Abstract
The design and synthesis of novel 3-D supramolecular dendrimers is described. Coordination-driven self-assembly of a 120° diplatinum acceptor and tritopic pyridyl donors bearing [G-0]–[G-3] Fréchet-type dendrons results in a series of supramolecular dendrimers under mild conditions, possessing a robust adamantanoid core of well-defined shape and size. The assemblies were identified using multinuclear (31P and 1H) NMR spectroscopy and electrospray ionization (ESI) mass spectrometry, as well as pulsed field gradient spin-echo (PGSE) NMR measurement together with computational simulations. Isotopically resolved mass spectral data support the existence of the [6 + 4] assembly of adamantanoid dendrimers, and the NMR results are consistent with the formation of these symmetrical assemblies. PGSE NMR measurements together with MMFF force-field modeling clearly reveal the structural feature of the 3-D supramolecular dendrimers with varying sizes.
Dendrimers of high structural complexity have emerged as one of the most important structures of significant interest in chemistry, biology, and medical research.1,2 While merging with self-assembly, complicated dendritic structures can be synthesized in a simple manner. One-dimensional (1-D) helical supramolecular dendrimers, for example, that vividly mimic natural pore-forming proteins can be efficiently self-assembled by a library of amphiphilic dendritic peptides.2a–c Recently, via coordination-driven self-assembly, a variety of two-dimensional (2-D), such as rhomboid, square, and hexagon, supramolecular dendrimers have been prepared.3,4 Most reported systems are limited to utilizing helical and 2-D structures. As compared with 1-D and 2-D structures, three-dimensional (3-D) assemblies exhibit a finite cavity of well-defined shape and size, and can be valuable in applications such as guest encapsulation, gas storage, catalysis, and drug delivery.5 However, 3-D supramolecular dendrimers are still rare,2d mainly because of the design constraint of rigid well-defined 3-D structures.
Coordination-driven self-assembly represents a successful methodology for constructing 3-D supramolecules, as indicated by a variety of elaborate supramolecular cages, prisms, and polydedra of well-defined shape and size.6 The self-assembled 3-D supramolecules have proven useful for capturing guest molecules, in supramolecular catalysis, and opening access to unusual reactions by virtue of 3-D nano-cavities.7 Covalent modifications of molecular subunits can endow assemblies with a wide variety of fascinating structural and functional properties.8 Recent examples reported by Fujita et al. demonstrated that functionalization of the interior and/or exterior of a M12L24 Pd-based supramolecular sphere can result in saccharide clusters, nanoscale fluoro-droplets, and confined polymerization.9 Because of the robust structure, unique confined space, and designable framework, the coordination-driven self-assembled 3-D metallosupramolecule is a promising candidate for constructing 3-D supramolecular dendrimers.
We have recently prepared 2-D hexagonal and 3-D cuboctahedral multifunctional scaffolds with a precise number, position, and orientation of functional groups by chemical tailoring of molecular subunits.10 Encouraged by the power and versatility of this methodology, we extend it to the construction of 3-D supramolecular dendrimers, and hereby present the self-assembly of 120° diplatinum acceptors 1 with tritopic donors 2a–d, substituted with Fréchet-type dendrons from [G-0] to [G-3].10 As shown in Scheme 1, coordination-driven self-assembly allows for quantitative generation of a series of supramolecular dendrimers 3a–d possessing a rigid 3-D adamantanoid core. These structures have been identified by multinuclear (31P and 1H) NMR spectroscopy and electrospray ionization (ESI) mass spectrometry, as well as pulsed field gradient spin-echo (PGSE) NMR measurement together with computational simulations.
Scheme 1.
