Summary
Vitamin D is a fundamental mediator of skeletal metabolism. It also has important non-skeletal actions. We hypothesized that vitamin D deficiency may play an important role in skeletal morbidity and clinical outcomes in MM. We studied 148 newly diagnosed MM patients from January 1, 2004 through December 31, 2008 who had a serum 25-hydroxyvitamin D [25(OH)D] obtained within 14 days of diagnosis. Subjects with vitamin D deficiency [25(OH)D level less than 50 nmol/L (20 ng/mL)] had higher mean values of serum C-reactive protein (CRP) (2.40 mg/L vs. 0.84 mg/L, p= 0.02) and creatinine (1.75 mg/dL vs. 1.24 mg/dL, p=0.03) and lower serum albumin values (3.12 g/dL vs. 3.39 g/dL, p=0.003) compared to subjects without vitamin D deficiency. The prevalence of vitamin D deficiency increased in parallel with International Staging System (ISS): 16% of subjects in Stage I, 20% in Stage II, and 37% in Stage III (p=0.03) were vitamin D deficient. No differences were detected between the two groups in terms of skeletal morbidity. Association of vitamin D deficiency with higher serum CRP, serum creatinine and ISS stage at time of diagnosis suggests that vitamin D deficiency may portend poorer outcomes in subjects with MM.
Keywords: Vitamin D, multiple myeloma, 25-hydroxyvitamin D, prognosis, vitamin D deficiency
Introduction
Skeletal complications are a major cause of morbidity in multiple myeloma (MM). These complications include hypercalcemia due to increased bone resorption, generalized bone loss, intractable bone pain due to lytic bone destruction, and pathologic fractures at skeletal sites compromised by osteolytic lesions (Roodman 2004, Roodman 2006). Vitamin D is a fundamental mediator of skeletal metabolism, due to its ability to stimulate the absorption of calcium and phosphate across the intestinal mucosa, and to promote bone mineralization. Vitamin D deficiency results in reduced intestinal calcium absorption and secondary hyperparathyroidism, leading to increased skeletal catabolism in order to maintain serum calcium levels within the normal range(Holick 2007).
In addition to its critical role in maintenance of skeletal homeostasis, several recent reports suggest that vitamin D modulates several other critical cellular processes, including inhibition of carcinogenesis by induction of differentiation, inhibition of proliferation and angiogenesis, and promotion of apoptosis (Bikle 2009). To date, evidence of an inverse association between 25(OH)D levels and cancer risk in humans is best documented for cancers of the gastrointestinal tract with emerging evidence in prostate cancer and breast cancer (Bikle 2009). Given these recognized roles of vitamin D, it is plausible that vitamin D deficiency may be both an important contributor to the spectrum of skeletal complications seen in subjects with MM and an important determinant of the prognosis and progression of MM. In vitro studies, in which 1,25(OH)2D3 analogues demonstrate anti-proliferative and pro-apoptotic effects in myeloma cell lines (Kumagai, et al 2003, Park, et al 2002, Park, et al 2000a, Park, et al 2000b), support this hypothesis. However, human studies on the relationship between vitamin D deficiency and MM are notably lacking. The need for such studies is even more urgent considering the current “pandemic” of vitamin D deficiency(Holick 2007); using current recommended minimum levels for serum 25(OH)D, recent studies suggest that a high proportion of community-dwelling men and women in both tropical and temperate climates are deficient in vitamin D (Holick and Chen 2008). In this study, we examined the relationship between vitamin D deficiency and the presentation of multiple myeloma at diagnosis. Our hypotheses were that vitamin D deficiency is associated with increased staging (International Staging System, ISS) (Greipp, et al 2005), predictors of MM disease progression, and greater skeletal disease at the time of diagnosis.
