Abstract
The postpartum period is associated with many behavioral changes, including a reduction in anxiety, which is thought to be necessary for mothers’ ability to appropriately care for infants. In laboratory rats, this reduction in anxiety requires recent contact with pups, but areas of the brain where infant contact influences neural activity to reduce anxiety are mostly unknown. We examined c-fos expression in lactating rats whose pups were removed for 4 hours to increase mothers’ anxiety, or not removed to maintain low anxiety in mothers, followed by exposure to the anxiogenic stimuli of either brief handling or handling followed by exposure to an elevated plus maze. Control animals had their litters removed or not, but no further stimulation. A large number of neural sites traditionally implicated in regulating anxiety in male rats were examined, and similar to what is found in male rats, most showed increased Fos expression after handling and/or elevated plus-maze exposure. Litter presence before testing affected Fos expression due to handling or elevated plus-maze exposure only in the ventral bed nucleus of the stria terminalis, dorsal and ventral preoptic area, ventromedial hypothalamus, lateral habenula, and supramammillary nucleus. Contrary to expectations, prior litter presence was associated with more Fos expression in most of these sites after handling and/or elevated plus maze stimulation, and only after such stimulation. These sites may be of particular importance for how sensory inputs from infants modulate anxiety and other mood states during the postpartum period.
Keywords: anxiety, elevated plus maze, immediate-early gene, lactation, postpartum, stress, suckling
1. Introduction
The postpartum period is a physically and emotionally demanding time of the reproductive cycle, and requires the coordination of numerous behavioral and physiological changes that maintain maternal homeostasis and promote offspring development. Reduced anxiety is one component of these changes, and occurs in both postpartum women and laboratory rats [for review see 43]. This reduction is critical for normal mother-infant interactions and infant development, because human or rodent mothers with high anxiety display altered or even completely aberrant maternal care [1,3,5,6,14,46–48,53,57,61,87–89,95].
The postpartum reduction in anxiety requires physical contact with offspring. In lactating women and rats, either suckling or non-suckling contact with infants can produce an anxiolytic effect [19,33,42]. Contact with infants must be recent, at least in rats, because anxiety-related behaviors in dams reverts back to the high levels found in diestrous virgin females if the litter is absent for even a few hours [42,59].
Areas of the brain affected by recent physical contact with infants to reduce anxiety in their mothers have not been explored in detail. Previous studies using peripheral or intracerebroventricular injections of receptor antagonists implicate numerous neurochemical systems, including GABA, norepinephrine, prolactin, and oxytocin [22,31,32,62,83,86], but do not address the specific sites of action. Lesion studies have been few, but reveal that the paraventricular hypothalamus is not involved in dams’ exploration of a novel environment or freezing in response to a startling noise [64], and that destruction of the ventrocaudal periaqueductal gray further decreases dams’ already low anxiety [45].
To explore where in the brain recent contact with pups might alter neural function in response to an anxiogenic experience, we examined c-fos expression in the brains of postpartum rats with low or high anxiety after they were subjected to brief handling or handing followed by exposure to an elevated plus maze. Low- and high-anxiety states were generated by permitting or preventing dams’ recent contact with pups, respectively. The literature on the neural regulation of anxiety in females at any stage of the reproductive cycle is relatively small. Therefore, to help determine sites to be analyzed, we turned to the large literature on the neural control of anxiety in males, which implicates sites including the midline cortex, numerous areas of the hypothalamus, bed nucleus of the stria terminalis, septum, hippocampus, amygdala, and periaqueductal gray [9,10,52]. Parsimony suggests that the fundamental neural networks mediating anxiety-related behaviors are similar between the sexes, but that males and females are susceptible to different endocrine and sensory influences, including those resulting from physical contact with infants.
2. Materials and Methods
2.1 Subjects
Subjects were 55 Long-Evans female rats born and raised in our colony, but descended from rats purchased from Harlan Laboratories (Indianapolis, IN). After weaning at 21 days old, subjects were housed in clear polypropylene cages (48 × 28 × 16 cm) with woods shavings for bedding in groups of 2–3 littermates per cage. Beginning at 70 days old, females’ estrous cycles were monitored daily with a vaginal impedance meter (Fine Science Tools, Foster City, CA), and females in proestrus placed overnight with a Long-Evans male from our colony. Mating was confirmed the next morning by copulatory plugs underneath the males’ cages. Mated females were removed from males’ cages and rehoused with one or two other recently inseminated females until 4–5 days before the expected day of parturition. Females were then individually housed and remained so with their litters throughout the remainder of the experiment. Litters were culled to four males and four females within 24 h after birth. The day of birth was assigned as day 0 postpartum. Food (Purina Rat Chow) and water were continuously available, lights were on a 12:12 light/dark cycle with onset at 0800 h daily, and the ambient temperature was ~ 22°C.
2.2 Experimental Design and Behavioral Testing
Dam and litter manipulation occurred between 0900–1000 hr on day 7 or 8 postpartum. To minimize disturbance before testing, anyone other than the experimenter was prohibited from entering the colony room on the day of testing. We used a between-subjects, 2-factor design with litter presence before testing as one factor (litter removed or not removed) and subsequent anxiogenic stimulation as the other factor (no stimulation, handling, or handling followed by exposure to an elevated plus maze). This resulted in six groups with final samples sizes of 8 animals in each group; an additional 7 dams fell off the elevated plus-maze and were immediately removed from the study. Dams whose pups were not removed had the experimenter’s hand placed in their home cage, and the pups picked up and immediately replaced, to control for perturbation caused by longer-term litter removal in females from the other condition. Four hours after litters were removed or not removed, experimental dams received one of two anxiogenic experiences - brief handling or handling followed by a 10-minute exposure to an elevated plus maze (see immediately below).
Handled subjects were carefully carried across the hallway from our colony room to a 10 × 10 ft testing room illuminated by a single 100-W bulb. Subjects were removed from their cage and very briefly suspended by their tail over an elevated plus maze. Subjects were immediately returned to their home cage, which contained the pups or not, and the cage then returned to the colony room.
For groups receiving exposure to an elevated plus maze, dams were carried in their home cage to the same testing room described above. The elevated plus maze was elevated 50 cm from the floor, and made of black plastic with four arms emerging from a 10 × 10 cm center square [68]. Arms measured 50 × 10 cm, two of which were open, while the other two had 40-cm-high walls. Illumination was ~28 lux on the open arms and ~2 lux at the end of enclosed arms. Each subject was removed from their home cage and placed in the central square of the maze facing an open arm and released. The home cage was then moved to an adjacent room. A Panasonic low-light-sensitive video camera relayed images of the plus maze from a mirror suspended above the maze to a Panasonic videocassette recorder and monitor in an adjacent room. An experimenter simultaneously recorded subjects’ behavior with a computerized data-acquisition system that allowed recording the time spent in the open arms and closed arms, and the frequency of entries into each arm. An entry was recorded when the female placed her head and both front paws into an arm. Time spent in none of the arms was scored as time spent in the center square. After testing, dams were gently removed from the maze, and returned to their home cage (which contained the pups or not), which was carried back to the colony. The elevated plus maze was cleaned with 70% ethanol and dried between subjects.
Groups of control dams had their pups removed in the morning, or not removed, but these dams were not handled or exposed to the plus maze. They instead remained in the colony room, unmanipulated in their home cages with or without their pups until sacrifice.
2.3 Immunocytochemistry
One hour after experimental dams were handled or exposed to the elevated plus maze, or 5 hours after the morning litter manipulation for the unstimulated controls, subjects were overdosed with sodium pentobarbital and perfused through the heart with 150 ml saline followed by 150 ml 4% paraformaldehyde in 0.1M sodium phosphate buffered saline (PBS, pH 7.4). This time point was based on the maximal Fos protein expression in the rat brain following exposure to the elevated plus maze [17]. Brains were removed and postfixed overnight in 4% paraformaldehyde/PBS. On the following day, brains were placed in a 20% sucrose/PBS solution until sectioning. Each brain was cut into 35 μm sections and stored in a sucrose-based cryoprotectant at −20°C until immunocytochemical processing.
