeled “s4,” and “s3” in this figure corresponds to the sum of hyoid mesoderm and somite 0 (see Kuratani et al. 1999 for a definition of somite 0). At this stage, the head mesoderm appears to be “segmented” only between s2 and s3. (B) Schematic illustration of segmentation during somitogenesis in vertebrate embryos. (C) The regionalization process typical of the vertebrate head mesoderm. The unsegmented mesoderm is simply regionalized by surrounding structures and no real segmentation is evident. (D) A scheme showing that parasagittal sections cannot discriminate between segmentation and regionalization.
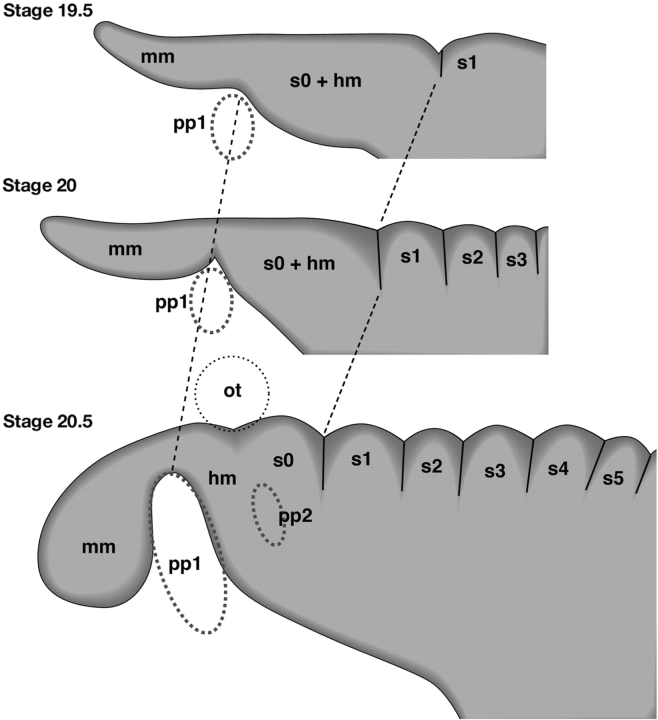
In the early development of lampreys, segmental boundaries appear only in the postotic paraxial mesoderm, and the initially unsegmented head mesoderm is only “regionalized” into domains by the protruding pharyngeal pouches and otic vesicle (Kuratani et al. 1999; Fig. 3). The premandibular mesoderm arises in later development from the prechordal plate, and for this reason the premandibular and mandibular mesoderm are separated by a clear boundary such as that between the prechordal plate and the mandibular mesoderm. It is noteworthy that the latter boundary is not formed in the initially continuous sheet of mesodermal cells, as is the case with somitogenesis, and thus is not identical to the somitic boundaries. Therefore, the “head somites” described by Koltzoff (1901) and Damas (1944) are most likely to have been histological exaggerations of a divided enterocoel and sulci on the surface of the head mesoderm (Fig. 2B–D).
Fig. 3.
Regionalization of the head mesoderm in embryos of L. japonicum. Embryos at stages 19.5, 20, and 20.5 (Tahara 1988) are schematically illustrated. As in other vertebrate embryos, the head mesoderm of the lamprey is primarily regionalized by pharyngeal pouches (pp1 and pp2) and the otic vesicle (ot); real somitic boundaries arise only in the postotic paraxial mesoderm. Redrawn from Kuratani et al. (1999).
The “enterocoelic” mesoderm of the lamprey described by Koltzoff (1901) does not constitute valid evidence for presence of segments. The term “enterocoely” refers to a mode of mesodermal development, not to a morphological pattern or process of segmentation in the mesoderm (the presence or absence of head cavities is discussed in a subsequent section). Although one of Koltzoff's parasagittal sections appears to show segmental organization of the paraxial mesoderm, only two mesodermal domains are depicted in the head (Fig. 2A). As discussed previously, the division of these two regions corresponds to a sulcus between the mandibular and hyoid mesodermal domains in L. japonicum (Kuratani et al. 1999; Fig. 3), which is not the real boundary.
Koltzoff's paper was strongly biased by the so-called “elasmobranch worship” that prevailed at the time (Gee 1996), and the mesodermal domains he called “head somites” are similar to those present in early elasmobranch pharyngula, in which head cavities and pharyngeal arch mesoderm are not well dissociated. Koltzoff's “second somite (s2)” corresponds to the mandibular arch mesoderm and mandibular cavity in well-formed elasmobranch pharyngula, and the “third somite (s3)” corresponds to the more posterior head mesodermal domain (hyoid mesoderm + somite 0 in the lamprey; Fig. 3) rostral to the real first somite that Koltzoff called the “fourth somite” (s4 in Fig. 2A). A histological pattern similar to that shown in Fig. 2A is also produced by regionalization in embryos of L. japonicum (Kuratani et al. 1999) (Fig. 2B–D).
From the preceding discussion, it is clear that it has never been proven that the head mesoderm of P. marinus is segmented and that it is merely regionalized by the growth of surrounding embryonic structures, as occurs in L. japonicum. Although the head mesoderm of the lamprey develops as an enterocoel, it never becomes segmented nor does it persist as head cavities surrounded by thin epithelium (many classical descriptions have confused enterocoelic head mesodermal domains with head cavities).
