Abstract
Although the etiology of essential hypertension is not clearly understood, endothelial dysfunction from chronic infection and/or impaired glucose metabolism may be involved. We hypothesized that salivary lysozyme, a marker for oral infection and hyperglycemia, might display a significant relationship with hypertension, an early stage of cardiovascular disease. Logistic regression analyses of the Kuopio Oral Health and Heart Study demonstrated that persons with higher lysozyme levels were more likely to have hypertension, after adjustment for age, gender, smoking, BMI, diabetes, the ratio of total cholesterol to HDL cholesterol, and C-reactive protein. The exposure to increasing quartiles of lysozyme was associated with adjusted Odds Ratios for the outcome, hypertension, 1.00 (referent), 1.25, 1.42, and 2.56 (linear trend p < 0.003). When we restricted the sample to the individuals without heart disease (N = 250), we observed a non-significant trend for increasing odds. Our hypothesis—"high salivary lysozyme levels are associated with the odds of hypertension"—was confirmed.
Keywords: salivary lysozyme, hypertension, cytokines, advanced glycation end-products, endothelial dysfunction
INTRODUCTION
Sixty-five million people in the US suffer from hypertension (AHA, 2004), and from 90 to 95% of these cases are idiopathic or essential hypertension where the exact cause is not clearly understood. Hypertension, a component of metabolic syndrome, is a risk factor for atherosclerosis, coronary heart disease, stroke, and heart failure (AHA, 2004). The etiology of essential hypertension may involve dyslipidemia, insulin resistance, vascular inflammation, and subsequent endothelial dysfunction (Reaven, 2002; Bautista, 2003; Grundy, 2003).
Salivary lysozyme is locally produced in the oral cavity, and is derived from neutrophils in response to oral infection (Klempner and Malech, 1998) and hyperglycemia (Karima et al., 2005). Lysozyme contains a domain that has strong affinity for advanced glycation end-products (AGE), and has been used for the removal of AGE in persons with diabetes (Zheng et al., 2001).
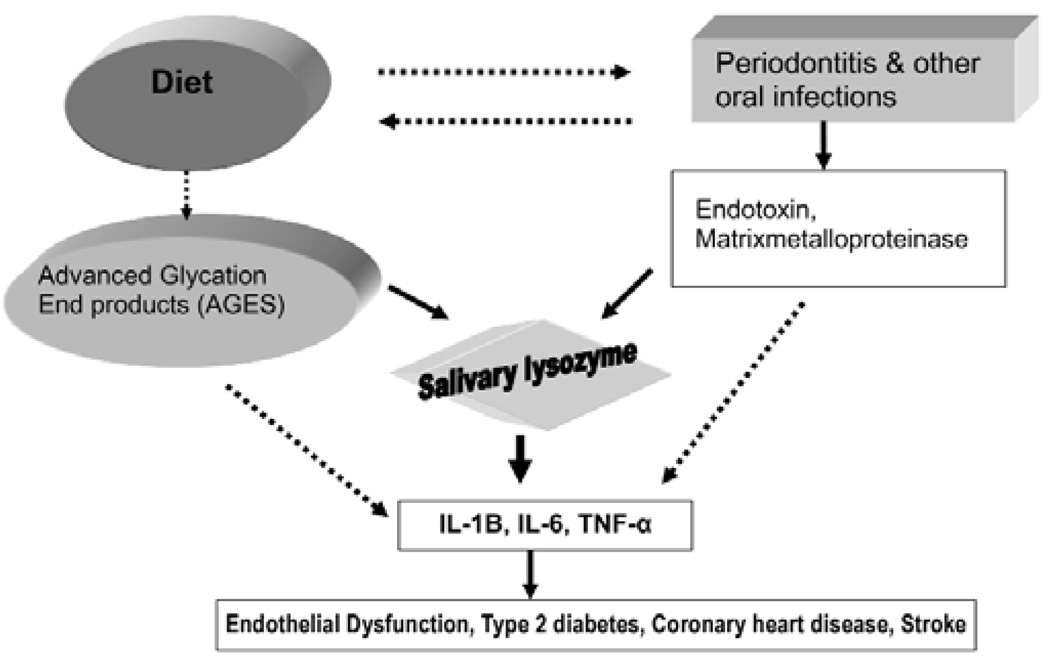
Although periodontitis has been presumed to be a source of systemic tissue necrosis factor-α (TNF-α), interleukin-6 (IL-6), and C-reactive protein (CRP), hyperglycemia is also known to be a powerful source of these inflammatory mediators (Liu and Willett, 2002; Vlassara et al., 2002; Vlassara and Palace, 2002, 2003; Pickup, 2004). Because salivary lysozyme is closely related to both infection and impaired glucose metabolism, we postulated that salivary lysozyme might assess(AQ) the pro-inflammatory process involved in atherogenesis from infection as well as from impaired glucose metabolism, as illustrated in the conceptual model (Fig. 1, solid arrows) (Janket et al., 2006, 2007a). Our previous results supported this thesis, with a much higher odds ratio (OR) for coronary artery disease (CAD) of 3.64 (Janket et al., 2006) than for periodontitis alone (Beck et al., 1996) or nutrition alone (Joshipura et al., 1996) (Fig. 1, dotted arrows).