Every third section through the brain was immunocytochemically processed similar to that previously described [39]. Free floating sections were rinsed between steps, with the exception of after blocking with normal goat serum, three times in Trisma-buffered saline (TBS; pH = 7.6). Sections were first incubated in a 0.1% sodium borohydride/TBS solution for 15 min, 1% H202/TBS for 10 min, 20% normal goat serum with 0.3% Triton X-100 in TBS for 45 minutes, and 2% goat serum with 0.3% Triton X-100/TBS solution containing rabbit polyclonal anti-c-fos primary antibody (1:10,000; Santa Cruz Biotechnology, T-4037) at 4°C for 48 hrs. Tissue was then incubated for one hour in biotinylated anti-rabbit secondary antibody (1:500; Vector Laboratories, Burlingame, CA) in 2% goat serum, a solution of avidin-biotin complex (Vectastain Elite; Vector Laboratories) for 60 min, and then a solution of 3,3′-diaminobenzidine (DAB), nickel ammonium sulfate, H202, in TBS. Sections were rinsed with TBS, mounted onto microscope slides, lightly counterstained with Neutral Red, dehydrated, and coverslipped.
2.4 Microscopic Analysis
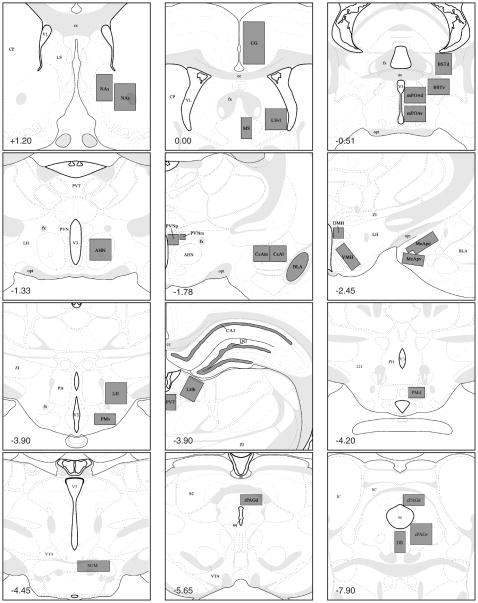
Slides were randomized and coded so that experimenters were blind to group designation. Fos-IR cells were quantified at 200X magnification with the aid of a reticle placed on one ocular lens on a Nikon E400 microscope. Brain sites analyzed are shown in Figure 1, and a few additional areas were examined but are not reported because there was no or very low Fos immunoreactivity in any of the groups (i.e., CA3, MHb, and MM). For each site, Fos-IR cells were counted bilaterally within a standardized area from one section for each subject, and sections were chosen based on their correspondence to the appropriate plate from Swanson’s atlas of the rat brain [81]. Cells with any level of immunoreactivity were quantified by a single observer (C.D.S.).
Figure 1.
Schematic representation of brain regions where Fos immunoreactive cells were quantified. Gray boxes indicate area of analysis, and numbers in bottom left of each panel indicate distance from bregma (in mm). All plates modified from Swanson (1998). Abbreviations: AHN -anterior hypothalamus; aq - cerebral aqueduct; BLAm - medial basolateral amygdala; BSTdm -dorsomedial bed nucleus of the stria teriminalis; BSTvm - ventromedial bed nucleus of the stria terminalis; CA1 - CA1 field of the hippocampus; cc - corpus callosum; CeAl - anterolateral central amygdala; CeAm - anteromedial central amygdala; CG - cingulate cortex; CP - caudate putamen; cPAGd - dorsocaudal periaqueductal gray; cPAGv - ventrocaudal periaqueductal gray; DG - dentate gyrus; DMH - dorsomedial hypothalamus; DR - dorsal raphe; fx - fornix; IC - inferior colliculus; LH - lateral hypothalamus; LHb - lateral habenula; LSvl - ventrolateral septum; MeApd - posterodorsal medial amygdala; MeApv - posteroventral medial amygdala; mPOAd - dorsal medial preoptic area; mPOAv - ventral medial preoptic area; MS - medial septum; NAc - nucleus accumbens core; NAs -nucleus accumbens shell; opt - optic tract; PMd - dorsal premammillary nucleus; PMv - ventral premammillary nucleus; PVNm - magnocellular paraventricular hypothalamus; PVNp -parvocellular paraventricular hypothalamus; PVT - paraventricular thalamic nucleus; rPAGd -dorsorostral periaqueductal gray; SC - superior colliculus; SUM - supramammillary nucleus; V3 -third ventricle; VL - lateral ventricle; VMH - ventromedial hypothalamus; VTA - ventral tegmental area; ZI - zona incerta.
2.5 Data analyses
Arm entries lasting <1 second were considered keystroke mistakes and removed from data analyses. The brain of one handled dam that remained with her pups was not analyzable, and she was removed from the study. One dam that had her pups removed before exposure to the elevated plus maze was found to be an outlier in the number of Fos-IR cells in the VMH (Dixon’s outlier test, p < 0.05), and was removed from the analysis of that site. Behavior in the elevated plus maze was analyzed with independent t-tests. The number of Fos-IR cells in each neural site was analyzed by a 2 × 3 ANOVA using pup condition (pup present or pups absent before testing) and test condition (no stimulation, handling, or handling followed by exposure to an elevated plus maze) as factors. Statistical significance for main effects and interactions was indicated by p ≤ 0.05. Main effects were followed by Bonferroni-corrected post-hoc analysis with p ≤ 0.016 indicating statistically significant differences between groups. Pearson’s r was used to examine correlations between Fos expression and behavior of the two groups of dams exposed to the elevated plus-maze.
3. Results
3.1 Elevated plus-maze behavior
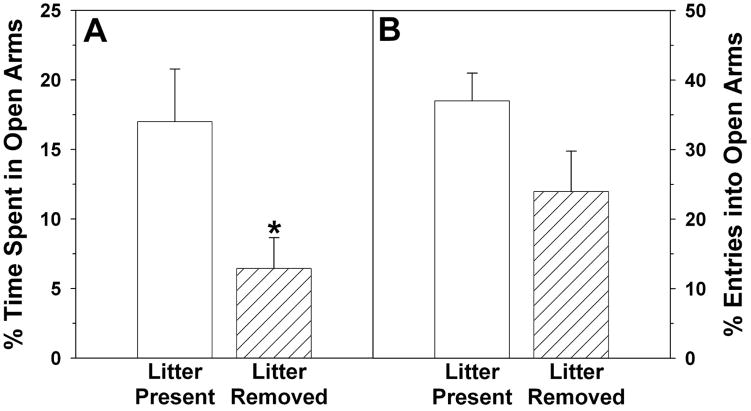
Similar to our previous results [42], removal of the pups four hours before testing increased dams’ anxiety-related behaviors in the elevated plus maze compared to dams allowed physical contact with pups until testing. Dams with their pups removed spent a significantly lower percentage of time in the open arms of the elevated plus maze (t14 = 2.36, p ≤ 0.05; Figure 2a), and tended to have a lower percentage of entries made into open arms (t14 = 1.84, p = 0.087; Figure 2b). The raw duration of time spent in the open arms was also reduced by litter removal (89 ± 20 vs. 33 ± 11 sec; t14 = 2.41, p ≤ 0.04), and the raw number of entries into open arms tended to be reduced (9 ± 2 vs. 5 ± 1; t14 = 2.11, p = 0.054). In contrast, the duration of time spent in the closed arms was somewhat higher for dams whose pups had been removed (445 ± 22 vs. 493 ± 18 sec; t14 = 1.71, p = 0.10). Removal of the litter did not significantly affect dams’ total time spent in the arms of the maze (open plus closed; 526 ± 9 vs. 534 ± 6 sec; t14 = 0.77, p ≥ 0.45), the number of entries into closed arms (11 ± 2 vs. 14 ± 1; t14 = 1.43, p ≥ 0.17), or the total number of arm entries - although this tended to be lower for dams who had their pups removed (23 ± 2 vs. 16 ± 3; t14 = 1.94, p = 0.07).
Figure 2.
Percentage of A) time spent and B) entries made in the open arms of an elevated plus maze by lactating rats that had their pups present for the four hours before testing (Litter Present) or had their pups removed (Litter Removed).
3.2 Neural Fos expression - Main effects of anxiogenic stimulus
In 30 of the 31 sites analyzed, handling alone or handling followed by exposure to the elevated plus maze increased the number of cells containing Fos immunoreactivity compared to the lower number found in Unstimulated controls. Numerous patterns of main effects for stimulus were found (see Table 1 for data and site abbreviations): 1) A pattern where Unstimulated < Handled < Plus Maze was found in the CG, DG, NA shell, and MeApd. 2) A pattern where Unstimulated < Handled = Plus Maze was found in the LSvl, MS, BSTd and BSTv, mPOAd and mPOAv, NA core, ANH, DMH, PVNp, CeAm, LHb, PVT, PMd, SUM, rPAGd, and cPAGv. 3) A pattern of expression where Unstimulated < Plus Maze, but neither group differed from the intermediate levels found for the Handled group was found in the VMH, LH, PVNm, CA1, BLA, CeAl, MeApv, and cPAGd, and finally, 4) a pattern where Unstimulated = Handled < Plus Maze was found in the PMv. No differences between groups were found in the DR.
