Abstract
To initiate embryo development, the sperm induces in the egg release of intracellular calcium ([Ca2+]i). During oocyte maturation, the inositol-1,4,5-trisphosphate receptor (IP3R1), the channel implicated, undergoes modifications that enhance its function. We found that IP3R1 becomes phosphorylated during maturation at an MPM-2 epitope and that this persists until the fertilization-associated [Ca2+]i responses cease. We also reported that maturation without ERK activity diminishes IP3R1 MPM-2 reactivity and [Ca2+]i responses. Here, we show that IP3R1 is a novel target for Polo-like kinase1 (Plk1), a conserved M-phase kinase, which phosphorylates it at an MPM-2 epitope. Plk1 and IP3R1 interact in an M-phase preferential manner, and they exhibit close co-localization in the spindle/spindle poles area. This co-localization is reduced in the absence of ERK activity, as the ERK pathway regulates spindle organization and IP3R1 cortical re-distribution. We propose that IP3R1 phosphorylation by Plk1, and possibly by other M-phase kinases, underlies the delivery of spatially and temporally regulated [Ca2+]i signals during meiosis/mitosis and cytokinesis.
Keywords: Calcium; mammalian eggs; ERK; inositol 1,4,5-trisphosphate receptor; phosphorylation; Polo
INTRODUCTION
The activation of the egg is the first stage in the initiation of embryo development. It comprises a series of events that unfold soon after interaction of the gametes and that ends with the completion of meiosis and progression into the mitotic cell cycles (Ducibella et al., 2002; Schultz and Kopf, 1995). In all species studied to date, egg activation requires a fertilization-associated increase in the intracellular concentration of calcium ([Ca2+]i) (Stricker, 1999). In mammals, the fertilizing [Ca2+]i signal consist of periodical rises, which are referred to as [Ca2+]i oscillations (Miyazaki et al., 1993).
The type 1 inositol 1,4,5-trisphosphate receptor (IP3R1) in mammals, or its homologue in other species, is responsible for the majority of Ca2+ release during fertilization (Miyazaki et al., 1993). IP3R1, the most widely expressed isoform (Taylor et al., 1999), is distributed along the membrane of the endoplasmic reticulum (ER), the main Ca2+ reservoir in the cell (reviewed in Berridge, 2002). Production of inositol 1,4,5-trisphosphate (IP3), the IP3R1 ligand, is detected during fertilization (Stith et al., 1993), and entails hydrolysis of phosphatidylinostitol (4,5)-bisphosphate by the action of a phospholipase C (PLC; Rebecchi and Pentyala, 2000). During mammalian fertilization, a sperm-specific PLC isoform, PLCζ (Saunders et al., 2002), is reportedly responsible for IP3 production.
During oocyte maturation, the capacity of IP3R1s to mediate Ca2+ release and the oocytes’ ability to mount [Ca2+]i oscillations are enhanced (Terasaki et al., 2001; Jones et al., 1995a). For instance, prior to maturation, at the germinal vesicle (GV) stage, oocytes display low amplitude spontaneous [Ca2+]i rises (Jones et al., 1995a), and show dampened responses to IP3 (Fujiwara et al., 1993). Nonetheless, IP3R1 sensitivity, which we define as the receptor’s ability to conduct Ca2+ in response to IP3, incrementally increases and becomes maximal by the time of fertilization (Fujiwara et al., 1993), which in mammals and in Xenopus eggs takes place at the metaphase of meiosis II (MII). Therefore, in eggs, maximal IP3R1 sensitivity and maximal ability to mount [Ca2+]i oscillations coexist. Fittingly, after fertilization and with progression into interphase both of these properties decline, suggesting an association between IP3R1-mediated [Ca2+]i oscillations and the M-phase stages of meiosis (Jellerette et al., 2004; Jones et al., 1995b).
Several subcellular events that develop simultaneously during maturation, such as an increased Ca2+ reservoir (Mehlmann and Kline, 1994; Tombes et al., 1992), a redistribution of the ER and IP3R1 (reviewed in Stricker, 2006; Shiraishi et al., 1995; Kume et al., 1997), and an increase in IP3R1 concentration and sensitivity (Fissore et al., 1999; Mehlmann et al., 1996) could explain the enhanced function of IP3R1 in eggs. Furthermore, IP3R1 becomes phosphorylated during maturation (Lee et al., 2006). Research shows that phosphorylation of IP3R1 mostly enhances its Ca2+ release (reviewed in Patterson et al., 2004), and that it can be phosphorylated by numerous kinases, including protein kinase A and protein kinase C (Vermassen et al., 2004; DeSouza et al., 2002), protein kinase G (Koga et al., 1994), Ca2+/calmodulin-dependent protein kinase II (CaMKII) (Ferris et al., 1991), the tyrosine kinases Fyn (Jayaraman et al., 1996) and Lyn (Yokoyama et al., 2002), Rho kinase (Singleton and Bourguignon, 2002) and protein kinase B (Khan et al., 2006). The aforementioned kinases do not display association with the cell cycle and are therefore unlikely to enhance IP3R1 function in MII eggs in a cell cycle-dependent manner. Recent studies on IP3R1, however, have identified phosphorylation consensus sites for Cyclin Dependent Kinase 1 (Cdk1), also known as Maturation Promoting Factor (MPF), and for Extracellular Signal-Regulated Kinase (ERK), also known as Mitogen Activated Protein Kinase (MAPK), both of which are pivotal regulators of oocyte maturation (reviewed in Masui, 2001). In vivo and in vitro studies have shown that Cdk1 (Malathi et al., 2003) and ERK (Lee et al., 2006; Bai et al., 2006), phosphorylate several of these conserved motifs, although their role in IP3R1 phosphorylation during fertilization remains to be demonstrated.
In a previous study (Lee et al., 2006), we used the MPM-2 antibody, which specifically recognizes M-phase phosphoproteins with phosphorylated serine (Ser)/threonine (Thr) next to proline (Pro) (Westendorf et al., 1994; Davis et al., 1983), the basic phosphorylation motif of Cdk and ERK kinases, to examine the possible involvement of M-phase kinases on IP3R1 phosphorylation in oocytes/eggs. We found that IP3R1 first becomes MPM-2 phosphorylated at the time of meiosis resumption, the germinal vesicle breakdown stage (GVBD), and remains phosphorylated at the MII stage. Following fertilization, MPM-2 IP3R1 phosphorylation decreases in a protracted manner, later than the decline in Cdk1 activity but in parallel with the decline in ERK activity. Consistent with this, inhibition of the ERK pathway during maturation reduced MPM-2 IP3R1 phosphorylation in MII eggs as well as IP3R1-mediated [Ca2+]i oscillations (Lee et al., 2006). Although these results suggested a role for the ERK pathway in IP3R1 MPM-2 phosphorylation in eggs, whether ERK is required for the initial phosphorylation and whether it directly phosphorylates the IP3R1 MPM-2 epitope was not elucidated.
Another MPM-2-epitope generating kinase that is activated at the time of meiosis resumption (reviewed in Liu and Maller, 2005b) is Polo-like kinase-1 (Plk1) (do Carmo Avides et al., 2001; Kumagai and Dunphy, 1996). Polo function was first identified in Drosophila melanogaster larvae that showed cellular abnormalities in the organization of the spindle and spindle poles (Sunkel and Glover, 1988). Since then, research shows that Plk1 and its orthologs are serine/threonine kinases that play crucial roles in almost every phase of mitosis and cytokinesis (Lowery et al., 2007; reviewed in Barr et al., 2004). Of relevance to our studies is that Plk1 becomes activated at the onset of oocyte maturation, the GVBD stage, and remains active throughout maturation (Okano-Uchida et al., 2003; Pahlavan et al., 2000; Qian et al., 1998). Moreover, after fertilization, Plk1 becomes de-phosphorylated/inactivated well after extrusion of the second polar body (Pahlavan et al., 2000), which mirrors the profile of IP3R1 MPM-2 reactivity during maturation and after fertilization (Lee et al., 2006). Collectively, these findings raise the prospect that Plk1 may be involved in MPM-2 IP3R1 phosphorylation in eggs. In this study, therefore, our goal was to unravel those kinases commonly associated with oocyte maturation that are responsible for IP3R1 MPM-2 phosphorylation, and elucidate the mechanism by which the ERK pathway regulates this phosphorylation. Our results show that IP3R1 is a novel and hitherto unknown substrate of Plk1, and that the IP3R1 MPM-2 epitope phosphorylation by Plk1 in MII eggs is regulated by the ERK pathway, which controls IP3R1 redistribution and affects Plk1 spindle localization during oocyte maturation.
MATERIALS AND METHODS
Collection of oocytes/eggs and culture conditions
GV and MII eggs were collected from the ovaries and oviducts of 5 to 8 weeks-old CD-1 female mice, respectively, as previously described (Lee et al., 2006). GV oocytes were recovered in HEPES-buffered Tyrode-Lactate solution (TL-HEPES) supplemented with 5 % heat-treated fetal calf serum (FCS; Gibco, Grand Island, NY) and 100 μM 3-isobutyl-1-methylxanthine (IBMX; Sigma, St. Louis, MO; all chemicals from Sigma unless otherwise specified). Oocytes were matured in vitro for 12–16 hr in a humidified atmosphere containing 6% CO2 in Chatot, Ziomek, and Bavister (CZB) medium (Chatot et al., 1990) containing 3 mg/ml polyvinyl alcohol (PVA) at 36.5°C. MII eggs were recovered in TL-HEPES and, after removal of cumulus cells, eggs were transferred into 50 μl drops of KSOM (Potassium Simplex Optimized Medium; Specialty Media, Phillipsburg, NJ) and cultured as above. Animal handling and procedures were approved by the University’s IACUC committee.
Microinjection procedures and preparation of Plk1 mRNA
Oocytes and eggs were microinjected as previously described (Lee et al., 2006). Reagents were diluted in injection buffer [100 mM KCl and 10 mM HEPES (pH= 7.0)], loaded into glass micropipettes by aspiration and delivered into the ooplasm by pneumatic pressure (PLI-100 picoinjector, Harvard Apparatus, Cambridge, MA); each egg received ~3–10 pl (1–3% of the total volume of the egg). Mouse Plk1 cDNA (Accession No. 18817) from mouse testis was amplified by PCR using previously reported primers (Clay et al., 1993) and cloned into the pCS2+ vector (Turner and Weintraub, 1994). A constitutively active (CA) form of the kinase was generated by substituting threonine (T) 210 by aspartate (D) (Jang et al., 2002) using the QuickChange mutagenesis kit (Stratagene, La Jolla, CA); a construct encoding only the polo box domain of Plk1 was produced as described by the same authors and cloned into pCS2+. The sequence encoding for Venus (a gift from Dr. Miyawaki, Riken, Japan to Dr. J. Ito), a yellow fluorescent protein variant (Nagai et al., 2002), was also subcloned into pCS2+. cDNAs were in vitro transcribed using the mMESSAGE mMACHINE capping Kit (Ambion, Austin, TX) and generated mRNAs handled as previously reported (Lee et al., 2006).
Antibodies
Two polyclonal antibodies raised against the same peptide sequence on the C-terminal end of the molecule were used to detect IP3R1. Rbt03 (Parys et al., 1995) was used for western blotting (WB) whereas CT1 (Wojcikiewicz et al., 1994), which was affinity purified, was used for immunolocalization studies. The MPM-2 monoclonal antibody (Upstate, Lake Placid, NY) was used to ascertain IP3R1 phosphorylation as previously reported (Jellerette et al., 2004). Detection of total and phosphorylated forms of Plk1 was accomplished using a cocktail of mouse monoclonal antibodies against mPlk1 (Zymed, San Francisco, CA) and an anti-phospho (T210) Plk1 polyclonal antibody (Rockland Immunochemicals, Gilbertsville, PA), respectively.
