Abstract
Background
Cabergoline is an ergotamine derivative that increases the expression of glial cell line-derived neurotrophic factor (GDNF) in vitro. We recently showed that GDNF in the ventral tegmental area (VTA) reduces the motivation to consume alcohol. We therefore set out to determine whether cabergoline administration decreases alcohol-drinking and -seeking behaviors via GDNF.
Methods
Reverse transcription polymerase chain reaction (RT-PCR) and Enzyme-Linked ImmunoSorbent Assay (ELISA) were used to measure GDNF levels. Western blot analysis was used for phosphorylation experiments. Operant self-administration in rats and a two-bottle choice procedure in mice were used to assess alcohol-drinking behaviors. Instrumental performance tested during extinction was used to measure alcohol-seeking behavior. The [35S]GTPγS binding assay was used to assess the expression and function of the dopamine D2 receptor (D2R).
Results
We found that treatment of the dopaminergic-like cell line SH-SY5Y with cabergoline and systemic administration of cabergoline in rats resulted in an increase in GDNF level and in the activation of the GDNF pathway. Cabergoline treatment decreased alcohol-drinking and -seeking behaviors including relapse, and its action to reduce alcohol consumption was localized to the VTA. Finally, the increase in GDNF expression and the decrease in alcohol consumption by cabergoline were abolished in GDNF heterozygous knockout mice.
Conclusions
Together, these findings suggest that cabergoline-mediated upregulation of the GDNF pathway attenuates alcohol-drinking behaviors and relapse. Alcohol abuse and addiction are devastating and costly problems worldwide. This study puts forward the possibility that cabergoline might be an effective treatment for these disorders.
Keywords: Addiction, alcohol, cabergoline, GDNF, growth factor, treatment
Although alcohol abuse and addiction are some of the most detrimental health problems worldwide (1), pharmacotherapies for alcohol-related phenotypes such as excessive drinking, craving, and relapse have been limited. Currently, only three approved medications are available to treat alcohol craving (disulfiram, acamprosate, and naltrexone), for which results are varied (2), largely due to high degrees of noncompliance and detrimental side-effects (3–5). Hence, the development of novel pharmacotherapeutic approaches to treat this psychiatric disorder remains critical.
Cabergoline (Dostinex, Cabaser) is a dopamine D2 receptor (D2R)-like agonist, which is also reported to act as an agonist at the dopamine D1, and serotonin receptors and to inhibit α2-adrenoceptors (6 – 8). In addition, incubation of cultured astrocytes with cabergoline increases the level of glial cell line-derived neurotrophic factor (GDNF) (9,10). GDNF is an essential growth factor for the development of the kidneys, and it exerts a wide range of effects on both developing and adult peripheral and central neurons (11). GDNF is also a potent trophic factor for midbrain dopaminergic neurons (12,13) and has an important neurorestorative role after lesions of the nigrostriatal system (14). As such, its potential as a therapeutic agent for the treatment of Parkinson’s disease, a condition characterized by the degradation of dopaminergic neurons in the midbrain, has been extensively explored. The GDNF receptors GFRα1 and Ret are highly expressed in the ventral tegmental area (VTA) (15,16), a brain region that is a critical component of the neural circuitry underlying drug- and alcohol-seeking behavior (17,18). We previously found that the upregulation of the GDNF pathway in the VTA is the mechanism by which the alkaloid Ibogaine reduces rat alcohol (ethanol)-drinking behaviors (19). Recently, we reported that intra-VTA application of GDNF produces a rapid and sustained reduction of rat operant self-administration of ethanol but not sucrose, an effect mediated by the activation of the mitogen-activated protein kinase (MAPK) signaling pathway (20). Importantly, we found that GDNF in the VTA blocks ethanol self-administration in a procedure that models relapse (20). Therefore, we postulated that drugs that increase GDNF expression in the VTA will also reduce ethanol consumption and, most importantly, relapse. To start addressing this possibility, we tested whether cabergoline treatment leads to the upregulation of GDNF expression and subsequent activation of GDNF pathway and whether cabergoline, via its actions on GDNF, might act as an inhibitor of ethanol-drinking and -seeking behaviors.
Methods and Materials
See Supplement 1 for details regarding materials, animals, cell culture, reverse transcription polymerase chain reaction (RT-PCR), Western blot analysis, Enzyme-Linked ImmunoSorbent Assay (ELISA), Quinpirole-stimulated D2R-like [35S]GTPγS binding assay, mice’s limited-access two-bottle choice drinking procedure, rats’ operant ethanol and sucrose self-administration procedures, histology, and statistical analyses.
Cabergoline Preparation and Treatment for In Vivo Studies
Doses for the in vivo studies were chosen according to previous studies (9,10,21,22). The 3-hour pretreatment time-point was based on the time needed for the upregulation of the GDNF pathway in vitro (Figures 1 and 2).
Figure 1.
Cabergoline increases GDNF messenger RNA (mRNA) levels in rats’ midbrain in vivo and in SH-SY5Y cells. (A) The GDNF expression in rat VTA and the substantia nigra (SN) 1.5 hours after an IP injection of cabergoline (1 mg/kg), n = 4 –5. (B) GDNF expression upon 1.5 hours of exposure of SH-SY5Y cells to vehicle or cabergoline (60 μmol/L), n = 5. The GDNF, glycerol-3-phosphate dehydrogenase (GPDH), and RACK1 mRNA expression were analyzed by RT-PCR. Results were expressed as mean ratios of GDNF/GPDH or GDNF/RACK1 ± SEM. *p < .05, **p < .01, ***p < .001.
Figure 2.
Cabergoline increases GDNF protein levels in SH-SY5Y cells and induces the activation of the GDNF pathway in vitro and in vivo. (A) GDNF protein levels upon 3 hours of exposure of SH-SY5Y cells to vehicle or cabergoline (60 μmol/L). The GDNF protein levels were detected by ELISA and expressed as percentage of control of the ratio GDNF/total protein ± SEM, n = 3. (B and C) Activation of the GDNF signaling pathway after 2 hours of treatment of SH-SY5Y cells with vehicle or cabergoline (60 μmol/L). GDNF (50 ng/mL, 15 min incubation) was used as a positive control. Data are shown as percentage of control of the ratios phospho-Ret/Ret and phospho-extracellular signal-regulated kinase (ERK)2/ERK2 ± SEM, n = 5. (D) ERK2 phosphorylation in rat midbrain 3 hours after an IP injection of vehicle or cabergoline (1 mg/kg). Data are shown as percentage of control of the ratio phospho-ERK2/ERK2, n = 6. *p < .05, ***p < .001.