Table 1.
Number (Mean ± SEM) of Fos-immunoreactive cells quantified bilaterally from one section per site in the brains of lactating rats that received no stimulation (Unstimulated), brief handling (Handled), or exposure to an elevated plus maze (Plus Maze) after their pups were removed or not removed four hours before testing. Sites are grouped according to pattern of significant differences between groups when collapsed across pup condition, with Bonferroni-corrected post-hoc p < 0.016 indicating statistical significance.
| Unstimulated | Handled | Plus Maze | F2,41 | p | ||||
|---|---|---|---|---|---|---|---|---|
| With pups | Pups removed | With pups | Pups removed | With pups | Pups removed | |||
| Unstimulated < Handled < Plus Maze | ||||||||
| CG | 7 ± 3 | 32 ± 14 | 73 ± 21 | 94 ± 15 | 207 ± 24 | 173 ± 34 | 35.95 | 0.0001 |
| DG | 11 ± 3 | 11 ± 3 | 25 ± 8 | 30 ± 6 | 49 ± 9 | 40 ± 7 | 13.99 | 0.0001 |
| NAs | 31 ± 6 | 56 ± 12 | 83 ± 15 | 95 ± 14 | 131 ± 12 | 116 ± 17 | 17.69 | 0.0001 |
| MeApd | 12 ± 6 | 19 ± 2 | 37 ± 8 | 40 ± 8 | 95 ± 7 | 84 ± 13 | 43.47 | 0.0001 |
| Unstimulated < Handled = Plus Maze | ||||||||
| LSvl | 12 ± 7 | 50 ± 20 | 149 ± 23 | 156 ± 25 | 184 ± 23 | 177 ± 33 | 22.30 | 0.0001 |
| MS | 4 ± 1 | 20 ± 8 | 41 ± 6 | 53 ± 10 | 66 ± 6 | 48 ± 9 | 20.31 | 0.0001 |
| BSTd | 11 ± 3 | 28 ± 9 | 35 ± 8 | 55 ± 10 | 61 ± 6 | 65 ± 13 | 11.94 | 0.0001 |
| NAc | 8 ± 2 | 20 ± 7 | 42 ± 9 | 46 ± 7 | 64 ± 10 | 56 ± 12 | 15.01 | 0.0001 |
| AHN | 35 ± 7 | 60 ± 8 | 85 ± 15 | 93 ± 15 | 128 ± 14 | 101 ± 19 | 11.82 | 0.0001 |
| DMH | 23 ± 5 | 25 ± 11 | 54 ± 8 | 51 ± 11 | 87 ± 14 | 46 ± 13 | 7.80 | 0.0013 |
| PVNp | 3 ± 1 | 11 ± 3 | 23 ± 6 | 32 ± 7 | 42 ± 6 | 45 ± 11 | 14.90 | 0.0001 |
| CeAm | 4 ± 2 | 4 ± 1 | 15 ± 5 | 12 ± 4 | 17 ± 3 | 10 ± 3 | 5.85 | 0.0058 |
| PVT | 16 ± 3 | 29 ± 4 | 37 ± 8 | 46 ± 8 | 36 ± 5 | 44 ± 10 | 4.58 | 0.0160 |
| PMd | 6 ± 2 | 8 ± 2 | 25 ± 11 | 16 ± 6 | 27 ± 6 | 25 ± 6 | 5.00 | 0.0114 |
| rPAGd | 6 ± 1 | 10 ± 3 | 17 ± 5 | 22 ± 5 | 30 ± 4 | 25 ± 6 | 10.03 | 0.0003 |
| cPAGv | 11 ± 3 | 14 ± 5 | 26 ± 8 | 31 ± 8 | 32 ± 6 | 29 ± 6 | 4.36 | 0.0191 |
| Unstimulated < Plus Maze* | ||||||||
| LH | 4 ± 2 | 11 ± 4 | 19 ± 6 | 27 ± 7 | 39 ± 5 | 35 ± 10 | 10.58 | 0.0002 |
| PVNm | 0 ± 0 | 1 ± 0 | 8 ± 3 | 3 ± 1 | 9 ± 4 | 6 ± 3 | 4.09 | 0.0241 |
| CA1 | 0 ± 0 | 1 ± 0 | 4 ± 2 | 3 ± 2 | 7 ± 2 | 7 ± 3 | 4.70 | 0.0145 |
| BLA | 1 ± 1 | 4 ± 2 | 19 ± 10 | 16 ± 2 | 47 ± 10 | 22 ± 9 | 9.88 | 0.0003 |
| CeAl | 2 ± 1 | 6 ± 3 | 7 ± 2 | 16 ± 3 | 12 ± 2 | 12 ± 5 | 4.15 | 0.0229 |
| MeApv | 11 ± 2 | 16 ± 3 | 36 ± 5 | 26 ± 6 | 70 ± 6 | 74 ± 14 | 33.55 | 0.0001 |
| cPAGd | 13 ± 3 | 16 ± 5 | 21 ± 6 | 26 ± 6 | 39 ± 5 | 25 ± 4 | 6.14 | 0.0047 |
| Unstimulated = Handled < Plus Maze | ||||||||
| PMv | 4 ± 1 | 8 ± 3 | 16 ± 4 | 18 ± 5 | 39 ± 9 | 32 ± 12 | 9.11 | 0.0005 |
| Unstimulated = Handled = Plus Maze | ||||||||
| DR | 0 ± 0 | 5 ± 5 | 12 ± 6 | 11 ± 4 | 5 ± 1 | 6 ± 2 | 2.39 | 0.1041 |
3.3 Neural Fos expression - Main effect of previous litter presence
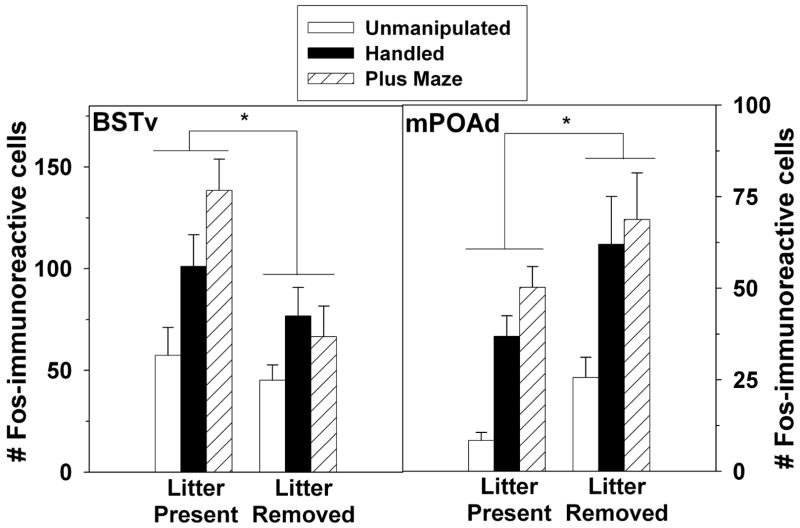
Two sites showed significant main effects of previous litter presence on Fos expression. In the BSTv, when collapsed across stimulus type, dams that had their pups before testing and/or sacrifice had greater Fos expression (F(1,41) = 10.27, p = .003; Figure 3). Conversely, in the adjacent mPOAd, dams had fewer Fos-IR cells if their pups were present before testing and/or sacrifice (F(1,41) = 8.21, p = .007; Figure 3).
Figure 3.
Number (Mean ± SEM) number of Fos-immunoreactive cells in the BSTv and mPOAd of postpartum rats exposed to no stimulation, handling, or an elevated plus maze. Subjects had their pups present for the four hours before testing (Litter Present) or had the pups removed (Litter Removed). Note that X axes differ across panels.