Immunoblotting
40 to 50 mouse oocytes/eggs or cell lysates from 0.5 to 6.0 Xenopus eggs were mixed with 2xLaemmli sample buffer (2xSB) (Laemmli, 1970), boiled and loaded onto NuPAGE Novex 3–8% Tris-Acetate gels (Invitrogen, Carlsbad, CA), or onto 7.5% gels made with 120:1 acrylamide:bisacrylamide mixture (Hamanaka et al., 1995). After electrophoresis, proteins were transferred onto nitrocellulose membranes (Micron Separations, Westboro, MA) and successive probing of both MPM-2/IP3R1 and phospho-Plk1/Plk1 was performed as described by our laboratory (Lee et al., 2006). Species-specific horseradish peroxidase-labeled secondary antibodies and chemiluminescence (NEN Life Science Products, Boston, MA) were used according to the manufacturer’s instructions. Each nitrocellulose membrane was digitally captured and quantified using an imaging system (Kodak Imaging Station 440 CF, Rochester, NY); quantification was performed on the TIFF files before any rendering was carried out and was performed only between lanes from the same blot membrane. The relative intensities of MPM-2 and phospho-Plk1 immunoreactive bands and of phosphorylated substrate bands in kinase assays were plotted using Sigma Plot (Jandel Scientific Software, San Rafael, CA) using MII eggs as the normalizing value. Figures were prepared using ImageJ software (NIH; http://rsb.info.nih.gov/ij/) and Microsoft PowerPoint. To improve the order of presentation, some lane(s) within the same membrane were cut and pasted and two short parallel lines denote this action.
Immunofluorescence
Oocytes/eggs were prepared for immunofluorescence (IF) by removing the zona pellucida (acid Tyrode solution (pH= 2.7)) followed by washes in 0.1% BSA supplemented Dulbecco’s PBS (DPBS-BSA). Cells were fixed in 3.7% paraformaldehyde + 0.02% Triton X-100, permeabilized with 0.1% Triton X-100 DPBS-BSA, and blocked in 5% normal goat serum-DPBS. Primary antibodies, mPlk1 antibodies and the CT1 antibody were incubated overnight at 4°C. Secondary antibodies were Alexa fluor 488 goat anti-mouse IgG and Alexa fluor 555 goat anti-rabbit IgG (Molecular Probes, Eugene, OR), respectively, and were incubated for 1hr at room temperature (RT). Fixed specimens were mounted with Vectashield Mounting Medium (Vector Laboratories, Burlingame, CA). Slides were examined at RT with a confocal laser-scanning microscope (510 META, Carl Zeiss Microimaging, Inc., Germany) using an Axiovert 2 microscope outfitted with a 63x 1.4 NA oil immersion objective lens. Z-stack images were collected from cortical to equatorial planes every 2 to 5 μm and exported as TIFF files using Zeiss software, and TIFF files were processed using Photoshop (Adobe) and assembled in PowerPoint (Microsoft). Negative control eggs were incubated without the primary antibody but with both secondary antibodies, or with the primary antibody (CT1) pre-treated with the antigenic peptide (10 μg/ml).
Preparation of pharmacological inhibitors
Wortmannin (WTMN), a commonly used PI 3-kinase inhibitor was used to inhibit Plk1 (Liu et al., 2005; Liu et al., 2007). Wortmannin was dissolved in DMSO and further diluted in CZB-PVA. Wortmannin-supplemented medium was replaced every 4 hr (Kimura et al., 1994). U0126 (Calbiochem, San Diego, CA), a MEK-specific inhibitor, was prepared in DMSO and used at 25 μM; the inactive analog U0124 was used as a negative control. Doxorubicin (DXR) was diluted in H2O and used at 1 μM in CZB-PVA (Jurisicova et al., 2006).
Histone 1 (H1) and myelin basic protein (MBP) kinase assays and kinase inhibitors
The activities of Cdk1 and ERK were assayed using lysates from 5 oocytes as described previously (Lee et al., 2006). Proteins were separated on 15% SDS-polyacrylamide gels, and H1 and MBP phosphorylations visualized by autoradiography. Autoradiographs were scanned and quantified as described for WB.
Preparation of Xenopus eggs/zygotes lysates and immunoprecipitation
Xenopus eggs were collected from mature females and after in vitro fertilization, as per standard protocols. For IP experiments, groups of 25 eggs were frozen on dry ice and solubilized with 500 μl cold embryo solubilization buffer containing 1.0% Triton X-100 (Cousin et al., 2000). After pelleting cellular debris, supernatants were incubated overnight at 4°C with non-immune serum, Rbt03 antibody, MPM-2 antibody or Plk1 antibody with head-over-head rotation. Incubation of protein A sepharose beads (Amersham) with immunocomplexes occurred for an additional 3 hr before several washes with PBS. Samples were denatured by addition of 2xSB and stored at −80°C until WB was performed.
Statistical analysis
Values from three or more experiments performed on different batches of oocytes, eggs or zygotes were used for evaluation of statistical significance. Statistical comparisons of band intensities after WB and kinase assays were performed using the Student’s t-test or one-way ANOVA and, if differences were found between groups, they were resolved using the Tukey/Kramer test using the JMP-IN software (SAS Institute, Cary, NC). Differences were considered to be significant when P<0.05. Significance among groups/treatments is denoted in bar graphs by different superscripts (WB) or by the presence of one or two asterisks (kinase assays).
RESULTS
The ERK pathway is not required for the initial IP3R1 MPM-2 epitope(s) phosphorylation during oocyte maturation
In a previous study we found that the IP3R1 MPM-2 phosphorylation during oocyte maturation was greatly diminished when oocytes were matured to the MII stage in the presence of the MEK inhibitor U0126 (Lee et al., 2006); these results implicate the ERK pathway in IP3R1 phosphorylation at the MPM-2 epitope(s). To determine whether the ERK pathway is required for IP3R1 MPM-2 phosphorylation at earlier stages, we cultured oocytes in the presence or absence of U0126 and collected samples for WB 8 hr after the initiation of maturation, the time at which oocytes had reached the metaphase stage of meiosis I (MI). Oocytes at the GV stage were devoid of IP3R1 MPM-2 reactivity and, as they matured, they became phosphorylated regardless of the presence of U0126 (Fig. 1A, upper panel), suggesting that the ERK pathway is not required for the initial MPM-2 IP3R1 phosphorylation; probing for IP3R1 after stripping of the membranes showed that receptor concentrations during this period remained stable (Fig. 1A., lower panel). Besides confirming that U0126 prevented ERK activity in these oocytes (data not shown), oocytes were also matured to the MII stage in the presence of U0126, replicating our previous experiments (Lee et al., 2006). Not unexpectedly, this procedure greatly decreased IP3R1 MPM-2 reactivity in MII eggs (Fig. 1B, upper panel) without affecting IP3R1 concentrations (Fig. 1B, lower panel); this last experiment was performed in the presence of colcemid to avoid the exit of metaphase stage that is known to occur in oocytes in which the ERK pathway is suppressed during maturation (Araki et al., 1996). Together, these results show that the ERK pathway is required to maintain IP3R1 MPM-2 phosphorylation during the MI to MII transition, but it is not necessary earlier during maturation to establish IP3R1 phosphorylation at the MPM-2 epitope(s).
Fig. 1. MPM-2 phosphorylation of IP3R1 is regulated independently of the ERK pathway during the early stages of maturation.
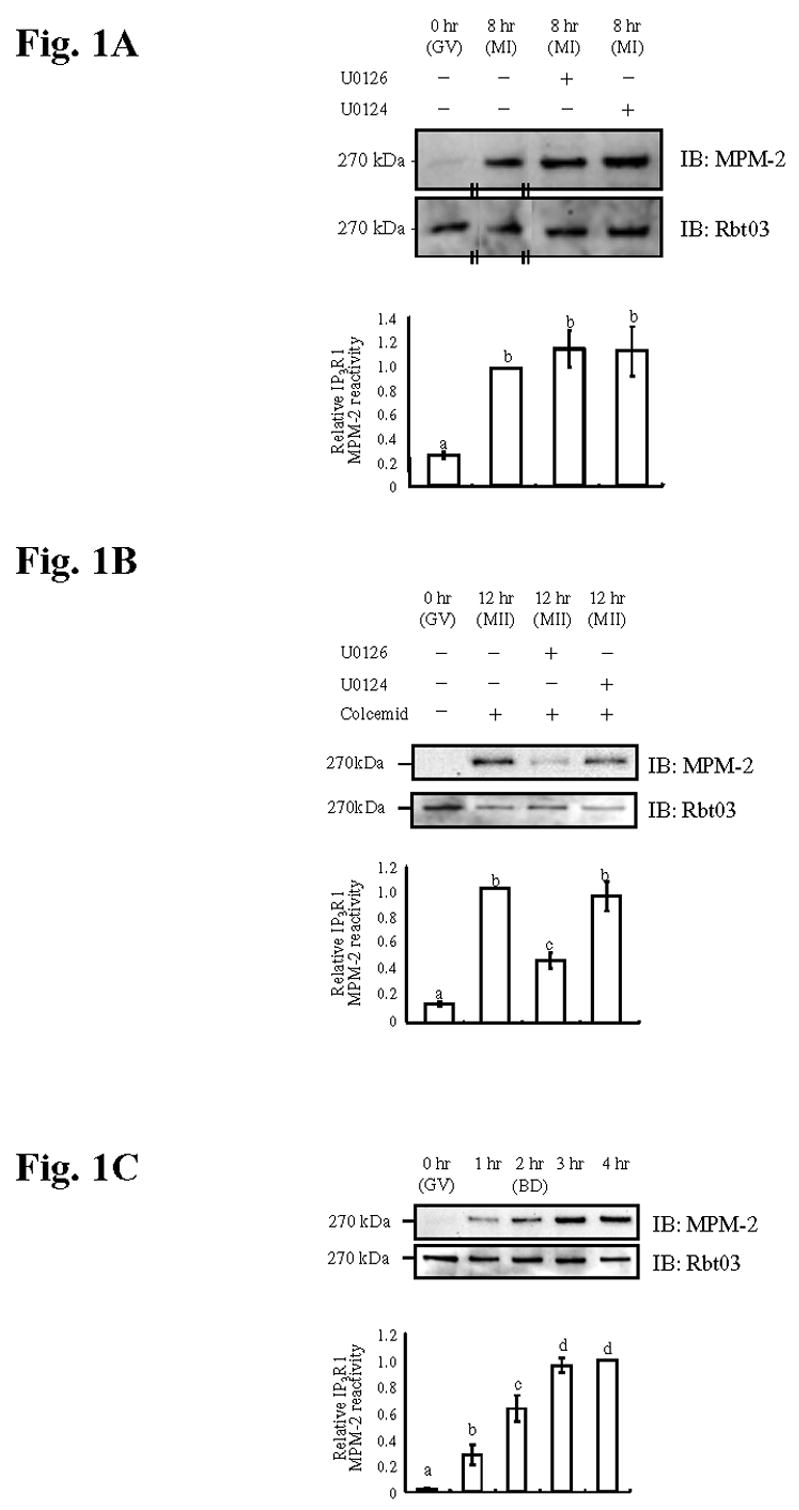
(A) Immunoblotting of oocyte lysates collected at GV (0hr), MI (8hr) and MI (8hr) after treatment with U0126 or U0124 for the indicated times and probed with the MPM-2 antibody (upper panel) and, after stripping of the blot, with the IP3R1 antibody Rbt03 (lower panel). Quantification of IP3R1 MPM-2 reactivity is shown in the graph below the immunoblotting panels. Data are presented as means ± s.e.m., and bars with different superscripts are significantly different (P<0.05) both here and elsewhere. The pair of short parallel lines indicates a lane that was moved from within the same blot. (B) Immunoblotting of lysates of GV, MII (12hr) eggs in the presence of colcemid and treated with U0126 or U0124 and (C) of oocytes cultured for 0 (GV), 1, 2 (BD), 3 and 4hr were probed as described above. For B, similar results were observed without colcemid.
To gain insight into the kinase responsible for IP3R1 MPM-2 phosphorylation, we investigated when this phosphorylation first occurred during oocyte maturation. The first noticeable IP3R1 MPM-2 immunoreactivity occurred 1 hr after initiation of maturation, continued to increase until the 2nd hr, the time at which all oocytes had undergone GVBD, and reached maximal levels by 3 hr (Fig. 1C; upper panel), all this time ERK activity remains basal (Verlhac et al., 1994) and the levels of IP3R1 remained unchanged (Fig. 1C; lower panel). The results therefore show that IP3R1 MPM-2 phosphorylation is set early in oocyte maturation prior to ERK activation.
