Abstract
This study aims to evaluate immunization with polymeric microparticles containing recombinant antigen 85B (rAg85B) delivered directly to the lungs to protect against tuberculosis. rAg85B was expressed in Escherichia coli and encapsulated in poly(lactic-co-glycolic acid) microparticles (P-rAg85B). These were delivered as dry powders to the lungs of guinea pigs in single or multiple doses of homologous and heterologous antigens. Bacille Calmette–Guérin (BCG) delivered subcutaneously was employed as the positive control and as part of immunization strategies. Immunized animals were challenged with a low-dose aerosol of Mycobacterium tuberculosis (MTB) H37Rv to assess the extent of protection measured as reduction in bacterial burden (CFU) in the lungs and spleens of guinea pigs. Histopathological examination and morphometric analysis of these tissues were also performed. The heterologous strategy of BCG prime–P-rAg85B aerosol boosts appeared to enhance protection from bacterial infection, as indicated by a reduction in CFU in both the lungs and spleens compared with untreated controls. Although the CFU data were not statistically different from the BCG and BCG–BCG groups, the histopathological and morphometric analyses indicated the positive effect of BCG–P-rAg85B in terms of differences in area of tissue affected and number and size of granulomas observed in tissues. P-rAg85B microparticles appeared to be effective in boosting a primary BCG immunization against MTB infection, as indicated by histopathology and morphometric analysis. These encouraging observations are relevant to boosting adults previously immunized with BCG or exposed to MTB, commonly the case in the developing world, and should be followed by further assessment of an appropriate immunization protocol for maximum protection.
Key words: antigen 85B, BCG, boost immunization, microparticles, pulmonary delivery
INTRODUCTION
Tuberculosis (TB) is the leading cause of death from a single infectious pathogen. Approximately 1.6 million people died of this disease in 2005 (1). Currently, the only approved TB vaccine is the attenuated strain of Mycobacterium bovis, Bacillus Calmette–Guérin (BCG). However, the protective effect of this vaccine has been shown to be highly variable and diminishes 10–15 years following administration (2). Therefore, new vaccines and immunizations strategies are needed urgently to prevent TB infection.
Since the majority of TB patients acquire their primary infection by the pulmonary route, vaccine administration to the lungs may elicit an immunologically relevant response resulting in protection from infection (3). Alveolar macrophages are antigen presenting, host cells for Mycobacterium tuberculosis (MTB) in the lungs and the principal target for antigen delivery. Depending on composition and physicochemical properties, particles <10 µm in aerodynamic diameter may be inhaled as aerosols and deposit in the lungs (4). They will reside at the site of deposition for extended periods of time prior to uptake by antigen presenting cells (APCs) (5). The combination of aerosol particle entry to the lungs and their subsequent uptake by macrophages increases the probability of eliciting a potent immune response (6). Therefore, immunization by the pulmonary route is a promising alternative to conventional approaches with a strong mechanistic rationale for the likelihood of success.
Poly (lactic-co-glycolic acid) (PLGA) copolymers have frequently been described as carriers for antigens (7,8). PLGA microparticles, in diameters ranging from 1 to 10 μm and loaded with antigenic peptides, interact with APCs to induce cell-mediated immunity (9,10). Antigen 85B (Ag85B), a secreted protein from M. tuberculosis, is one of the most promising vaccine candidates for prevention of tuberculosis. It induces both humoral and cell-mediated immune responses (CD4 and CD8 T cell responses) in MTB-infected patients (11,12). The immunoprotective effect of Ag85B has been demonstrated in the highly relevant low-dose aerosol inoculation guinea pig model of pulmonary tuberculosis (13). Furthermore, the recombinant Ag85B (rAg85B) encapsulated in respirable PLGA microparticles (designed P-rAg85B in this study) enhanced the CD4 Th1 cell response after being processed by human macrophage-like THP-1 cells and presented to the DB-1, T hybridoma cell, which recognizes an epitope of Ag85B (14). Production of sufficient quantities of Ag85B from bacterial cell cultures is limited, but the antigen can be generated for vaccine development by recombinant methods using Escherichia coli overexpression (15).