Systemic Intraperitoneal Injections
Cabergoline was suspended in a saline solution containing .25% methylcellulose and 3% tween 80 for rats or .25% methylcellulose and .6% tween 80 for mice. Systemic injections were given in a volume of 2 mL/kg for rats and 10 mL/kg for mice. Mice and rats were injected with cabergoline (.12, .25, .5, or 1 mg/kg) or vehicle 3 hours before the beginning of the behavioral session. For the RT-PCR, rats and mice were injected with cabergoline (1 mg/kg) or vehicle 1.5 hours before the dissection of the midbrain.
Microinjections into the VTA or the Substantia Nigra Compacta
Cabergoline was dissolved in phosphate buffered saline containing 5% dimethyl sulfoxide. Cabergoline (.5 μL/side; 30, 60, or 120 μmol/L) or vehicle was microinjected 3 hours before the beginning of the behavioral session.
Operant Self-Administration Test in Extinction
Cabergoline’s effects on ethanol-seeking were tested as follow: after 2 months of training as described in Supplement 1, rats experienced a single 1-hour session of extinction/week in which responses on the ethanol lever were counted but no programmed events occurred. Cabergoline (.25, .5, or 1 mg/kg) or vehicle was injected IP 3 hours before the beginning of the session.
Reacquisition of Ethanol Self-Administration
After 2 months of training, rats underwent daily 60-min extinction sessions (no reward after active lever responses). After 17 days of extinction, rats were injected IP with cabergoline (1 mg/kg) or vehicle 3 hours before the reacquisition test session. To trigger memory retrieval of the operant responding for ethanol, a prime (.2 mL of 10% ethanol) was delivered at the beginning of the test session, into the reward port, noncontingent to the lever press (20). Subsequently, three lever presses on the active lever resulted in the delivery of .1 mL of the reinforcer, as during the self-administration procedure. After 1 week of reacquisition of ethanol self-administration followed by 10 further extinction sessions, a second reacquisition test session was conducted with the drug treatment reversed.
Operant Responding During Extinction After Abstinence in Rats with a History of High Voluntary Ethanol Consumption
High voluntary ethanol consumption was induced as described in previous studies (20,23,24). A stable level of ethanol consumption of 5.5 ± .4 g/kg in 24 hours was obtained after 6 weeks (21 sessions). Rats were then trained to self-administer a 20% ethanol solution in operant chambers, and the length of the sessions was 30 min. After 3 months of training, rats experienced a withdrawal period of 10 days. The motivation to seek ethanol after a period of abstinence was assessed in the operant self-administration chamber during a 15-min extinction session, 3 hours after an IP injection of cabergoline (1 mg/kg) or vehicle. After this test, animals had 1 week of access to ethanol in the self-administration chambers followed by 10 days of withdrawal. A second session of extinction was then conducted with the drug treatment reversed.
Surgery and Microinjection
Rats were anesthetized continuously with isoflurane. Bilateral guide cannulae (C235G-2.0, 26 ga, Plastics One, Roanoke, Virginia) were aimed dorsal to the VTA (5.6 mm posterior to bregma, 1.0 mm mediolateral, 8.0 mm ventral to the skull surface) or the substantia nigra compacta (SNc) (5.4 mm posterior to bregma, 2.6 mm mediolateral, 6.8 mm ventral to the skull surface), according to Paxinos and Watson, 1998 (25). The coordinates were identical to those used in a previous study (20) and allowed us to target mainly the posterior part of the VTA, which is preferentially involved in rewarding processes and mediation of the reinforcing effects of ethanol (26,27). One week after recovery, subjects returned to self-administration training, and microinjections began when the responding was stable. Three hours before the beginning of the session, cabergoline or vehicle was infused over 2 min to gently restrained rats via injection cannulae extending .5 mm beyond the guide cannula tip. Injection cannulae were left in place for an additional 2 min. All subjects received each treatment in a counterbalanced manner, with one injection/week, allowing the lever responding for ethanol to return to baseline between treatments.
Results
Cabergoline Increases GDNF Levels and Activates the GDNF Pathway In Vitro and In Vivo
We first tested whether systemic treatment of rats with cabergoline results in increased GDNF expression in the mid-brain. Cabergoline was systemically administered to rats, and GDNF expression in the VTA and the substantia nigra was measured 1.5 hours after injection. As shown in Figure 1A, treatment of rats with cabergoline significantly increased GDNF messenger RNA (mRNA) in both regions of the midbrain.
To test whether the increase in GDNF expression by cabergoline leads to a subsequent increase in protein level of GDNF, we used the dopaminergic-like SH-SY5Y cell line as a model system. After differentiation, SH-SY5Y cells develop a neuron-like morphology and express high level of the GDNF receptors. We found that treatment of SH-SY5Y cells with cabergoline resulted in a significant increase in GDNF mRNA levels (Figure 1B), which correlated with a significant increase in the level of the protein (Figure 2A). Next, we tested whether the upregulation in GDNF levels by cabergoline corresponded with the activation of the GDNF pathway. To do so, we measured the phosphorylation and thus activation level of the GDNF receptor tyrosine kinase, Ret. As shown in Figure 2B (upper panel) and Figure 2C (left panel), treatment of SH-SY5Y cells with cabergoline resulted in a significant increase in the phosphorylation level of Ret. Activation of Ret leads to the activation of downstream signaling cascades such as the MAPK pathway (11), which mediates the effect of GDNF on ethanol-drinking behaviors (20). As shown in Figure 2B (second panel from bottom) and Figure 2C (right panel), the phosphorylation and thus activation of the MAPK, extracellular signal-regulated kinase (ERK)1/2, was also increased upon exposure of cells to cabergoline. Importantly, systemic administration of cabergoline resulted in the activation of the MAPK pathway in the midbrain (Figure 2D). Taken together, these results suggest that cabergoline treatment results in the upregulation of GDNF levels and in the activation of the GDNF pathway in the VTA.