IP3R1 MPM-2 phosphorylation during oocyte maturation coincides with activation of Plk1
The early appearance of IP3R1 MPM-2 reactivity in oocytes leads to the expectation that the responsible kinase(s) must play a role early in oocyte maturation. Plk1 has been implicated in the G2-M transition in oocytes by virtue of phosphorylating and activating Cdc25C (Qian et al., 1998; Kumagai and Dunphy, 1996), the phosphatase responsible for Cdk1 activation, which in turn orchestrates the initiation of oocyte maturation (Nebreda and Ferby, 2000). Further, Plk1, or its Xenopus homologue Plx1, has been shown to generate MPM-2 reactive epitopes on target proteins (do Carmo Avides et al., 2001; Kumagai and Dunphy, 1996). To ascertain Plk1 activity in oocytes and zygotes, we evaluated two properties of Plk1 that correspond with its activation. First, because Plk1 activation coincides with its phosphorylation (Mundt et al., 1997), which in turn retards its migration during SDS-polyacrylamide gel electrophoresis (Hamanaka et al., 1995), we examined whether or not Plk1 undergoes mobility shifts during oocyte maturation and after egg activation. Second, specific phosphorylation on Thr210 of Plk1 in mammals, or the equivalent Thr201 residue of Plx1 in Xenopus, is indispensable for activation and in vitro kinase activity of Plk1 (Jang et al., 2002; Qian et al., 1999); we therefore analyzed phosphorylation at this residue using an anti-phospho (Thr210) Plk1 antibody. By either method, Plk1 appeared dephosphorylated and therefore inactive at the GV stage, but showed initial signs of phosphorylation by 1 hr of maturation and became progressively more phosphorylated for the next 3 hr of culture (Fig. 2A). Thereafter, Plk1 remained phosphorylated until the MII stage (Fig. 2B, left and center panels), although it underwent dephosphorylation after exposure to SrCl2, which induced egg activation and PN formation (Fig. 2B, right panel). Our results therefore show that the profiles of Plk1 activity and IP3R1 MPM-2 reactivity in oocytes and zygotes closely correspond.
Fig. 2. IP3R1 MPM-2 reactivity and Plk1 phosphorylation correspond during oocyte maturation and the early zygotic stage.
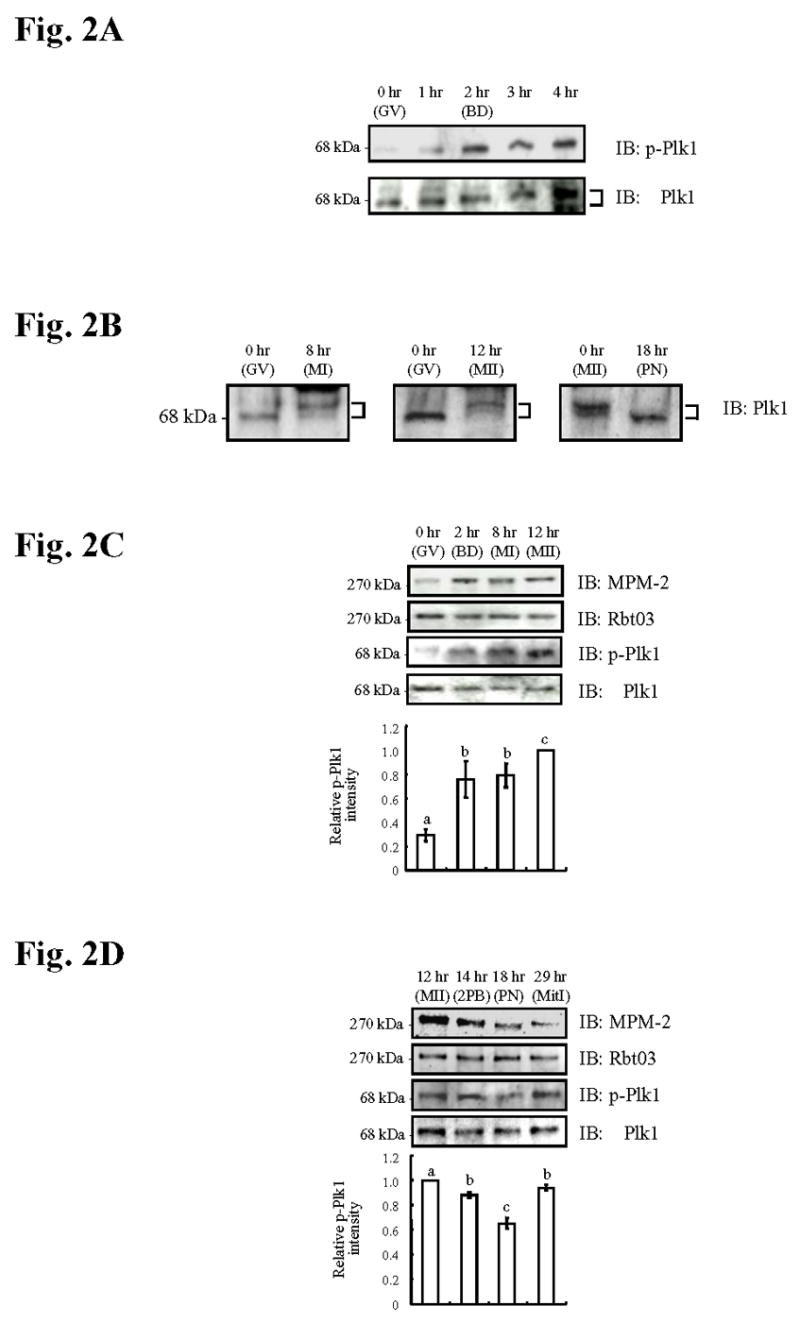
(A) Immunoblotting of oocyte lysates collected at 0 (GV), 1, 2 (BD), 3 and 4 hr probed with a phospho-Plk1 (p-Plk1) antibody (upper panel) and, after stripping, re-probed with a Plk1 antibody (lower panel). (B) Immunoblotting of lysates of GV (0hr), MI (8hr), MII (12hr) oocytes and PN (18hr) zygotes were probed with a Plk1 antibody. In A and B, proteins were separated on 7.5% gels made with 120:1 acrylamide:bisacrylamide mixture, and upper arm of the bracket at the right side of the blot points to the phosphorylated Plk1 variant. (C) Immunoblotting of lysates of GV, BD, MI and MII oocytes and (D) of MII (12hr), 2PB (14hr), PN (18hr) and MitI (29hr) eggs/zygotes show MPM-2 reactivity (upper panel) and p-Plk1 reactivity (3th panel). After stripping, IP3R1 reactivity (Rbt03; 2nd panel) and Plk1 reactivity (lower panel) was demonstrated. p-Plk1 intensity is shown in the graph below the immunoblotting panels.
To more precisely determine whether IP3R1 MPM-2 epitope phosphorylation and Plk1 phosphorylation/activity temporally overlap, we simultaneously examined IP3R1 MPM-2 reactivity and Thr210 Plk1 phosphorylation in the same groups of oocytes, eggs and zygotes. GV stage oocytes were devoid of both MPM-2 reactivity and phosphorylated Plk1 although, by the time of GVBD and through the MI and MII stages, high IP3R1 MPM-2 reactivity was accompanied by increased levels of phospho-Plk1 reactivity (Fig. 2C); neither protein showed alteration in total concentrations (Fig. 2C). Following egg activation, both IP3R1 MPM-2 and phospho-Plk1 reactivity decreased until the time of PN formation (Fig. 2D), although, while MPM-2 IP3R1 reactivity remained low at mitosis I, phospho-Plk1 reactivity rebounded (Fig. 2D). Collectively, these results show that throughout meiosis and the early zygote stage, IP3R1 MPM-2 reactivity corresponds with Plk1 phosphorylation/activity suggesting a role for Plk1 in IP3R1 MPM-2 phosphorylation.
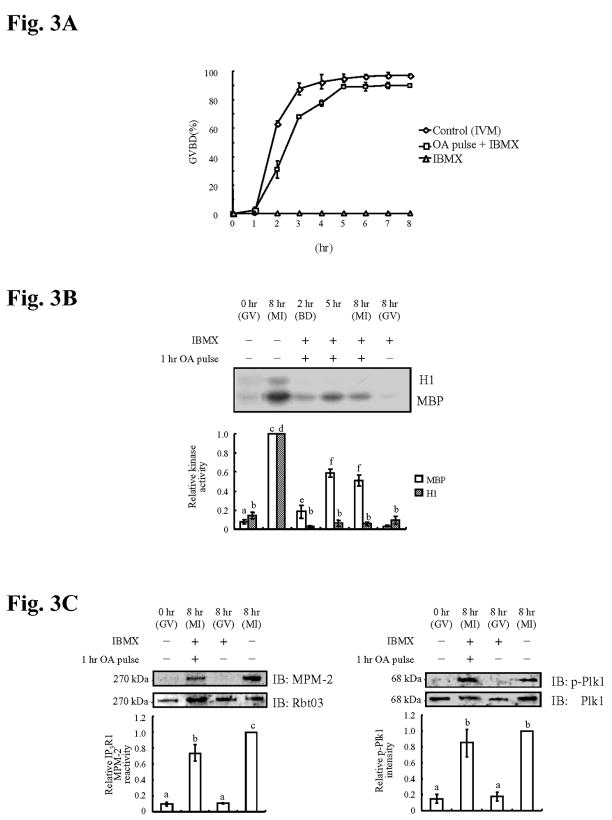
IP3R1 MPM-2 phosphorylation occurs in oocytes matured in the absence of CDK1 activity
Cdk1/MPF activity also operates early during oocyte maturation (reviewed in Masui, 2001). Thus, to discern the possible impact of this kinase on IP3R1 MPM-2 reactivity, we induced oocyte maturation while precluding Cdk1/MPF activity. Accordingly, oocytes were incubated with IBMX, a phosphodiesterase inhibitor that maintains the GV arrest, and treated for 1 hr with okadaic acid (OA), a phosphatase inhibitor; it is known that under these conditions oocytes undergo GVBD in the presence of negligible levels of Cdk1 activity (Phillips et al., 2002; de Vantery Arrighi et al., 2000). Our results indeed show that IBMX-imposed GV arrest can be relieved by OA treatment (Fig. 3A), and that resumption of meiosis is accomplished in the absence of Cdk1 activity as evidenced by the lack of H1 phosphorylation (Fig. 3B). Even under these conditions, IP3R1s showed near normal levels of MPM-2 reactivity (Fig. 3C, left panel), suggesting that a kinase other than Cdk1 is responsible for this phosphorylation. While ERK undergoes robust and premature activation in IBMX-OA-treated oocytes (Fig. 3B), given our previous results, it is unlikely that it would be responsible for the IP3R1 MPM-2 reactivity. Moreover, treatment of somatic cells with OA has been shown to lead to Plk1 activation in an ERK pathway-dependent manner (Liu et al., 2004a). Consistent with this observation, IBMX-OA-treated oocytes showed maximal Plk1 activation (Fig. 3C, right panel). Together, our results show that Cdk1 is not responsible for the IP3R1 MPM-2 phosphorylation in oocytes and that, instead, Plk1 activity (as indicated by the p-Plk1 levels) seems more intimately associated with this phosphorylation.
Fig. 3. Completion of GVBD and IP3R1 MPM-2 reactivity occur in the absence of Cdk1 activity. OA activates ERK and Plk1 in the presence of IBMX.
(A) Percentage (%) of oocytes (control, 1hr OA pulse + IBMX, and IBMX) that underwent GVBD at different times of in vitro maturation; at least 30 oocytes examined per treatment and time point. (B) Kinase assays. H1 and MBP phosphorylation indicate Cdk1 and ERK activities, respectively. Oocytes were cultured for 0, 2, 5, 8hr ± IBMX and ± 1hr OA pulse. Quantification of kinase activities is shown below the autoradiograph. BD under the 2 hr time point stands for GVBD. (C) Immunoblotting of oocyte lysates collected at 0hr (GV), 8hr (MI) treated with IBMX + 1hr OA pulse, 8hr (GV) with IBMX and 8hr control (MI) and probed with MPM-2 antibody (left panel) and p-Plk1 antibody (right panel) and after stripping re-probed with IP3R1 (Rbt03) and Plk1 antibodies, respectively. Quantification of IP3R1 MPM-2 reactivity and p-Plk1 intensity is shown in the graph below the immunoblotting panels.