Antigen subunit proteins necessarily present a much smaller number of epitopes required for immunization than the original whole organism. This frequently results in a weak immune response. In order to amplify the response, the antigen may be administered several times in a regimen designated a prime-boost immunization strategy. The boost that follows the priming dose may be homologous or heterologous which reflects dosing with the same or different antigens to the prime, respectively. Repeated immunization with the same antigen is expected to result in higher levels of antibodies than following a single dose. Homologous boosting is sufficient to protect from organisms where humoral response is the dominant element of immunity (16). Many pathogens, including MTB, need T cell-mediated responses for protective immunity. Pathogen-specific T cells are induced in higher levels in response to a heterologous, rather than a homologous, boost in a variety of animal models of TB (16). Moreover, adult populations in many developing countries have been immunized previously with BCG or exposed to MTB, which constitutes the priming exposure, and a boost approach is more appropriate for these populations. Therefore, there is a need to develop an effective boosting immunization that could enhance and prolong the protective immunity based on BCG, or prior MTB, initiated immunity.
The objective of the present study was to evaluate a prime-boost immunization strategy with polymeric microparticles containing rAg85B delivered as aerosols to the lungs in comparison or combination with BCG to protect against tuberculosis.
MATERIALS AND METHODS
Recombinant Antigen 85B
rAg85B protein was produced from E. coli strain of JM109DE3, containing Ag85B gene with His tag (courtesy of Dr. Douglas Kernodle, Vanderbilt University). Flasks containing 1 L Luria–Bertani broth and 50 μg of carbenicillin per milliliter were inoculated with 20 ml of an overnight culture of E. coli JM109DE3 and incubated at 37°C until the OD600 reading reached 0.4–0.5. Recombinant protein expression was then induced by the addition of isopropyl-β-thiogalactopyranoside (IPTG, 1 mM) and incubation at 22°C overnight. The E. coli cells were pelleted and then probe-sonicated. The supernatant was passed through a nickel-affinity column (Ni Sepharose™ 6 Fast Flow, Amersham Biosciences, Piscataway, NJ). The eluted fractions with His-tag proteins were further purified in a Superdex 75 peptide column (Amersham Biosciences, Piscataway, NJ) using Tris (20 mM, pH 7.5)–sodium chloride (1 M), as the eluting buffer. The rAg85B was quantified at UV 280 nm with extinction coefficient of UV280 of 1.0 for a 1.0 mg/mL protein solution (15).
Endotoxin in the protein preparations was removed with Detoxin-Gel™ Endotoxin Removing Gel (Pierce, Rockford, IL). The endotoxin level was quantified by QCL-Chromogenic LAL (Cambrex Bio Science Walkersville, Inc., Walkersville, MD) and determined to be <0.025 ng/mg after purification. Proteins were dialyzed in 0.1 M ammonium bicarbonate overnight and then lyophilized for 48 h.
Microparticle Manufacture
PLGA polymer (700 mg; MW 84.7 kDa, L/G 75:25, intrinsic viscosity 0.68 dL/g in chloroform, Durect Corp., Pelham, AL) was dissolved in 200 ml methylene chloride (organic phase). The aqueous phase consisted of either 4 or 16 mg rAg85B dissolved in 2.4 ml of 20 mM sodium phosphate buffer, pH 7.4 with or without trehalose dibehenate (TDB, Sigma, St. Louis, MO), a component of the MTB cell wall, the molecular structure, and adjuvancy is well documented (17). The aqueous and organic phases were probe-sonicated for three 10-s periods on an ice bath immediately prior to spray drying. Microparticles were manufactured using a spray-drier (Buchi Mini Spray-drier B-191, Buchi, Flawil, Switzerland) with optimized conditions of liquid feed rate 4.5 mL/min, atomization pressure 3.0 bar, inlet temperature 65°C, outlet temperature 41–43°C, and nitrogen flow 600 L/h.
Encapsulation Efficiency and Antigen Stability
The amount of rAg85B was determined as follows: Two milliliter of DMSO was added to a vial containing 10–20 mg of P-rAg85B microspheres and periodical stirring for an hour. Ten milliliter of 0.05 N NaOH with 0.5% SDS was then added, and the contents of the vial gently mixed and let stand for 1 h. The contents of the vial were centrifuged (10 min, 12,000 rpm, 4°C) and the supernatant assayed for protein content of Ag85B by Lowry’s method. The functionality of rAg85B after particle manufacture was evaluated by the T hybridoma cell recognition assay (14).
Aerodynamic Performance of PLGA Microparticles
The aerodynamic performance of the microparticles when delivered from an Insufflator DP-3 (Penncentury, PA) was evaluated with the USP apparatus B and the nonviable Andersen cascade impactor (ACI) under a vacuum of 60LPM for 4 s. Emitted dose was determined by the USP apparatus B, whereas the mass median aerodynamic diameter (MMAD) and the fine particle fractions (fraction of the particles sizing <5 μm in emitted dose) were calculated after powder evaluation with the ACI.