Methods
Subjects
We used a well-characterized cohort of newly diagnosed MM patients seen at Mayo Clinic from January 1, 2004 through December 31, 2008 and included subjects who had a serum 25-hydroxyvitamin D [(25(OH)D] obtained within 14 days of MM diagnosis. In total, 148 subjects met these criteria. Subjects on renal replacement therapy were excluded. All corresponding baseline investigations (biochemical and imaging studies) used in this analysis were also obtained at the time of diagnosis. All the data were extracted from patient medical records and from the prospectively maintained Mayo Hematologic Malignancies database. The study was approved by the Mayo Foundation Institutional Review Board and all patients consented to have their medical records reviewed according to institutional review board practices and Health Insurance Portability and Accountability Act (HIPAA) guidelines.
Determination of serum 25(OH) Vitamin D levels
Serum 25(OH)D levels were measured by liquid chromatography tandem mass spectrometry (LC-MS/MS) (Singh, et al 2006) in all subjects with the exception of 4 which were measured by high-performance liquid chromatography (HPLC) (Kao and Heser 1984). The correlation between the 2 methods is excellent, with a value of R= 0.99 in our laboratory (unpublished data).
Outcome measures
We defined vitamin D deficiency as a serum 25(OH)D level < 50 nmol/L (20 ng/mL). Although consensus guidelines for the diagnosis of vitamin D deficiency have not been established, experts increasing accept this level for the establishment of hypovitaminosis D, as poorer skeletal and non-skeletal outcomes have been shown to occur with values below this threshold (Bischoff-Ferrari, et al 2006).
MM subjects were staged using the International Staging System (ISS) as previously described (Greipp, et al 2005). We were able to establish the ISS stage for 138 subjects; 10 subjects had either missing beta-2 microglobulin and/or albumin levels.
The free light chain (FLC) assay measures free κ and λ light chains. The FLC ratio is calculated as κ/λ; that is, free κ concentration divided by free λ concentration. Based on earlier work from our MM cohort, an FLC ratio of < 0.03 or > 32 independently (of other prognostic variables) confers a poorer prognosis compared to an FLC ratio between 0.03 and 32 (Snozek, et al 2008). As such, these FLC ratio cut-offs were also used to categorize our subjects.
The burden of skeletal morbidity at diagnosis was assessed by skeletal surveys. This was performed in all subjects except one, in whom imaging was not performed. Assessment for the presence of lytic lesions, major long bone fractures and vertebral compression fractures was undertaken by the clinical bone radiology service and confirmed by the consulting hematologist in each case.
Statistical analysis
Calculations were performed using JMP version 8 (SAS Institute Inc., Cary, NC). The statistical significance of differences in categorical variables associated with vitamin D deficiency was assessed using Pearson's Chi-Square/Fisher's Exact test, while continuous variables were assessed using t-tests. The relationship between vitamin D status and ISS stage was assessed using Wilcoxon rank sum test, to take into account the ordinal nature of the staging system. Similarly, the relationship between vitamin D levels and ISS stage was assessed using Spearman's rank correlation coefficient. Multivariate logistic regression analysis was used to assess the variables with P-values < 0.05 in the univariate analysis, which were adjusted for age, gender and body mass index (BMI). All statistical tests were two-sided, and P-values of less than 0.05 were considered to be significant.
Results
Between January 1, 2004 to December 31, 2008, 148 subjects with newly diagnosed MM were determined to have had a serum 25(OH)D drawn within 14 days of diagnosis, and were included in the study (Table I). Of these, 35 (24%) were vitamin D deficient [defined as a serum 25(OH)D level of less than 50 nmol/L (20 ng/mL)]. Vitamin D deficient and non-vitamin D deficient subjects had generally similar demographics, with the exception of race/ethnicity. This is not surprising, given that vitamin D levels can be significantly affected by skin pigmentation, sun-protection habits, geographic residency and diet. No seasonal variation in vitamin D status was noted (Table I and Supplemental Figure 1).