Pharmacological inhibition of Plk1 activation precludes IP3R1 MPM-2 phosphorylation
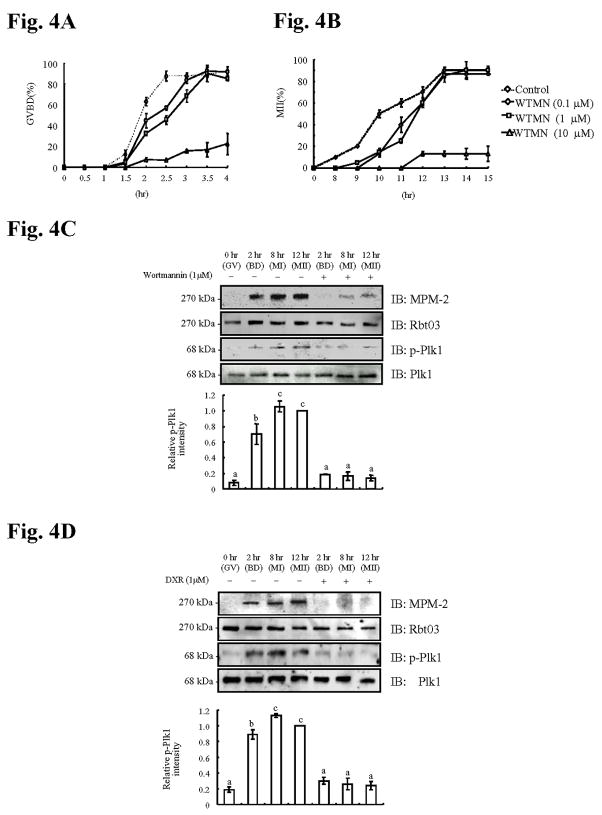
Two pharmacological inhibitors were used to ascertain whether IP3R1 MPM-2 phosphorylation requires active Plk1. The first inhibitor tested was wortmannin, a well-known and broadly used phosphatidylinositol 3-kinase (PI3K) inhibitor (Cross et al., 1995). Research shows that at the concentrations routinely used to block PI3K in somatic cells, wortmannin also efficiently inhibits Plk1 activity (Liu et al., 2005). We therefore examined whether wortmannin was effective at blocking Plk1 activity in oocytes. First, a concentration-dependent study was performed to investigate the effects of wortmannin on meiotic progression. Wortmannin delayed initiation of GVBD (Fig. 4A) and progression to the MII stage in a concentration-dependent manner (Fig. 4B), although 0.1 and 1 μM wortmannin did not affect the total number of oocytes completing maturation (Fig. 4B); 10 μM wortmannin affected both progression to GVBD and MII and, therefore, it was not used further (Figs. 4A, B). Consistent with the effect on meiotic progression, in vitro kinase assays revealed that 1 μM wortmannin delayed activation of Cdk1 and ERK, although both kinase activities fully recovered by the time oocytes had reached the MII stage (data not shown). Remarkably, under similar conditions, wortmannin prevented phosphorylation and activation of Plk1, and the acquisition of MPM-2 reactivity by IP3R1 (Fig. 4C). As the PI3K/PKB pathway plays a role in meiotic progression (Hoshino et al., 2004) the latter effects of wortmannin could in theory be attributed to its inhibition of PI3K activity. Nevertheless, this is unlikely given that during oocyte maturation PKB activation is transient (Kalous et al., 2006), and that its phosphorylation consensus motif (Lawlor and Alessi, 2001) differs greatly from the MPM-2 epitope.
Fig. 4. Inhibition of Plk1 activity by Wortmannin (WTMN), or Doxorubicin (DXR) abolishes IP3R1 MPM-2 reactivity and delays progression to GVBD and MII.
Percentage of oocytes (control, 0.1, 1 or 10μM WTMN) that underwent progression to GVBD (A) and MII (B) during maturation; at least 30 oocytes were examined per concentration and time point. Immunoblotting of oocyte lysates collected at 0, 2, 8 and 12h ± 1μM WTMN (C) or ± 1μM DXR (D) were probed with MPM-2 (upper panel) antibody and p-Plk1 antibody (3th panel) and, after stripping, re-probed with IP3R1 antibody (Rbt03; 2nd panel) and Plk1 (lower panel), respectively. Relative p-Plk1 intensity is shown for both (4C and 4D) in the graphs below the immunoblotting panels.
The second inhibitor used was the anti-tumor antibiotic doxorubicin, which induces DNA damage and triggers the DNA-damage checkpoint in somatic cells, reportedly inactivating and dephosphorylating Plk1 (Jang et al., 2007). We therefore examined the effect of 1μM doxorubicin on maturation-associated IP3R1 MPM-2 phosphorylation. Although doxorubicin -treated oocytes resumed and progressed through meiosis, doxorubicin inhibited IP3R1 MPM-2 phosphorylation as well as the occurrence of phospho-Plk1 at all of the time points examined (Fig. 4D). Together, the data provide evidence that active Plk1 is required for IP3R1 MPM-2 phosphorylation during oocyte maturation.
Molecular manipulations of Plk1 influence IP3R1 MPM-2 phosphorylation
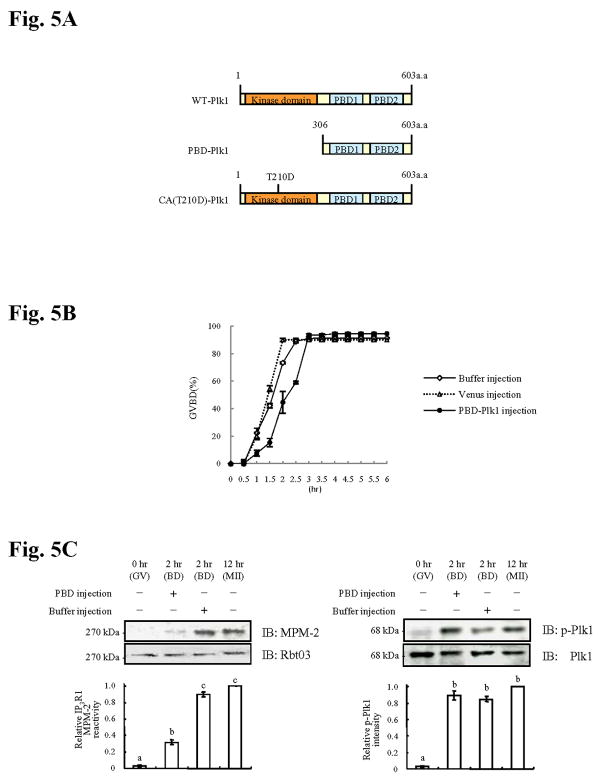
Plk1, like other family members, consist of a canonical Ser/Thr kinase domain at the N terminus and two signature motifs, the polo boxes, at the C terminus, which form the polo box domain (PBD) (reviewed in Barr et al., 2004; Kumagai and Dunphy, 1996) (Fig. 5A). The PBD has been implicated in the regulation of the catalytic activity of the enzyme as well as in the targeting of Plks to specific cellular structures (Lee et al., 1998). Recent research showed that the PBD acts as a phosphopeptide-binding motif (Elia et al., 2003a) recognizing “primed phosphoepitopes” on Plk1 target proteins. It stands to reason that disruption of PBD function should interfere with the progression/completion of Plk1-controlled cellular events. Consistent with this notion, over-expression of a kinase dead Plx1 protein delayed activation of Cdc25C and initiation of GVBD in Xenopus oocytes (Liu et al., 2004b). Accordingly, we over-expressed in mouse oocytes a PBD-Plk1 mRNA to compete with endogenous Plk1 (PBD-Plk1; Fig. 5A). We found that while injection of PBD-Plk1 mRNA markedly delays GVBD, it does not affect the percent of oocytes that are able to complete this process (Fig. 5B). More specifically, we observed that while ~40% of buffer control injected oocytes or of the oocytes injected with mRNA encoding for the Venus fluorescent protein had undergone GVBD by 1.5 hr of maturation and nearly all oocytes had undergone GVBD by 2.5 hr, only 5% and 50%, respectively, of PBD-Plk1 mRNA injected oocytes had completed GVBD at those times of maturation (Fig. 5B). Injection of PBD-Plk1 mRNA also greatly prevents IP3R1 MPM-2 phosphorylation by 2 hr of maturation (Fig. 5C, left panel), although it did not affect the levels of phospho-Plk1 (Fig. 5C, right panel) or the total amounts of IP3R1 and Plk1 proteins (Fig. 5C, lower panels).
Fig. 5. Overexpression of PBD-Plk1 delays GVBD and inhibits IP3R1 MPM-2 reactivity.
(A) Schematic overview of the different Plk1 constructs. Wild type Plk1 (WT-Plk1) consists of a kinase domain and two Polo Boxes. PBD-Plk1 encodes only for the 2 Polo Boxes, and the CA Plk1 has residue Thr 210 substituted by Asp (CA(T210D)-Plk1). (B) Percentage of oocytes injected with buffer, venus mRNA (control) or with PBD-Plk1 mRNA that underwent GVBD after initiation of maturation; at least 20 oocytes were examined per group and time point. (C) Immunoblotting of oocyte lysates obtained after injection with PBD-Plk1 mRNA or buffer and collected at GVBD (2hr) or from buffer-injected GV and MII oocytes. Immunoblottings were probed with MPM-2 (left panel) or p-Plk1 antibody (right panel) and after stripping with IP3R1 (Rbt03) or Plk1 antibody, respectively. Quantification of MPM-2 or p-Plk1 intensity is shown in graph below the immunoblotting.
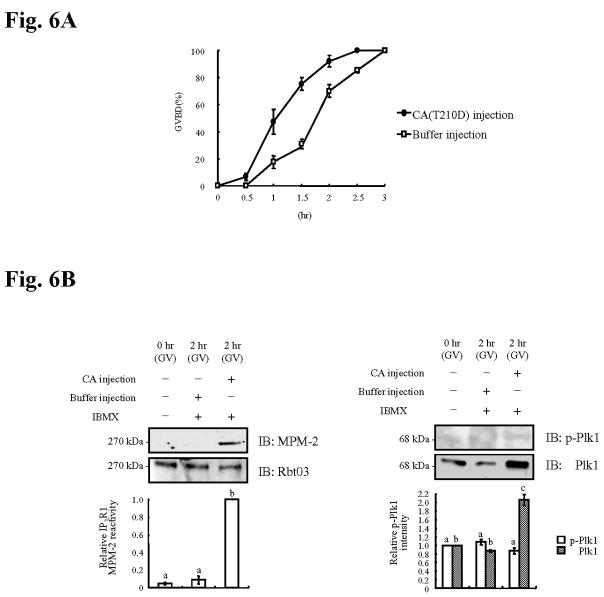
To further establish the role of Plk1 on IP3R1 phosphorylation, we over-expressed the constitutively active (CA) form of Plk1, CA(T210D)-Plk1 (Fig. 5A) (Jang et al., 2002). Previously, injection of a similar construct into Xenopus oocytes induced premature activation of Cdc25C and GVBD (Qian et al., 1999). Similarly, injection of the CA(T210D)-Plk1 mRNA into mouse oocytes evoked premature GVBD (Fig. 6A). We next examined whether CA(T210D)-Plk1 mRNA injection induced IP3R1 MPM-2 epitope phosphorylation. To circumvent the confounding effect of phosphorylation by endogenous kinases, all oocytes were kept in IBMX-supplemented media such that GVBD and activation of Cdk1 and ERK activities were precluded (de Vantery Arrighi et al., 2000). In the presence of IBMX, all groups remained at the GV stage (Fig. 6B). Nonetheless, despite the arrest at the GV stage, CA(T210D)-Plk1-injected oocytes showed significant IP3R1 MPM-2 reactivity, which was not the case for buffer injected oocytes (Fig. 6B, left panel). Injection of CA(T210D)-Plk1 mRNA did not alter the expression of IP3R1 or phospho-Plk1, although it increased the total amount of Plk1, demonstrating that the CA(T210D)-Plk1 mRNA underwent in vivo translation in the oocyte (Fig. 6B, right panel). Collectively, these data suggest that manipulation of Plk1 activity influences IP3R1 MPM-2 phosphorylation, directly implicating this kinase in IP3R1 phosphorylation.
Fig. 6. Overexpression of CA(T210D)-Plk1 accelerates GVBD and induces IP3R1 MPM-2 reactivity.
(A) Percentage of oocytes (buffer-injected and injected with CA(T210D)-Plk1 mRNA) that underwent GVBD after initiation of maturation; at least 20 oocytes were examined per group and time point. (B) Immunoblotting of lysates from uninjected GV oocytes, or from GV oocytes injected with CA(T210D)-Plk1 mRNA or buffer (all in IBMX) and collected at 2hr. Immunoblottings were probed with MPM-2 (left panel) or p-Plk1 antibody (right panel) and after stripping with IP3R1 (Rbt03) or Plk1 antibody, respectively. Quantification of MPM-2 or p-Plk1/Plk1 intensity is shown in graph below the immunoblotting.