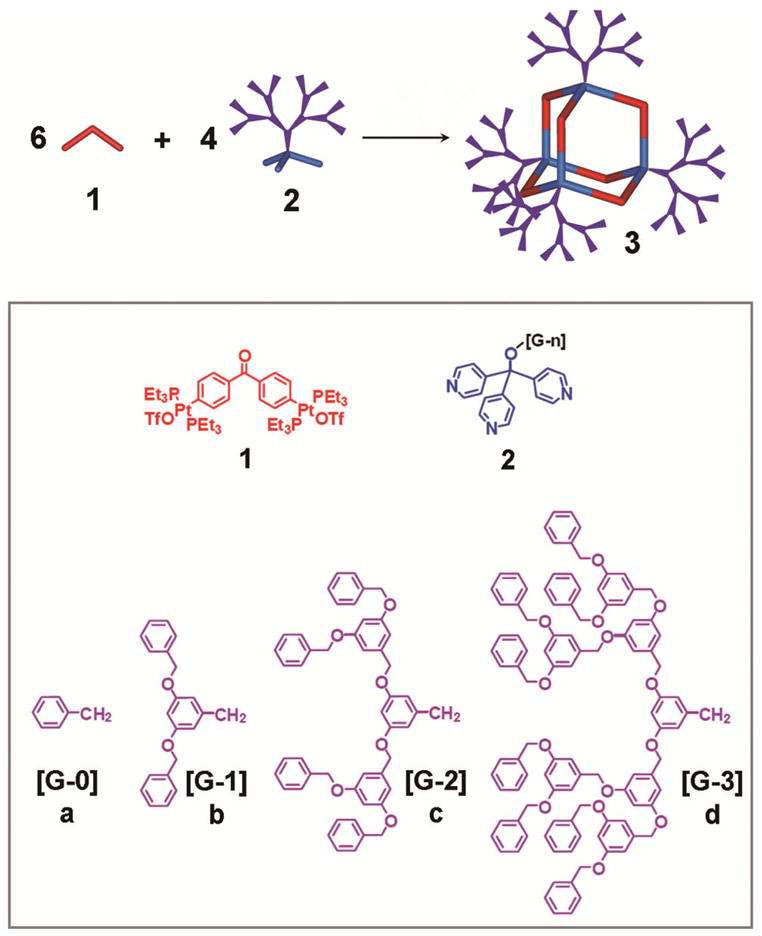
Representation of the [6 + 4] Self-Assembly of Adamantanoid Supramolecular [G-0]–[G-3] Dendrimers 3a–d.
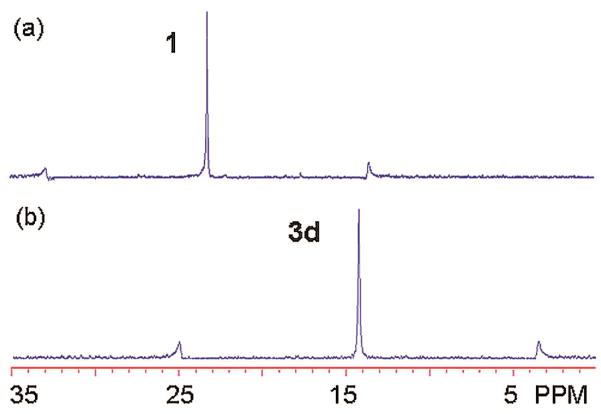
[G-0]–[G-3] tripyridyl donors 2a–d were prepared by substituting the tripyridin-4-yl methanol with Fréchet-type dendrons. The CD2Cl2 solution of tripyridyl donors 2 was slowly added into an acetone-d6 solution of 120° organoplatinum acceptor 1 in a 2:3 ratio, and after stirring for 15 min at R.T., [6 + 4] self-assembly of adamantanoid dendrimers 3 of different sizes was quantitatively obtained. In the 31P{1H} NMR spectra (Figure 1a and b and Supporting Information) of these mixtures, only a sharp singlet (3a: 14.06 ppm; 3b: 14.06 ppm; 3c: 14.05 ppm; 3d: 14.01 ppm) with concomitant 195Pt satellites can be found. Due to the formation of the Pt-N coordination bond, the signals are shifted upfield from that of the organoplatinum acceptor 1 by approximately 9.0 ppm. Likewise, the 1H NMR spectra (see Supporting Information) exhibit sharp signals for the pyridyl protons with small shifts downfield (ΔδPyα-H: 0.30 ppm; ΔδPyβ-H: 0.75 ppm) due to the loss of electron density that occurs upon coordination. The well assigned sharp NMR signals in both the 31P and 1H NMR agree with the high symmetry of 3 and rule out the formation of oligomers.
Figure 1.
31P{1H} NMR (300 MHz, Acetone-d6/CD2Cl2: 1/1) Spectra of (a) 120° Organoplatinum Acceptor 1 and (b) [G-3] Supramolecular Dendrimer 3d.