Figure 1.
Conceptual model for the relationship of salivary lysozyme and endothelial dysfunction. TNF-α, Tumor necrosis factor-alpha; IL-1B, Interleukin-1B; IL-6, Interleukin-6. **Reproduced with permission (Janket et al., 2007a).
We postulated that the dual pro-inflammatory contribution assessed(AQ) by salivary lysozyme might be evident even in hypertension, an early stage of CAD. The hypothetical relationship of hypertension to cardiovascular disease (CVD) is shown in the APPENDIX Figure.
The objective of the current study was to test the hypothesis that "salivary lysozyme is associated with hypertension" in the Kuopio Oral Health and Heart Study cohort.
MATERIALS & METHODS
Ethical and Human Participants' Protection Consideration
This is a secondary data analysis of the Kuopio Oral Health and Heart (KOHH) Study. The Joint Ethical Committee of the Kuopio University Hospital and the University of Kuopio approved the study protocol. All participants provided written informed consent, and the KOHH study adhered to the guidelines set forth by the Declaration of Helsinki and the Belmont Accord, to ensure the safety of individuals participating in the research.
Study Population
We recruited 250 consecutive cardiac patients at Kuopio University Hospital who were referred for coronary angiography and confirmed as having CAD. Potential participants were excluded if they took antibiotics during the previous 30 days or had chronic infection other than dental disease. Also recruited were 250 age- and gender-matched control individuals from the same catchment area without any evidence of coronary heart disease as determined from the pre-admission tests. The control individuals were admitted to general surgery or otorhinolaryngology departments at the same hospital for elective surgery. The same exclusion and inclusion criteria were applied to non-cardiac patients. Additional exclusion criteria were: (1) those who needed emergency coronary by-pass surgery or valvular replacement surgery; (2) those whose disease status was so grave that a dental examination or dental x-ray could not be performed safely; and (3) those who needed antibiotic prophylaxis prior to periodontal probing.
Data Collection
(i) Ascertainment of Outcome
In 1998–1999, 169 individuals diagnosed as hypertensive or under physicians' care for hypertension were identified via medical record review and categorized as hypertensive=1. If no records of diagnosis or treatment for hypertension were documented, they were considered non-hypertensive (hypertensive=0). These data were subsequently validated for reliability and validity.
(ii) Saliva Collection
To avoid diurnal fluctuation, we collected saliva samples from the patients between 7 and 9 a.m. The patients had been advised not to eat or smoke 1 hr before collection. Using the free-flow method, we collected saliva into a 10-mL test tube for 5 min after the patient's initial swallowing.
(iii) Lysozyme Analysis
Salivary lysozyme from fresh saliva was quantified at the Kuopio University research laboratory by a modified lysoplate method, with Micrococcus lysodeikticus (Sigma Chemical Co., St. Louis, MO, USA) and human milk lysozyme (Sigma) and bovine serum albumin (Sigma) as standards, according to the methods suggested previously (Rudney and Smith, 1985).
(iv) Vascular Risk Factors
All blood samples were analyzed immediately in the hospital laboratory. The analyses were performed in batches including both cases and control individuals, so that any potential environmental changes and measurement errors would be evenly distributed. Total cholesterol (TC), triglyceride (TG), and high-density lipoprotein cholesterol (HDL) were measured by an automated enzymatic technique, and the 'Total to HDL cholesterol' (T/H) ratio was calculated by statistical function. C-reactive protein (CRP) was measured by high-sensitivity immunoturbidometry assay in a Hitachi 717 analyzer.
(v) Other Confounding Factors
Age was recorded in yrs, gender was labeled 0 for males and 1 for females, and smoking was categorized as 0 if the person had never smoked, 1 if he/she smoked in the past, and 2 if he/she was currently smoking. We calculated body mass index (BMI) by dividing weight in kilograms by squared height in meters. Diabetes mellitus (DM) status was ascertained by medical record review; a person was labeled 0 for the absence of disease, and 1 if he/she had been diagnosed or was receiving dietary advice, oral hypoglycemic agents, or insulin. 'Years of education' was used as a socioeconomic indicator.