Segments as generative constraints
The head segmental theories were aimed at formulating the morphological patterns of the vertebrate head and referred not only to mesodermal segments but also indirectly to mechanisms responsible for the developmental patterns of the skeletal, nervous, and vascular systems. In developmental biology, for example, many of the segments in the neural tube (neuromeres) represent developmental compartments whose cell lineages are separated from those in other compartments (Fraser et al. 1990; Figdor and Stern 1993). They are often centers of cell proliferation and impose domain restrictions on regulatory gene expression, as observed in the hindbrain and forebrain. They represent typical epithelial segments that develop autonomously (Källen 1956). The spinal cord, on the other hand, contains a different type of neuromeres (myelomeres) whose development is largely dependent on the presence of somites (Lim et al. 1991). Although Neal (1918) did not realize the morphogenetic (segmental) significance of neuromeres, he was aware that neuromeres arise for different reasons at each level of the neural tube (Neal 1898). Neal (1918) recognized the primary role of somites in the development of myelomeres, which is now termed “generative constraint” (Wagner 1994). Thus, modern segmental theory should indicate the nature and distribution of generative constraints in vertebrate head development.
As has been recognized by several experimental embryologists, the anlage of the spinal cord is not primarily segmented, but the segmental patterns of dorsal root ganglia and motor roots are imposed secondarily by the presence of somites. Experimental removal or addition of somites causes a loss or an increase of peripheral nerve elements in a manner similar to changes in mesodermal segments (Lehmann 1927; Detwiler 1934; Tosney 1982; Keynes and Stern 1984; Rickmann et al. 1985; Lim et al. 1987, 1991; Teillet et al. 1987; Sechrist et al. 1993). In amniotes, the segmental pattern of the nerve is ascribed to the fact that neither the motor axons nor the neural crest cells are segmented per se and can only penetrate or migrate into the rostral half of the somites during development. Thus, the pattern of spinal nerves is “constrained” to develop into a somitomeric pattern and the primary factor responsible for their segmental pattern is the presence of somites (the somitomeric constraint).
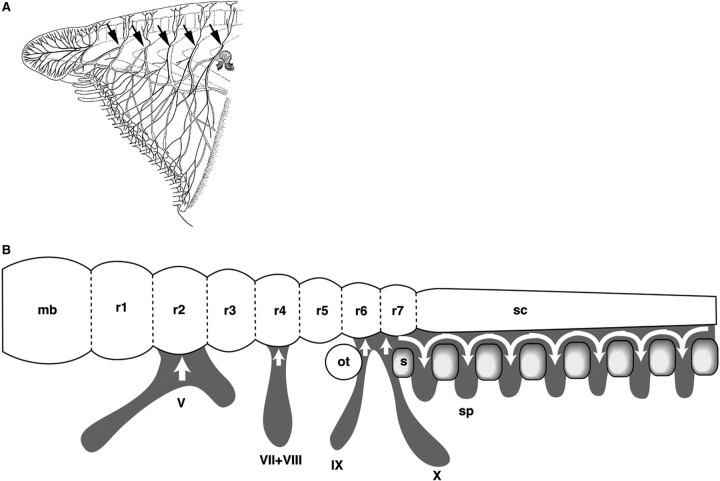
In contrast, it is not the head mesoderm but the rhombomeres that determine the position of nerve root formation in the hindbrain (Lumsden et al. 1991; Kuratani and Eichele 1993; Kuratani and Aizawa 1995; Niederländer and Lumsden 1996). In the hindbrain, unlike the spinal cord, the segmental pattern of peripheral nerves is constrained to the segmental pattern of the neurectoderm, and selective adhesion of the crest cells to even-numbered rhombomeres prefigures the developmental pattern of the nerves (Moody and Heaton 1983a, 1983b, 1983c), as was first recognized by Froriep (1902). Thus, even-numbered and odd-numbered rhombomeres serve as prepatterns for the formation of the roots of cranial nerves (rhombomeric constraint; Kuratani 1991; Guthrie et al. 1992; Graham et al. 1993; Inoue et al. 1997), and this is likely to prevail in the lamprey (Horigome et al. 1999). By considering the mechanism responsible for pattern formation, “primary segments” responsible for generation of secondarily segmented (constrained) structures can be identified. In the ventral part of the head, mesoderm, and crest cells (ectomesenchyme) of the pharyngeal arch and epibranchial placodes may be considered secondarily segmented structures that are constrained or induced by primarily segmented endodermal protrusions, namely, the pharyngeal pouches (branchiomeric constraint; see Begbie et al. 1999 and Holzschuh et al. 2005 for induction of epibranchial placodes through signals from endodermal pouches).
Because comparative embryology and morphology depends solely on the observation of shapes, it is incapable of identifying generative constraints. Thus, in the classical segmental theories, cranial nerves (rhombomeric and branchiomeric) were often regarded as highly modified somitomeric spinal nerves. Only amphioxus (Fig. 4A) fits such a scheme—vertebrates do not.
Fig. 4.
Patterning and evolution of the peripheral nerves in chordates. (A) Peripheral nerves of the amphioxus “head.” As indicated by arrows, rostral peripheral nerves are likely to be patterned segmentally by the presence of somites, as spinal nerves are in vertebrates. This does not mean that amphioxus nerves are identical to vertebrate spinal nerves. Redrawn from Hatschek (1892). (B) Developmental logics responsible for the spatial distribution patterns of crest cells (peripheral nerve primordia) in vertebrate embryos. In all the vertebrate embryos examined, the cephalic crest cell streams adhere to the even-numbered rhombomeres (r2, r4, r6, and posterior) of the hindbrain, whereas in the trunk, crest cell streams are subdivided into a somitomeric pattern by the presence of somites (s). Abbreviations: mb, midbrain; ot, otic vesicle; sc, spinal cord; sp, spinal nerve primordia; V, VII + VIII, IX, and X, cranial nerve primordia. Modified from Kuratani (2004).