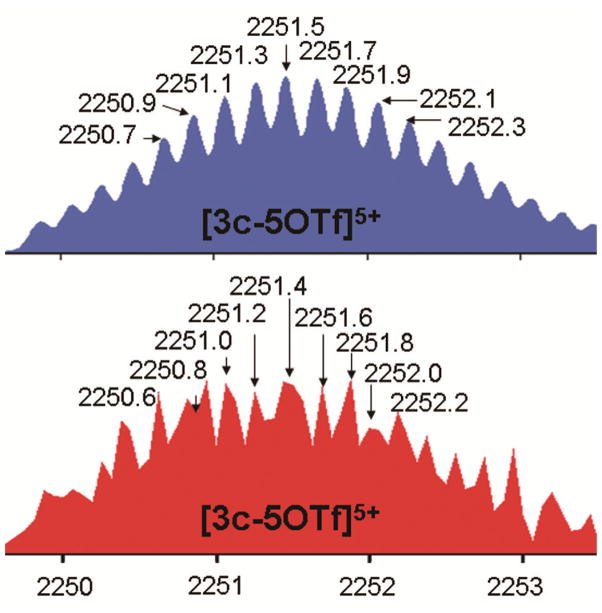
ESI mass spectrometry further confirmed the [6 + 4] structures (Supporting Information). In the ESI mass spectrum of the [G-0] adamantanoid dendrimer 3a, peaks corresponding to [M – 4OTf]4+ and [M – 5OTf]5+ can be found at m/z = 1742.4 and m/z = 1427.3. The ESI mass peak for loss of triflate anions from [G-1] adamantanoid dendrimer 3b at m/z = 1912.2 [M – 5OTf]5+ is observed, likewise for the [G-2] adamantanoid dendrimer 3c at m/z = 2251.6 [M – 5OTf]5+ (Figure 2). All ESI mass peaks are isotopically resolved and agree with their theoretical distributions. However, for the [G-3] adamantanoid dendrimer 3d, because of its high molecular weight, an isotopically resolved ESI mass signal was not obtained.
Figure 2.
Calculated (blue, top) and Experimental (red, bottom) ESI Mass Spectra of [G-2] Supramolecular Dendrimer 3c.
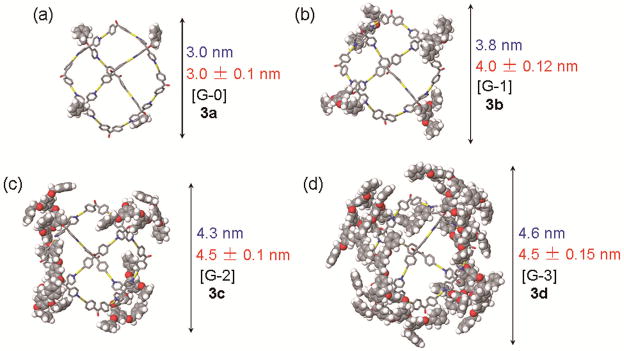
As suitable X-ray quality crystals could not be obtained, a computational study was carried out to gain insight into the structural characteristics of these assemblies. A 1.0 ns molecular dynamics simulation (MMFF force field, 300K, gas phase) was used to equilibrate each supramolecule, and the output of the simulation was then minimized to full convergence. As shown in Figure 3, each assembly has a 3.0 nm adamantanoid core with Td symmetry, and the size of the overall structure is increased from 3.0 nm (for [G-0] 3a) to 4.6 nm (for [G-3] 3d). In 3d, the adamantanoid core is partially covered by a dendritic shell.
Figure 3.
Computational simulations (MMFF force field) of 3-D dendrimers 3a–d (protons and triethyl phosphrous moieties of adamantanoid core omitted for clarity) and their calculated (blue) sizes as well as experimental (red) values from PGSE NMR measurements.
PGSE NMR measurements were used to characterize these structures by determination of the translational self-diffusion coefficient, and in conjunction with the Stokes-Einstein equation, the “effective size” of the overall assembly in solution was obtained.11 The 1H PGSE for all assemblies in acetone-d6 (see Supporting Information) were determined: 3a: 3.0 ± 0.1 nm; 3b: 4.0 ± 0.12 nm; 3c: 4.5 ± 0.1 nm; 3d: 4.5 ± 0.15 nm. As indicated in Figure 3, they are in good agreement with the computational values (3a: 3.0 nm; 3b: 3.8 nm; 3c: 4.3 nm; 3d: 4.6 nm) from the molecular force field modeling. Since the dendrons on the exterior are spreading around the adamantanoid core instead of spreading out, the size increase from 3c to 3d is minor resulting in a similar size in the PGSE NMR measurement.