Table I. Characteristics of study population.
| Vitamin D Deficient N = 35 N(%) - nominal mean(95% CI) - continuous |
Non-Vitamin D Deficient N = 113 N(%) mean(95% CI) - continuous |
P value | |
|---|---|---|---|
| Age, years | 60.3 (56.6 – 63.9) | 63.0 (61.3 – 64.7) | 0.14 |
| Gender | |||
| Male | 23 (25) | 69 (75) | 0.62 |
| Female | 12 (21) | 44 (79) | |
| Race/Ethnicity | |||
| Caucasian non-Hispanic | 30 (22) | 109 (78) | |
| African American | 2 (40) | 3 (60) | 0.01 |
| Hispanic | 0 (0) | 1 (100) | |
| Asian | 3 (100) | 0 (0) | |
| BMI, kg/m2 | 28.9 (26.4 -31.3) | 27.8 (26.7 - 28.9) | 0.38 |
| Season of diagnosis | |||
| Spring | 9 (24) | 28 (76) | |
| Summer | 8 (17) | 39 (83) | 0.58 |
| Winter | 9 (26) | 25 (74) | |
| Autumn | 9 (30) | 21 (70) |
Vitamin D status: Deficiency is defined as 25(OH)D < 20 ng/mL.
As seen in Table II, vitamin D deficient subjects had a higher mean serum values for C-reactive protein (CRP) (2.40 mg/L vs. 0.84 mg/L, p=0.02) and creatinine (1.75 mg/dL vs. 1.24 mg/dL, p=0.03), and lower serum albumin values (3.12 g/dL vs. 3.39 g/dL, p=0.003) as compared to non-vitamin D deficient subjects. The OR for having vitamin D deficiency was 1.15 (95 % CI: 1.01 - 1.40, p=0.04) per 1 mg/L increase in serum CRP, and 1.35 (95% CI: 1.01 - 1.85, p=0.04) per 1 mg/dL increase in serum creatinine, which remained statistically significant after adjustment for age, gender and BMI (Table III). Other serum biomarkers, as well as hematological indices, were not different between the 2 groups, although a trend (p=0.1) for higher alkaline phosphatase levels was seen in vitamin D deficient subjects, perhaps reflecting hyperparathyroidism secondary to hypovitaminosis D with resultant increased skeletal turnover.
Table II. Laboratory parameters.
| Vitamin D Deficient N = 35 N(%) - nominal mean(95% CI) - continuous |
Non-Vitamin D Deficient N = 113 N(%) mean(95% CI) - continuous |
P value | |
|---|---|---|---|
| Albumin-corrected calcium, mg/dL | 9.87 (9.44-10.30) | 10.19 (9.94-10.44) | 0.20 |
| Phosphate, mg/dL | 3.61 (3.27 – 3.95) | 3.71 (3.51-3.90) | 0.64 |
| Alkaline phosphatase, U/L | 114.8 (38.2 – 191.4) | 79.5 (59.4 – 99.6) | 0.10 |
| Serum protein electrophoresis, g/dL | 2.37 (1.72 – 3.03) | 2.13 (1.78 – 2.49) | 0.52 |
| Urine M spike, mg/24 hours | 1.09 (0.14 -2.04) | 1.01 (0.55 – 1.47) | 0.88 |
| Free light chain ratio | |||
| <0.03 or >32 | 24 (26) | 70 (74) | 0.76 |
| 0.03-32 | 3 (30) | 7 (70) | |
| Beta-2 microglobulin, mcg/mL | 6.05 (4.00- 8.10) | 4.95 (3.90 – 6.00) | 0.32 |
| Lactate dehydrogenase, U/L | 202.9 (162.1 – 243.7) | 191.2 (175.6 – 206.8) | 0.51 |
| C-reactive protein, mg/L | 2.40 (0.09 – 4.72) | 0.84 (0.54 – 1.14) | 0.02 |
| Albumin, g/dL | 3.12 (0.10 – 2.91) | 3.39 (0.04 – 3.32) | 0.0029 |
| Creatinine, mg/dL | 1.75 (1.17 – 2.35) | 1.24 (1.04 – 1.43) | 0.03 |
| Hemoglobin, g/dL | 11.7 (11.0 – 12.3) | 11.6 (11.3 – 11.9) | 0.80 |
| Platelet count, × 10 (9)/L | 229.5 (199.1 – 260.0) | 235.3 (215.7 – 255.0) | 0.77 |
| Leukocyte count, × 10 (9)/L | 6.0 (5.2 – 6.8) | 5.6 (5.1 – 6.1) | 0.40 |
Vitamin D status: Deficiency is defined as 25(OH)D < 20 ng/mL.