Statistical Methods
Using Statistical Analysis System version 9.1, t tests, chi-square tests, or Wilcoxon non-parametric tests, depending on the variable distributions, we compared the basic characteristics such as mean age, gender, smoking status, body mass index, number of teeth, and cholesterol levels of individuals with and without hypertension.
Using logistic regression methods, we explored the relationship between the probability of having hypertension and salivary lysozyme levels. All P values were calculated as two-tailed, and all confidence intervals were computed at the 95% level. The predictor lysozyme level was highly skewed and was transformed into natural log. Since one unit increase in log-transformed SLZ level was equivalent to one quartile increase (Table 1, column heading), we analyzed the data in quartiles for easier clinical interpretation. We calculated odds ratios (OR) for each quartile compared with the lowest quartile as the reference group, controlling for established confounding variables such as age, gender, smoking, BMI, diabetes, 'total cholesterol to HDL cholesterol' ratio as a continuous variable, and CRP dichotomized at 3 mg/L or 10 mg/L as has been suggested (Ridker et al., 1998). However, we used only CRP dichotomized at 10 mg/L in our final models, because CRP at the 3 mg/L cut-off was not useful as a predictor of CAD, due to a high false-positive rate (Janket et al., 2007b).
Table 1.
Distribution of CHD Risk Factors According to the Quartiles of Lysozyme
| Lysozyme Quartile 1 Log-transformed mean = 1.24 |
Lysozyme Quartile 2 Log-transformed mean = 2.48 |
Lysozyme Quartile 3 Log-transformed mean = 3.40 |
Lysozyme Quartile 4 Log-transformed mean = 4.32 |
|
|---|---|---|---|---|
| Age (mean ± SD) | 63.2 ± 7.8 | 59.7 ± 9.9 | 58.5 ± 9.6 | 57.7 ± 9.8 |
| BMI (mean ± SD) Kg/M2 | 25.2 (± 3.4) | 25.9 (± 4.3) | 24.5 (± 3.3) | 24.8 (± 3.4) |
| Number of teeth (mean ± SD) |
15.5 (± 11.01) | 12.2 (± 10.33) | 13.0 (± 10.59) | 11.10 (± 10.08) |
| Asymptotic dental score (mean ± SD) |
6.87 (± 7.96) | 8.37 (± 8.02) | 7.77 (± 7.96) | 9.81 (± 9.77) |
| Edentulism, N (%) | 26 (21.7%) | 31 (24.8%) | 29 (22.5%) | 38 (30.7%) |
| Diabetes, N (%) | 9 (7.4%) | 14 (12.0%) | 9 (7.3%) | 17 (14.4%) |
| Smoking, N (%) | ||||
| • Never smokers | 97 (79.5%) | 76 (63.9%) | 89 (70.1%) | 68 (56.6%) |
| • Past smokers | 21 (17.2%) | 27 (22.6%) | 25 (19.7%) | 36 (30.0%) |
| • Current smokers | 4 (3.3%) | 16 (13.4%) | 13 (10.2%) | 16 (13.3%) |
| Total/HDL cholesterol ratio (mean ± SD) |
4.85 (± 1.93) | 5.02 (± 2.35) | 5.08 (± 2.46) | 5.22 (± 1.43) |
| HDL > = 1.14 mmol/L (median), N (%) |
73 (58.3%) | 60 (48.4%) | 64 (49.6%) | 55 (44.7%) |
| Triglyceride > = 1.72 mmol/L (median), N (%) |
56 (45.1%) | 60 (48.0%) | 67 (51.9%) | 69 (56.6%) |
| C-reactive protein ≥ 10 mg/L, N (%) |
31 (24.8%) | 39 (31.2%) | 40 (31.0%) | 43 (34.7%) |
| C-reactive protein ≥ 3 mg/L, N (%) |
101 (80.8%) | 99 (79.2%) | 108 (83.7%) | 108 (87.1%) |
Due to missing values, not all variables have N = 500.
Because lysozyme is associated with inflammation originating from both infection and hyperglycemia, to assess the hyperglycemia-related lysozyme effect, independent of oral infection, we controlled for CRP as well as high leukocyte count dichotomized at the level where risk for cardiac events was reported to increase (6.7 × 109) (Margolis et al., 2005).