Cabergoline Decreases Ethanol Self-Administration and Ethanol-Seeking in Rats
Next, we evaluated the effects of cabergoline on ethanol consumption by using a rat ethanol operant self-administration procedure. Self-administration of ethanol was measured 3 hours after systemic injection of cabergoline. As shown in Figure 3A, cabergoline dose-dependently reduced self-administration for ethanol [F(4,28) = 4.87, p < .01]. Importantly, cabergoline administration did not significantly alter operant responding for sucrose [F (4,32) = 1.40, p = .26] (Figure 3B). Hence, these results suggest that the decrease in ethanol self-administration induced by cabergoline was not due to a general attenuation of motivation or a change in locomotor activity. Next, we tested the motivation of rats to seek ethanol upon cabergoline administration. To do so, instrumental performance was tested in extinction (ethanol was not delivered during the test). As shown in Figure 3C, cabergoline administration resulted in a significant decrease in the number of lever presses on the ethanol lever [F (3,21) = 15.60, p < .001]. Taken together, these data suggest that cabergoline reduces ethanol-drinking and -seeking behaviors in rats.
Figure 3.
Cabergoline decreases operant ethanol self-administration–related behaviors in rats. Vehicle or cabergoline (.12, .25, .5, or 1 mg/kg) was injected IP 3 hours before the beginning of the test sessions. (A) Cabergoline decreased the number of ethanol deliveries, n = 8. (B) Cabergoline did not decrease self-administration for sucrose, n = 9. (C) Cabergoline decreased the number of presses on the ethanol lever during an extinction session, n = 8. (D) Cabergoline reduced reacquisition of operant ethanol self-administration after a period of extinction. Baseline represents the mean lever presses for the last 4 days of self-administration training, and extinction represents the mean lever presses during the final extinction session, n = 8. (E) Cabergoline decreased the number of presses on the ethanol lever during an extinction session of 15 min that followed 10 days of abstinence from ethanol, in rats with a history of high voluntary ethanol consumption, n = 8. Data are shown as mean ± SEM. *p < .05, **p < .01, and ***p < .001 compared with vehicle and #p < .05 and ###p < .001 compared with extinction.
Cabergoline Reduces Reacquisition of Ethanol Self-Administration and Ethanol-Seeking After a Period of Abstinence
We also tested whether cabergoline reduces relapse to ethanol consumption. First, we assessed the effect of a systemic administration of cabergoline (1 mg/kg) or its vehicle on reacquisition of ethanol self-administration. Reacquisition, a rapid return of responding when the outcome is made available again after a period of extinction, is especially relevant for therapies that seek to extinguish drug-related behaviors (28), and reacquisition of ethanol self-administration is reduced by GDNF (20). As shown in Figure 3D, a rapid albeit partial reacquisition of ethanol self-administration was observed in the control vehicle-injected rats. However, the reacquisition of operant responding for ethanol was markedly reduced in rats treated with cabergoline [Figure 3D, F(3,21) = 20.40, p < .001]. Second, we examined the effect of cabergoline on instrumental performance during an extinction session after 10 days of withdrawal from ethanol, in rats with a history of high levels of voluntary ethanol consumption. This procedure allowed us to evaluate the motivation of rats to seek ethanol after a period of abstinence (29,30). In control vehicle-injected rats, re-exposure to the self-administration chambers after a period of abstinence resulted in a high rate of responding within a short time period (15 min) (Figure 3E). The increase was selective for the lever associated with ethanol, whereas the responding on the inactive lever remained extremely low (3.13 ± .72, data not shown). Importantly, cabergoline administration resulted in a significant decrease in the number of presses on the ethanol lever [T(7) = 3.66, p < .01]. Together, these results suggest that cabergoline reduced the motivation to consume and to seek ethanol in models of relapse.
Microinjection of Cabergoline into the VTA Decreases Ethanol Operant Self-Administration in Rats
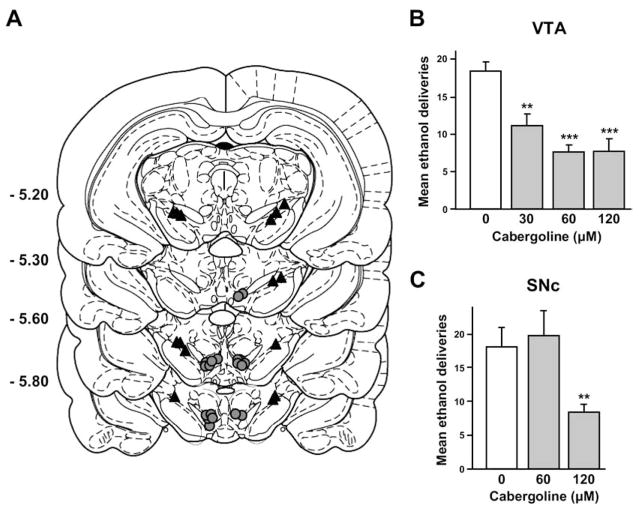
Because systemic administration of cabergoline increases GDNF expression in the VTA (Figure 1A), a structure in which GDNF infusion decreases ethanol self-administration (20), we set out to determine whether cabergoline would act directly within the VTA (Figure 4A) to reduce ethanol self-administration. As shown in Figure 4B, intra-VTA infusion of cabergoline dose-dependently decreased operant responding for ethanol [F(3,27) = 12.61, p < .001]. To determine the site specificity of GDNF’s action, we infused cabergoline into the neighboring midbrain dopaminergic region, the SNc. As shown in Figure 4C, only the highest concentration of cabergoline infused into the SNc (120 μmol/L) altered lever-press responding for ethanol [F(2,16) = 6.35, p < .01]. Therefore, the main site of action of cabergoline to reduce ethanol-drinking behavior is likely to be the VTA.
Figure 4.