Free light chain ratio is calculated as κ/λ; that is, free κ concentration divided by free λ concentration
Table III. Multivariate logistic regression analysis of the association between MM prognostic variables and vitamin D deficiency before and after adjustment for potential demographic confounding variables.
| Unadjusted Odds Ratio (95 % CI) |
P value | Adjusted* Odds Ratio (95 % CI) |
P value | |
|---|---|---|---|---|
|
ISS stage, per 1 stage increase |
1.89 (1.05 - 3.50) | 0.03 | 2.14 (1.08 - 4.53) | 0.03 |
|
C-reactive protein, per 1 mg/dl increase |
1.15 (1.01 - 1.40) | 0.04 | 1.26 (1.03 - 1.61) | 0.03 |
|
Creatinine, per 1 mg/dl increase |
1.35 (1.01 - 1.85) | 0.04 | 1.46 (1.06 - 2.08) | 0.02 |
Each model is adjusted for age, gender and BMI.
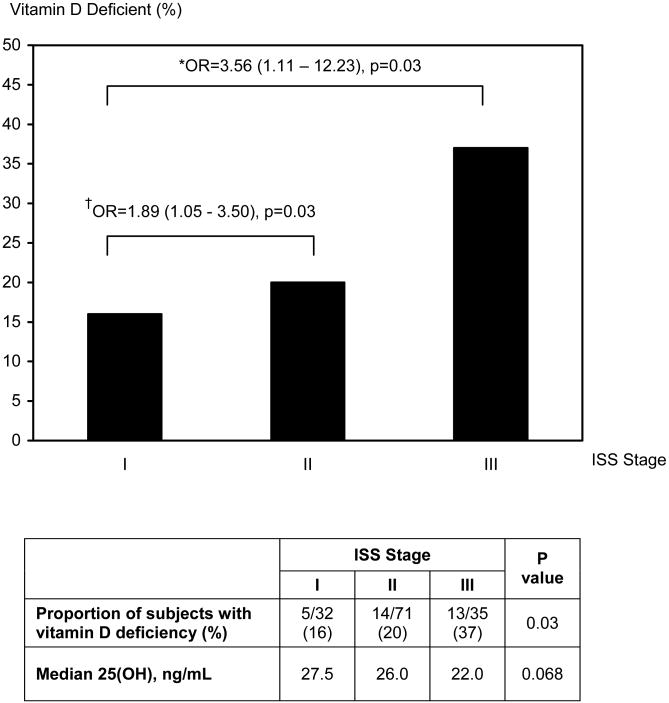
The proportion of subjects with vitamin D deficiency increased with progressively higher ISS staging (Figure 1); vitamin D deficiency was found in 16% of subjects in Stage I, 20% of subjects in Stage II and 37% of subjects categorized as stage III (p=0.03). Median vitamin D levels decreased with successive ISS staging (Spearman's rho = -0.16, p=0.068). The unadjusted odds ratio (OR) for having vitamin D deficiency was 1.89 (95% CI: 1.05 - 3.50, p=0.03) for ISS Stage II vs. Stage I, and increased to 3.56 (95% CI: 1.11 -12.23, p=0.03) for ISS Stage III vs. Stage I (Figure 1). ISS remained statistically significant in predicting vitamin D deficiency after adjustment for age, gender and BMI (Table III).
Figure 1. Proportion of subjects with vitamin D deficiency at successive ISS stages.
† Refers to the unadjusted odds ratio (OR) for having vitamin D deficiency in ISS Stage II vs. Stage I.
* Refers to the unadjusted odds ratio (OR) for having vitamin D deficiency in ISS Stage III vs. Stage I.