In conclusion, we have developed a convergent synthesis of 3-D metallosupramolecular dendrimers via coordination-driven self-assembly, where the exterior dendrons were introduced by pre-modification of the molecular subunits. These supramolecular dendrimers show a unique 3-D adamantanoid core, which is partially covered by the dendritic exterior, and exploration of the interior environment would be interesting and valuable. Furthermore, functionalization of dendrons and/or the supramolecular core should allow access to 3-D hollow supramolecular functional systems with potential applications such as guest encapsulation, nanoreactor, and drug delivery.1
Supplementary Material
Acknowledgments
P.J.S. thanks the NIH (GM-057052) for financial support. H.-B.Y. thanks the NSFC (20902027), Shanghai Pujiang Program (09PJ1404100), Shanghai Shuguang Program (09SG25), and Innovation Program of SMEC (10ZZ32) for financial support.
Footnotes
Supporting Information Available: General detailed experimental procedures for synthesis and characterization of dendritic tripyridyl donors 2a–d and supramolecular dendrimers 3a–d. (http://pubs.acs.org/paragonplus/submission/jacsat/).
References
- 1.(a) Hawker CJ, Fréchet JMJ. J Am Chem Soc. 1990;112:7638. [Google Scholar]; (b) Fréchet JMJ, Tomalia DA. Dendrimers and Other Dendritic Polymers. VCH-Wiley; New York: 2000. [Google Scholar]; (c) Grayson SM, Fréchet JMJ. Chem Rev. 2001;101:3819. doi: 10.1021/cr990116h. [DOI] [PubMed] [Google Scholar]; (d) Hecht S, Fréchet JMJ. Angew Chem, Int Ed. 2001;40:74–91. doi: 10.1002/1521-3773(20010105)40:1<74::aid-anie74>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]; (e) Hecht S, Vladimirov N, Fréchet JMJ. J Am Chem Soc. 2001;123:18. doi: 10.1021/ja003304u. [DOI] [PubMed] [Google Scholar]; (f) Fréchet JMJ. Proc Natl Acad Sci USA. 2002;99:4782. doi: 10.1073/pnas.082013899. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Landskron K, Ozin GA. Science. 2004;306:1529. doi: 10.1126/science.1104555. [DOI] [PubMed] [Google Scholar]; (h) Lee CC, MacKay JA, Fréchet JMJ, Szoka FC. Nature Biotech. 2005;23:1517. doi: 10.1038/nbt1171. [DOI] [PubMed] [Google Scholar]; (i) Medina SH, El-Sayed MEH. Chem Rev. 2009;109:3141. doi: 10.1021/cr900174j. [DOI] [PubMed] [Google Scholar]
- 2.(a) Percec V, Dulcey AE, Balagurusamy VSK, Miura Y, Smidrkal J, Peterca M, Nummelin S, Edlund U, Hudson SD, Heiney PA, Duan H, Magonov SN, Vinogradov SA. Nature. 2004;430:764–768. doi: 10.1038/nature02770. [DOI] [PubMed] [Google Scholar]; (b) Percec V, Dulcey AE, Peterca M, Ilies M, Sienkowska MJ, Heiney PA. J Am Chem Soc. 2005;127:17902. doi: 10.1021/ja056313h. [DOI] [PubMed] [Google Scholar]; (c) Percec V, Dulcey AE, Peterca M, Adelman P, Samant R, Balagurusamy VSK, Heiney PA. J Am Chem Soc. 2007;129:5992. doi: 10.1021/ja071088k. [DOI] [PubMed] [Google Scholar]; (d) Percec V, Peterca M, Dulcey AE, Imam MR, Hudson SD, Nummelin S, Adelman P, Heiney PA. J Am Chem Soc. 2008;130:13079. doi: 10.1021/ja8034703. [DOI] [PubMed] [Google Scholar]; (e) Rudick JG, Percec V. Acc Chem Res. 2008;41:1641. doi: 10.1021/ar800086w. [DOI] [PubMed] [Google Scholar]; (f) Rosen BM, Wilson CJ, Wilson DA, Peterca M, Imam MR, Percec V. Chem Rev. 2009;109:6275. doi: 10.1021/cr900157q. [DOI] [PubMed] [Google Scholar]