IP3R1 and Plx1 interact in a cell cycle-stage preferential manner in Xenopus eggs/zygotes
We next performed immunoprecipitation (IP) studies to investigate whether Plk1 and IP3R1 interact. For these studies we chose Xenopus egg extracts because of the large availability of material in this species, and because in Xenopus eggs Plx1 also becomes dephosphorylated/inactivated after fertilization and transition into interphase (Descombes and Nigg, 1998) (Fig. 7A). IP of Xenopus MII egg extracts using an anti-IP3R1 antibody brought down Plx1 (Fig. 7B, left part), demonstrating direct association of these two molecules; IP of the same extracts using non-immune serum (far left lane) or with beads alone (far right lane) failed to do so. We next examined whether this interaction occurred in a cell cycle-preferential manner. We found that association of IP3R1 and Plx1 was higher at the MII stage than at the interphase stage (Fig. 7B, bar graphs), which is similar to the preferential association between Plk1 and other substrates during M-phase stages of the cell cycle (Elia et al., 2003b). In addition, we found that the Plk1 antibody precipitated equal amounts of Plx1 during the cell cycle (Fig. 7B), suggesting that Plx1 concentrations remain stable in zygotes and therefore, per se, cannot account for the cell cycle-specific IP3R1 MPM-2 phosphorylation. Together, these results suggest that Plk1 binds and phosphorylates IP3R1 in vertebrate oocytes/eggs in a cell cycle preferential manner.
Fig. 7. Plx1 is active and interacts maximally with IP3R1 in MII Xenopus egg extracts.
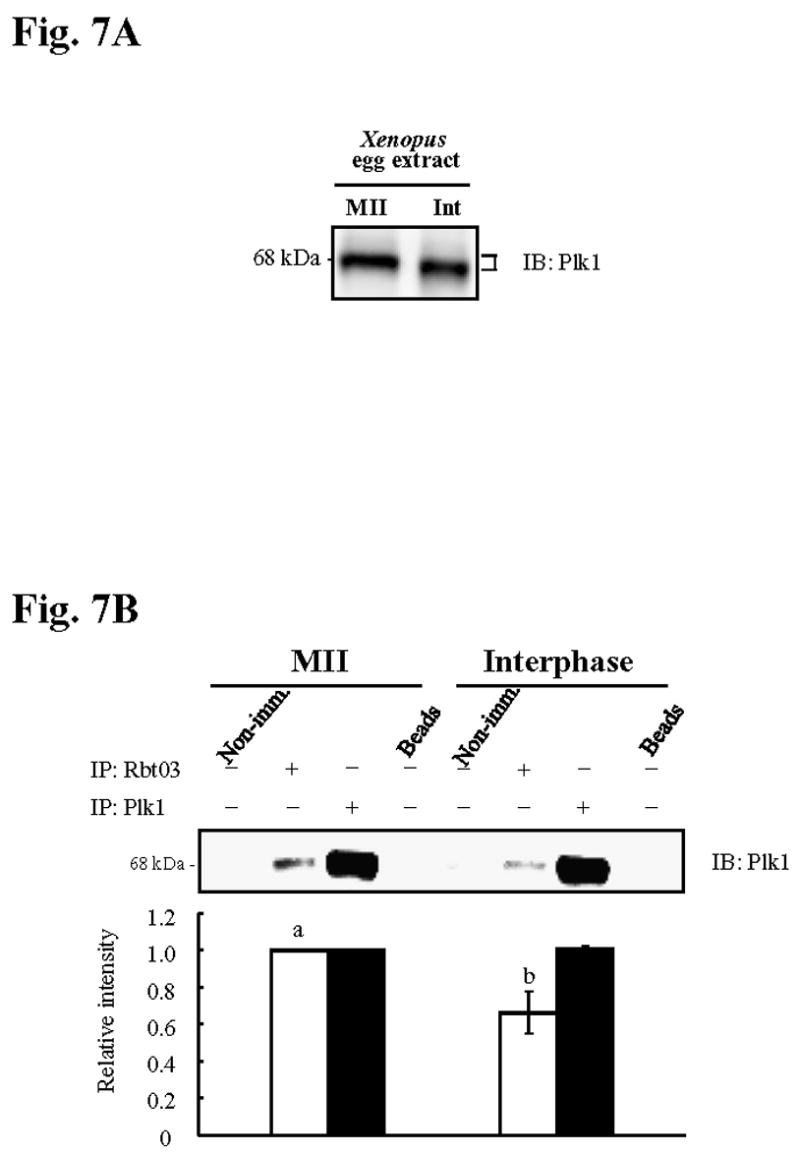
(A) Immunoblotting of MII and Interphase (Int) extracts of Xenopus eggs probed with Plk1 antibody. Arms of the bracket at the right of immunoblotting denote upper and lower location of Plx1 bands (B) IP of Plx1 (~68kDa) with IP3R1 (Rbt03; lanes 2 and 6) or Plk1 antibody (lanes 3 and 7) in both MII (left) and Int (right) extracts; IP with non-immune (Non-imm) serum (lanes 1 and 5) or beads alone (lanes 4 and 8) were used as controls. Quantification of Plx1 after IP is shown in graph below the immunoblotting.
Inactivation of the ERK pathway prevents IP3R1 cortical cluster formation and MPM-2 IP3R1 phosphorylation at the MII stage
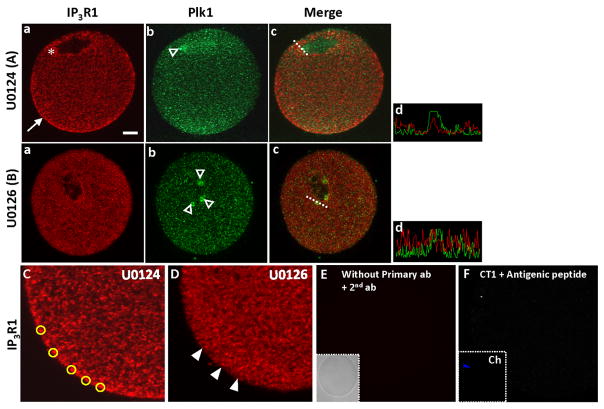
While our data collectively show that Plk1 is the IP3R1 MPM-2 generating kinase, the finding that abrogation of the ERK pathway inhibits IP3R1 MPM-2 phosphorylation after the MI stage suggests that the ERK pathway has another, novel regulatory role on IP3R1 phosphorylation. For this purpose, we examined whether its inhibition prevented phosphorylation/activation of Plk1. Contrary to previous observations in starfish oocytes (Okano-Uchida et al., 2003), inactivation of the ERK pathway using U0126 did not affect Plk1 phosphorylation (data not shown). We therefore examined whether inactivation of the ERK pathway might affect IP3R1 and Plk1 localization, and by doing so might compromise receptor phosphorylation. We considered this possibility because research shows that the ERK pathway is required for the egg’s cortical reorganization (Deng et al., 2005), for the migration of the meiotic spindle to the oocyte cortex (Verlhac et al., 2000), and because several ERK substrates contribute to maintain the configuration of the MII spindle (Terret et al., 2003; Lefebvre et al., 2002). Given that IP3R1 attains prominent cortical cluster organization in MII eggs (Melhmann et al., 1996; Shiraishi et al., 1995) and that Plk1 accumulates to the spindle poles in these cells (Wianny et al., 1998), it is possible that abrogation of the ERK pathway during maturation might interfere with the normal distribution of these molecules and therefore decrease their functional association. Accordingly, oocyte maturation in the presence of U0124 did not alter IP3R1 distribution, cortical cluster formation (Fig. 8Aa; arrows; C; circles), or accumulation of Plk1 to the spindle poles (Fig. 8Ab; arrowheads). Nevertheless, oocyte maturation in the presence of U0126 diminished the numbers of IP3R1 clusters in the ooplasm and prevented IP3R1 cortical cluster formation (Fig. 8Ba; D; arrowheads); U0126 treatment also altered Plk1 localization, as several additional Plk1 accumulations were seen around the spindle, leading to a generally decreased IP3R1 and Plk1 overlap (Fig. 8Bb, c; and panels displaying signal quantification (Ad and Bd). IP3R1 and Plk1 signals were absent in negative control samples, which were treated without primary antibodies but with both secondary antibodies (E), or with the CT1 antibody pretreated with the appropriate antigenic peptide (F). These results reveal that during maturation the ERK pathway plays a novel regulatory role in the localization of IP3R1 and Plk1, which is essential for IP3R1 MPM-2 phosphorylation in MII eggs.
Fig. 8. The ERK pathway regulates IP3R1 redistribution and formation of IP3R1 cortical clusters during oocyte maturation.
IF confocal images of IP3R1 (a; red; CT1 antibody), Plk1 (b; green), and merged images (c) in MII oocytes treated with U0124 (A, C) or U0126 (B, D). Asterisks and open arrowheads denote accumulations of IP3R1 and Plk1, respectively. White arrows denote IP3R1 cortical clusters (Aa), and closed arrowheads denote their absence in U0126 matured eggs (D). Broken white lines in merged images denote the traces used for intensity quantification of IP3R1 and Plk1 signals (intensity panels on the right side of the figure; Ad, U0124, and Bd, U0126). Negative controls were stained without primary antibody but in the presence of both secondary antibodies (E; inset shows bright field image), or with the CT1 antibody pre-incubated with antigenic peptide (F). Yellow circles encircle representative IP3R1 clusters, which we defined as having an area of ≥0.7μm2 or a diameter of ≥1μm. Ch in (F) denotes chromosomes (DNA staining with TO-PRO-3 (blue)). Scale bar (Aa) indicates 10μm.
DISCUSSION
A consequence of the extensive biochemical and morphological modifications that oocytes of vertebrate species undergo during maturation is an enhancement of the egg’s [Ca2+]i releasing mechanisms required for fertilization. Here we show that one of these modifications involves active MPM-2 epitope(s) phosphorylation of IP3R1, and that Plk1 is the kinase responsible for this phosphorylation. We found that IP3R1 and Plk1 associate in a cell cycle-preferential manner, that they display diffuse distribution throughout the ooplasm, and that they co-localize more prominently on areas around the spindle/spindle poles. We demonstrate that while the ERK pathway is dispensable for IP3R1 MPM-2 phosphorylation during the early stages of maturation, it is required for this phosphorylation at the MII stage. We also determined that the ERK pathway regulates IP3R1 cellular distribution during the late stages of meiosis. Altogether, our results in oocytes show that Plk1 and possibly other M-phase kinases involved in the reinitiation of meiosis are also involved in its completion via regulation of the Ca2+ release channels necessary for fertilization.
Plk1 is the MPM-2 epitope IP3R1 kinase
The precise nature of the molecular mechanisms underlying the increased sensitivity of IP3R1s during oocyte maturation remains to be elucidated. Nonetheless, IP3R1 phosphorylation is likely to play a pivotal role in this process, as it has been associated with increased Ca2+ release in numerous other cell systems (reviewed in Patterson et al., 2004; Krizanova and Ondrias, 2003). In this study we extend our previous findings (Lee et al., 2006) by showing that the temporal development of IP3R1 MPM-2 phosphorylation coincides with the progressive increase in IP3R1 sensitivity that occurs during oocyte maturation (Mehlmann and Kline, 1994; Fujiwara et al., 1993). Because a number of M-phase kinases are activated in close succession during oocyte maturation, we used a combination of pharmacological and molecular reagents to ascertain the kinase responsible for IP3R1 MPM-2 phosphorylation. Here we show that IP3R1 is a novel target for Plk1, that it phosphorylates IP3R1 at an MPM-2 epitope, and that both proteins interact in a cell-cycle preferential manner. The association of Plk1 and IP3R1 is likely to be mediated by the PBD of Plk1, which binds phosphorylated epitopes and reportedly targets Plk1 to its substrates (Elia et al., 2003b). The preferred binding epitope of Plk1 is Ser-pSer/pThr-Pro/X (Elia et al., 2003a), and a Ser-Ser-Pro motif is present in IP3R1 (amino acids 1491–1493). This Ser-Ser-Pro site lies within a larger consensus sequence remarkably reminiscent of the epitope recognized by the MPM-2 antibody (Yaffe et al., 1997), raising the prospect that Plk1 might phosphorylate this site. If this were so, it would imply that Plk1 acts as its own priming kinase, which has been shown to be the case with other Plk1 substrates (Neef et al., 2003). Our finding that injection of CA(T210D)-Plk1 mRNA induces IP3R1 MPM-2 reactivity in the absence of other M-phase kinases supports this possibility. Nonetheless, in the absence of Cdk1 activity, such as it occurs in OA-IBMX induced maturation, IP3R1 MPM-2 phosphorylation is noticeably lower than in control oocytes, suggesting that other kinases assist Plk1-dependent phosphorylation of IP3R1.