3.4 Neural Fos expression - Interactions between anxiogenic stimulus and previous litter presence
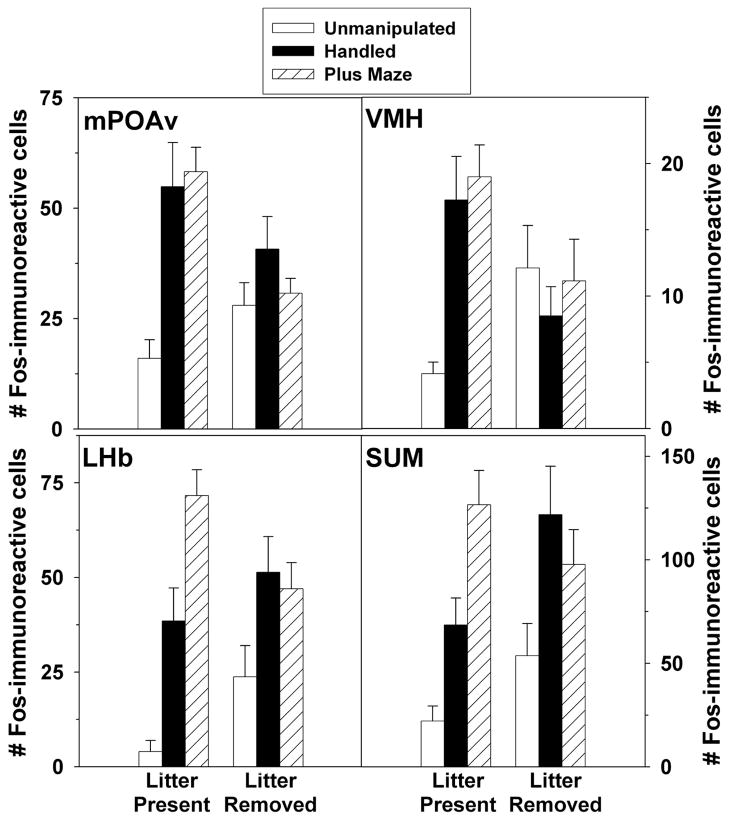
Statistically significant stimulus by litter presence interactions were found in the mPOAv (F(2,41) = 4.82, p = .013), VMH (F(2,41) = 6.041, p = 0.005), LHb (F(2,41) = 4.93, p = .012) and SUM (F(2,41) = 3.40, p = 0.043; Figure 4).
Figure 4.
Number (Mean ± SEM) number of Fos-immunoreactive cells in the mPOAv, VMH, LHb, and SUM of postpartum rats exposed to no stimulation, handling, or an elevated plus maze. Subjects had their pups present for the four hours before testing (Litter Present) or had the pups removed (Litter Removed). Note that X-axes differ across panels.
3.5 Correlations between Fos expression and elevated plus-maze behavior
Significant positive correlations between the number of Fos-IR cells and the percentage of time spent in the open arms of the elevated plus maze were found in the LHb (r = 0.55, p ≤ 0.03) and cPAGd (r = 0.56, p ≤ 0.03; Figure 5). These positive correlations were found only when both groups of dams exposed to the elevated plus maze (i.e., dams with pups before testing and dams without pups before testing) were included in the analysis, but not when each group was analyzed separately.
Figure 5.
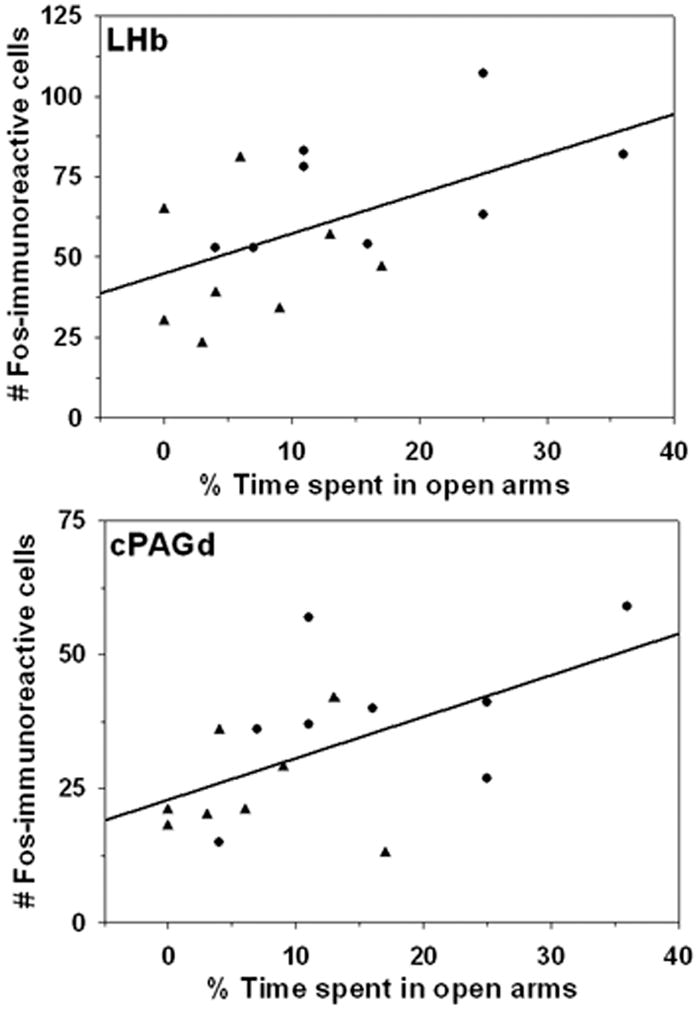
Correlations between the number of Fos-immunoreactive cells in the LHb (top) and cPAGd (bottom) and the percentage of time dams spent in open arms of an elevated plus maze. Circles represent dams with pups before testing, and triangles indicate dams whose pups were removed before testing.
4. Discussion
Numerous experiments have compared neural immediate-early gene activity in male rats differing in their responsiveness to anxiogenic stimuli, in order to provide insight into how the brain generates low- and high-anxiety states [36,37,70,71]. Using a similar strategy, our experiment compared Fos expression between postpartum rats showing low anxiety-related behavior due to the presence of pups prior to an anxiogenic experience, and dams showing higher anxiety-related behavior due to a lack of recent contact with pups. We identified a handful of neural sites that differed between these groups, and these sites may critical places of interface between the neural pathways processing sensory cues from infants and those modulating anxiety-related behaviors.
4.1 Fos expression in response to anxiogenic stimuli
The moderately anxiogenic and stressful experiences of handling, or handling followed by exposure to an elevated plus maze, significantly increased c-fos activity in most of the neural sites we analyzed. We consider these experiences moderately anxiogenic and stressful because they elicit lower hypothalamic-pituitary-adrenal (HPA) response compared to more severe stimuli such as physical restraint, hypoxia, or forced swimming [40,54,60,62,93]. Our study is one of the few to examine immediate early-gene activity in the lactating rat brain after exposure to anxiogenic or stressful stimuli [12,72,94], and it is notable that almost all sites showing increased Fos expression in our mother rats have also been reported to show increased immediate early-gene expression when male rats are exposed to anxiogenic stimuli or anxiety-modulating drugs [13,15,17,18,25,30,49,51,69,71,73,74,76,77,82]. The neural networks underlying fundamental responses to anxiogenic stimuli, therefore, are probably quite similar in male and female rats. In females, though, activity of these networks can be uniquely modified by hormones and other neurochemicals fluctuating across the reproductive cycle [58,59,84,85]. Furthermore, these fluctuations increase maternal motivation and behavior [44,63], which compels mothers to interact with their infants, thereby providing an additional factor that can lower mothers’ anxiety.
4.2 Fos expression in response to anxiogenic stimuli is modulated by recent infant contact
If mother rats do not have recent contact with their infants, the percentage of time they spend in the open arms of an elevated plus maze is significantly reduced [42,59], and we replicate that finding here. We have recently demonstrated that the percentage of time spent in the open arms of an elevated plus maze is an appropriate reflection of anxiety in lactating rats, because it is reduced by anxiogenic drugs [67]. Interestingly, the percentage of open arm entries was not significantly affected by litter removal, although it approached statistical significance. Similarly, a previous experiment within an entire series of experiments from our lab found that the time spent in open arms significantly differed between lactating and diestrus virgin females, but that the frequency of entries into open arms did not [42]. Other experiments on lactating rats have also reported that open arm time and frequency of entries into open arms do not always have the same sensitivity to an experimental manipulation [62,83]. In fact, the classic studies of male rat behavior in an elevated plus maze by Pellow and colleagues [68] demonstrated that the time spent in open arms is more sensitive to anxiety-modulating drugs than is the frequency of entries into open arms, indicating that time spent in opens arms is a more reliable measure of anxiety-related behavior in this paradigm.