Interestingly and contrary to one of our original hypotheses, there were no differences in skeletal morbidity (lytic lesions, long bone fractures, and vertebral compression fractures) detected between subjects with vitamin D deficiency or sufficiency (Table IV).
Table IV. Skeletal Involvement in vitamin D deficient and non-vitamin D deficient subjects.
| Vitamin D Deficient N = 34 N(%) |
Non-Vitamin D Deficient N = 113 N(%) |
P value | |
|---|---|---|---|
| Lytic lesions | |||
| Present | 13 (19) | 54 (81) | 0.33 |
| Absent | 21 (26) | 59 (74) | |
| Long bone fractures | |||
| Present | 5 (23) | 17 (77) | 0.96 |
| Absent | 29 (23) | 96 (77) | |
| Vertebral compression fractures | |||
| Present | 11 (20) | 45 (80) | 0.43 |
| Absent | 23 (25) | 68 (75) |
Discussion
In this study population of newly diagnosed MM patients, 24% of subjects had vitamin D deficiency, as defined by having a serum 25(OH)D level of less than 50 nmol/L (20 ng/mL). This is slightly lower than the prevalence determined in National Health and Nutrition Examination Survey (NHANES) 2000-2004 data, in which approximately 30% of subjects age 50 years and above had 25(OH)D levels below this value (Yetley 2008). Whether this small difference reflects higher rates of vitamin supplementation among patients seen at our institution compared to the general population is unknown, as rigorous information about dietary supplements was not available in this retrospective study. Regardless of differences in rates of supplementation, however, serum 25 (OH)D levels are considered to accurately reflect vitamin D stores (Holick 2007). As seasonal variation can be an important determinant of cutaneous vitamin D3 synthesis (Holick 2008), we examined whether seasonal differences (defined by calendar dates appropriate for the Northern Hemisphere) in serum 25(OH)D existed in our population. Importantly, we did not find any significant seasonal variation in vitamin D status in our study population.
Contrary to one of our study hypotheses, we did not detect differences in skeletal morbidity between subjects who had vitamin D deficiency and those who did not at time of diagnosis. While this is consistent with a recently published report by Badros et al (Badros, et al 2008), it does not preclude the possibility that vitamin D deficiency may have an important role in the subsequent development of new skeletal events or in progression of MM bone disease following diagnosis.
In our analysis, we found higher serum CRP and creatinine levels in subjects with vitamin D deficiency. Both CRP (Barlogie, et al 1999, Vesole, et al 1996) and creatinine (Kyle, et al 2003) levels have been shown to predict prognosis in MM, with higher levels of both CRP and creatinine shown to portend poorer outcomes and survival. Vitamin D deficiency has been associated with increased circulating CRP levels in healthy subjects (Timms, et al 2002), and vitamin D supplementation has been shown to decrease CRP levels (Timms, et al 2002, Van den Berghe, et al 2003), supporting the anti-inflammatory role of vitamin D.
Interestingly, the recent study by Badros and colleagues (Badros, et al 2008) failed to show higher serum creatinine levels in subjects with lower vitamin D levels. Differences in study design, however, are likely to account for differences between our findings and their results. Accordingly, their study subjects were recruited successively from all patients seen in a clinical practice and thus represented a broad spectrum of time points of MM diagnosis and treatment. Only 9 of 100 subjects in their study had a new diagnosis of MM, whereas all 148 subjects in our study were newly diagnosed. In addition, their study included patients who were on renal replacement therapy (Badros, et al 2008), whereas these subjects were excluded from our study.
We also found a ‘step-wise’ association between ISS staging and vitamin D deficiency, such that there was an increasing likelihood that a subject would be vitamin D deficient with higher ISS staging at time of diagnosis. As reported previously, ISS staging also has important prognostic significance (Greipp, et al 2005). Together, these findings suggest that vitamin D deficiency may be predictive of a poorer outcome in MM.