Head cavities and head mesodermal segmentation in vertebrates
If the head mesoderm in vertebrates differs from somites in capability for developmental patterning, is there evidence of the remnants of head mesodermal segments in, for example, the head cavities? Balfour (1878) first described three pairs of epithelial cavities in the head of early pharyngula shark embryos. They were termed, from anterior to posterior, the premandibular, mandibular, and hyoid cavities (reviewed by G. Northcutt in this issue) but were not clearly demarcated from the mesoderm in the pharynegal arches. The pharynegal arches are tube-like epithelial cavities ventrally confluent with the primordial pericardium. Van Wijhe (1882) redefined the head cavities as the dorsal paraxial moiety of the epithelial cavity, which he compared with the paraxial mesoderm, and the more ventrally located pharyngeal mesoderm was compared with the lateral mesodermal derivatives that are visceral. Such head cavities are evident in mid-pharyngula as epithelial cysts floating in a loose mesenchyme in the paraxial domain of the head (van Wijhe 1882; Kuratani and Horigome 2000; Kuratani et al. 2000). Of the head cavities, the premandibular mesoderm (and the Platt's vesicle) has no ventral counterpart. Goodrich (1930) explained that the Platt's vesicle represented the ventral counterpart of the premandibular cavity (Fig. 1A and B).
Historically, there has often been confusion about the definition of head cavities and the concept of head mesodermal domains. The mandibular cavity is thought to be the paraxial portion of the mandibular mesoderm and to be ventrally attached to the mandibular “arch” mesoderm. Thus, in many vertebrate embryos, it corresponds to the ventral, mandibular arch mesoderm that Engrailed cognates are expressed (Hatta et al. 1990; Holland et al. 1993; reviewed by Hall 1998). This expression persists in some of the muscles of the mandibular arch in gnathostomes and lampreys. By definition, this En expression cannot be regarded as a mandibular cavity homologue (paraxial part) in the amphioxus as was once held (Holland et al. 1997). In the lamprey, the mandibular mesoderm mostly represents the pharyngeal arch of the mesoderm, and if this animal possesses a mandibular cavity-equivalent portion, it will be found only in a small dorsal portion of this mesoderm, which has not yet been identified. En expression in the mandibular mesoderm of the lamprey is, thus, most likely to represent a signal for specification of the mandibular arch muscle, as in gnathostomes, not for mesodermal segmentation. Expression of a T-box gene, AmphiTbx15/18/22, in amphioxus somites (Beaster-Jones et al. 2006) appears to induce segmentation in the paraxial mesoderm, similar to AmphiEn expression. In vertebrates, similar expression of these gene cognates occurs in somites in the trunk. However, no pseudosegmental expression patterns of T-box genes have been reported for head mesoderm in vertebrates, although these genes are often reported to be expressed in crest-derived ectomesenchyme (Haenig et al. 2002; Herr et al. 2003).
Expression of Hox gene cognates by vertebrates and amphioxus may indicate that their mesodermal domains or neuraxial levels are similar (Holland 1988, 1996a, 1996b, 2000). At that taxonomic level, however, morphological homology cannot be definitively established because the morphological identities of structures may not be tightly linked to Hox gene expression. Even among vertebrates, Hox gene expressions along the neuraxis are not always identical, although morphological homologies can be established among rhombomeres and cranial nerves (Murakami et al. 2004). It is difficult to rule out the possibility of a vertebrate ancestor whose rostral paraxial mesoderm may have been segmented in a similar fashion to that of somites. However, animals with a common Hox code does not necessarily mean that an identical set of morphological patterns will be established downstream of the code, as shown by Fritzsch and Northcutt (1993). Thus, it does not seem possible at present to support a homology between rostral somites in amphioxus and head cavities in gnathostomes, as was first implied by van Wijhe (1906) (cited by Franz 1927) (for incomplete homology, see Owen 1866; Gegenbaur 1898 and Tautz 1998; see also Yasui et al. 1998 and Kaji et al. 2001 for the homology of peripheral nerves between amphioxus and vertebrates).
Head cavities in the form of the epithelial cysts in elasmobranch embryos are also present in holocephalans, some actinopterygeans (premandibular and mandibular cavities in Amia and sturgeon; de Beer 1924; Kuratani et al. 2000) and in amniotes, including humans (usually only the premandibular cavity; reviewed by Kuratani 2003). No such epithelial cysts appear in the head mesoderm of the lamprey after disappearance of the original enterocoel. The presence of an enterocoel does not dictate a head cavity as the premandibular cavity of the chicken is not preceded by any enterocoelic precursor. It seems reasonable to assume that on the phylogenetic tree, head cavities represent a gnathostome synapomorphy and disappeared in a caudal to rostral direction in the more crown groups of vertebrates (reviewed by Kuratani 2003, 2004). In this regard, there exists little information on development of the extrinsic eye muscles of the lamprey. Although eye muscle primordia in the lamprey embryo were illustrated by Koltzoff, they appear to be mesenchymal and no clear epithelial cysts were described to be associated with these muscle anlagen (Koltzoff 1901). I have been unable to detect primordia of this particular eye muscle in L. japonicum embryos or larvae so far.