To control for the effect of CAD, we adjusted CAD status in a multivariate model and also conducted stratified analyses dividing the cohort by CAD status. However, stratified analyses ignore the fact that CAD and non-CAD groups may have different references. Therefore, we stratified the whole cohort into four groups: (1) non-CAD group with low lysozyme levels (reference); (2) non-CAD group with high lysozyme levels; (3) CAD group with low lysozyme levels; and (4) CAD group with high lysozyme levels. We then compared their risks of having hypertension. Additionally, we substituted high triglyceride levels and low HDL levels for 'Total to HDL cholesterol' ratio. to control for the effects of metabolic dyslipidemia.
RESULTS
Most CAD risk factors were more prevalent in the hypertensive group. However, unlike the US population, BMI, years of education, smoking status, and the ratio of total to HDL cholesterol were not significantly different among the groups. These basic characteristics of this cohort have been reported previously (Janket et al., 2004). As reported in our previous study, that edentulous persons favored soft carbohydrates that might elevate glycated protein level (Johansson et al., 1994), our results demonstrated proportionate increases in edentulism and salivary lysozyme levels (Table 1) (Janket et al., 2006).
In multivariate analyses controlled for age in yrs, gender, smoking in 3 categories (never, past, current), diabetes, total/HDL cholesterol ratio, education as a socio-economic indicator, and CRP levels, each unit increase in log-transformed SLZ resulted in roughly a 50% increase in OR for hypertension (Model 1 in Table 2). Similarly, each quartile increase of salivary lysozyme resulted in an increased OR (95% confidence interval) of 1.00 (referent), 1.25 (0.68 , 2.32), 1.42 (0.78 , 2.60), and 2.56 (1.39 , 4.70), p-value for linear trend < 0.003, presented in Model 2 (Table 2). This association remained independent of leukocyte counts or CRP (Model 3 in Table 2).
Table 2.
Multivariate Models for the Full Cohort and Subcohorts Stratified by CAD
| Multivariate Models | OR (95% confidence interval) | p-value for Parameter Estimates | |
|---|---|---|---|
| Model 1* (N = 500) |
Natural log-transformed lysozyme and covariates* |
1.47 (1.16 – 1.87) | < 0.001 |
| Model 2† (N = 500) |
Quartiled lysozyme levels and covariates | ||
| • Quartile 1 | 1.00 | < 0.003 | |
| • Quartile 2 | 1.25 (0.68 – 2.32) | (linear trend) | |
| • Quartile 3 | 1.42 (0.78 – 2.60) | ||
| • Quartile 4 | 2.56 (1.39 – 4.70) | ||
| Model 3‡ (N = 500) |
Quartiled lysozyme levels and covariates† | ||
| • Quartile 1 | 1.00 | < 0.004 | |
| • Quartile 2 | 1.16 (0.63 – 2.15) | (linear trend) | |
| • Quartile 3 | 1.32 (0.72 – 2.41) | ||
| • Quartile 4 | 2.49 (1.35 – 4.58) | ||
| Model 4§ | Quartiled lysozyme levels and covariates |
Non-CAD group (N = 250) |
CAD group (N = 250) |
| • Quartile 1 | 1.00 | 1.00 | |
| • Quartile 2 | 1.23 (0.53 – 2.90) | 1.14 (0.48, 2.74) | |
| • Quartile 3 | 1.38 (0.55 – 3.45) | 1.17 (0.51, 2.66) | |
| • Quartile 4 | 2.31 (0.87 – 6.18) | 1.86 (0.82, 4.21) | |
| P-value for trend < 0.16 | P-value for trend < 0.12 | ||
All models adjusted for age in yrs, gender, smoking in 3 categories (never, past, current), diabetes, total/HDL cholesterol ratio, education as a socio-economic indicator, and CRP ≥ 10 mg/L.
Model 1: natural log-transformed lysozyme as predictors and covariates listed above.
Model 2: Quartiles of lysozyme and covariates.
Model 3: Quartiles of lysozyme and covariates plus high leukocyte count (≥ 6.7 × 109 per L).
Model 4: Analyses stratified by CAD status.