Our experiment is the first to address where in the brain recent sensory cues from pups affect neural modulation in response to an anxiogenic experience, and possibly influence mothers’ anxiety-related behaviors. This was accomplished by examining Fos expression after manipulating the presence of the litter, and thereby mothers’ anxiety, before exposing dams to anxiogenic stimuli. This procedure allowed us to compare high and low-anxiety female rats otherwise identical in their reproductive experience and previous hormonal and neurochemical milieu, an advantage that would be lost if anxiety and Fos expression were compared between lactating and virgin females. Studies examining Fos expression after a stressor (restraint, urethane, or endotoxin) find that mothers have fewer Fos-containing cells compared to virgins in numerous sites, including the PVN, supraoptic nucleus, medial amygdala, ventrolateral septum, and cingulate cortex [12,72,94]. We found that Fos expression in none of these sites differed between low-anxiety and high-anxiety dams. This could be due to differences in the specific stressful and anxiogenic stimulus used, but could also imply that reproductive state and recent contact with infants have unique influences on anxiety- and stress-induced Fos expression in some neural sites.
Although removal of pups increases dams’ anxiety-related behaviors, Fos expression in most neural sites we examined was not affected by whether or not the pups were present before testing. Two neural sites showed significant main effects of prior litter presence on Fos expression (mPOAd and BSTv), and four had a significant litter presence by stimulus interaction (mPOAv, LHb, VMH, SUM). This is consistent with the finding that low- or high-anxiety males differ in Fos expression after presentation of anxiogenic stimuli in some, but not most, neural sites implicated in anxiety [36,37,70,71]. The sites differing between low- and high-anxiety males (CG, LSvl, PVN, AHN, LH, CA1, CA3, DG, PVT, and rPAGd) were not the same as those that differed between our low- and high-anxiety females. Thus, even if the fundamental neural networks responding to anxiogenic stimuli are the same in male and female rats, the sites specifically responsible for generating low-and high-anxiety responses within each sex may differ between males and females. Indeed, the six sites we found affected by litter presence seem uniquely suited to modulate anxiety in postpartum females. These sites are directly or indirectly connected [8,16,20,28,75], are responsive to sensory cues from pups and necessary for the display of maternal behaviors [63,92], as well as influence emotional responses in rodents [2,4,26,27,34,56,65,91]. They might be important sites of interface between the neural networks balancing dams’ maternal and anxiety-related behaviors.
We believe there are two points of particular interest regarding these six sites. First, in five of them (mPOAd, mPOAv, LHb, SUM, VMH), and even in some sites that did not have significant main effects of or interactions with litter presence (e.g., CG, BSTd, MS, LSvl, PVNp, NAc, LH), a comparison of the unstimulated controls reveals that simply removing the litter produced a 2–6-fold increase in Fos expression. This is not the result of a physiological stress response, because temporary removal of the litter actually decreases maternal glucocorticoid levels, not increases them [24,78,90]. One of the largest increases in Fos after removal of the litter was in the LHb. The LHb is necessary for the onset of maternal behavior in rats [20,50], and shows increased Fos expression after mothers and litters are reunited following a 2-day separation [45]. This increase occurs only if the litter is removed again before dams are sacrificed [45], but not if the litter and dam remain together until sacrifice [79]. Our data reveal that Fos expression also increases in the LHb after litter removal even following otherwise undisturbed mother-litter interactions. The relevance of increased immediately early-gene activity in the LHb or any other sites after dams are separated from their infants remains to be determined, but it could be related to short-term behavioral and physiological changes that normally occur when mothers leave their nest, including anxiety that increases when dams spend time away from their infants.
The second interesting finding was that the presence of pups had a generally permissive effect on dams’ Fos expression in response to elevated plus-maze exposure, even though these dams displayed less-anxious behavior. We also found only positive correlations, two of which were statistically significant, between the number of Fos-IR cells in any site and the percentage of time spent in the open arms of the maze. The need to collapse across plus-maze groups to find the significant correlations is probably because we needed a sample large enough to provide sufficient variability, particularly on the behavioral variable, before a correlation of any strength could be revealed. This relationship between high Fos expression and low-anxiety behaviors was not completely unexpected, and quite a few neural sites (CG, PVT, LHb, CA1, CA3, and DG) show higher Fos expression after anxiogenic experiences in male rats that are less anxious compared to males that are more anxious [36,37,70,71].
It is valuable to note that, even though Fos expression in some sites increased in controls simply by removing the litter, it was the groups with their pups present before handling or plus-maze exposure that had the highest Fos expression in most areas. This permissive effect of infant presence on Fos expression was greatest in the BSTv, where dams with their litter present before exposure to the elevated plus maze had twice as many Fos-IR cells compared to dams who did not have their litter present before exposure to the maze Importantly, mothers with or without pups that remained unstimulated had a similarly low number of Fos-IR cells in the BSTv, so Fos expression was not due to the performance of maternal behaviors during the four hours before handling or exposure to the elevated plus maze. Anxiety-induced Fos expression in the BSTv, and the other sites where there was a permissive effect of prior litter presence, may occur selectively in GABAergic neurons. The BSTv is an important relay between hypothalamic, amygdalar, and brainstem sites mediating anxiety and stress [91]. It is highly sensitive to norepinephrine [23], and most of its neurons are GABAergic [11,55,80]. Reduced noradrenergic tone when mother are in contact with infants [86] might disinhibit GABA cells in the BSTv [23], thereby suppressing downstream neural activity, and conveying a sense of “safety” that reduces anxiety-related behaviors [7]. Future research is required to determine the phenotype of Fos-immunoreactive cells in the BSTv, and elsewhere in the brain, after lactating rats are exposed to anxiogenic stimuli to help reveal the possibility of such mechanisms.
The exception to the facilitatory effect of pup presence on anxiety-induced Fos expression was the mPOAd, where previous litter presence had a generally suppressive effect. The majority of cells in the mPOAd are also GABAergic [55], but some of its projection neurons to neural areas modulating anxiety and stress responses - such as the posterior hypothalamus and PAG - are not [29,35,66]. The simplest hypothesis is that these excitatory projections need to be suppressed in low-anxiety mothers, which is reflected by low c-fos activity when mothers are faced with anxiogenic stimuli. Again, determining the phenotype of dams’ Fos-immunoreactive cells will be necessary to support this possibility.
In conclusion, lactating rats subjected to an anxiogenic experience show increased Fos expression in many neural areas traditionally implicated in regulating anxiety and stress. Recent contact with pups influences this Fos expression in a handful of sites that could be important starting points to examine how infant contact produces an anxiolytic effect in their mothers. A variety of neurochemicals released in or by these sites - including GABA, norepinephrine, oxytocin, prolactin -might be involved in this process [38,43,58]. Understanding how infant contact modulates the variety of neurochemicals capable of modulating anxiety in postpartum rats will likely contribute to a better understanding of anxiety disorders in postpartum women, and how these disorders can be alleviated by physical contact with infants.
Acknowledgments
The authors would like to thank Ray Figueira for his assistance with this project. This work was supported by a grant for new faculty provided by Michigan State University to J.S. Lonstein.