The developmental fate of head cavities is not well understood except that they appear to differentiate into extrinsic eye muscles at histological levels, which is consistent with the one-to-one relationships between the eye-moving cranial nerves and the head cavities (van Wijhe 1882). In avian embryos, the prechordal plate, the putative precursor of the premandibular cavity, has been shown by labeling of cells to differentiate into extrinsic eye muscle (Jacob et al. 1984; Wachtler et al. 1984; Wachtler and Jacob, 1986; also see Couly et al. 1992). It is not known why the head cavities are only well developed in chondrichthys. It was suggested that their function is to regulate the rapid growth of the eye in some gnathostomes, but this does not explain why they are poorly developed in some amniotes that have larger eye primordia than do the chondrichthys (reviewed by Kuratani 2003).
It is intriguing that the head cavities of elasmobranch embryos occupy positions similar to those of the head mesodermal domains of the lamprey. The premandibular cavities and premandibular mesoderm are both located rostral to the notochord, the right and left moieties are united in the midline, the dorsal part of the mandibular mesoderm in the lamprey, and the head cavity are dorsal to the mandibular arch between the ophthalmic and maxillomandibular branches of the trigeminal nerve, and the dorsal hyoid mesoderm and hyoid cavity are dorsal to the hyoid arch (Kuratani et al. 1999, 2000; Kuratani and Horigome 2000). This implies that the development of head cavities is under the same constraint that causes regionalization of the head mesoderm in the lamprey. Therefore, head cavities appear to be partially branchiomeric. In this sense, it seems appropriate to name the cavities after the pharyngeal arches. It is inappropriate to compare head cavities with somitomeres that are somitomeric by definition.
Conclusion
Because of the absence of a proper out-group, it is not easy to speculate about the ancestral state of the mesoderm of the vertebrate head. From the perspective of generative constraint, the head mesoderm of all the vertebrate species (even if it develops into head cavities) appears incapable of metamerical patterning of peripheral nerves as serial homologues of spinal nerves. This appears to be a synapomorphy that defines vertebrates. Thus, the primary pattern of the vertebrate head, which was explained by an archetypal segmental concept, is not determined by vestigial somites in the head. Rather, a neurepithelial segmental mechanism (rhombomeres) and pharyngeal pouches determine the iterating pattern of the branchiomeric cranial nerves. All the vertebrate species, including cyclostomes and elasmobranchs, share the basic morphology of branchial nerves at embryonic stages (Kuratani 1997).
In the search for an ancestral somitomeric pattern in the mesoderm, the vertebrate head should be conceptualized as a modified plesiomorphic pattern similar to that of amphioxus, whose peripheral nerves are mostly patterned under somitomeric generative constraint (Yasui et al. 1998; Kaji et al. 2001; Fig. 4A). This does not imply, however, that the rostral nerves of amphioxus are homologous to spinal nerves. Without rhombomeres and epibranchial placodes, this animal simply cannot have peripheral nerves with branchiomeric nerve morphology. It is, therefore, logically impossible to include vertebrate-like branchiomeric nerves and amphioxus-like rostral somites in segmentalist schemes, all of which are rather similar to Goodrich's scheme (Jefferies 1986) (Fig. 1A). To formulate the developmental patterning of vertebrates as a crown group of chordates, the absence of mesodermally derived, segmental generative constraint should be emphasized, not vice versa.
Developmentally, the expression patterns and functions of vertebrate Hox genes, conspicuous in pharyngeal arch ectomesenchyme, and rhombomeres in the head and in somites in the trunk (reviewed by Hunt and Krumlauf 1991; Hunt et al. 1991; Kessel 1992; Rijli et al. 1993; Graham 2001), as well as the contrasting homeotic responses to all-trans retinoic acid (pharyngeal arch-derivatives in the head, vertebral elements in the trunk) strongly imply that the vertebrate body plan is clearly and characteristically dissociated into head and trunk. This simultaneously emphasizes the lack of overt somitomerism (absence of the source of generative constraint) in the head. The head cavities are paraxial mesodermal structures that become evident secondarily in mid-pharyngula in concert with the regionalization pattern of the head mesoderm. Thus, the cavities can exhibit branchiomeric regionalization to some extent. However, histologically overt head cavities are only observed in gnathostomes and are likely to be a synapomorphy that defines this group. Mesodermal segmentation in the head, which has not even been shown to exist, cannot be regarded as a trait for defining vertebrates as a crown group of chordates.