Proteins displaying a PBD binding motif are likely to host Plk1 phosphorylation sites (Lowery et al., 2007). The consensus sequence for Plk1 phosphorylation was first described by Nakajima et al. (2003) and consists of the following residues E/D-X-Ser/Thr-ψ-X-D/E, where D/E stand for either of the acidic amino acids and ψ stands for a hydrophobic amino acid; an expanded motif has recently been described by Johnson et al. (2007). Examination of the IP3R1 sequence reveals that it contains three highly conserved Plk1 phosphorylation sites centered on residues Thr1048, Ser1890, and Thr2656. Moreover, several minimal consensus sites for Plk1 (Lowery et al., 2007) are scattered throughout the receptor sequence. It is therefore likely that besides the MPM-2 epitope(s), Plk1 phosphorylates one or several of these sites in oocytes and eggs. Nonetheless, further research will be needed to ascertain how many sites are phosphorylated in vivo by Plk1, and how this affects IP3R1 function. It is worth noting that while Cdk1 or ERK do not phosphorylate the IP3R1 MPM-2 epitope in eggs, they could still act on IP3R1 by phosphorylating their own target sites (Lee et al., 2006; Bai et al., 2006; Malathi et al., 2003). If this were the case, it would underscore the importance of IP3R1 phosphorylation to promote maximal Ca2+ release at fertilization to ensure completion and exit of the meiotic program.
IP3R1 MPM-2 phosphorylation, Plk1 and IP3R1 cellular distribution and the role of ERK
A second mechanism that might underlie the increased IP3R1 sensitivity in oocytes after maturation is the differential redistribution of the receptor that culminates with the formation of conspicuous cortical clusters (Stricker et al., 2006; reviewed in Kline, 2000). Here, we show that the ERK pathway is required for IP3R1 cortical redistribution, as eggs matured in the presence of the MEK inhibitor U0126 were devoid of IP3R1 cortical clusters, although the receptor dispersed from its spindle localization at MI. Inhibition of the ERK pathway also reduced the overlap of Plk1 and IP3R1 signals on the spindle poles, and this might explain, at least in part, the decreased IP3R1 MPM-2 phosphorylation found in these eggs (present data and Lee et al., 2006). Whether the reduced IP3R1 MPM-2 phosphorylation is due to ERK’s ability to impose priming phosphorylation(s) on IP3R1, or is merely due to this pathway’s regulatory role on cytoskeletal/cortical re-organization (Deng et al., 2005; Verlhac et al., 1994) remains to be elucidated. Likewise, whether the reported inability of eggs maturated in the presence of U0126 to mount normal [Ca2+]i oscillations (Matson and Ducibella, 2007; Lee et al., 2006) is due to reduced IP3R1 phosphorylation, or to the absence of cortical clusters or to both remains to be determined. It is worth noting that these effects of U0126 likely reflect specific suppression of the ERK pathway, as during mitosis I, which is naturally devoid of ERK activity, IP3R1s show minor MPM-2 reactivity (this study and Lee et al., 2006), the ER does not organize in cortical clusters (FitzHarris et al., 2003), and zygotes display limited [Ca2+]i oscillatory ability (Jellerette et al., 2004). Therefore, our results provide the framework for future studies to discern the contribution of cortical clusters to IP3R1 sensitivity and [Ca2+]i oscillatory capacity.
Plk1, Ca2+ release, cell cycle progression and cytokinesis
Plk1 plays essential roles in almost every phase of the cell cycle, from promoting G2/M transition in oocytes, to making possible the completion of meiosis after fertilization (Liu and Maller, 2005b; Rauh et al., 2005), to being a critical regulator of mitosis and cytokinesis (Liu and Maller, 2005a; reviewed in Barr et al., 2004). Remarkably, the unfolding of several of these Plk1-regulated events is accompanied by Ca2+ release, and requires activation of Ca2+-dependent kinases. Nevertheless, whether Plk1 function and Ca2+ release are interdependent as well as the molecular underpinning of this association remain unexplored. Here, we offer the first demonstration that Plk1 associates with and phosphorylates IP3R1 thereby providing a direct molecular link between the cell cycle machinery and the Ca2+ release system. For instance, it is well known that the function of Plx1, a Plk ortholog, is required for MII exit in Xenopus eggs (Descombes and Nigg, 1998), although earlier studies had shown that this event also requires Ca2+ release and activation of CaMKII (Lorca et al., 1993). The association between these kinases, however, remained unclear until the recent demonstration that CaMKII introduces a priming phosphorylation for Plx1 on Emi2, the Anaphase Promoting Complex/Cyclosome (APC/C) egg inhibitor (Madgwick et al., 2006; Tung et al., 2005). Following Plx1 phosphorylation, Emi2 is degraded, which is necessary for eggs to escape the MII arrest (Liu and Maller, 2005b; Rauh et al., 2005). It is therefore logical to envision a regulatory role for Plx1 on the Ca2+ channel responsible for the priming phosphorylation of a pivotal substrate. Similarly, the distinct nuclear-associated IP3-mediated [Ca2+] rises observed in syncytial Drosophila embryos (Parry et al., 2005) might be under the regulation of Polo, given that in these embryos as mitosis approaches both the ER (Parry et al., 2005) and Polo (Moutinho-Santos et al., 1998) gather on the spindle poles, which we show are sites of IP3R1 and Plk1 co-localization. Lastly, the demand for Ca2+ release during cytokinesis in Drosophila spermatoocytes (Wong et al., 2005) and in Xenopus embryos (Muto and Mikoshiba, 1998) might also be under a similar regulation, as both the presence and function of Plk1/Polo and IP3R1 are required for cleavage furrow formation and stability (Muto and Mikoshiba, 1998; Mitsuyama and Sawai, 2001; Wong et al., 2005; Santamaria, et al., 2007) and, by this late stage of mitosis, most other M-phase kinases have been inactivated (Neef et al., 2003).
In summary, our results reveal that IP3R1, a widespread Ca2+ channel responsible for the majority of [Ca2+]i release in non-muscle cells is a novel substrate for Plk1, a M-phase kinase with a broad range of functions during meiosis/mitosis and cytokinesis. We propose that IP3R1 phosphorylation by Plk1, and possibly by other M-phase kinases, is responsible for the delivery of spatially and temporally regulated [Ca2+]i signals that ensure faithful progression and completion of meiosis/mitosis and cytokinesis.
Acknowledgments
We thank Ms. Changli He for technical assistance, and Drs. M. Kurokawa and J. Smyth for reading the manuscript. This work was supported in part by a grant HD051872 from the NIH to R.A.F. The Confocal facility was supported by a grant from the National Science Foundation (BBS-8714235). Work in the laboratory of H.D.S. and J.B.P was supported by Research Programs G.0604.07 and G.0210.03 from FWO, and by grant G.O.A. 2004/07 from K.U.Leuven; V.V. was the recipient of a FWO Travel Grant. J.I. was supported in part by a Fellowship of the JSPS for the promotion of Science for Young Scientists. Work in the laboratories of R.W. and D.A. was supported by NIH grants DK49194 and DE 016289, respectively.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Araki K, Naito K, Haraguchi S, Suzuki R, Yokoyama M, Inoue M, Aizawa S, Toyoda Y, Sato E. Meiotic abnormalities of c-mos knockout mouse oocytes: activation after first meiosis or entrance into third meiotic metaphase. Biol Reprod. 1996;55:1315–24. doi: 10.1095/biolreprod55.6.1315. [DOI] [PubMed] [Google Scholar]
- Bai GR, Yang LH, Huang XY, Sun FZ. Inositol 1,4,5-trisphosphate receptor type 1 phosphorylation and regulation by extracellular signal-regulated kinase. Biochem Biophys Res Commun. 2006;348:1319–27. doi: 10.1016/j.bbrc.2006.07.208. [DOI] [PubMed] [Google Scholar]
- Barr FA, Sillje HH, Nigg EA. Polo-like kinases and the orchestration of cell division. Nat Rev Mol Cell Biol. 2004;5:429–40. doi: 10.1038/nrm1401. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium. 2002;32:235–49. doi: 10.1016/s0143416002001823. [DOI] [PubMed] [Google Scholar]
- Chatot CL, Lewis JL, Torres I, Ziomek CA. Development of 1-cell embryos from different strains of mice in CZB medium. Biol Reprod. 1990;42:432–40. doi: 10.1095/biolreprod42.3.432. [DOI] [PubMed] [Google Scholar]
- Clay FJ, McEwen SJ, Bertoncello I, Wilks AF, Dunn AR. Identification and cloning of a protein kinase-encoding mouse gene, Plk, related to the polo gene of Drosophila. Proc Nat Acad Sci USA. 1993;90:4882–4886. doi: 10.1073/pnas.90.11.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin H, Gaultier A, Bleux C, Darribere T, Alfandari D. PACSIN2 is a regulator of the metalloprotease/disintegrin ADAM13. Dev Biol. 2000;227:197–210. doi: 10.1006/dbio.2000.9871. [DOI] [PubMed] [Google Scholar]
- Cross MJ, Stewart A, Hodgkin MN, Kerr DJ, Wakelam MJ. Wortmannin and its structural analogue demethoxyviridin inhibit stimulated phospholipase A2 activity in Swiss 3T3 cells. Wortmannin is not a specific inhibitor of phosphatidylinositol 3-kinase. J Biol Chem. 1995;270:25352–5. doi: 10.1074/jbc.270.43.25352. [DOI] [PubMed] [Google Scholar]
- Davis FM, Tsao TY, Fowler SK, Rao PN. Monoclonal antibodies to mitotic cells. Proc Nat Acad Sci USA. 1983;80:2926–30. doi: 10.1073/pnas.80.10.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vantery Arrighi C, Campana A, Schorderet-Slatkine S. A role for the MEK-MAPK pathway in okadaic acid-induced meiotic resumption of incompetent growing mouse oocytes. Biol Reprod. 2000;63:658–65. doi: 10.1095/biolreprod63.2.658. [DOI] [PubMed] [Google Scholar]
- Deng M, Williams CJ, Schultz RM. Role of MAP kinase and myosin light chain kinase in chromosome-induced development of mouse egg polarity. Dev Biol. 2005;278:358–66. doi: 10.1016/j.ydbio.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Descombes P, Nigg EA. The polo-like kinase Plx1 is required for M phase exit and destruction of mitotic regulators in Xenopus egg extracts. Embo J. 1998;17:1328–35. doi: 10.1093/emboj/17.5.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSouza N, Reiken S, Ondrias K, Yang YM, Matkovich S, Marks AR. Protein kinase A and two phosphatases are components of the inositol 1,4,5-trisphosphate receptor macromolecular signaling complex. J Biol Chem. 2002;277:39397–400. doi: 10.1074/jbc.M207059200. [DOI] [PubMed] [Google Scholar]
- do Carmo Avides M, Tavares A, Glover DM. Polo kinase and Asp are needed to promote the mitotic organizing activity of centrosomes. Nat Cell Biol. 2001;3:421–4. doi: 10.1038/35070110. [DOI] [PubMed] [Google Scholar]
- Ducibella T, Huneau D, Angelichio E, Xu Z, Schultz RM, Kopf GS, Fissore R, Madoux S, Ozil JP. Egg-to-embryo transition is driven by differential responses to Ca2+ oscillation number. Dev Biol. 2002;250:280–91. [PubMed] [Google Scholar]