References
- 1.Adam EK, Gunnar MR, Tanaka A. Adult attachment, parent emotion, and observed parenting behavior: mediator and moderator models. Child Dev. 2004;75:110–122. doi: 10.1111/j.1467-8624.2004.00657.x. [DOI] [PubMed] [Google Scholar]
- 2.Aranda L, Santin LJ, Begega A, Aguirre JA, Arias Supramammillary and adjacent nuclei lesions impair spatial working memory and induce anxiolitic-like behavior. Behav Brain Res. 2006;167:156–64. doi: 10.1016/j.bbr.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Barnett B, Parker G. Possible determinants, correlates, and consequences of high levels of anxiety in primiparous mothers. Psychol Med. 1986;16:177–185. doi: 10.1017/s0033291700002610. [DOI] [PubMed] [Google Scholar]
- 4.Beck CH, Fibiger HC. Conditioned fear-induced changes in behavior and in the expression of the immediate early gene c-fos: with and without diazepam pretreatment. J Neurosci. 1995;15:709–720. doi: 10.1523/JNEUROSCI.15-01-00709.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosch OJ, Meddle SL, Beiderbeck DI, Douglas AJ, Neumann ID. Brain oxytocin correlates with maternal aggression: link to anxiety. J Neurosci. 2005;25:6807–6815. doi: 10.1523/JNEUROSCI.1342-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campeau S, Falls WA, Cullinan WE, Helmreich DL, Davis M, Watson SJ. Elicitation and reduction of fear: behavioural and neuroendocrine indices and brain induction of the immediate-early gene c-fos. Neuroscience. 1997;78:1087–1104. doi: 10.1016/s0306-4522(96)00632-x. [DOI] [PubMed] [Google Scholar]
- 8.Canteras NS, Simerly RB, Swanson LW. Organization of projections from the ventromedial nucleus of the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol. 1994;348:41–79. doi: 10.1002/cne.903480103. [DOI] [PubMed] [Google Scholar]
- 9.Charney DS. Neuroanatomical circuits modulating fear and anxiety behaviors. Acta Psychiatr Scand Suppl. 2003;417:38–50. doi: 10.1034/j.1600-0447.108.s417.3.x. [DOI] [PubMed] [Google Scholar]
- 10.Charney DS, Deutch A. A functional neuroanatomy of anxiety and fear: implications for the pathophysiology and treatment of anxiety disorders. Crit Rev Neurobiol. 1996;10:419–46. doi: 10.1615/critrevneurobiol.v10.i3-4.70. [DOI] [PubMed] [Google Scholar]
- 11.Cullinan WE, Herman JP, Watson SJ. Ventral subicular interaction with the hypothalamic paraventricular nucleus: evidence for a relay in the bed nucleus of the stria terminalis. J Comp Neurol. 1993;332:1–20. doi: 10.1002/cne.903320102. [DOI] [PubMed] [Google Scholar]
- 12.Da Costa AP, Wood S, Ingram CD, Lightman SL. Region-specific reduction in stress-induced c-fos mRNA expression during pregnancy and lactation. Brain Res. 1996;742:177–184. doi: 10.1016/s0006-8993(96)00962-6. [DOI] [PubMed] [Google Scholar]
- 13.Day HE, Masini CV, Campeau S. The pattern of brain c-fos mRNA induced by a component of fox odor, 2,5-dihydro-2,4,5-trimethylthiazoline (TMT), in rats, suggests both systemic and processive stress characteristics. Brain Res. 2004;1025:139–151. doi: 10.1016/j.brainres.2004.07.079. [DOI] [PubMed] [Google Scholar]
- 14.De Bellis MD, Broussard ER, Herring DJ, Wexler S, Moritz G, Benitez JG. Psychiatric co-morbidity in caregivers and children involved in maltreatment: a pilot research study with policy implications. Child Abuse Negl. 2001;25:923–944. doi: 10.1016/s0145-2134(01)00247-2. [DOI] [PubMed] [Google Scholar]
- 15.Dielenberg RA, Hunt GE, McGregor IS. “When a rat smells a cat”: the distribution of Fos immunoreactivity in rat brain following exposure to a predatory odor. Neuroscience. 2001;104:1085–1097. doi: 10.1016/s0306-4522(01)00150-6. [DOI] [PubMed] [Google Scholar]
- 16.Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, magnocellular nucleus: implications for cerebral hemisphere regulation of micturition, defecation, and penile erection. J Comp Neurol. 2006;494:108–41. doi: 10.1002/cne.20789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duncan GE, Knapp DJ, Breese GR. Neuroanatomical characterization of Fos induction in rat behavioral models of anxiety. Brain Res. 1996;713:79–91. doi: 10.1016/0006-8993(95)01486-1. [DOI] [PubMed] [Google Scholar]
- 18.Emmert MH, Herman JP. Differential forebrain c-fos mRNA induction by ether inhalation and novelty: evidence for distinctive stress pathways. Brain Res. 1999;845:60–67. doi: 10.1016/s0006-8993(99)01931-9. [DOI] [PubMed] [Google Scholar]
- 19.Feijo L, Hernandez-Reif M, Field T, Burns W, Valley-Gray S, Simco E. Mothers’ depressed mood and anxiety levels are reduced after massaging their preterm infants. Infant Behav Dev. 2006;29:476–80. doi: 10.1016/j.infbeh.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Felton TM, Linton L, Rosenblatt JS, Morrell JI. Intact neurons of the lateral habenular nucleus are necessary for the nonhormonal, pup-mediated display of maternal behavior in sensitized virgin female rats. Behav Neurosci. 1998;112:1458–65. doi: 10.1037//0735-7044.112.6.1458. [DOI] [PubMed] [Google Scholar]
- 21.Felton TM, Linton L, Rosenblatt JS, Morell JI. First and second order maternal behavior related afferents of the lateral habenula. Neuroreport. 1999;10:883–7. doi: 10.1097/00001756-199903170-00039. [DOI] [PubMed] [Google Scholar]
- 22.Ferreira A, Hansen S, Nielsen M, Archer T, Minor BG. Behavior of mother rats in conflicts tests sensitive to antianxiety agents. Behav Neurosci. 1989;103:193–201. doi: 10.1037//0735-7044.103.1.193. [DOI] [PubMed] [Google Scholar]
- 23.Forray MI, Gysling K. Role of noradrenergic projections to the bed nucleus of the stria terminalis in the regulation of the hypothalamic-pituitary-adrenal axis. Brain Res Rev. 2004;47:145–60. doi: 10.1016/j.brainresrev.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Fukushima A, Yin P, Ishida M, Sugiyama N, Arita J. Pup removal suppresses estrogen-induced surges of LH secretion and activation of GnRH neurons in lactating rats. J Endocrinol. 2006;191:339–348. doi: 10.1677/joe.1.06728. [DOI] [PubMed] [Google Scholar]
- 25.Funk D, Amir S. Circadian modulation of fos responses to odor of the red fox, a rodent predator, in the rat olfactory system. Brain Res. 2000;866:262–267. doi: 10.1016/s0006-8993(00)02249-6. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez MI, Baker BI, Hole DR, Wilson CA. Behavioral effects of neuropeptide E-I (NEI) in the female rat: interactions with alpha-MSH, MCH and dopamine. Peptides. 1998;19:1007–1016. doi: 10.1016/s0196-9781(98)00045-x. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez MI, Vaziri S, Wilson CA. Behavioral effects of alpha-MSH and MCH after central administration in the female rat. Peptides. 1996;17:171–177. doi: 10.1016/0196-9781(95)02092-6. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalo-Ruiz A, Alonso A, Sanz JM, Llinas RR. Afferent projections to the mammillary complex of the rat, with special reference to those from surrounding hypothalamic regions. J Comp Neurol. 1992;321:277–99. doi: 10.1002/cne.903210208. [DOI] [PubMed] [Google Scholar]
- 29.Gritti I, Mainville L, Jones BE. Projections of GABAergic and cholinergic basal forebrain and GABAergic preoptic-anterior hypothalamic neurons to the posterior lateral hypothalamus of the rat. J Comp Neurol. 1994;339:251–268. doi: 10.1002/cne.903390206. [DOI] [PubMed] [Google Scholar]
- 30.Handa RJ, Nunley KM, Bollnow MR. Induction of c-fos mRNA in the brain and anterior pituitary gland by a novel environment. Neuroreport. 1993;4:1079–1082. [PubMed] [Google Scholar]
- 31.Hansen S. Mechanisms involved in the control of punished responding in mother rats. Horm Behav. 1990;24:186–197. doi: 10.1016/0018-506x(90)90004-h. [DOI] [PubMed] [Google Scholar]