References
- Anderson CB, Meier S. The influence of the metameric pattern in the mesoderm on migration of cranial neural crest cells in the chick embryo. Dev Biol. 1981;85:385–402. doi: 10.1016/0012-1606(81)90270-0. [DOI] [PubMed] [Google Scholar]
- Balfour FM. The development of the elasmobranchial fishes. J Anat Physiol. 1878;11:405–706. [Google Scholar]
- Beaster-Jones L, Horton AC, Gibson-Brown JJ, Holland ND, Holland LZ. The amphioxus T-box gene, AmphiTbx15/18/22, illuminates the origins of chordate segmentation. Evol Dev. 2006;8:119–129. doi: 10.1111/j.1525-142X.2006.00083.x. [DOI] [PubMed] [Google Scholar]
- Begbie J, Brunet JF, Rubenstein JL, Graham A. Induction of the epibranchial placodes. Development. 1999;126:895–902. doi: 10.1242/dev.126.5.895. [DOI] [PubMed] [Google Scholar]
- Bjerring HC. A contribution to structual analysis of the head of craniate animals. Zool Scr. 1977;6:127–183. [Google Scholar]
- Couly GF, Colty PM, Le Douarin NM. The developmental fate of the cephalic mesoderm in quail – chick chimeras. Development. 1992;114:1–15. doi: 10.1242/dev.114.1.1. [DOI] [PubMed] [Google Scholar]
- Damas H. Recherches sur le développement de Lampetra fluviatilis L. – contribution à l’étude de la cephalogénèse des vertébrés. Arch Biol. 1944;55:1–289. [Google Scholar]
- de Beer GR. The segmentation of the head in Squalus acanthias. Q J Microsc Sci. 1922;66:458–474. [Google Scholar]
- de Beer GR. The prootic somites of Heterodontus and of Amia. Q J Microsc Sci. 1924;68:17–38. [Google Scholar]
- de Beer GR. The development of the vertebrate skull. London: Oxford University Press; 1937. [Google Scholar]
- Detwiler SR. An experimental study of spinal nerve segmentation in Amblystoma with reference to the plurisegmental contribution to the brachial plexus. J Exp Zool. 1934;67:395–441. [Google Scholar]
- Figdor MC, Stern CD. Segmental organization of embryonic diencephalon. Nature. 1993;363:630–634. doi: 10.1038/363630a0. [DOI] [PubMed] [Google Scholar]
- Franz V. Morphologie der Akranier. Z Ges Anat. 1927;27:465–692. [Google Scholar]
- Fraser S, Keynes R, Lumsden A. Segmentation in the chick embryo hindbrain is defined by cell lineage restriction. Nature. 1990;344:431–435. doi: 10.1038/344431a0. [DOI] [PubMed] [Google Scholar]
- Freund R, Dörfler D, Popp W, Wachtler F. The metameric pattern of the head mesoderm – does it exist? Anat Embryol. 1996;193:73–80. doi: 10.1007/BF00186835. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Northcutt G. Cranial and spinal nerve organization in amphioxus and lampreys: Evidence for an ancestral craniate pattern. Acta Anat. 1993;148:96–109. doi: 10.1159/000147529. [DOI] [PubMed] [Google Scholar]
- Froriep A. Über ein Ganglion des Hypoglossus und Wirbelanlagen in der Occipitalregion. Arch Anat Physiol. 1882;1882:279–302. [Google Scholar]
- Froriep A. Über Anlagen von Sinnesorganen am Facialis, Glossopharyngeus und Vagus, über die genetische Stellung des Vagus zum Hypoglossus, und über die Herkunft der Zungenmusculatur. Arch Anat Physiol. 1885;1885:1–55. [Google Scholar]
- Froriep A. Zur Entwicklungsgeschichte des Wirbeltierkopfes. Verh Anat Ges. 1902;1902:34–46. [Google Scholar]
- Gaupp E. Die Metamerie des Schädels. Erg Anat Ent-ges. 1898;7:793–885. [Google Scholar]
- Gee H. Before the backbone. London: Chapman & Hall; 1996. [Google Scholar]
- Gegenbaur C. Vergleichende Anatomie der Wirbeltihiere mit Berücksichtung der Wirbellosen. Leipzig (Germany): Verlag von Wilhelm Engelmann; 1898. [Google Scholar]
- Goethe JW. Zur Naturwissenschaft überhaupt, besonders zur Morphologie. 1790. Das Schädelgrüt aus sechs Wirbelknochen aufgebaut. II 2 (cited in Gaupp 1898) [Google Scholar]
- Goethe JW. Zur Naturwissenschaft überhaupt, besonders zur Morphologie. Stuttgart and Tübingen (Germany): I, J. G. Cotta; 1820. [Google Scholar]
- Goodrich ES. Structure and development of vertebrates. London: Macmillan; 1930. [Google Scholar]
- Graham A. The development and evolution of the pharyngeal arches. J Anat. 2001;199:133–141. doi: 10.1046/j.1469-7580.2001.19910133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A, Heyman I, Lumsden A. Even-numbered rhombomeres control the apoptotic elimination of neural crest cells from odd-numbered rhombomeres in the chick hindbrain. Development. 1993;119:233–245. doi: 10.1242/dev.119.1.233. [DOI] [PubMed] [Google Scholar]
- Guthrie S, Muchamore I, Kuroiwa A, Marshall H, Krumlauf R, Lumsden A. Neuroectodermal autonomy of Hox-2.9 expression revealed by rhombomere transpositions. Nature. 1992;356:157–159. doi: 10.1038/356157a0. [DOI] [PubMed] [Google Scholar]