- 3.(a) Yang HB, Das N, Huang F, Hawkridge AM, Muddiman DC, Stang PJ. J Am Chem Soc. 2006;128:10014. doi: 10.1021/ja063377z. [DOI] [PubMed] [Google Scholar]; (b) Yang HB, Hawkridge AM, Huang SD, Das N, Bunge SD, Muddiman DC, Stang PJ. J Am Chem Soc. 2007;129:2120. doi: 10.1021/ja066804h. [DOI] [PubMed] [Google Scholar]; (c) Yang HB, Northrop BH, Zheng YR, Ghosh K, Lyndon MM, Muddiman DC, Stang PJ. J Org Chem. 2009;74:3524. doi: 10.1021/jo900067v. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Yang HB, Northrop BH, Zheng YR, Ghosh K, Stang PJ. J Org Chem. 2009;74:7067. doi: 10.1021/jo9013589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baytekin HT, Sahre M, Rang A, Engeser M, Schulz A, Schalley CA. Small. 2008;4:1823. doi: 10.1002/smll.200800135. [DOI] [PubMed] [Google Scholar]
- 5.(a) Rosi NL, Eckert J, Eddaoudi M, Vodak DT, Kim J, O’Keeffe M, Yaghi OM. Science. 2003;300:1127. doi: 10.1126/science.1083440. [DOI] [PubMed] [Google Scholar]; (b) Trembleau Laurent, Rebek Julius., Jr Science. 2003;301:1219. doi: 10.1126/science.1086644. [DOI] [PubMed] [Google Scholar]; (c) Yoshizawa M, Tamura M, Fujita M. Science. 2006;312:251. doi: 10.1126/science.1124985. [DOI] [PubMed] [Google Scholar]; (d) Pluth Michael D, Bergman Robert G, Raymond Kenneth N. Science. 2007;316:85. doi: 10.1126/science.1138748. [DOI] [PubMed] [Google Scholar]
- 6.(a) Stang PJ, Olenyuk B. Acc Chem Res. 1997;30:502. [Google Scholar]; (b) Leininger S, Olenyuk B, Stang PJ. Chem Rev. 2000;100:853. doi: 10.1021/cr9601324. [DOI] [PubMed] [Google Scholar]; (c) Seidel SR, Stang PJ. Acc Chem Res. 2002;35:972. doi: 10.1021/ar010142d. [DOI] [PubMed] [Google Scholar]; (d) Fiedler D, Leung DH, Bergman RG, Raymond KN. Acc Chem Res. 2005;38:351. doi: 10.1021/ar040152p. [DOI] [PubMed] [Google Scholar]; (e) Fujita M, Tominaga M, Hori A, Therrien B. Acc Chem Res. 2005;38:369. doi: 10.1021/ar040153h. [DOI] [PubMed] [Google Scholar]
- 7.(a) Pluth MD, Bergman RG, Raymond KN. Acc Chem Res. 2009;42:1650. doi: 10.1021/ar900118t. [DOI] [PubMed] [Google Scholar]; (b) Yoshizawa M, Klosterman JK, Fujita M. Angew Chem, Int Ed. 2009;48:3418. doi: 10.1002/anie.200805340. [DOI] [PubMed] [Google Scholar]
- 8.Northrop BH, Yang H-B, Stang PJ. Chem Commun. 2008:5896. doi: 10.1039/b811712h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Sato S, Iida J, Suzuki K, Kawano M, Ozeki T, Fujita M. Science. 2006;313:1273. doi: 10.1126/science.1129830. [DOI] [PubMed] [Google Scholar]; (b) Kamiya N, Tominaga M, Sato S, Fujita M. J Am Chem Soc. 2007;129:3816. doi: 10.1021/ja0693082. [DOI] [PubMed] [Google Scholar]; (c) Murase T, Sato S, Fujita M. Angew Chem, Int Ed. 2007;46:5133. doi: 10.1002/anie.200700793. [DOI] [PubMed] [Google Scholar]; (d) Suzuki K, Kawano M, Sato S, Fujita M. J Am Chem Soc. 2007;129:10652. doi: 10.1021/ja073629b. [DOI] [PubMed] [Google Scholar]; (e) Suzuki K, Sato S, Fujita M. Nature Chem. 2010;2:25. doi: 10.1038/nchem.446. [DOI] [PubMed] [Google Scholar]
- 10.(a) Yang HB, Ghosh K, Northrop BH, Zheng YR, Lyndon MM, Muddiman DC, Stang PJ. J Am Chem Soc. 2007;129:14187. doi: 10.1021/ja073744m. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yang HB, Ghosh K, Zhao Y, Northrop BH, Lyndon MM, Muddiman DC, White HS, Stang PJ. J Am Chem Soc. 2008;130:839. doi: 10.1021/ja710349j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ghosh K, Hu J, White HS, Stang PJ. J Am Chem Soc. 2009;131:6695. doi: 10.1021/ja902045q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caskey DC, Yamamoto T, Addicott C, Shoemaker RK, Vacek J, Hawkridge AM, Muddiman DC, Kottas GS, Michl J, Stang PJ. J Am Chem Soc. 2009;130:7620. doi: 10.1021/ja710715e. and references therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.