Animals
Dunkin-Hartley guinea pigs weighing 368.9 ± 19.4 g were employed (Hilltop Laboratory Animals, Inc., Scottsdale, PA). The animals were housed in a 12-h light/12-h dark cycle and constant temperature environment of 22°C with free access to food and water. All animal protocols were approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill.
Aerosol Immunization
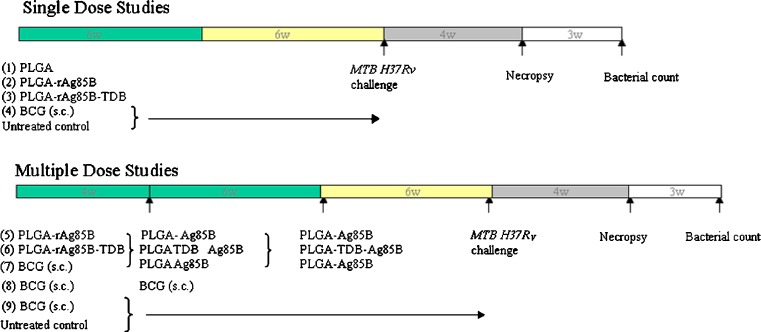
Immunization regimens consisted of single and multiple-dosed groups and are shown in Fig. 1. Five groups (n = 6) were employed in a single-dose regimen: BCG (1 × 103 CFU, Tice strain, Organon USA Inc., Roseland, NJ) was subcutaneously (s.c.) administered to guinea pigs as positive control; negative controls consisted of untreated animals and those treated with PLGA microparticles alone; PLGA-rAg85B (P-rAg85B, 0.57% w/w rAg85B) and PLGA-rAg85B-TDB (P-rAg85B-TDB, 0.57% w/w rAg85B, 0.1% w/w TDB) were the microparticle vaccine formulations, containing subunit antigens, administered by insufflation as follows. Animals were anesthetized with a mixture of ketamine (50 mg/kg), xylazine (5 mg/kg), intubated and approximately 10 mg of microparticle formulations were administered with a small animal insufflator (Model DP-3, Penn Century, PA) into the airways of guinea pigs. In a multiple-dose regimen, the animals were primed with P-rAg85B (0.57% w/w r-Ag85B, 10 mg by insufflation), P-rAg85B-TDB (0.57% w/w rAg85B, 0.1% w/w TDB, 10 mg by insufflation), and BCG (s.c. 1 × 103 CFU). Animals were boosted 4 weeks later by insufflation with approximately 10 mg of P-rAg85B (0.57% w/w r-Ag85B, for P-rAg85B and BCG groups) and P-rAg85B-TDB (0.57% w/w rAg85B, 0.1% w/w TDB, for P-rAg85B-TDB group), or BCG (s.c. 1 × 103 CFU). The second boosting dose was administered 10 weeks after initial prime vaccination by insufflation of approximately 3 mg of P-rAg85B (2.28% w/w rAg85B; for P-rAg85B and BCG groups) and P-rAg85B-TDB (2.28% w/w rAg85B, 0.1% w/w TDB; for P-rAg85B-TDB group). The schedule for aerosol immunization in multiple-dose groups was selected based on previous studies employing BCG and Ag85B (13,18,19). Animal weights were recorded throughout the study.
Fig. 1.
Immunization regimens for single and multiple-dosed groups
Cutaneous Delayed-Type Hypersensitivity
Intradermal injection of purified protein derivative (PPD) allows the detection of individuals sensitized by mycobacteria, either due to TB infection (20) or to assess the degree of immunity conferred by BCG vaccine (21). Upon inoculation into the skin of sensitized individuals, a delayed-type hypersensitivity (DTH) response is evoked (22). This test was performed in immunized animals, the day prior to bacterial challenge. PPD (100TU, from MTB H37Rv, Mycos Research, Loveland, CO) and rAg85B (50 μg in 100 μl phosphate-buffered saline (PBS)) were injected intradermally (i.d.) on the two flanks of the animals. The injection sites were measured with a pair of calipers 24 h after i.d. injection for mean diameter of induration or erythema. It should be noted that the gene sequence for very few guinea pig cytokines and chemokines are available (23). Consequently, insufficient reagents exist to conduct a thorough assessment of immunogenicity as would be familiar to those working with other species for which many cytokine ELISA assays are commercially available (24–26).