- 32.Hansen S, Ferreira A, Selart ME. Behavioural similarities between mother rats and benzodiazepine-treated non-maternal animals. Psychopharmacology. 1985;86:344–347. doi: 10.1007/BF00432226. [DOI] [PubMed] [Google Scholar]
- 33.Heinrichs M, Meinlschmidt G, Neumann I, Wagner S, Kirschbaum C, Ehlert U, Hellhammer DH. Effects of suckling on hypothalamic-pituitary-adrenal axis response to psychosocial stress in postpartum women. J Endocrinol Metab. 2001;86:4798–4804. doi: 10.1210/jcem.86.10.7919. [DOI] [PubMed] [Google Scholar]
- 34.Jardim MC, Aguiar DC, Moreira FA, Guimaraes FS. Role of glutamate ionotropic and benzodiazepine receptors in the ventromedial hypothalamic nucleus on anxiety. Pharmacol Biochem Behav. 2005;82:182–189. doi: 10.1016/j.pbb.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 35.Jiang M, Behbehani MM. Physiological characteristics of the projection pathway from the medial preoptic to the nucleus raphe magnus of the rat and its modulation by the periaqueductal gray. Pain. 2001;94:139–47. doi: 10.1016/S0304-3959(01)00348-7. [DOI] [PubMed] [Google Scholar]
- 36.Kabbaj M, Akil H. Individual differences in novelty-seeking behavior in rats: a c-fos study. Neuroscience. 2001;106:535–545. doi: 10.1016/s0306-4522(01)00291-3. [DOI] [PubMed] [Google Scholar]
- 37.Kalisch R, Salome N, Platzer S, Wigger A, Czisch M, Sommer W, Singewald N, Heilig M, Berthele A, Holsboer F, Landgraf R, Auer DP. High trait anxiety and hyporeactivity to stress of the dorsomedial prefrontal cortex: a combined phMRI and Fos study in rats. Neuroimage. 2004;23:382–391. doi: 10.1016/j.neuroimage.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 38.Landgraf R. Neuropeptides in anxiety modulation. Handb Exp Pharmacol. 2005;169:335–369. doi: 10.1007/3-540-28082-0_12. [DOI] [PubMed] [Google Scholar]
- 39.Lansing SW, Lonstein JS. Tyrosine hydroxylase-synthesizing cells in the hypothalamus of prairie voles (Microtus ochrogaster): sex differences in the anteroventral periventricular preoptic area and effects of adult gonadectomy or neonatal gonadal hormones. J Neurobiol. 2006;66:197–204. doi: 10.1002/neu.20212. [DOI] [PubMed] [Google Scholar]
- 40.Liebsch G, Linthorst AC, Neumann ID, Reul JM, Holsboer F, Landgraf R. Behavioral, physiological, and neuroendocrine stress responses and differential sensitivity to diazepam in two Wistar rat lines selectively bred for high- and low-anxiety-related behavior. Neuropsychopharmacology. 1998;19:381–396. doi: 10.1016/S0893-133X(98)00042-6. [DOI] [PubMed] [Google Scholar]
- 41.Lightman SL, Young WS. Lactation inhibits stress-mediated secretion of corticosterone and oxytocin and hypothalamic accumulation of corticotropin-releasing factor and enkephalin messenger ribonucleic acids. Endocrinology. 1989;124:2358–2364. doi: 10.1210/endo-124-5-2358. [DOI] [PubMed] [Google Scholar]
- 42.Lonstein JS. Reduced anxiety during lactation requires recent interactions with pups, but not their suckling or peripheral sources of hormones. Horm Behav. 2005;47:241–255. doi: 10.1016/j.yhbeh.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 43.Lonstein JS. Regulation of anxiety during the postpartum period. Front Neuroendocrinol. 2007;28:115–41. doi: 10.1016/j.yfrne.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 44.Lonstein JS, Morrell JI. Neuropharmacology and neuroendocrinology of maternal behavior and motivation. In: Blaustein JD, editor. Behavioral Neurobiology. Handbook of Neurochemistry and Molecular Biology. Vol. 18. New York: Kluwer Press; 2006. pp. 195–245. [Google Scholar]
- 45.Lonstein JS, Simmons DA, Stern JM. Functions of the caudal periaqueductal gray in lactating rats: kyphosis, lordosis, maternal aggression, and fearfulness. Behav Neurosci. 1998;112:1502–1518. doi: 10.1037//0735-7044.112.6.1502. [DOI] [PubMed] [Google Scholar]
- 46.Maestripieri D. Maternal anxiety in rhesus macaques (Macaca mulatta). II. Emotional bases of individual differences in mothering style. Ethology. 1993;95:32–42. [Google Scholar]
- 47.Maestripieri D. Parenting styles of abusive mothers in group-living rhesus macaques. Anim Behav. 1998;55:1–11. doi: 10.1006/anbe.1997.0578. [DOI] [PubMed] [Google Scholar]
- 48.Maestripieri D. The biology of human parenting: insights from nonhuman primates. Neurosci Biobehav Rev. 1999;23:411–422. doi: 10.1016/s0149-7634(98)00042-6. [DOI] [PubMed] [Google Scholar]
- 49.Masini CV, Sauer S, Campeau S. Ferret odor as a processive stress model in rats: neurochemical, behavioral, and endocrine evidence. Behav Neurosci. 2005;119:280–292. doi: 10.1037/0735-7044.119.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matthews-Felton T, Corodimas KP, Rosenblatt JS, Morrell JI. Lateral habenula neurons are necessary for the hormonal onset of maternal behavior and for the display of postpartum estrus in naturally parturient female rats. Behav Neurosci. 1995;109:1172–1188. doi: 10.1037//0735-7044.109.6.1172. [DOI] [PubMed] [Google Scholar]
- 51.McGregor IS, Hargreaves GA, Apfelbach R, Hunt GE. Neural correlates of cat odor-induced anxiety in rats: region-specific effects of the benzodiazepine midazolam. J Neurosci. 2004;24:4134–4144. doi: 10.1523/JNEUROSCI.0187-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol. 2003;70:183–244. doi: 10.1016/s0301-0082(03)00087-x. [DOI] [PubMed] [Google Scholar]
- 53.Moore PS, Wiley SE, Sigman M. Interactions between mothers and children: impacts of maternal and child anxiety. J Abnormal Psych. 2004;113:471–476. doi: 10.1037/0021-843X.113.3.471. [DOI] [PubMed] [Google Scholar]
- 54.Mueller NK, Dolgas CM, Herman JP. Stressor-selective role of the ventral subiculum in regulation of neuroendocrine stress responses. Endocrinology. 2004;145:3763–3768. doi: 10.1210/en.2004-0097. [DOI] [PubMed] [Google Scholar]
- 55.Mugnaini E, Oertel WH. An atlas of the distribution of GABAergic neurons and terminals in the rat CNS as revealed by GAD immunohistochemistry. In: Bjorklund A, Hokfelt T, editors. GABA and Neuropeptides in the CNS, part I. Handbook of Chemical Neuroanatomy. Vol. 4. Amsterdam: Elsevier; 1985. pp. 436–608. [Google Scholar]
- 56.Murphy CA, DiCamillo AM, Haun F, Murray M. Lesion of the habenular efferent pathway produces anxiety and locomotor hyperactivity in rats: a comparison of the effects of neonatal and adult lesions. Behav Brain Res. 1996;81:43–52. doi: 10.1016/s0166-4328(96)00041-1. [DOI] [PubMed] [Google Scholar]
- 57.Nayak MB, Milner JS. Neuropsychological functioning: comparison of mothers at high- and low-risk for child physical abuse. Child Abuse Negl. 1998;22:687–703. doi: 10.1016/s0145-2134(98)00052-0. [DOI] [PubMed] [Google Scholar]
- 58.Neumann ID. Alterations in behavioral and neuroendocrine stress coping strategies in pregnant, parturient and lactating rats. Prog Brain Res. 2001;133:143–152. doi: 10.1016/s0079-6123(01)33011-x. [DOI] [PubMed] [Google Scholar]
- 59.Neumann ID. Brain mechanisms underlying emotional alterations in the peripartum period in rats. Depress Anxiety. 2003;17:111–121. doi: 10.1002/da.10070. [DOI] [PubMed] [Google Scholar]
- 60.Neumann ID, Johnstone HA, Hatzinger M, Liebsch G, Shipston M, Russell JA, Landgraf R, Douglas AJ. Attenuated neuroendocrine responses to emotional and physical stressors in pregnant rats involve adenohypophysial changes. J Physiol. 1998;508:289–300. doi: 10.1111/j.1469-7793.1998.289br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Neumann ID, Kromer SA, Bosch OJ. Effects of psycho-social stress during pregnancy on neuroendocrine and behavioural parameters in lactation depend on the genetically determined stress vulnerability. Psychoneuroendocrinology. 2005;30:791–806. doi: 10.1016/j.psyneuen.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 62.Neumann ID, Torner L, Wigger A. Brain oxytocin: differential inhibition of neuroendocrine stress responses and anxiety-related behaviour in virgin, pregnant, and lactating rats. Neuroscience. 2000;95:567–575. doi: 10.1016/s0306-4522(99)00433-9. [DOI] [PubMed] [Google Scholar]