Intra-VTA injections of cabergoline decreases operant ethanol self-administration in rats. Vehicle or cabergoline (30, 60, or 120 μmol/L) was infused into the VTA or the SNc 3 hours before the test sessions. (A) Schematic representation of the injection cannulae placements in coronal sections (25). The location of the injector tips is represented by gray circles, and black triangles show the VTA and SNc microinjection sites. Numbers indicate the distance anterior to bregma in millimeters. (B) Cabergoline infused into the VTA decreased ethanol self-administration, n = 10. (C) High concentration of cabergoline decreased ethanol self-administration when infused into the SNc, n = 9. Data are shown as mean ±SEM. **p < .01, ***p < .001 compared with vehicle.
Cabergoline Decreases Ethanol Consumption in Mice
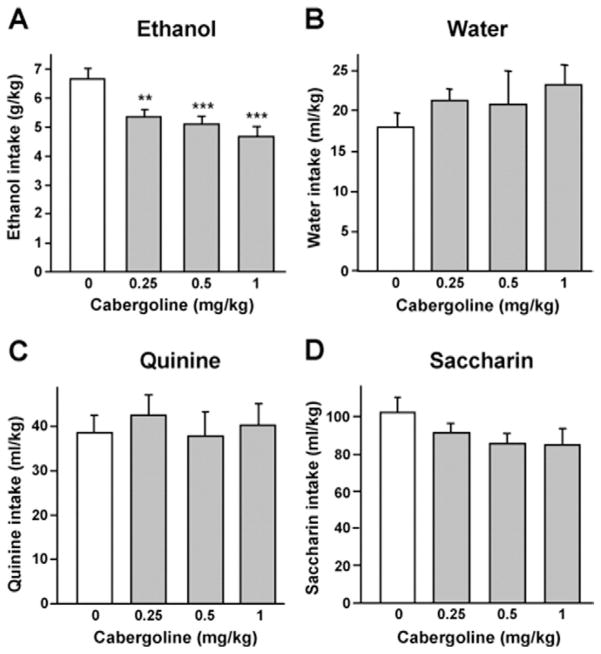
Next, we tested the effect of systemic injection of cabergoline on voluntary ethanol intake in mice, with a limited ethanol access two-bottle choice procedure. This paradigm ensures a high-level of voluntary ethanol-drinking (6–7 g/kg) during a short time period (Figure 5A). As shown in Figures 5A and 5B, systemic administration of cabergoline decreased ethanol but not water intake [F(3,33) = 11.43, p < .001 and F(3,33) = .85, p = .48; for ethanol and water intake, respectively]. Importantly, cabergoline did not alter the intake of a bitter solution of quinine [F(3,33) = .18, p = .91] (Figure 5C). Cabergoline did not significantly change the consumption of saccharin [F(3,33) = 1.38, p = .26], although a trend toward a reduction of saccharin intake was observed (Figure 5D). Taken together, these results suggest that the decrease in ethanol intake induced by cabergoline at the concentrations used in this study is not due to locomotor impairments or a change in taste palatability.
Figure 5.
Cabergoline decreases ethanol consumption in mice. Vehicle or cabergoline (.25, .5, or 1 mg/kg) was injected IP 3 hours before test session. (A–D) Cabergoline dose-dependently decreased voluntary ethanol intake during a 4-hour access period (A) but not water (B), quinine (C), or saccharin intake (D). For each experiment, n = 12. Data are shown as mean ±SEM. **p < .01, ***p < .001 compared with vehicle.
Cabergoline Fails to Increase Midbrain GDNF Expression and to Decrease Ethanol Consumption in GDNF Heterozygous Knockout Mice
As described in the preceding text, we found that cabergoline increased GDNF expression and decreased ethanol-drinking behaviors. Therefore, we hypothesized that cabergoline reduces ethanol consumption by GDNF. Consequently, we tested whether cabergoline administration would result in an increase in GDNF expression and a decrease in ethanol intake in GDNF heterozygous knock-out mice (GDNF+/−), in which GDNF levels are significantly reduced compared with wild-type (GDNF+/+) mice (Figure 6A, white bars). Systemic administration of cabergoline increased GDNF mRNA in the midbrain of the GDNF+/+ but not of the GDNF+/− littermate mice [Genotype, F(1,32) = 21.83, p < .001; Treatment, F(1,32) = 2.28, p = .14; Genotype × Treatment interaction, F(1,32) = 5.50, p < .05] (Figure 6A). Importantly, as shown in Figure 6B, systemic administration of cabergoline decreased ethanol intake in GDNF+/+ mice, which was not observed in the GDNF+/− litter-mate mice. In the absence of interaction [Genotype, F(1,27) = 18.67, p < .001; Treatment, F(1,27) = 8.26, p < .01; Interaction: F(1,27) = 1.03, p = .32], the data were analyzed by the method of contrasts that showed a significant difference between the vehicle and the cabergoline treatment for the GDNF+/+ [T(13) = 4.28, p < .001] but not for the GDNF+/− mice [T(13) = 1.01, p = .33].
Figure 6.

Cabergoline fails to increase midbrain GDNF mRNA levels and to decrease ethanol consumption in GDNF heterozygous knockout mice. (A) Cabergoline (1 mg/kg) injected IP 1.5 hours before dissection of the brain increased GDNF expression in the midbrain of the GDNF+/+ but not of the GDNF+/− mice. The GDNF expression was analyzed by RT-PCR and expressed as ratios of GDNF/GPDH, n = 8 –10. (B) Cabergoline (1 mg/kg) injected IP 3 hours before the test session decreased ethanol intake in the GDNF+/+ but not in the GDNF+/− mice, n = 14. (C and D) Quinpirole-stimulated dopamine D2 receptor (D2R)-like [35S]GTPγS binding measured in midbrain (C) and striatal (D) membranes homogenates prepared from GDNF+/+ and GDNF+/− mice. The Emax and EC50 values were similar for GDNF+/+ and GDNF+/− (midbrain: Emax, 28 ± 2% and 24 ± 2%; EC50, 59 ± 19 μmol/L and 85 ± 17 μmol/L; striatum: Emax, 23 ± 3% and 21 ± 2%; EC50, 3.8± .2 μmol/L and 3.6 ± .3 μmol/L; for GDNF+/+ and GDNF+/− mice, respectively), n = 3. Data are shown as mean ± SEM. *p < .05, ***p < .001 compared with vehicle.