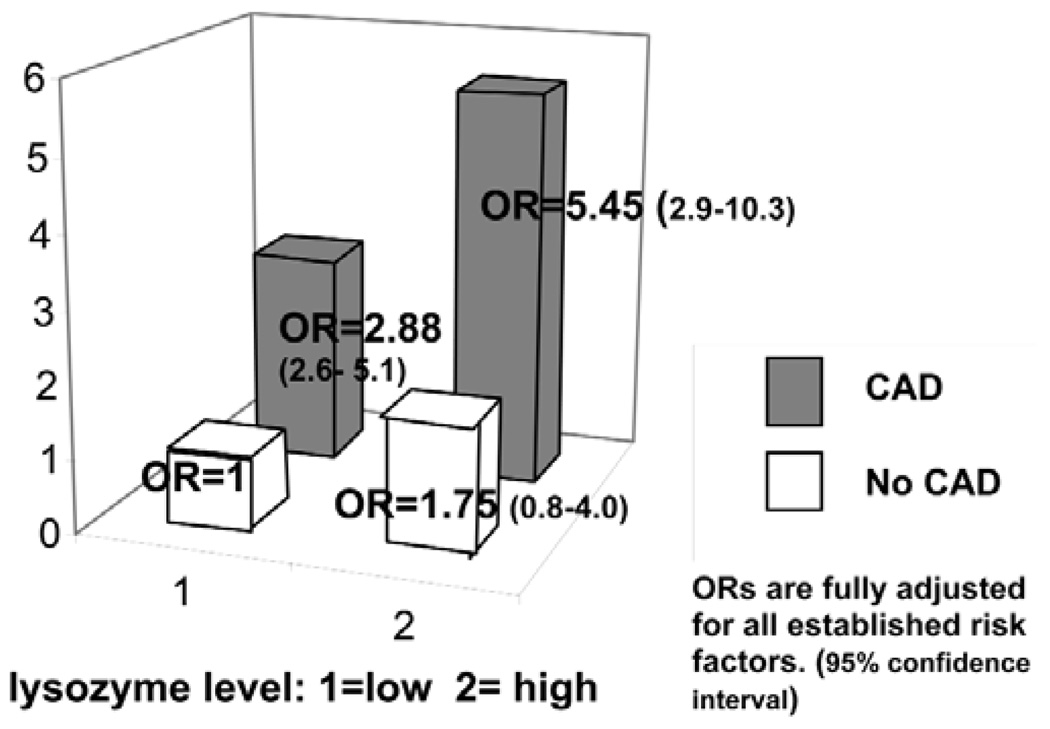
Among the four groups' combined risk of dichotomies of CAD (yes/no) and lysozyme levels (high/low), CAD patients with high lysozyme levels had the highest OR (95% confidence interval) of 5.5 (2.8 –10.3), followed by CAD patients with low lysozyme levels [2.88 (1.6 – 5.1)], and individuals without CAD with high lysozyme levels [1.75 (0.77–3.99)], compared with the reference group, those without CAD who had low lysozyme levels (Fig. 2). The odds ratio difference between the CAD group with high and low lysozyme (5.45–2.88 = 2.57) was attributable to lysozyme levels (Fig. 2), which is similar to the OR (2.56) we observed in a multivariate analysis adjusted for CAD (Model 3 in Table 2). In the stratified analyses, the trend of increasing odds for hypertension with increasing lysozyme levels was evident, but the p-value did not reach the significance level of 0.05 (Model 4 in Table 2).
Figure 2.
Odds ratios for hypertension stratified by CAD status and salivary lysozyme levels.
The substitution of total/HDL cholesterol ratio with high triglyceride levels, a marker for carbohydrate metabolism, and low HDL cholesterol did not alter results.
DISCUSSION
Our results, derived from 500 Finnish men and women with or without CAD, suggest that the top quartile of salivary lysozyme levels was significantly associated with prevalent hypertension, yielding a fully adjusted OR of 2.56 with a linear trend p-value < 0.004. Thus, our hypothesis was confirmed.
This is in agreement with the general paradigm that inflammation is an important part of pathogenesis of atherosclerosis, and also with our previous report that high salivary lysozyme was associated with a much stronger increase in odds for CAD (OR = 3.64) (Janket et al., 2006).
The present study is the first to assess the combined oral health impact, via oral infection and nutritional pathways, on hypertension in a mixed cohort including men and women. Among individuals who did not have CAD, log-transformed SLZ (OR = 1.41, p = 0.045) was a significant predictor of prevalent hypertension, while log-transformed CRP was not (OR = 0.92, p = 0.34; data not presented). However, when CAD was modeled as an outcome, the OR associated with CRP > = 10 mg/L increased dramatically (OR = 24.1). Indirectly, this suggests that CRP may be a marker for the inflammation surrounding established atheroma, rather than inflammation in the pre-atheroma stage. In contrast, lysozyme appears to be associated with the inflammatory process, even in the pre-atheroma stage, suggesting a possible causal relationship. However, prospective studies are needed to corroborate this finding.