- Haenig B, Schmidt C, Kraus F, Pfordt M, Kispert A. Cloning and expression analysis of the chick ortholog of TBX22, the gene mutated in X-linked cleft palate and ankyloglossia. Mech Dev. 2002;117:321–325. doi: 10.1016/s0925-4773(02)00196-x. [DOI] [PubMed] [Google Scholar]
- Hall BK. Evolutionary developmental biology. 2nd. London: Chapman & Hall; 1998. [Google Scholar]
- Hatschek B. Die Metamerie des Amphioxus und des Ammocoetes. Verh Anat Ges. 1892;6:136–162. [Google Scholar]
- Hatta K, Schilling T, BreMille RA, Kimmel CB. Specification of jaw muscle identity in Zebrafish: Correlation with engrailed-homeoprotein expression. Science. 1990;250:802–805. doi: 10.1126/science.1978412. [DOI] [PubMed] [Google Scholar]
- Herr A, Meunier D, Muller I, Rump A, Fundele R, Ropers HH, Nuber UA. Expression of mouse Tbx22 supports its role in palatogenesis and glossogenesis. Dev Dyn. 2003;226:579–586. doi: 10.1002/dvdy.10260. [DOI] [PubMed] [Google Scholar]
- Holland PW. Homeobox genes and the vertebrate head. Development. 1988;103(Suppl):17–24. [PubMed] [Google Scholar]
- Holland ND. Homology, homeobox genes, and the early evolution of the vertebrates. Mem Calif Acad Sci. 1996a:2063–2069. [Google Scholar]
- Holland PWH. Molecular biology of lancelets: insights into development and evolution. Isr J Zool. 1996b;42:247–272. [Google Scholar]
- Holland PW. Embryonic development of heads, skeletons and amphioxus: Edwin S. Goodrich revisited. Int J Dev Biol. 2000;44:29–34. [PubMed] [Google Scholar]
- Holland ND, Holland LZ, Honma Y, Fujii T. Engrailed expression during development of a lamprey, Lampetra japonica: a possible clue to homologies between agnathan and gnathostome muscles of the mandibular arch. Dev Growth Differ. 1993;35:153–160. doi: 10.1111/j.1440-169X.1993.00153.x. [DOI] [PubMed] [Google Scholar]
- Holland LZ, Kene M, Williams NA, Holland ND. Sequence and embryonic expression of the amphioxus engrailed gene (AmphiEn): The metameric pattern of transcription resembles that of its segment-polarity homolog in Drosophila. Development. 1997;124:1723–1732. doi: 10.1242/dev.124.9.1723. [DOI] [PubMed] [Google Scholar]
- Holzschuh J, Wada N, Wada C, Schaffer A, Javidan Y, Tallafuss A, Bally-Cuif L, Schilling TF. Requirements for endoderm and BMP signaling in sensory neurogenesis in zebrafish. Development. 2005;132:3731–3742. doi: 10.1242/dev.01936. [DOI] [PubMed] [Google Scholar]
- Horigome N, Myojin M, Hirano S, Ueki T, Aizawa S, Kuratani S. Development of cephalic neural crest cells in embryos of Lampetra japonica, with special reference to the evolution of the jaw. Dev Biol. 1999;207:287–308. doi: 10.1006/dbio.1998.9175. [DOI] [PubMed] [Google Scholar]
- Hunt P, Krumlauf R. Deciphering the Hox code: Clues to patterning branchial regions of the head. Cell. 1991;66:1075–1078. doi: 10.1016/0092-8674(91)90029-x. [DOI] [PubMed] [Google Scholar]
- Hunt P, Gulisano M, Cook M, Sham M-H, Faiella A, Wilkinson D, Boncinelli E, Krumlauf R. A distinct Hox code for the branchial region of the vertebrate head. Nature. 1991;353:861–864. doi: 10.1038/353861a0. [DOI] [PubMed] [Google Scholar]
- Inoue T, Chisaka O, Matsunami H, Takeichi M. Cadherin-6 expression transiently delineates specific rhombomeres, other neural tube subdivisions, and neural crest subpopulations in mouse embryos. Dev Biol. 1997;183:183–194. doi: 10.1006/dbio.1996.8501. [DOI] [PubMed] [Google Scholar]
- Jacob M, Jacob HJ, Wachtler F, Christ B. Ontogeny of avian extrinsic ocular muscles. I. A light- and electron-microscopic study. Cell Tissue Res. 1984;237:549–557. doi: 10.1007/BF00228439. [DOI] [PubMed] [Google Scholar]
- Jacobson AG. Somitomeres:Mesodermal segments of vertebrate embryos. Development. 1988;104(Suppl):209–220. doi: 10.1242/dev.104.Supplement.209. [DOI] [PubMed] [Google Scholar]
- Jacobson AG. Somitomeres: Mesodermal segments in the head and trunk. In: Hanken J, Hall BK, editors. The vertebrate skull. Vol. 1. Chicago (IL): University of Chicago Press; 1993. pp. 42–76. [Google Scholar]
- Jacobson AG, Meier S. Morphogenesis of the head of the newt: Mesodermal segments, neuromeres, and distribution of neural crest. Dev Biol. 1984;106:181–193. doi: 10.1016/0012-1606(84)90074-5. [DOI] [PubMed] [Google Scholar]
- Jarvik E. Basic structure and evolution of vertebrates. London: Academic Press; 1980. [Google Scholar]
- Jefferies RPS. The ancestry of the vertebrates. London: British Museum (Natural History); 1986. [Google Scholar]
- Jouve C, Iimura T, Pourquie O. Onset of the segmentation clock in the chick embryo: Evidence for oscillations in the somite precursors in the primitive streak. Development. 2002;129:1107–1117. doi: 10.1242/dev.129.5.1107. [DOI] [PubMed] [Google Scholar]