Cabergoline is a D2R-like agonist, and D2R agonists were shown to modulate ethanol-drinking behaviors (31). Therefore, changes in the expression or function of the D2R in the GDNF+/− mice might account for the attenuated effect of cabergoline on ethanol consumption. We therefore measured the level of [35S]GTPγS binding to the D2R-like in midbrain and striatal membrane homogenates treated with the dopamine D2R-like agonist, quinpirole. As shown in Figures 6C and 6D, the dose-dependent increase in [35S]GTPγS binding to the receptor upon D2R-like agonist treatment was similar in the GDNF+/− and GDNF+/+ mice [for midbrain: Treatment, F (6,122) = 12.53, p < .001; Genotype, F (6,122) = 2.24, p = .14; Treatment × Genotype interaction, F (6,122) = .18, p = .98; and for striatum: Treatment, F (6,123) = 59.37, p < .001), Genotype, F (1,123) = .62, p = .16), Interaction, F (6,123) = 2.10, p = .35], suggesting that reduction of the GDNF level in the GDNF+/− mice does not lead to alterations in the level or activity of the D2R-like. Hence, the effects of cabergoline on ethanol-drinking and -seeking behaviors are very likely to be mediated by GDNF.
Discussion
Here we show that cabergoline treatment results in an increase in GDNF level and in the subsequent activation of the GDNF pathway. Furthermore, we found that cabergoline selectively decreased ethanol-drinking and -seeking behaviors in rodents, including relapse. These actions of cabergoline are likely to be mediated by the midbrain, specifically by the VTA, because microinjection of cabergoline into this brain area was highly effective in reducing operant ethanol self-administration in rats. Finally, cabergoline failed to increase GDNF levels and to reduce ethanol consumption in GDNF heterozygous knockout mice. Together, these results suggest that cabergoline decreases ethanol-drinking and -seeking behaviors and that these effects are mediated via upregulation of the GDNF pathway in the mesolimbic system.
Previous studies showed that cabergoline treatment increases GDNF levels and secretion of GDNF in cultured astrocytes (9,10), and we found that cabergoline upregulates GDNF mRNA and protein levels in the dopaminergic-like SH-SY5Y neuroblastoma cell line. Moreover, the increase in GDNF expression was followed by the activation of the GDNF pathway, as shown by an increase in Ret and ERK1/2 phosphorylation. Importantly, the increase in GDNF expression and ERK1/2 phosphorylation induced by cabergoline was also observed in vivo. The source of GDNF induced by cabergoline treatment is yet to be determined and could be neurons and/or astrocytes, because GDNF is expressed in both types of cells (11). The mechanism by which cabergoline increases GDNF synthesis is also unknown. However, cabergoline is a dopaminergic receptors agonist (7), and in vitro studies showed that blockade of the D2Rs partially inhibited the increase in GDNF levels induced by cabergoline (9). In addition, activation of dopaminergic receptors by the nonselective agonist apomorphine or selective D3R agonists was found to stimulate GDNF synthesis in mesencephalic neuronal cultures (32,33). Therefore, activation of the dopaminergic receptors might contribute to the upregulation of GDNF levels by cabergoline.
Cabergoline did not significantly alter the self-administration of sucrose, a natural reward, in rats. These data suggest that cabergoline acts on processes induced by exposure to ethanol rather than on general rewarding and/or motivational mechanisms. Similarly, we previously reported that systemic injection of Ibogaine reduced ethanol but not sucrose consumption in rats (19), and intra-VTA infusion of GDNF did not affect sucrose self-administration (20). This relative selectivity of cabergoline’s action is in contrast to the approved drugs for alcohol craving, acamprosate and naltrexone, which have been shown to reduce water and sucrose consumption in rodents (34,35). This lack of selectivity in naltrexone’s and acamprosate’s actions suggests a general effect on motivation that could be related, for example, to the dysphoria induced by naltrexone (36,37) and the problem of compliance for both medications (4,5). Furthermore, we found that cabergoline also decreased lever-responding during an extinction period when ethanol was not available, suggesting that cabergoline reduces the motivation to seek ethanol. Importantly, cabergoline was effective in two procedures that model relapse (28–30). Specifically, we found that cabergoline administration reduced reacquisition of ethanol self-administration after a period of extinction and the motivation to seek ethanol after a period of abstinence. Similarly, we found that Ibogaine and GDNF decreased ethanol self-administration in models of relapse (19,20). Thus, cabergoline seems to have an improved selectivity for ethanol compared with the current medications available for the treatment of alcohol dependence and relapse.
Our results suggest that the VTA is a primary site of action for cabergoline. However, a high concentration of cabergoline also decreased ethanol self-administration when the drug was micro-injected in the SNc, however, diffusion of the drug from the SNc to the VTA could account for this effect. The possibility that the SNc is not a primary site of cabergoline-mediated modulation of ethanol-drinking behaviors is also supported by our recent finding showing that GDNF infused in the SNc does not alter ethanol self-administration (20). However, we cannot exclude the possible contribution of the SNc to cabergoline’s actions, because SNc neurons project to the dorsal striatum, a structure involved in the self-administration of drugs of abuse and alcohol (38–40) and is thought to also be implicated in addictive behaviors (41,42).
Cabergoline is an agonist of the dopaminergic adrenergic and serotoninergic receptors (6–8), which were shown to decrease ethanol consumption in rodents (31,43). We show here that the effect of cabergoline on ethanol consumption was detected in the GDNF+/+ mice but was abolished in GDNF+/− mice in which cabergoline was also unable to increase GDNF expression in the midbrain. Moreover, the GDNF+/− mice do not differ from the wild-type mice in their number of D1R-like (44) and in number or functionality of D2R-like (present study), suggesting that the change we observed is specific for GDNF and not due to compensatory mechanisms resulting in a differential dopaminergic response between the GDNF+/+ and the GDNF+/− mice to cabergoline. Hence, although the pharmacological action of cabergoline on other systems might contribute to the inhibitory effect of this drug on ethanol-related behaviors, the present results suggest that GDNF is a crucial component in the action of cabergoline to reduce ethanol-drinking and -seeking.