Another noteworthy fact is that, in diabetic patients with hyperglycemia, the prevalence of periodontitis is extremely high (Miller et al., 1992; Tervonen et al., 1994; Yalda et al., 1994; Nishimura et al., 1996; Salvi et al., 1997; Collin et al., 1998, 2000; Cutler et al., 1999; Kurtis et al., 1999; Losche et al., 2000; Sandberg et al., 2000). Thus, whether the increased risk of CVD among diabetic patients is attributable to hyperglycemia or to periodontitis is yet to be determined. We postulate that salivary lysozyme may be an appropriate marker for the inflammation initiated by the combination of oral infection and hyperglycemia (Janket et al., 2007a).
The strength of the current study is that this ethnically and socioeconomically homogeneous cohort prevented any confounding by these factors. Additionally, confounding by smoking, BMI, and other demographic factors is also unlikely, because these factors were distributed similarly in the two compared groups. Obesity is an important risk factor for hypertension in other cohorts (Doll et al., 2002). However, in this homogeneously slender Nordic population, the mean BMI in the hypertensive and non-hypertensive groups was 25.2 and 25.0 kg/M, respectively.
Although these data were drawn from a Finnish cohort, these results may be applicable to the US population. This is supported by the fact that the lipid profile is comparable with that of the US national data recorded in the National Health and Nutrition Examination Survey (NHANES III) (Clearfield et al., 2000). For example, in the NHANES III, the mean total cholesterol, mean LDL-C level, and median triglyceride levels were 5.71 mmol/L, 3.89 mmol/L, and 1.78 mmol/L. The Finnish counterparts of these values were 5.73 mmol/L, 3.64 mmol/L, and 1.96 mmol/L, respectively.
Several limitations of our present study should be mentioned. Although the outcome was ascertained 3 yrs after the baseline, we consider our study as a cross-sectional investigation with two selected groups, cases and control individuals. Thus, potential selection bias is possible. However, the central location of Kuopio University Hospital in the region might have alleviated potential biases, because the control individuals came from the same population base. Second, because we determined the outcome, hypertensive status, by review of medical records, it is possible that some misclassification might have occurred. However, the validity and reliability tests with 80 randomly selected persons displayed good agreement rates (0.81 and 0.93, respectively), and misclassification did not appear to be a major problem. Third, due to the design of our study, the results should not be interpreted as causal association between salivary lysozyme and hypertension.
From data derived from 500 Finnish persons, we observed that a high salivary lysozyme level was significantly associated with prevalent hypertension, after adjusting for established cardiovascular risk factors, as well as leukocyte count or CRP levels. This trend was evident in the subgroup analyses stratified by CAD status. Further prospective investigations are warranted to establish whether this relationship is causal.
Supplementary Material
ACKNOWLEDGMENTS
The funding sources listed here had no influence on our results. Dr. Janket is a recipient of The National Scientist Development Grant from the American Heart Association. Dr. Baird is supported by the intramural program of the National Institutes of Health. Dr. Meurman was supported by grant TYH 3245 from the Helsinki University Central Hospital, Helsinki, Finland, the Ulf Nilsonne Foundation (SalusAnsvar Prize), Stockholm, Sweden, and the Päivikki and Sakari Sohlberg Foundation, Helsinki, Finland. Dr. Jones is supported by Boston University. Dr. Van Dyke is supported by NIH grant DE13191 and USPHS grant DE15566.
Drs. Janket and Meurman mentored Dr. Qvarnstrom in preparing this manuscript, as partial fulfillment of the requirements for the PhD degree at the University of Helsinki.
The authors express their appreciation to Susan Schur for her editorial contributions.
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.iadrjournals.org/cgi/content/full/87/5/480/DC1.