- Kaji T, Aizawa S, Uemura M, Yasui K. Establishment of left-right asymmetric innervation in the lancelet oral region. J Comp Neurol. 2001;435:394–405. doi: 10.1002/cne.1039. [DOI] [PubMed] [Google Scholar]
- Källen B. Experiments on neuromery in Ambystoma punctatum embryos. J Embryol Exp Morphol. 1956;4:66–72. [Google Scholar]
- Kessel M. Respecification of vertebral identities by retinoic acid. Development. 1992;115:487–501. doi: 10.1242/dev.115.2.487. [DOI] [PubMed] [Google Scholar]
- Keynes RJ, Stern CD. Segmentation in the vertebrate nervous system. Nature. 1984;310:786–789. doi: 10.1038/310786a0. [DOI] [PubMed] [Google Scholar]
- Koltzoff NK. Entwicklungsgeschichte des Kopfes von Petromyzon planeri. Bull Soc Nat Moscou. 1901;15:259–289. [Google Scholar]
- Kuratani S. Alternate expression of the HNK-1 epitope in rhombomeres of the chick embryo. Dev Biol. 1991;144:215–219. doi: 10.1016/0012-1606(91)90493-m. [DOI] [PubMed] [Google Scholar]
- Kuratani S. Distribution of postotic crest cells in the chick embryo defines the trunk/head interface: Embryological interpretation of crest cell distribution and evolution of the vertebrate head. Anat Embryol. 1997;195:1–13. doi: 10.1007/s004290050020. [DOI] [PubMed] [Google Scholar]
- Kuratani S. Evolutionary developmental biology and vertebrate head segmentation: A perspective from developmental constraint. Theory Biosci. 2003;122:230–251. [Google Scholar]
- Kuratani S. Evolutionary morphology: Bauplan and embryonic development of vertebrates. (in Japanese) Tokyo (Japan): Tokyo University Press; 2004. [Google Scholar]
- Kuratani S, Aizawa S. Patterning of the cranial nerve in the chick embryo is dependent on cranial mesoderm and rhombomeric metamerism. Dev Growth Differ. 1995;37:717–731. doi: 10.1046/j.1440-169X.1995.t01-5-00010.x. [DOI] [PubMed] [Google Scholar]
- Kuratani SC, Eichele G. Rhombomere transplantation repatterns the segmental organization of cranial nerves and reveals cell-autonomous expression of a homeodomain protein. Development. 1993;117:105–117. doi: 10.1242/dev.117.1.105. [DOI] [PubMed] [Google Scholar]
- Kuratani S, Horigome N. Development of peripheral nerves in a cat shark, Scyliorhinus torazame, with special reference to rhombomeres, cephalic mesoderm, and distribution patterns of crest cells. Zool Sci. 2000;17:893–909. [Google Scholar]
- Kuratani S, Horigome N, Hirano S. Developmental morphology of the cephalic mesoderm and re-evaluation of segmental theories of the vertebrate head: Evidence from embryos of an agnathan vertebrate, Lampetra japonica. Dev Biol. 1999;210:381–400. doi: 10.1006/dbio.1999.9266. [DOI] [PubMed] [Google Scholar]
- Kuratani S, Nobusada Y, Saito H, Shigetani Y. Morphological development of the cranial nerves and mesodermal head cavities in sturgeon embryos from early pharyngula to mid-larval stages. Zool Sci. 2000;17:911–933. [Google Scholar]
- Lehmann F. Further studies on the morphogenetic role of somites in the development of the nervous system of amphibians. J Exp Zool. 1927;49:93–131. [Google Scholar]
- Lim TM, Jaques KF, Stern CD, Keynes RJ. An evaluation of myelomeres and segmentation of the chick embryo spinal cord. Development. 1991;113:227–238. doi: 10.1242/dev.113.1.227. [DOI] [PubMed] [Google Scholar]
- Lim TM, Lunn ER, Keynes RJ, Stern CD. The differing effects of occipital and trunk somites on neural development in the chick embryo. Development. 1987;100:525–533. doi: 10.1242/dev.100.3.525. [DOI] [PubMed] [Google Scholar]
- Lumsden A, Keynes R. Segmental patterns of neuronal development in the chick hindbrain. Nature. 1989;337:424–428. doi: 10.1038/337424a0. [DOI] [PubMed] [Google Scholar]
- Lumsden A, Sprawson N, Graham A. Segmental origin and migration of neural crest cells in the hindbrain region of the chick embryo. Development. 1991;113:1281–1291. doi: 10.1242/dev.113.4.1281. [DOI] [PubMed] [Google Scholar]
- Meier S. Development of the chick mesoblast. Formation of the embryonic axis and establishment of the metameric pattern. Dev Biol. 1979;73:25–45. doi: 10.1016/0012-1606(79)90135-0. [DOI] [PubMed] [Google Scholar]
- Meier S. Development of the chick embryo mesoblast: Morphogenesis of the prechordal plate and cranial segments. Dev Biol. 1981;83:49–61. doi: 10.1016/s0012-1606(81)80007-3. [DOI] [PubMed] [Google Scholar]
- Meier S, Packard DS., Jr Morphogenesis of the cranial segments and distribution of neural crest in embryos of the snapping turtle, Chelydra serpentina. Dev Biol. 1984;102:309–323. doi: 10.1016/0012-1606(84)90196-9. [DOI] [PubMed] [Google Scholar]
- Meier S, Tam PPL. Metatmeric pattern development in the embryonic axis of the mouse. I. Differentiation of the cranial segments. Differentiation. 1982;21:95–108. doi: 10.1111/j.1432-0436.1982.tb01202.x. [DOI] [PubMed] [Google Scholar]