Our findings are in agreement with a growing body of evidence suggesting a modulatory role for GDNF in addiction (45). For example, VTA dopaminergic neurons are selectively vulnerable to neuroadaptations induced by repeated long-term exposure to drugs of abuse and ethanol (46,47), and administration of GDNF into the VTA blocks these biochemical adaptations to cocaine and morphine exposure (48). In addition, we recently reported that GDNF reverses the alteration of tyrosine hydroxylase immunoreactivity induced by prolonged ethanol exposure (45). Furthermore, GDNF+/− mice exhibit an increased sensitivity to morphine and psychostimulants as assessed by psychomotor sensitization, place conditioning, and self-administration procedures (45,48). Conversely, intra-VTA infusion of GDNF reduces cocaine place conditioning (48), and sustained administration of GDNF into the striatum impedes the acquisition of cocaine self-administration (45). We previously found that the upregulation of the GDNF pathway in the VTA by Ibogaine reduces rat ethanol-drinking behaviors (19). Similarly, Niwa et al. (49) reported that increasing endogenous GDNF expression in the brain blocks methamphetamine place conditioning and psychomotor sensitization. Finally, we showed that direct infusion of GDNF into the VTA results in a rapid and sustained reduction of ethanol self-administration (20). Importantly, GDNF blocks the ability of an ethanol prime to induce reacquisition of ethanol self-administration after an extinction period (20), suggesting that GDNF inhibits relapse to ethanol consumption. These studies and the present findings support the idea that upregulation of the GDNF pathway in the mesolimbic system by agents such as cabergoline might be a valuable strategy to combat alcoholism and other forms of addiction.
Cabergoline is approved for marketing in several countries including the U.S. for the treatment of hyperprolactinemia (50,51) and has also been used as adjunctive or monotherapy for Parkinson’s disease (52,53). In Parkinsonian patients, cabergoline is used at very high doses (2–6 mg/day) that were reported to increase the risk of cardiac valvulopathy (reviewed in [54]). However, in hyperprolactinemia, in which cabergoline is used at much lower doses (.25 to 3.5 mg/week) (51,55), six recent cross-sectional studies of patients treated for several years with the drug (45–79 months) found an association between moderate valvular regurgitation only at the highest cumulative doses of cabergoline (54,56,57). We found that systemic administration of a low dose of cabergoline (.25–.5 mg/kg) (21,58) was sufficient to significantly reduce ethanol-consumption and -seeking in rodents. Furthermore, a pilot study conducted on cocaine addicts reported that cabergoline significantly reduced cocaine use, as evaluated by analysis of cocaine metabolite levels in urine samples and self-report of substance use, with a weekly dose of only .5 mg (59). Therefore, these data suggest that low doses of cabergoline that circumvent the increasing prevalence of cardiac valvulopathy might be effective in reducing drug and alcohol abuse.
In conclusion, we found that cabergoline selectively reduces ethanol-drinking and -seeking behaviors. Moreover, we identified GDNF as the mechanism that mediates cabergoline’s actions to reduce ethanol-drinking behaviors, supporting the idea that upregulation of the GDNF pathway might be a valuable strategy for the treatment of addiction. Importantly, although in-depth clinical investigations are needed, our data suggest that cabergoline might be used as a selective medication for the treatment of alcohol addiction.
Supplementary Material
Acknowledgments
This research was supported by funds provided by National Institutes of Health-National Institute on Alcohol Abuse and Alcoholism RO1 AA014366 (DR) and the State of California for Medical Research on Alcohol and Substance Abuse through the University of California San Francisco (DR, PHJ, and SEB). We thank Pfizer for supplying us with cabergoline and Drs. Barry Hoffer and Andreas Tomac (National Institute on Drug Abuse) for the GDNF transgenic mice. We also thank Emily King, Quinn Yowell, and Madeline Ferwerda for technical support.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online.
References
- 1.World Health Organization. WHO global status report on alcohol 2004. Geneva: World Health Organization; 2004. [Google Scholar]
- 2.Heilig M, Egli M. Pharmacological treatment of alcohol dependence: Target symptoms and target mechanisms. Pharmacol Ther. 2006;111:855–876. doi: 10.1016/j.pharmthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Assanangkornchai S, Srisurapanont M. The treatment of alcohol dependence. Curr Opin Psychiatry. 2007;20:222–227. doi: 10.1097/YCO.0b013e3280fa837d. [DOI] [PubMed] [Google Scholar]
- 4.Johnson BA. Update on neuropharmacological treatments for alcoholism: Scientific basis and clinical findings. Biochem Pharmacol. 2008;75:34–56. doi: 10.1016/j.bcp.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouza C, Angeles M, Munoz A, Amate JM. Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: A systematic review. Addiction. 2004;99:811–828. doi: 10.1111/j.1360-0443.2004.00763.x. [DOI] [PubMed] [Google Scholar]
- 6.Millan MJ, Maiofiss L, Cussac D, Audinot V, Boutin JA, Newman-Tancredi A. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. I. A multivariate analysis of the binding profiles of 14 drugs at 21 native and cloned human receptor subtypes. J Pharmacol Exp Ther. 2002;303:791–804. doi: 10.1124/jpet.102.039867. [DOI] [PubMed] [Google Scholar]
- 7.Newman-Tancredi A, Cussac D, Audinot V, Nicolas JP, De Ceuninck F, Boutin JA, et al. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. II. Agonist and antagonist properties at subtypes of dopamine D(2)-like receptor and alpha(1)/alpha(2)-adrenoceptor. J Pharmacol Exp Ther. 2002;303:805–814. doi: 10.1124/jpet.102.039875. [DOI] [PubMed] [Google Scholar]