- Elia AE, Cantley LC, Yaffe MB. Proteomic screen finds pSer/pThr-binding domain localizing Plk1 to mitotic substrates. Science. 2003a;299:1228–31. doi: 10.1126/science.1079079. [DOI] [PubMed] [Google Scholar]
- Elia AE, Rellos P, Haire LF, Chao JW, Ivins FJ, Hoepker K, Mohammad D, Cantley LC, Smerdon SJ, Yaffe MB. The molecular basis for phosphodependent substrate targeting and regulation of Plks by the Polo-box domain. Cell. 2003b;115:83–95. doi: 10.1016/s0092-8674(03)00725-6. [DOI] [PubMed] [Google Scholar]
- Ferris CD, Huganir RL, Bredt DS, Cameron AM, Snyder SH. Inositol trisphosphate receptor: phosphorylation by protein kinase C and calcium calmodulin-dependent protein kinases in reconstituted lipid vesicles. Pro Natl Acad Sci USA. 1991;88:2232–5. doi: 10.1073/pnas.88.6.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fissore RA, Longo FJ, Anderson E, Parys JB, Ducibella T. Differential distribution of inositol trisphosphate receptor isoforms in mouse oocytes. Biol Reprod. 1999;60:49–57. doi: 10.1095/biolreprod60.1.49. [DOI] [PubMed] [Google Scholar]
- FitzHarris G, Marangos P, Carroll J. Cell Cycle-dependent Regulation of Structure of Endoplasmic Reticulum and Inositol 1,4,5-Trisphosphate-induced Ca2+ Release in Mouse Oocytes and Embryos. Mol. Biol. Cell. 2003;14:288–301. doi: 10.1091/mbc.E02-07-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T, Nakada K, Shirakawa H, Miyazaki S. Development of inositol trisphosphate-induced calcium release mechanism during maturation of hamster oocytes. Dev Biol. 1993;156:69–79. doi: 10.1006/dbio.1993.1059. [DOI] [PubMed] [Google Scholar]
- Hamanaka R, Smith MR, O’Connor PM, Maloid S, Mihalic K, Spivak JL, Longo DL, Ferris DK. Polo-like kinase is a cell cycle-regulated kinase activated during mitosis. J Biol Chem. 1995;270:21086–91. doi: 10.1074/jbc.270.36.21086. [DOI] [PubMed] [Google Scholar]
- Hoshino Y, Yokoo M, Yoshida N, Sasada H, Matsumoto H, Sato E. Phosphatidylinositol 3-kinase and Akt participate in the FSH-induced meiotic maturation of mouse oocytes. Mol Reprod Dev. 2004;69:77–86. doi: 10.1002/mrd.20150. [DOI] [PubMed] [Google Scholar]
- Jang YJ, Lin CY, Ma S, Erikson RL. Functional studies on the role of the C-terminal domain of mammalian polo-like kinase. Proc Natl Acad Sci USA. 2002;99:1984–9. doi: 10.1073/pnas.042689299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang YJ, Ji JH, Choi YC, Ryu CJ, Ko SY. Regulation of Polo-like kinase 1 by DNA damage in mitosis. Inhibition of mitotic PLK-1 by protein phosphatase 2A. J Biol Chem. 2007;282:2473–82. doi: 10.1074/jbc.M605480200. [DOI] [PubMed] [Google Scholar]
- Jayaraman T, Ondrias K, Ondriasova E, Marks AR. Regulation of the inositol 1,4,5-trisphosphate receptor by tyrosine phosphorylation. Science. 1996;272:1492–4. doi: 10.1126/science.272.5267.1492. [DOI] [PubMed] [Google Scholar]
- Jellerette T, Kurokawa M, Lee B, Malcuit C, Yoon SY, Smyth J, Vermassen E, De Smedt H, Parys JB, Fissore RA. Cell cycle-coupled [Ca2+]i oscillations in mouse zygotes and function of the inositol 1,4,5-trisphosphate receptor-1. Dev Biol. 2004;274:94–109. doi: 10.1016/j.ydbio.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Johnson EF, Stewart KD, Woods KW, Giranda VL, Luo Y. Pharmacological and functional comparison of the polo-like kinase family: insight into inhibitor and substrate specificity. Biochem J. 2007;46:9551–63. doi: 10.1021/bi7008745. [DOI] [PubMed] [Google Scholar]
- Jones KT, Carroll J, Whittingham DG. Ionomycin, thapsigargin, ryanodine, and sperm induced Ca2+ release increase during meiotic maturation of mouse oocytes. J Biol Chem. 1995a;270:6671–7. doi: 10.1074/jbc.270.12.6671. [DOI] [PubMed] [Google Scholar]
- Jones KT, Carroll J, Merriman JA, Whittingham DG, Kono T. Repetitive sperm-induced Ca2+ transients in mouse oocytes are cell cycle dependent. Development. 1995b;121:3259–66. doi: 10.1242/dev.121.10.3259. [DOI] [PubMed] [Google Scholar]
- Jurisicova A, Lee HJ, D’Estaing SG, Tilly J, Perez GI. Molecular requirements for doxorubicin-mediated death in murine oocytes. Cell Death Differ. 2006;13:1466–74. doi: 10.1038/sj.cdd.4401819. [DOI] [PubMed] [Google Scholar]
- Kalous J, Solc P, Baran V, Kubelka M, Schultz RM, Motlik J. PKB/AKT is involved in resumption of meiosis in mouse oocytes. Biol Cell. 2006;98:111–23. doi: 10.1042/BC20050020. [DOI] [PubMed] [Google Scholar]
- Khan MT, Wagner L, Yule DI, Bhanumathy C, Joseph SK. Akt kinase phosphorylation of inositol 1,4,5-trisphosphate receptors. J Biol Chem. 2006;281:3731–7. doi: 10.1074/jbc.M509262200. [DOI] [PubMed] [Google Scholar]
- Kimura K, Hattori S, Kabuyama Y, Shizawa Y, Takayanagi J, Nakamura S, Toki S, Matsuda Y, Onodera K, Fukui Y. Neurite outgrowth of PC12 cells is suppressed by wortmannin, a specific inhibitor of phosphatidylinositol 3-kinase. J Biol Chem. 1994;269:18961–7. [PubMed] [Google Scholar]
- Kline D. Attributes and dynamics of the endoplasmic reticulum in mammalian eggs. Curr Top Dev Biol. 2000;50:125–54. doi: 10.1016/s0070-2153(00)50007-6. [DOI] [PubMed] [Google Scholar]
- Koga T, Yoshida Y, Cai JQ, Islam MO, Imai S. Purification and characterization of 240-kDa cGMP-dependent protein kinase substrate of vascular smooth muscle. Close resemblance to inositol 1,4,5-trisphosphate receptor. J Biol Chem. 1994;269:11640–7. [PubMed] [Google Scholar]
- Krizanova O, Ondrias K. The inositol 1,4,5-trisphosphate receptor--transcriptional regulation and modulation by phosphorylation. Gen Physiol Biophys. 2003;22:295–311. [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG. Purification and molecular cloning of Plx1, a Cdc25-regulatory kinase from Xenopus egg extracts. Science. 1996;273:1377–80. doi: 10.1126/science.273.5280.1377. [DOI] [PubMed] [Google Scholar]
- Kume S, Yamamoto A, Inoue T, Muto A, Okano H, Mikoshiba K. Developmental expression of the inositol 1,4,5-trisphosphate receptor and structural changes in the endoplasmic reticulum during oogenesis and meiotic maturation of Xenopus laevis. Dev Biol. 1997;182:228–39. doi: 10.1006/dbio.1996.8479. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawlor MA, Alessi DR. PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J. Cell Sci. 2001;114:2903–10. doi: 10.1242/jcs.114.16.2903. [DOI] [PubMed] [Google Scholar]
- Lee B, Vermassen E, Yoon SY, Vanderheyden V, Ito J, Alfandari D, De Smedt H, Parys JB, Fissore RA. Phosphorylation of IP3R1 and the regulation of [Ca2+]i responses at fertilization: a role for the MAP kinase pathway. Development. 2006;133:4355–65. doi: 10.1242/dev.02624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Grenfell TZ, Yarm FR, Erikson RL. Mutation of the polo-box disrupts localization and mitotic functions of the mammalian polo kinase Plk. Proc Natl Acad Sci U S A. 1998;95:9301–6. doi: 10.1073/pnas.95.16.9301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre C, Terret ME, Djiane A, Rassinier P, Maro B, Verlhac MH. Meiotic spindle stability depends on MAPK-interacting and spindle-stabilizing protein (MISS), a new MAPK substrate. J Cell Biol. 2002;157:603–13. doi: 10.1083/jcb.200202052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Yan S, Zhou T, Terada Y, Erickson RL. The MAP kinase pathway is required for entry into mitosis and cell survival. Oncogene. 2004a;23:763–76. doi: 10.1038/sj.onc.1207188. [DOI] [PubMed] [Google Scholar]
- Liu J, Lewellyn AL, Chen LG, Maller JL. The polo box is required for multiple functions of Plx1 in mitosis. J Biol Chem. 2004b;279:21367–73. doi: 10.1074/jbc.M400482200. [DOI] [PubMed] [Google Scholar]
- Liu J, Maller JL. Calcium elevation at fertilization coordinates phosphorylation of XErp1/Emi2 by Plx1 and CaMK II to release metaphase arrest by cytostatic factor. Curr Biol. 2005a;15:1458–68. doi: 10.1016/j.cub.2005.07.030. [DOI] [PubMed] [Google Scholar]
- Liu J, Maller JL. Xenopus Polo-like kinase Plx1: a multifunctional mitotic kinase. Oncogene. 2005b;24:238–47. doi: 10.1038/sj.onc.1208220. [DOI] [PubMed] [Google Scholar]
- Liu Y, Shreder KR, Gai W, Corral S, Ferris DK, Rosenblum JS. Wortmannin, a widely used phosphoinositide 3-kinase inhibitor, also potently inhibits mammalian polo-like kinase. Chem Biol. 2005;12:99–107. doi: 10.1016/j.chembiol.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Liu Y, Jiang N, Wu J, Dai W, Rosenblum JS. Polo-like kinases inhibited by wortmannin. Labeling site and downstream effects. J Biol Chem. 2007;282:2505–11. doi: 10.1074/jbc.M609603200. [DOI] [PubMed] [Google Scholar]
- Lorca T, Cruzalegui FH, Fesquet D, Cavadore JC, Mery J, Means A, Doree M. Calmodulin-dependent protein kinase II mediates inactivation of MPF and CSF upon fertilization of Xenopus eggs. Nature. 1993;366:270–3. doi: 10.1038/366270a0. [DOI] [PubMed] [Google Scholar]
- Lowery DM, Clauser KR, Hjerrild M, Lim D, Alexander J, Kishi K, Ong SE, Gammeltoft S, Carr SA, Yaffe MB. Proteomic screen defines the Polo-box domain interactome and identifies Rock2 as a Plk1 substrate. Embo J. 2007;26:2262–73. doi: 10.1038/sj.emboj.7601683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madgwick S, Hansen DV, Levasseur M, Jackson PK, Jones KT. Mouse Emi2 is required to enter meiosis II by reestablishing cyclin B1 during interkinesis. J Cell Biol. 2006;174:791–801. doi: 10.1083/jcb.200604140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malathi K, Kohyama S, Ho M, Soghoian D, Li X, Silane M, Berenstein A, Jayaraman T. Inositol 1,4,5-trisphosphate receptor (type 1) phosphorylation and modulation by Cdc2. J Cell Biochem. 2003;90:1186–96. doi: 10.1002/jcb.10720. [DOI] [PubMed] [Google Scholar]
- Masui Y. From oocyte maturation to the in vitro cell cycle: the history of discoveries of Maturation-Promoting Factor (MPF) and Cytostatic Factor (CSF) Differentiation. 2001;69:1–17. doi: 10.1046/j.1432-0436.2001.690101.x. [DOI] [PubMed] [Google Scholar]
- Matson S, Ducibella T. The MEK inhibitor, U0126, alters fertilization-induced [Ca2+]i oscillation parameters and secretion: differential effects associated with in vivo and in vitro meiotic maturation. Dev Biol. 2007;306:538–48. doi: 10.1016/j.ydbio.2007.03.029. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Kline D. Regulation of intracellular calcium in the mouse egg: calcium release in response to sperm or inositol trisphosphate is enhanced after meiotic maturation. Biol Reprod. 1994;51:1088–98. doi: 10.1095/biolreprod51.6.1088. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Mikoshiba K, Kline D. Redistribution and increase in cortical inositol 1,4,5-trisphosphate receptors after meiotic maturation of the mouse oocyte. Dev Biol. 1996;180:489–98. doi: 10.1006/dbio.1996.0322. [DOI] [PubMed] [Google Scholar]
- Mitsuyama F, Sawai T. The redistribution of Ca2+ stores with inositol 1,4,5-trisphosphate receptor to the cleavage furrow in a microtubule-dependent manner. Int J Dev Biol. 2001;45:861–8. [PubMed] [Google Scholar]