- Moody SA, Heaton MB. Developmental relationships between trigeminal ganglia and trigeminal motoneurons in chick embryos. I. Ganglion development is necessary for motoneuron migration. J Comp Neurol. 1983a;213:327–343. doi: 10.1002/cne.902130308. [DOI] [PubMed] [Google Scholar]
- Moody SA, Heaton MB. Developmental relationships between trigeminal ganglia and trigeminal motoneurons in chick embryos. II. Ganglion axon ingrowth guides motoneuron migration. J Comp Neurol. 1983b;213:344–349. doi: 10.1002/cne.902130309. [DOI] [PubMed] [Google Scholar]
- Moody SA, Heaton MB. Developmental relationships between trigeminal ganglia and trigeminal motoneurons in chick embryos. III. Ganglion perikarya direct motor axon growth in the periphery. J Comp Neurol. 1983c;213:350–364. doi: 10.1002/cne.902130310. [DOI] [PubMed] [Google Scholar]
- Murakami Y, Pasqualetti M, Takio Y, Hirano S, Rijli F, Kuratani S. Segmental development of reticulospinal and branchiomotor neurons in the lamprey: Insights into evolution of the vertebrate hindbrain. Development. 2004;131:983–995. doi: 10.1242/dev.00986. [DOI] [PubMed] [Google Scholar]
- Neal HV. The segmentation of the nervous system in Squalus acanthias. Bull Mus Comp Zool. 1898;31:147–294. [Google Scholar]
- Neal HV. Neuromeres and metameres. J Morphol. 1918;31:293–315. [Google Scholar]
- Niederländer C, Lumsden A. Late emigrating neural crest cells migrate specifically to the exit points of cranial branchiomotor nerves. Development. 1996;122:2367–2374. doi: 10.1242/dev.122.8.2367. [DOI] [PubMed] [Google Scholar]
- Olsson L, Ericsson R, Cerny R. Vertebrate head development: Segmentation, novelties, and homology. Theory Biosci. 2005;124:145–163. doi: 10.1007/BF02814481. [DOI] [PubMed] [Google Scholar]
- Owen R. On the anatomy of vertebrates. Vol. 1. London: Longmans, Green & Co; 1866. [Google Scholar]
- Rickmann M, Fawcett JW, Keynes R. Ther migration of neural crest cells and the growth of motor axons through the rostral half of the chick somite. J Embryol Exp Morphol. 1985;90:437–455. [PubMed] [Google Scholar]
- Rijli FM, Mark M, Lakkaraju S, Dierich A, Dollé P, Chambon P. Homeotic transformation is generated in the rostral branchial region of the head by disruption of Hoxa-2, which acts as a selector gene. Cell. 1993;75:1333–1349. doi: 10.1016/0092-8674(93)90620-6. [DOI] [PubMed] [Google Scholar]
- Romer AS. The vertebrate as a dual animal – somatic and visceral. Evol Biol. 1972;6:121–156. [Google Scholar]
- Sechrist J, Serbedzija GN, Sherson T, Fraser S, Bronner-Fraser M. Segmental migration of the hindbrain neural crest does not arise from segmental generation. Development. 1993;118:691–703. doi: 10.1242/dev.118.3.691. [DOI] [PubMed] [Google Scholar]
- Tahara Y. Normal stages of development in the lamprey, Lampetra reissneri (Dybowski) Zool Sci. 1988;5:109–118. [Google Scholar]
- Tautz D. Debatable homologies. Nature. 1998;395:17–19. doi: 10.1038/25604. [DOI] [PubMed] [Google Scholar]
- Teillet AM, Kalcheim K, LeDouarin NM. Formation of the dorsal root ganglia in the avian embryo: Segmental origin and migratory behavior of neural crest progenitor cells. Dev Biol. 1987;120:329–347. doi: 10.1016/0012-1606(87)90236-3. [DOI] [PubMed] [Google Scholar]
- Tosney KW. The segregation and early migration of cranial neural crest cells in the avian embryo. Dev Biol. 1982;89:13–24. doi: 10.1016/0012-1606(82)90289-5. [DOI] [PubMed] [Google Scholar]
- Veit O. Über das Problem Wirbeltierkopf. Kempen-Niederrhein (Germany): Thomas; 1947. [Google Scholar]
- Wachtler F, Jacob M. Origin and development of the cranial skeletal muscles. Biblthka Anat. 1986;29:24–46. [PubMed] [Google Scholar]
- Wachtler F, Jacob HJ, Jacob M, Christ B. The extrinsic ocular muscles in birds are derived from the prechordal mesoderm. Naturewiss. 1984;71:379–380. doi: 10.1007/BF00410750. [DOI] [PubMed] [Google Scholar]
- Wagner GP. Homology and the mechanisms of development. In: Hall BK, editor. Homology: The hierarchical basis of comparative biology. San Diego (CA): Academic Press; 1994. pp. 273–299. [Google Scholar]
- van Wijhe JW. Über die Mesodermsegmente und die Entwicklung der Nerven des Selachierkopfes. Ver Akad Wiss. 1882;22:1–50. [Google Scholar]
- van Wijhe JW. Die Homologisierung des Mundes des Amphioxus und die primitive Leibesgliederung der Wirbelthiere. 1906. Petrus Camper 4, 1. Auflage (cited by Franz 1927) [Google Scholar]
- Yasui K, Tabata S, Ueki T, Uemura M, Zhang SC. Early development of the peripheral nervous system in a lancelet species. J Comp Neurol. 1998;393:415–425. [PubMed] [Google Scholar]