REFERENCES
- AHA. Statistical fact sheets. Dallas, TX: American Heart Association; 2004. Heart disease and stroke statistics—2005 update. [Google Scholar]
- Bautista LE. Inflammation, endothelial dysfunction, and the risk of high blood pressure: epidemiologic and biological evidence. J Hum Hypertens. 2003;17:223–230. doi: 10.1038/sj.jhh.1001537. [DOI] [PubMed] [Google Scholar]
- Beck J, Garcia R, Heiss G, Vokonas PS, Offenbacher S. Periodontal disease and cardiovascular disease. J Periodontol. 1996;67(10 Suppl):1123S–1137S. doi: 10.1902/jop.1996.67.10s.1123. [DOI] [PubMed] [Google Scholar]
- Clearfield M, Whitney EJ, Weis S, Downs JR, Shapiro DR, Stein EA, et al. Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS): baseline characteristics and comparison with USA population. J Cardiovasc Risk. 2000;7:125–133. doi: 10.1177/204748730000700207. [DOI] [PubMed] [Google Scholar]
- Collin HL, Uusitupa M, Niskanen L, Kontturi-Närhi V, Markkanen H, Koivisto AM, et al. Periodontal findings in elderly patients with non-insulin dependent diabetes mellitus. J Periodontol. 1998;69:962–966. doi: 10.1902/jop.1998.69.9.962. [DOI] [PubMed] [Google Scholar]
- Collin HL, Sorsa T, Meurman JH, Niskanen L, Salo T, Ronka H, et al. Salivary matrix metalloproteinase (MMP-8) levels and gelatinase (MMP-9) activities in patients with type 2 diabetes mellitus. J Periodontal Res. 2000;35:259–265. doi: 10.1034/j.1600-0765.2000.035005259.x. [DOI] [PubMed] [Google Scholar]
- Cutler CW, Machen RL, Jotwani R, Iacopino AM. Heightened gingival inflammation and attachment loss in type 2 diabetics with hyperlipidemia. J Periodontol. 1999;70:1313–1321. doi: 10.1902/jop.1999.70.11.1313. [DOI] [PubMed] [Google Scholar]
- Doll S, Paccaud F, Bovet P, Burnier M, Wietlisbach V. Body mass index, abdominal adiposity and blood pressure: consistency of their association across developing and developed countries. Int J Obes Relat Metabol Disord. 2002;26:48–57. doi: 10.1038/sj.ijo.0801854. [DOI] [PubMed] [Google Scholar]
- Grundy SM. Inflammation, hypertension, and the metabolic syndrome. J Am Med Assoc. 2003;290:3000–3002. doi: 10.1001/jama.290.22.3000. [DOI] [PubMed] [Google Scholar]
- Janket SJ, Qvarnstrom M, Meurman JH, Baird AE, Nuutinen P, Jones JA. Asymptotic dental score and prevalent coronary heart disease. Circulation. 2004;109:1095–1100. doi: 10.1161/01.CIR.0000118497.44961.1E. [DOI] [PubMed] [Google Scholar]
- Janket SJ, Meurman JH, Nuutinen P, Qvarnstrom M, Nunn ME, Baird AE, et al. Salivary lysozyme and prevalent coronary heart disease: possible effects of oral health on endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2006;26:433–434. doi: 10.1161/01.ATV.0000198249.67996.e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janket SJ, Jones JA, Meurman JH, Baird AE, Van Dyke TE. Oral infection, hyperglycemia and endothelial dysfunction. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007a;105:173–179. doi: 10.1016/j.tripleo.2007.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janket SJ, Shen Y, Meurman JH. "Use and misuse of receiver operating characteristic (ROC) curve" revisited (comments/letter) Circulation. 2007b;116:133. doi: 10.1161/CIRCULATIONAHA.107.714360. comment on Circulation 115:928–935. [DOI] [PubMed] [Google Scholar]
- Johansson I, Tidehag P, Lundberg V, Hallmans G. Dental status, diet and cardiovascular risk factors in middle-aged people in northern Sweden. Community Dent Oral Epidemiol. 1994;22:431–436. doi: 10.1111/j.1600-0528.1994.tb00792.x. [DOI] [PubMed] [Google Scholar]
- Joshipura KJ, Rimm EB, Douglass CW, Trichopoulos D, Ascherio A, Willett WC. Poor oral health and coronary heart disease. J Dent Res. 1996;75:1631–1636. doi: 10.1177/00220345960750090301. [DOI] [PubMed] [Google Scholar]
- Karima M, Kantarci A, Ohira T, Hasturk H, Jones VL, Nam BH, et al. Enhanced superoxide release and elevated protein kinase C activity in neutrophils from diabetic patients: association with periodontitis. J Leukoc Biol. 2005;78:862–870. doi: 10.1189/jlb.1004583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempner MS, Malech HL. Phagocytes: normal and abnormal neutrophil host defenses. In: Gorbach SL, Bartlett JG, Blacklow NR, editors. Infectious diseases. Philadelphia: WB Saunders Co.; 1998. pp. 41–47. [Google Scholar]