- 63.Numan M, Insel TR. The Neurobiology of Parental Behavior. New York: Springer-Verlag; 2003. [Google Scholar]
- 64.Olazabal DE, Ferreira A. Maternal behavior in rats with kainic acid-induced lesions of the hypothalamic paraventricular nucleus. Physiol Behav. 1997;61:779–784. doi: 10.1016/s0031-9384(96)00567-7. [DOI] [PubMed] [Google Scholar]
- 65.Pan WX, McNaughton N. The role of the medial supramammillary nucleus in the control of hippocampal theta activity and behaviour in rats. Eur J Neurosci. 2002;16:1797–1809. doi: 10.1046/j.1460-9568.2002.02267.x. [DOI] [PubMed] [Google Scholar]
- 66.Parry DM, Johns N, Semenenko FM, Snowball RK, Hudson PM, Lumb BM. Glutamatergic projections from the rostral hypothalamus to the periaqueductal grey. Neuroreport. 1996;7:1536–1540. doi: 10.1097/00001756-199606170-00020. [DOI] [PubMed] [Google Scholar]
- 67.Peabody MF, Lonstein JS. GABAA and oxytocin receptor activity in the midbrain periaqueductal gray influence anxiety during lactation in rats. Society for Neuroscience; Washington DC. November 12–16, 2005. [Google Scholar]
- 68.Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 69.Rosen JB, Adamec RE, Thompson BL. Expression of egr-1 (zif268) mRNA in select fear-related brain regions following exposure to a predator. Behav Brain Res. 2005;162:279–288. doi: 10.1016/j.bbr.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 70.Salchner P, Sartori SB, Sinner C, Wigger A, Frank E, Landgraf R, Singewald N. Airjet and FG-7142-induced fos expression differs in rats selectively bred for high and low anxiety-related behavior. Neuropharmacology. 2006;50:1048–1058. doi: 10.1016/j.neuropharm.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 71.Salome N, Salchner P, Viltart O, Sequeira H, Wigger A, Landgraf R, Singewald N. Neurobiological correlates of high (HAB) versus low anxiety-related behavior (LAB): differential fos expression in HAB and LAB rats. Biol Psychiatry. 2004;55:715–723. doi: 10.1016/j.biopsych.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 72.Shanks N, Windle RJ, Perks P, Wood S, Ingram CD, Lightman SL. The hypothalamic-pituitary-adrenal axis response to endotoxin is attenuated during lactation. J Neuroendocrinol. 1999;11:857–865. doi: 10.1046/j.1365-2826.1999.00400.x. [DOI] [PubMed] [Google Scholar]
- 73.Silveira MC, Graeff FG, Sandner G. Regional distribution of Fos-like immunoreactivity in the rat brain after exposure to fear-inducing stimuli. Braz J Med Biol Res. 1994;4:1077–1081. [PubMed] [Google Scholar]
- 74.Silveira MC, Sandner G, Graeff FG. Induction of fos immunoreactivity in the brain by exposure to the elevated plus-maze. Behav Brain Res. 1993;56:115–118. doi: 10.1016/0166-4328(93)90028-o. [DOI] [PubMed] [Google Scholar]
- 75.Simerly RB, Swanson LW. Projections of the medial preoptic nucleus: a Phaseolus vulgaris leucoagglutinin anterograde tract-tracing study in the rat. J Comp Neurol. 1988;270:209–42. doi: 10.1002/cne.902700205. [DOI] [PubMed] [Google Scholar]
- 76.Singewald N, Salchner P, Sharp T. Induction of c-Fos expression in specific areas of the fear circuitry in rat forebrain by anxiogenic drugs. Biol Psychiatry. 2003;53:275–283. doi: 10.1016/s0006-3223(02)01574-3. [DOI] [PubMed] [Google Scholar]
- 77.Singewald N, Sharp T. Neuroanatomical targets of anxiogenic drugs in the hindbrain as revealed by fos immunocytochemistry. Neuroscience. 2000;98:759–770. doi: 10.1016/s0306-4522(00)00177-9. [DOI] [PubMed] [Google Scholar]
- 78.Smotherman WP, Wiener SG, Mendoza SP, Levine S. Pituitary--adrenal responsiveness of rat mothers to noxious stimuli and stimuli produced by pups. Ciba Found Symp. 1976;45:5–25. doi: 10.1002/9780470720271.ch2. [DOI] [PubMed] [Google Scholar]
- 79.Stack EC, Numan M. The temporal course of expression of c-fos and fos b within the medial preoptic area and other brain regions of postpartum female rats during prolonged mother--young interactions. Behav Neurosci. 2000;114:609–622. doi: 10.1037//0735-7044.114.3.609. [DOI] [PubMed] [Google Scholar]
- 80.Stefanova N, Bozhilova-Pastirova A, Ovtscharoff W. Sex and age differences of neurons expressing GABA-immunoreactivity in the rat bed nucleus of the stria terminalis. Int J Dev Neurosci. 1998;16:443–448. doi: 10.1016/s0736-5748(98)00056-2. [DOI] [PubMed] [Google Scholar]
- 81.Swanson LW. Brain Maps: Structure of the Rat Brain. Amsterdam: Elsevier; 1998. [Google Scholar]
- 82.Thompson BL, Rosen JB. Immediate-early gene expression in the central nucleus of the amygdala is not specific for anxiolytic or anxiogenic drugs. Neuropharmacology. 2006;50:57–68. doi: 10.1016/j.neuropharm.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 83.Torner L, Toschi N, Nava G, Clapp C, Neumann ID. Increased hypothalamic expression of prolactin in lactation: involvement in behavioural and neuroendocrine stress responses. Eur J Neurosci. 2002;15:1381–1389. doi: 10.1046/j.1460-9568.2002.01965.x. [DOI] [PubMed] [Google Scholar]
- 84.Toufexis DJ. Region- and sex-specific modulation of anxiety behaviours in the rat. J Neuroendocrinol. 2007;19:461–73. doi: 10.1111/j.1365-2826.2007.01552.x. [DOI] [PubMed] [Google Scholar]
- 85.Toufexis DJ, Myers KM, Davis M. The effect of gonadal hormones and gender on anxiety and emotional learning. Horm Behav. 2006;50:539–549. doi: 10.1016/j.yhbeh.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 86.Toufexis DJ, Rochford J, Walker CD. Lactation induced reduction in rats’ acoustic startle is associated with changes in noradrenergic neurotransmission. Behav Neurosci. 1999;113:176–184. doi: 10.1037//0735-7044.113.1.176. [DOI] [PubMed] [Google Scholar]
- 87.Troisi A, D’Amato FR. Anxiety in the pathogenesis of primate infant abuse: a pharmacological study. Psychopharmacology. 1991;103:571–572. doi: 10.1007/BF02244261. [DOI] [PubMed] [Google Scholar]
- 88.Troisi A, Schino G, D’Antoni M, Pandolfi N, Aureli F, D’Amato FR. Scratching as a behavioral index of anxiety in macaque mothers. Behav Neural Biol. 1991;56:307–313. doi: 10.1016/0163-1047(91)90469-7. [DOI] [PubMed] [Google Scholar]
- 89.Turner SM, Beidel DC, Roberson-Nay R, Tervo K. Parenting behaviors in parents with anxiety disorders. Behav Res Ther. 2002;41:541–554. doi: 10.1016/s0005-7967(02)00028-1. [DOI] [PubMed] [Google Scholar]
- 90.Walker CD, Lightman SL, Steele MK, Dallman MF. Suckling is a persistent stimulus to the adrenocortical system of the rat. Endocrinology. 1992;130:115–125. doi: 10.1210/endo.130.1.1309321. [DOI] [PubMed] [Google Scholar]
- 91.Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- 92.Wehr R, Mansouri A, de Maeyer T, Gruss P. Fkh5-deficient mice show dysgenesis in the caudal midbrain and hypothalamic mammillary body. Development. 1997;124:4447–4456. doi: 10.1242/dev.124.22.4447. [DOI] [PubMed] [Google Scholar]
- 93.Wigger A, Neumann ID. Periodic maternal deprivation induces gender-dependent alterations in behavioral and neuroendocrine responses to emotional stress in adult rats. Physiol Behav. 1999;66:293–302. doi: 10.1016/s0031-9384(98)00300-x. [DOI] [PubMed] [Google Scholar]
- 94.Woodside B, Amir S. Lactation reduces fos induction in the paraventricular and supraoptic nuclei of the hypothalamus after urethane administration in rats. Brain Res. 1997;752:319–323. doi: 10.1016/s0006-8993(97)00044-9. [DOI] [PubMed] [Google Scholar]
- 95.Zelkowitz P, Papageorgiou A. Maternal anxiety: an emerging prognostic factor in neonatology. Acta Paediatrica. 2005;94:1704–1705. doi: 10.1111/j.1651-2227.2005.tb01840.x. [DOI] [PubMed] [Google Scholar]