It is credible that vitamin D deficiency can contribute to a poorer prognosis in subjects with MM, due to both the skeletal and non-skeletal actions of vitamin D. An alternative explanation would be that subjects with a poorer prognosis at the time of MM diagnosis may have alterations in their physiological milieu which lead to lower serum vitamin D levels. As example, subjects with a poorer prognosis at diagnosis may be more affected by malaise which could limit their sunlight exposure. Alternatively, subjects with worsened disease at diagnosis may have reduced caloric intake which could lead to reduced dietary vitamin D intake.
The strengths of our study include the inclusion of a large, well-characterized cohort of subjects with newly diagnosed MM who have not yet undergone treatment for their MM, and the use of LC-MS/MS methods for the measurement of serum 25(OH)D levels, a technique which is considered to be the ‘gold-standard’ for 25(OH)D determination (Binkley, et al 2008). Although our study provides only a cross-sectional perspective, the results are supportive of an important role for vitamin D in the natural history of MM. One limitation is that our study population is predominantly Caucasian; whether these results are generally applicable to other ethnic groups requires further investigation. In addition, as this is a retrospective study we did have serum intact parathyroid hormone or bone turnover markers available, which would provide further biochemical correlates of skeletal metabolism.
In sum, our study provides intriguing data on the relationship between vitamin D and prognosis in MM, and suggests a need for larger population-based studies both to confirm our findings and to prospectively assess the role of vitamin D deficiency in disease progression, overall survival, and quality of life in subjects with newly diagnosed MM.
Supplementary Material
Acknowledgments
This work was supported by a Mayo Hematologic Malignancies Program grant and a Mayo Career Development Award to Dr. Drake. Statistical analyses were supported by Grant Number 1 UL1 RR024150-01* from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. We thank Dr. Sundeep Khosla for critical reading of this manuscript.
Footnotes
Authorship: Contribution: All authors contributed to the analysis of the data. A.C.N. and M.T.D. co-wrote the paper. S.V.R and S.K.K. critically reviewed and contributed to the writing of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References
- Badros A, Goloubeva O, Terpos E, Milliron T, Baer MR, Streeten E. Prevalence and significance of vitamin D deficiency in multiple myeloma patients. Br J Haematol. 2008 doi: 10.1111/j.1365-2141.2008.07214.x. [DOI] [PubMed] [Google Scholar]
- Barlogie B, Jagannath S, Desikan KR, Mattox S, Vesole D, Siegel D, Tricot G, Munshi N, Fassas A, Singhal S, Mehta J, Anaissie E, Dhodapkar D, Naucke S, Cromer J, Sawyer J, Epstein J, Spoon D, Ayers D, Cheson B, Crowley J. Total therapy with tandem transplants for newly diagnosed multiple myeloma. Blood. 1999;93:55–65. [PubMed] [Google Scholar]
- Bikle D. Nonclassic actions of vitamin d. J Clin Endocrinol Metab. 2009;94:26–34. doi: 10.1210/jc.2008-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binkley N, Krueger D, Gemar D, Drezner MK. Correlation among 25-hydroxy-vitamin D assays. J Clin Endocrinol Metab. 2008;93:1804–1808. doi: 10.1210/jc.2007-2340. [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Blade J, Boccadoro M, Child JA, Avet-Loiseau H, Kyle RA, Lahuerta JJ, Ludwig H, Morgan G, Powles R, Shimizu K, Shustik C, Sonneveld P, Tosi P, Turesson I, Westin J. International staging system for multiple myeloma. J Clin Oncol. 2005;23:3412–3420. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D: a D-Lightful health perspective. Nutr Rev. 2008;66:S182–194. doi: 10.1111/j.1753-4887.2008.00104.x. [DOI] [PubMed] [Google Scholar]
- Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S–1086S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- Kao PC, Heser DW. Simultaneous determination of 25-hydroxy- and 1,25-dihydroxyvitamin D from a single sample by dual-cartridge extraction. Clin Chem. 1984;30:56–61. [PubMed] [Google Scholar]
- Kumagai T, O'Kelly J, Said JW, Koeffler HP. Vitamin D2 analog 19-nor-1,25-dihydroxyvitamin D2: antitumor activity against leukemia, myeloma, and colon cancer cells. J Natl Cancer Inst. 2003;95:896–905. doi: 10.1093/jnci/95.12.896. [DOI] [PubMed] [Google Scholar]
- Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, Fonseca R, Rajkumar SV, Offord JR, Larson DR, Plevak ME, Therneau TM, Greipp PR. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- Park WH, Seol JG, Kim ES, Binderup L, Koeffler HP, Kim BK, Lee YY. The induction of apoptosis by a combined 1,25(OH)2D3 analog, EB1089 and TGF-beta1 in NCI-H929 multiple myeloma cells. Int J Oncol. 2002;20:533–542. [PubMed] [Google Scholar]
- Park WH, Seol JG, Kim ES, Hyun JM, Jung CW, Lee CC, Binderup L, Koeffler HP, Kim BK, Lee YY. Induction of apoptosis by vitamin D3 analogue EB1089 in NCI-H929 myeloma cells via activation of caspase 3 and p38 MAP kinase. Br J Haematol. 2000a;109:576–583. doi: 10.1046/j.1365-2141.2000.02046.x. [DOI] [PubMed] [Google Scholar]
- Park WH, Seol JG, Kim ES, Jung CW, Lee CC, Binderup L, Koeffler HP, Kim BK, Lee YY. Cell cycle arrest induced by the vitamin D(3) analog EB1089 in NCI-H929 myeloma cells is associated with induction of the cyclin-dependent kinase inhibitor p27. Exp Cell Res. 2000b;254:279–286. doi: 10.1006/excr.1999.4735. [DOI] [PubMed] [Google Scholar]
- Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- Roodman GD. New potential targets for treating myeloma bone disease. Clin Cancer Res. 2006;12:6270s–6273s. doi: 10.1158/1078-0432.CCR-06-0845. [DOI] [PubMed] [Google Scholar]
- Singh RJ, Taylor RL, Reddy GS, Grebe SK. C-3 epimers can account for a significant proportion of total circulating 25-hydroxyvitamin D in infants, complicating accurate measurement and interpretation of vitamin D status. J Clin Endocrinol Metab. 2006;91:3055–3061. doi: 10.1210/jc.2006-0710. [DOI] [PubMed] [Google Scholar]
- Snozek CLH, Katzmann JA, Kyle RA, Dispenzieri A, Larson DR, Therneau TM, Melton LJ, III, Kumar S, Greipp PR, Clark RJ, Rajkumar SV. Prognostic value of the serum free light chain ratio in newly diagnosed myeloma: proposed incorporation into the international staging system. 2008;22:1933–1937. doi: 10.1038/leu.2008.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timms PM, Mannan N, Hitman GA, Noonan K, Mills PG, Syndercombe-Court D, Aganna E, Price CP, Boucher BJ. Circulating MMP9, vitamin D and variation in the TIMP-1 response with VDR genotype: mechanisms for inflammatory damage in chronic disorders? Qjm. 2002;95:787–796. doi: 10.1093/qjmed/95.12.787. [DOI] [PubMed] [Google Scholar]
- Van den Berghe G, Van Roosbroeck D, Vanhove P, Wouters PJ, De Pourcq L, Bouillon R. Bone turnover in prolonged critical illness: effect of vitamin D. J Clin Endocrinol Metab. 2003;88:4623–4632. doi: 10.1210/jc.2003-030358. [DOI] [PubMed] [Google Scholar]
- Vesole DH, Tricot G, Jagannath S, Desikan KR, Siegel D, Bracy D, Miller L, Cheson B, Crowley J, Barlogie B. Autotransplants in multiple myeloma: what have we learned? Blood. 1996;88:838–847. [PubMed] [Google Scholar]
- Yetley EA. Assessing the vitamin D status of the US population. Am J Clin Nutr. 2008;88:558S–564S. doi: 10.1093/ajcn/88.2.558S. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.