- 8.Newman-Tancredi A, Cussac D, Quentric Y, Touzard M, Verriele L, Carpentier N, et al. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. III. Agonist and antagonist properties at serotonin, 5-HT(1) and 5-HT(2), receptor subtypes. J Pharmacol Exp Ther. 2002;303:815–822. doi: 10.1124/jpet.102.039883. [DOI] [PubMed] [Google Scholar]
- 9.Ohta K, Fujinami A, Kuno S, Sakakimoto A, Matsui H, Kawahara Y, et al. Cabergoline stimulates synthesis and secretion of nerve growth factor, brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor by mouse astrocytes in primary culture. Pharmacology. 2004;71:162–168. doi: 10.1159/000077451. [DOI] [PubMed] [Google Scholar]
- 10.Ohta K, Kuno S, Mizuta I, Fujinami A, Matsui H, Ohta M. Effects of dopamine agonists bromocriptine, pergolide, cabergoline, and SKF-38393 on GDNF, NGF, and BDNF synthesis in cultured mouse astrocytes. Life Sci. 2003;73:617–626. doi: 10.1016/s0024-3205(03)00321-7. [DOI] [PubMed] [Google Scholar]
- 11.Airaksinen MS, Saarma M. The GDNF family: Signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- 12.Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: A glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 13.Pascual A, Hidalgo-Figueroa M, Piruat JI, Pintado CO, Gomez-Diaz R, Lopez-Barneo J. Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat Neurosci. 2008;11:755–761. doi: 10.1038/nn.2136. [DOI] [PubMed] [Google Scholar]
- 14.Beck KD, Valverde J, Alexi T, Poulsen K, Moffat B, Vandlen RA, et al. Mesencephalic dopaminergic neurons protected by GDNF from axotomy-induced degeneration in the adult brain. Nature. 1995;373:339–341. doi: 10.1038/373339a0. [DOI] [PubMed] [Google Scholar]
- 15.Sarabi A, Hoffer BJ, Olson L, Morales M. GFRalpha-1 mRNA in dopaminergic and nondopaminergic neurons in the substantia nigra and ventral tegmental area. J Comp Neurol. 2001;441:106–117. doi: 10.1002/cne.1400. [DOI] [PubMed] [Google Scholar]
- 16.Trupp M, Belluardo N, Funakoshi H, Ibanez CF. Complementary and overlapping expression of glial cell line-derived neurotrophic factor (GDNF), c-ret proto-oncogene, and GDNF receptor-alpha indicates multiple mechanisms of trophic actions in the adult rat CNS. J Neurosci. 1997;17:3554–3567. doi: 10.1523/JNEUROSCI.17-10-03554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21:467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- 18.McBride WJ, Murphy JM, Ikemoto S. Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies. Behav Brain Res. 1999;101:129–152. doi: 10.1016/s0166-4328(99)00022-4. [DOI] [PubMed] [Google Scholar]
- 19.He DY, McGough NN, Ravindranathan A, Jeanblanc J, Logrip ML, Phamluong K, et al. Glial cell line-derived neurotrophic factor mediates the desirable actions of the anti-addiction drug ibogaine against alcohol consumption. J Neurosci. 2005;25:619–628. doi: 10.1523/JNEUROSCI.3959-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carnicella S, Kharazia V, Jeanblanc J, Janak PH, Ron D. GDNF is a fast-acting potent inhibitor of alcohol consumption and relapse. Proc Natl Acad Sci U S A. 2008;105:8114–8119. doi: 10.1073/pnas.0711755105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyagi M, Arai N, Taya F, Itoh F, Komatsu Y, Kojima M, et al. Effect of cabergoline, a long-acting dopamine D2 agonist, on reserpine-treated rodents. Biol Pharm Bull. 1996;19:1499–1502. doi: 10.1248/bpb.19.1499. [DOI] [PubMed] [Google Scholar]
- 22.Miyagi M, Itoh F, Taya F, Arai N, Isaji M, Kojima M, et al. Dopamine receptor affinities in vitro and stereotypic activities in vivo of cabergoline in rats. Biol Pharm Bull. 1996;19:1210–1213. doi: 10.1248/bpb.19.1210. [DOI] [PubMed] [Google Scholar]
- 23.Carnicella S, Amamoto R, Ron D. Excessive alcohol consumption is blocked by GDNF. Alcohol. 2009;43:35–43. doi: 10.1016/j.alcohol.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wise RA. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia. 1973;29:203–210. doi: 10.1007/BF00414034. [DOI] [PubMed] [Google Scholar]
- 25.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. San Diego: Academic Press; 1998. [Google Scholar]
- 26.Ikemoto S. Dopamine reward circuitry: Two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodd ZA, Melendez RI, Bell RL, Kuc KA, Zhang Y, Murphy JM, et al. Intracranial self-administration of ethanol within the ventral tegmental area of male Wistar rats: Evidence for involvement of dopamine neurons. J Neurosci. 2004;24:1050–1057. doi: 10.1523/JNEUROSCI.1319-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bouton ME. Context, ambiguity, and unlearning: Sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- 29.Le A, Shaham Y. Neurobiology of relapse to alcohol in rats. Pharmacol Ther. 2002;94:137–156. doi: 10.1016/s0163-7258(02)00200-0. [DOI] [PubMed] [Google Scholar]
- 30.Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: An assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vengeliene V, Bilbao A, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. Br J Pharmacol. 2008;154:299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo H, Tang Z, Yu Y, Xu L, Jin G, Zhou J. Apomorphine induces trophic factors that support fetal rat mesencephalic dopaminergic neurons in cultures. Eur J Neurosci. 2002;16:1861–1870. doi: 10.1046/j.1460-9568.2002.02256.x. [DOI] [PubMed] [Google Scholar]
- 33.Du F, Li R, Huang Y, Li X, Le W. Dopamine D3 receptor-preferring agonists induce neurotrophic effects on mesencephalic dopamine neurons. Eur J Neurosci. 2005;22:2422–2430. doi: 10.1111/j.1460-9568.2005.04438.x. [DOI] [PubMed] [Google Scholar]
- 34.Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an {alpha}4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci U S A. 2007;104:12518–12523. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Escher T, Mittleman G. Schedule-induced alcohol drinking: Non-selective effects of acamprosate and naltrexone. Addict Biol. 2006;11:55–63. doi: 10.1111/j.1369-1600.2006.00004.x. [DOI] [PubMed] [Google Scholar]