- Miyazaki S, Shirakawa H, Nakada K, Honda Y. Essential role of the inositol 1,4,5-trisphosphate receptor/Ca2+ release channel in Ca2+ waves and Ca2+ oscillations at fertilization of mammalian eggs. Dev Biol. 1993;158:62–78. doi: 10.1006/dbio.1993.1168. [DOI] [PubMed] [Google Scholar]
- Moutinho-Santos T, Sampaio P, Amorim I, Costa M, Sunkel CE. In vivo localization of the mitotic POLO kinase shows a highly dynamic association with the mitotic apparatus during early embryogenesis in Drosophila. Biol Cell. 1998;91:585–96. [PubMed] [Google Scholar]
- Mundt KE, Golsteyn RM, Lane HA, Nigg EA. On the regulation and function of human polo-like kinase 1 (PLK1): effects of overexpression on cell cycle progression. Biochem Biophys Res Commun. 1997;239:377–85. doi: 10.1006/bbrc.1997.7378. [DOI] [PubMed] [Google Scholar]
- Muto A, Mikoshiba K. Activation of inositol 1,4,5-trisphosphate receptors induces transient changes in cell shape of fertilized Xenopus eggs. Cell Motil Cytoskeleton. 1998;39:201–8. doi: 10.1002/(SICI)1097-0169(1998)39:3<201::AID-CM3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Nagai T, Ibata K, Park ES, Kubota M, Mikoshiba K, Miyawaki A. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat Biotechnol. 2002;20:87–90. doi: 10.1038/nbt0102-87. [DOI] [PubMed] [Google Scholar]
- Nakajima H, Toyoshima-Morimoto F, Taniguchi E, Nishida E. Identification of a consensus motif for Plk (Polo-like kinase) phosphorylation reveals Myt1 as a Plk1 substrate. J Biol Chem. 2003;278:25277–80. doi: 10.1074/jbc.C300126200. [DOI] [PubMed] [Google Scholar]
- Nebreda AR, Ferby I. Regulation of the meiotic cell cycle in oocytes. Curr Opin Cell Biol. 2000;12:666–75. doi: 10.1016/s0955-0674(00)00150-2. [DOI] [PubMed] [Google Scholar]
- Neef R, Preisinger C, Sutcliffe J, Kopajtich R, Nigg EA, Mayer TU, Barr FA. Phosphorylation of mitotic kinesin-like protein 2 by polo-like kinase 1 is required for cytokinesis. J Cell Biol. 2003;162:863–75. doi: 10.1083/jcb.200306009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano-Uchida T, Okumura E, Iwashita M, Yoshida H, Tachibana K, Kishimoto T. Distinct regulators for Plk1 activation in starfish meiotic and early embryonic cycles. Embo J. 2003;22:5633–42. doi: 10.1093/emboj/cdg535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahlavan G, Polanski Z, Kalab P, Golsteyn R, Nigg EA, Maro B. Characterization of polo-like kinase 1 during meiotic maturation of the mouse oocyte. Dev Biol. 2000;220:392–400. doi: 10.1006/dbio.2000.9656. [DOI] [PubMed] [Google Scholar]
- Parry H, McDougall A, Whitaker M. Microdomains bounded by endoplasmic reticulum segregate cell cycle calcium transients in syncytial Drosophila embryos. J Cell Biol. 2005;171:47–59. doi: 10.1083/jcb.200503139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parys JB, De Smedt H, Missiaen L, Bootman MD, Sienaert I, Casteels R. Rat basophilic leukemia cells as model system for inositol 1,4,5-trisphosphate receptor IV, a receptor of the type II family: functional comparison and immunological detection. Cell Calcium. 1995;17:239–49. doi: 10.1016/0143-4160(95)90070-5. [DOI] [PubMed] [Google Scholar]
- Patterson RL, Boehning D, Snyder SH. Inositol 1,4,5-trisphosphate receptors as signal integrators. Annu Rev Biochem. 2004;73:437–65. doi: 10.1146/annurev.biochem.73.071403.161303. [DOI] [PubMed] [Google Scholar]
- Phillips KP, Petrunewich MA, Collins JL, Baltz JM. The intracellular pH-regulatory HCO3-/Cl- exchanger in the mouse oocyte is inactivated during first meiotic metaphase and reactivated after egg activation via the MAP kinase pathway. Mol Biol Cell. 2002;13:3800–10. doi: 10.1091/mbc.E02-04-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian YW, Erikson E, Li C, Maller JL. Activated polo-like kinase Plx1 is required at multiple points during mitosis in Xenopus laevis. Mol Cell Biol. 1998;18:4262–71. doi: 10.1128/mcb.18.7.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian YW, Erikson E, Maller JL. Mitotic effects of a constitutively active mutant of the Xenopus polo-like kinase Plx1. Mol Cell Biol. 1999;19:8625–32. doi: 10.1128/mcb.19.12.8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh NR, Schmidt A, Bormann J, Nigg EA, Mayer TU. Calcium triggers exit from meiosis II by targeting the APC/C inhibitor XErp1 for degradation. Nature. 2005;437:1048–52. doi: 10.1038/nature04093. [DOI] [PubMed] [Google Scholar]
- Rebecchi MJ, Pentyala SN. Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol Rev. 2000;80:1291–335. doi: 10.1152/physrev.2000.80.4.1291. [DOI] [PubMed] [Google Scholar]
- Santamaria A, Neef R, Eberspächer U, Eis K, Husemann M, Mumberg D, Prechtl S, Schulze V, Siemeister G, Wortmann L, Barr FA, Nigg EA. Use of the novel Plk1 inhibitor ZK-thiazolidinone to elucidate functions of Plk1 in early and late stages of mitosis. Mol Biol Cell. 2007;18:4024–36. doi: 10.1091/mbc.E07-05-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, Swann K, Lai FA. PLCζ: a sperm-specific trigger of Ca2+ oscillations in eggs and embryo development. Development. 2002;129:3533–44. doi: 10.1242/dev.129.15.3533. [DOI] [PubMed] [Google Scholar]
- Schultz RM, Kopf GS. Molecular basis of mammalian egg activation. Curr Top Dev Biol. 1995;30:21–62. doi: 10.1016/s0070-2153(08)60563-3. [DOI] [PubMed] [Google Scholar]
- Shiraishi K, Okada A, Shirakawa H, Nakanishi S, Mikoshiba K, Miyazaki S. Developmental changes in the distribution of the endoplasmic reticulum and inositol 1,4,5-trisphosphate receptors and the spatial pattern of Ca2+ release during maturation of hamster oocytes. Dev Biol. 1995;170:594–606. doi: 10.1006/dbio.1995.1239. [DOI] [PubMed] [Google Scholar]
- Singleton PA, Bourguignon LY. CD44v10 interaction with Rho-kinase (ROK) activates inositol 1,4,5-triphosphate IP3 receptor-mediated Ca2+ signaling during hyaluronan (HA)-induced endothelial cell migration. Cell Motil. Cytoskeleton. 2002;53:293–316. doi: 10.1002/cm.10078. [DOI] [PubMed] [Google Scholar]
- Stith BJ, Goalstone M, Silva S, Jaynes C. Inositol 1,4,5-trisphosphate mass changes from fertilization through first cleavage in Xenopus laevis. Mol Biol Cell. 1993;4:435–43. doi: 10.1091/mbc.4.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker SA. Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev Biol. 1999;211:157–76. doi: 10.1006/dbio.1999.9340. [DOI] [PubMed] [Google Scholar]
- Stricker SA. Structural reorganizations of the endoplasmic reticulum during egg maturation and fertilization. Sem Cell Dev Biol. 2006;17:303–13. doi: 10.1016/j.semcdb.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Sunkel CE, Glover DM. Polo, a mitotic mutant of Drosophila displaying abnormal spindle poles. J Cell Sci. 1988;89:25–38. doi: 10.1242/jcs.89.1.25. [DOI] [PubMed] [Google Scholar]
- Taylor CW, Genazzani AA, Morris SA. Expression of inositol trisphosphate receptors. Cell Calcium. 1999;26:237–51. doi: 10.1054/ceca.1999.0090. [DOI] [PubMed] [Google Scholar]
- Terasaki M, Runft LL, Hand AR. Changes in organization of the endoplasmic reticulum during Xenopus oocyte maturation and activation. Mol Biol Cell. 2001;12:1103–16. doi: 10.1091/mbc.12.4.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terret ME, Lefebvre C, Djiane A, Rassinier P, Moreau J, Maro B, Verlhac MH. DOC1R: a MAP kinase substrate that control microtubule organization of metaphase II mouse oocytes. Development. 2003;130:5169–77. doi: 10.1242/dev.00731. [DOI] [PubMed] [Google Scholar]
- Tombes RM, Simerly C, Borisy GG, Schatten G. Meiosis, egg activation, and nuclear envelope breakdown are differentially reliant on Ca2+, whereas germinal vesicle breakdown is Ca2+ independent in the mouse oocyte. J Cell Biol. 1992;117:799–811. doi: 10.1083/jcb.117.4.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung JJ, Hansen DV, Ban KH, Loktev AV, Summers MK, Adler JR, 3rd, Jackson PK. A role for the anaphase-promoting complex inhibitor Emi2/XErp1, a homolog of early mitotic inhibitor 1, in cytostatic factor arrest of Xenopus eggs. Proc Natl Acad Sci U S A. 2005;102:4318–23. doi: 10.1073/pnas.0501108102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner DL, Weintraub H. Expression of achaete-scute homolog 3 in Xenopus embryos converts ectodermal cells to a neural fate. Genes Dev. 1994:8,1434–47. doi: 10.1101/gad.8.12.1434. [DOI] [PubMed] [Google Scholar]
- Verlhac MH, Kubiak JZ, Clarke HJ, Maro B. Microtubule and chromatin behavior follow MAP kinase activity but not MPF activity during meiosis in mouse oocytes. Development. 1994;120:1017–25. doi: 10.1242/dev.120.4.1017. [DOI] [PubMed] [Google Scholar]
- Verlhac MH, Lefebvre C, Guillaud P, Rassinier P, Maro B. Asymmetric division in mouse oocytes: with or without Mos. Curr Biol. 2000;10:1303–6. doi: 10.1016/s0960-9822(00)00753-3. [DOI] [PubMed] [Google Scholar]
- Vermassen E, Fissore RA, Nadif Kasri N, Vanderheyden V, Callewaert G, Missiaen L, Parys JB, De Smedt H. Regulation of the phosphorylation of the inositol 1,4,5-trisphosphate receptor by protein kinase C. Biochem Biophys Res Commun. 2004;319:888–893. doi: 10.1016/j.bbrc.2004.05.071. [DOI] [PubMed] [Google Scholar]
- Westendorf JM, Rao PN, Gerace L. Cloning of cDNAs for M-Phase Phosphoproteins Recognized by the MPM2 Monoclonal Antibody and Determination of the Phosphorylated Epitope. Proc Nat Acad Sci USA. 1994;91:714–718. doi: 10.1073/pnas.91.2.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wianny F, Tavares Á, Evans MJ, Glover DM, Zernicka-Goetz M. Mouse polo-like kinase 1 associates with the acentriolar spindle poles, meiotic chromosomes and spindle midzone during oocyte maturation. Chromosoma. 1998;107:430–39. doi: 10.1007/s004120050327. [DOI] [PubMed] [Google Scholar]
- Wojcikiewicz RJ, Furuichi T, Nakade S, Mikoshiba K, Nahorski SR. Muscarinic receptor activation down-regulates the type I inositol 1,4,5- trisphosphate receptor by accelerating its degradation. J Biol Chem. 1994;269:7963–9. [PubMed] [Google Scholar]
- Wong R, Hadjiyanni I, Wei HC, Polevoy G, McBride R, Sem KP, Brill JA. PIP2 hydrolysis and calcium release are required for cytokinesis in Drosophila spermatocytes. Curr Biol. 2005;15:1401–6. doi: 10.1016/j.cub.2005.06.060. [DOI] [PubMed] [Google Scholar]
- Yaffe MB, Schutkowski M, Shen M, Zhou XZ, Stukenberg PT, Rahfeld JU, Xu J, Kuang J, Kirschner MW, Fischer G, Cantley LC, Lu KP. Sequence-Specific and Phosphorylation-Dependent Proline Isomerization: A Potential Mitotic Regulatory Mechanism. Science. 1997;278:1957–1960. doi: 10.1126/science.278.5345.1957. [DOI] [PubMed] [Google Scholar]
- Yokoyama K, Su IH, Tezuka T, Yasuda T, Mikoshiba K, Tarakhovsky A, Yamamoto T. BANK regulates BCR-induced calcium mobilization by promoting tyrosine phosphorylation of IP3 receptor. Embo J. 2002;21:83–92. doi: 10.1093/emboj/21.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]