Bacterial Challenge with MTB H37Rv
A whole body exposure chamber was employed for bacterial challenge of the immunized animals by inhalation. A nebulizer (Collison, Venturi unit, Waltham, MA) was employed to atomize the suspension of MTB, strain H37Rv (ATCC, Manassas, VA), at a concentration of 5 × 104 CFU in PBS, mixed with secondary air prior to delivery to an exposure chamber (University of Wisconsin, Mechanical Engineering Workshop, Madison, WI) under vacuum (27,28). After 5 min of nebulization of the bacterial suspension, the primary air supply was stopped and airflow through the chamber continued for 10 min. This procedure, has been shown to result in the inhalation and retention of 10–15 viable virulent microorganisms in the lungs of guinea pigs (29,30).
Necropsy and Bacteriology
Necropsy was performed 4 weeks after bacterial challenge when the bacterial burden is known to plateau in the lungs of infected animals (31). Guinea pigs were euthanized with a lethal dose of sodium pentobarbital (175 mg/kg) and exsanguinated prior to necropsy. The peritoneal and chest cavities were exposed and lungs resected, weighed, and inspected for grossly visible primary lesions. Spleen and liver were also resected aseptically and the weight recorded, as an indirect measurement of the degree of inflammation. The lower left lobe of the lung and a piece of the spleen were homogenized separately using Teflon glass homogenizers. Tissue homogenates were diluted with sterile saline, and aliquots were inoculated onto duplicate Middlebrook 7H10 agar plates. After being sealed with oxygen permeable tape, plates were incubated at 37°C, and the number of bacterial colonies (CFU) was counted after 21 days. Bacteriology is reported as Log10 protection = [(CFU/ml untreated control) − (CFU/ml treatment)].
Histopathological analysis
In the day of necropsy, the right lower lung lobe and a portion of the spleen were preserved in 10% neutral-buffered formalin solution. These tissues were later embedded in paraffin and sectioned with a microtome (Leica Microsystems, Bannockburn, IL, USA) at 5 mm. The sections were mounted on a glass slide and stained with hematoxylin–eosin for microscopic evaluation by a pathologist that was blinded with respect to the treatments received by each group.
For selected treatment groups, the area of each tissue affected by granulomas was further quantified by morphometric analysis. The internal structure of “true granulomas” containing a central core of aggregated histiocytes (and in some cases degenerate neutrophils) surrounded by a band of lymphocytes was measured using “ImageJ” software (Rasband, W.S., ImageJ, US National Institutes of Health, Bethesda, Maryland, USA, http://rsb.info.nih.gov/ij/, 1997–2008). Image measurements were calibrated with a certified stage micrometer provided by Klarmann Rulings, Inc. Data points were subsequently entered into an Excel spreadsheet where the total area of individual granulomas was calculated and reported in square meters.
Statistics
One-way ANOVA and the least-squares significant difference multiple comparison method were used to compare the multiple groups. The statistical significance was set at p < 0.05.
RESULTS
Microparticle Manufacture and Characterization
PLGA, P-rAg85, and P-rAg85B-TDB microspheres were manufactured under optimized conditions as described previously (14). The resulting microspheres had porous structure with an encapsulation efficiency of 91.3% for rAg85B. The antigen appeared to be functional after spray drying of microspheres as evidenced by their ability to induce DB-1 cells to produce IL-2 in the T hybridoma cell recognition assay (14). The emitted dose was determined to be 92.9% (±8.1) by the USP apparatus B. Microspheres had desirable aerosol properties for pulmonary delivery (32), as indicated by a MMAD for the P-rAg85B of 2.8 ± 0.3 μm and fine particle fraction of 68.9% ± 8.4.
DTH Response in Treated Animals
Currently, PPD is the only diagnostic tool to assess exposure or infection with tuberculosis (20) and is also used to assess the degree of immunity conferred by the only approved tuberculosis vaccine BCG (21). Upon inoculation into the skin of sensitized individuals, a DTH response is evoked (22). However, DTH reaction does not correlate well with vaccine-derived protection from infection (33). Thus, the specific antigen used for vaccination can also be used in the skin test to detect the systemic cell-mediated immunity. In this study, PPD and rAg85B were used for detection of DTH responses prior to challenge.