- Kurtis B, Develioglu H, Taner IL, Balos K, Tekin IO. IL-6 levels in gingival crevicular fluid (GCF) from patients with non-insulin dependent diabetes mellitus (NIDDM), adult periodontitis and healthy subjects. J Oral Sci. 1999;41:163–167. doi: 10.2334/josnusd.41.163. [DOI] [PubMed] [Google Scholar]
- Liu S, Willett WC. Dietary glycemic load and atherothrombotic risk. Curr Atheroscler Rep. 2002;4:454–461. doi: 10.1007/s11883-002-0050-2. [DOI] [PubMed] [Google Scholar]
- Losche W, Karapetow F, Pohl A, Pohl C, Kocher T. Plasma lipid and blood glucose levels in patients with destructive periodontal disease. J Clin Periodontol. 2000;27:537–541. doi: 10.1034/j.1600-051x.2000.027008537.x. [DOI] [PubMed] [Google Scholar]
- Margolis KL, Manson JE, Greenland P, Rodabough RJ, Bray PF, Safford M, et al. Leukocyte count as a predictor of cardiovascular events and mortality in postmenopausal women: the Women's Health Initiative Observational Study. Arch Intern Med. 2005;165:500–508. doi: 10.1001/archinte.165.5.500. [DOI] [PubMed] [Google Scholar]
- Miller LS, Manwell MA, Newbold D, Reding ME, Rasheed A, Blodgett J, et al. The relationship between reduction in periodontal inflammation and diabetes control: a report of 9 cases. J Periodontol. 1992;63:843–848. doi: 10.1902/jop.1992.63.10.843. [DOI] [PubMed] [Google Scholar]
- Nishimura F, Terranova V, Foo H, Kurihara M, Kurihara H, Murayama Y. Glucose-mediated alteration of cellular function in human periodontal ligament cells. J Dent Res. 1996;75:1664–1671. doi: 10.1177/00220345960750090801. [DOI] [PubMed] [Google Scholar]
- Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27:813–823. doi: 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- Reaven G. Metabolic syndrome: pathophysiology and implications for management of cardiovascular disease. Circulation. 2002;106:286–288. doi: 10.1161/01.cir.0000019884.36724.d9. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98:731–733. doi: 10.1161/01.cir.98.8.731. [DOI] [PubMed] [Google Scholar]
- Rudney JD, Smith QT. Relationships between levels of lysozyme, lactoferrin, salivary peroxidase, and secretory immunoglobulin A in stimulated parotid saliva. Infect Immun. 1985;49:469–475. doi: 10.1128/iai.49.3.469-475.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi GE, Yalda B, Collins JG, Jones BH, Smith FW, Arnold RR, et al. Inflammatory mediator response as a potential risk marker for periodontal diseases in insulin-dependent diabetes mellitus patients. J Periodontol. 1997;68:127–135. doi: 10.1902/jop.1997.68.2.127. [DOI] [PubMed] [Google Scholar]
- Sandberg GE, Sundberg HE, Fjellstrom CA, Wikblad KF. Type 2 diabetes and oral health: a comparison between diabetic and non-diabetic subjects. Diabetes Res Clin Pract. 2000;50:27–34. doi: 10.1016/s0168-8227(00)00159-5. [DOI] [PubMed] [Google Scholar]
- Tervonen T, Oliver RC, Wolff LF, Bereuter J, Anderson L, Aeppli DM. Prevalence of periodontal pathogens with varying metabolic control of diabetes mellitus. J Clin Periodontol. 1994;21:375–379. doi: 10.1111/j.1600-051x.1994.tb00732.x. [DOI] [PubMed] [Google Scholar]
- Vlassara H, Palace MR. Diabetes and advanced glycation endproducts. J Intern Med. 2002;251:87–101. doi: 10.1046/j.1365-2796.2002.00932.x. [DOI] [PubMed] [Google Scholar]
- Vlassara H, Palace MR. Glycoxidation: the menace of diabetes and aging. Mt Sinai J Med. 2003;70:232–241. [PubMed] [Google Scholar]
- Vlassara H, Cai W, Crandall J, Goldberg T, Oberstein R, Dardaine V, et al. Inflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic angiopathy. Proc Natl Acad Sci USA. 2002;99:15596–15601. doi: 10.1073/pnas.242407999. erratum in Proc Natl Acad Sci USA 100:763, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalda B, Offenbacher S, Collins JG. Diabetes as a modifier of periodontal disease expression. Periodontol 2000. 1994;6:37–49. doi: 10.1111/j.1600-0757.1994.tb00025.x. [DOI] [PubMed] [Google Scholar]
- Zheng F, Cai W, Mitsuhashi T, Vlassara H. Lysozyme enhances renal excretion of advanced glycation endproducts in vivo and suppresses adverse age-mediated cellular effects in vitro: a potential AGE sequestration therapy for diabetic nephropathy? Mol Med. 2001;7:737–747. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.