- 36.Hollister LE, Johnson K, Boukhabza D, Gillespie HK. Aversive effects of naltrexone in subjects not dependent on opiates. Drug Alcohol Depend. 1981;8:37–41. doi: 10.1016/0376-8716(81)90084-3. [DOI] [PubMed] [Google Scholar]
- 37.Crowley TJ, Wagner JE, Zerbe G, Macdonald M. Naltrexone-induced dysphoria in former opioid addicts. Am J Psychiatry. 1985;142:1081–1084. doi: 10.1176/ajp.142.9.1081. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Carnicella S, Phamluong K, Jeanblanc J, Ronesi JA, Chaudhri N, et al. Ethanol induces long-term facilitation of NR2B-NMDA receptor activity in the dorsal striatum: Implications for alcohol drinking behavior. J Neurosci. 2007;27:3593–3602. doi: 10.1523/JNEUROSCI.4749-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vanderschuren LJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. J Neurosci. 2005;25:8665–8670. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeanblanc J, He DY, McGough NN, Logrip ML, Phamluong K, Janak PH, et al. The dopamine D3 receptor is part of a homeostatic pathway regulating ethanol consumption. J Neurosci. 2006;26:1457–1464. doi: 10.1523/JNEUROSCI.3786-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 42.Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, et al. Cocaine cues and dopamine in dorsal striatum: Mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weinshenker D, Schroeder JP. There and back again: A tale of norepinephrine and drug addiction. Neuropsychopharmacology. 2007;32:1433–1451. doi: 10.1038/sj.npp.1301263. [DOI] [PubMed] [Google Scholar]
- 44.Airavaara M, Planken A, Gaddnas H, Piepponen TP, Saarma M, Ahtee L. Increased extracellular dopamine concentrations and FosB/DeltaFosB expression in striatal brain areas of heterozygous GDNF knockout mice. Eur J Neurosci. 2004;20:2336–2344. doi: 10.1111/j.1460-9568.2004.03700.x. [DOI] [PubMed] [Google Scholar]
- 45.Carnicella S, Ron D. GDNF—A potential target to treat addiction [published online ahead of print December 24] Pharmacol Ther. 2008 doi: 10.1016/j.pharmthera.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: The role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 47.Ortiz J, Fitzgerald LW, Charlton M, Lane S, Trevisan L, Guitart X, et al. Biochemical actions of chronic ethanol exposure in the mesolimbic dopamine system. Synapse. 1995;21:289–298. doi: 10.1002/syn.890210403. [DOI] [PubMed] [Google Scholar]
- 48.Messer CJ, Eisch AJ, Carlezon WA, Jr, Whisler K, Shen L, Wolf DH, et al. Role for GDNF in biochemical and behavioral adaptations to drugs of abuse. Neuron. 2000;26:247–257. doi: 10.1016/s0896-6273(00)81154-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Niwa M, Nitta A, Yamada Y, Nakajima A, Saito K, Seishima M, et al. An inducer for glial cell line-derived neurotrophic factor and tumor necrosis factor-alpha protects against methamphetamine-induced rewarding effects and sensitization. Biol Psychiatry. 2007;61:890–901. doi: 10.1016/j.biopsych.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 50.Colao A, Di Sarno A, Guerra E, De Leo M, Mentone A, Lombardi G. Drug insight: Cabergoline and bromocriptine in the treatment of hyperprolactinemia in men and women. Nat Clin Pract Endocrinol Metab. 2006;2:200–210. doi: 10.1038/ncpendmet0160. [DOI] [PubMed] [Google Scholar]
- 51.Webster J, Piscitelli G, Polli A, Ferrari CI, Ismail I, Scanlon MF. A comparison of cabergoline and bromocriptine in the treatment of hyperprolactinemic amenorrhea. Cabergoline Comparative Study Group. N Engl J Med. 1994;331:904–909. doi: 10.1056/NEJM199410063311403. [DOI] [PubMed] [Google Scholar]
- 52.Jankovic J, Stacy M. Medical management of levodopa-associated motor complications in patients with Parkinson’s disease. CNS Drugs. 2007;21:677–692. doi: 10.2165/00023210-200721080-00005. [DOI] [PubMed] [Google Scholar]
- 53.Bonuccelli U. Comparing dopamine agonists in Parkinson’s disease. Curr Opin Neurol. 2003;16(Suppl 1):S13–19. doi: 10.1097/00019052-200312001-00004. [DOI] [PubMed] [Google Scholar]
- 54.Kars M, Pereira AM, Bax JJ, Romijn JA. Cabergoline and cardiac valve disease in prolactinoma patients: Additional studies during long-term treatment are required. Eur J Endocrinol. 2008;159:363–367. doi: 10.1530/EJE-08-0611. [DOI] [PubMed] [Google Scholar]
- 55.De Rosa M, Ciccarelli A, Zarrilli S, Guerra E, Gaccione M, Di Sarno A, et al. The treatment with cabergoline for 24 month normalizes the quality of seminal fluid in hyperprolactinaemic males. Clin Endocrinol (Oxf) 2006;64:307–313. doi: 10.1111/j.1365-2265.2006.02461.x. [DOI] [PubMed] [Google Scholar]
- 56.Colao A, Galderisi M, Di Sarno A, Pardo M, Gaccione M, D’Andrea M, et al. Increased prevalence of tricuspid regurgitation in patients with prolactinomas chronically treated with cabergoline. J Clin Endocrinol Metab. 2008;93:3777–3784. doi: 10.1210/jc.2007-1403. [DOI] [PubMed] [Google Scholar]
- 57.Bogazzi F, Buralli S, Manetti L, Raffaelli V, Cigni T, Lombardi M, et al. Treatment with low doses of cabergoline is not associated with increased prevalence of cardiac valve regurgitation in patients with hyperprolactinaemia. Int J Clin Pract. 2008 doi: 10.1111/j.1742-1241.2008.01779.x. [DOI] [PubMed] [Google Scholar]
- 58.Benedetti MS, Dostert P, Barone D, Efthymiopoulos C, Peretti G, Roncucci R. In vivo interaction of cabergoline with rat brain dopamine receptors labelled with [3H]N-n-propylnorapomorphine. Eur J Pharmacol. 1990;187:399–408. doi: 10.1016/0014-2999(90)90367-f. [DOI] [PubMed] [Google Scholar]
- 59.Shoptaw S, Watson DW, Reiber C, Rawson RA, Montgomery MA, Majewska MD, et al. Randomized controlled pilot trial of cabergoline, hydergine and levodopa/carbidopa: Los Angeles Cocaine Rapid Efficacy Screening Trial (CREST) Addiction. 2005;100(suppl 1):78–90. doi: 10.1111/j.1360-0443.2005.00991.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.