Six weeks after single-dose vaccination, animals vaccinated with BCG (group 4, Fig. 1) exhibited DTH induration in responses to PPD but no apparent response to i.d. rAg85B solution. Animals vaccinated only with microparticle formulations containing rAg85B (groups 1–3, Fig. 1) showed no response to i.d. rAg85B solution or PPD. Likewise, in multiple-dose groups, the homologous P-rAg85B and P-rAg85B-TDB groups (Fig. 1, groups 5 and 6, respectively) did not respond to PPD or rAg85B. In contrast, groups of BCG prime: P-rAg85B boost, BCG, and BCG–BCG (groups 7, 8, and 9, Fig. 1) responded to PPD with induration diameters of 16.8 ± 0.5, 17.7 ± 0.9, and 18.3 ± 0.7 mm, respectively, and did not respond to i.d. rAg85B solution. A lack of DTH-induced response by MTB culture filtrate protein has been observed previously in guinea pigs, despite protective effects obtained in the lungs (34). Pais et al. has also reported that the specificity of T cells involved in the classical tuberculin reaction to PPD were different from those involved in protective immunity (35). Consequently, despite the apparent absence of immunity observed in some of the experimental groups, as indicated by negative PPD test, challenge studies were conducted in all groups.
Bacteriology and Histopathology
Single-Dose Studies
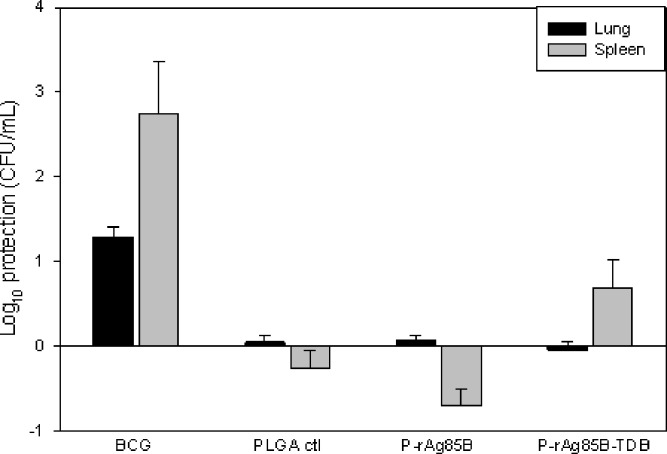
Figure 2 presents the bacteriology data for each of the treatment groups with respect to the untreated control in lungs and spleen.
Fig. 2.
Bacterial burden in lungs and spleens of guinea pigs immunized with single doses of the different formulations after challenge with MTB H37Rv (BCG = BCG solution; PLGA ctl = blank microparticles; P-rAg85B = microparticles containing rAg85B; P-rAg85B-TDB = microparticles containing rAg85B and adjuvant TDB (average ± SD, n = 6)
Among lungs of animals receiving single-dose immunization, BCG was the only treatment (log CFU = 4.13 ± 0.35) that demonstrated a statistically significantly decrease in the bacterial burden compared to untreated control (log CFU = 5.42 ± 0.32). This observation was supported by the histopathological observation of a smaller proportion of lung lobes containing granulomas (Table I and Fig. 4b). Likewise, BCG was the only treatment (log CFU 2.24 ± 2.0) that demonstrated a statistically significantly decrease in the bacterial burden in the spleens of single-dose-immunized animals compared to untreated control (log CFU = 4.97 ± 0.66). Histopathological analysis in these groups also indicated that animals treated with BCG exhibited a smaller proportion of white pulp affected by granulomas than any other treatment (Table I, Fig. 5b).
Table I.
Histopathology of Lung and Spleen Samples from Studied Guinea Pigs in Different Vaccination Groups (n = 4)
| Treatment Group | % Lung lobe affected by granulomas | Granuloma size | % Granulomas affected by caseous necrosis | Amount of caseous necrosis |
| A. Lung samples | ||||
| Untreated Ctl | 10–25 | Med–large | 10–50 | Min–moderate |
| Single P-rAg85B | 15–25 | Med–large | 10–30 | Mild–moderate |
| P-rAg85B-P-rAg85B | 10–20 | Med–large | 30–50 | Mild |
| BCG | 5–10 | Small–med | 0–10 | None–minimal |
| BCG–P-rAg85B | 5 | Small | 0–10 | None–minimal |
| BCG–BCG | 5–10 | Medium | 0–10 | None–minimal |
| Treatment Group | % White pulp affected by granulomas | Granuloma size | % Granulomas affected by caseous necrosis | Amount of caseous necrosis |
| B. Spleen samples | ||||
| Untreated Ctl | 5–30 | Med | 50 | Mild |
| Single P-rAg85B | 10–30 | Med | 5 | Minimal–mild |
| P-rAg85B-P-rAg85B | 0–30 | None–med | 0–10 | None–mild |
| BCG | 5 | Small–med | 0 | None |
| BCG–P-rAg85B | 0 | None | 0 | None |
| BCG–BCG | 30 | Coalescing | 0 | None |
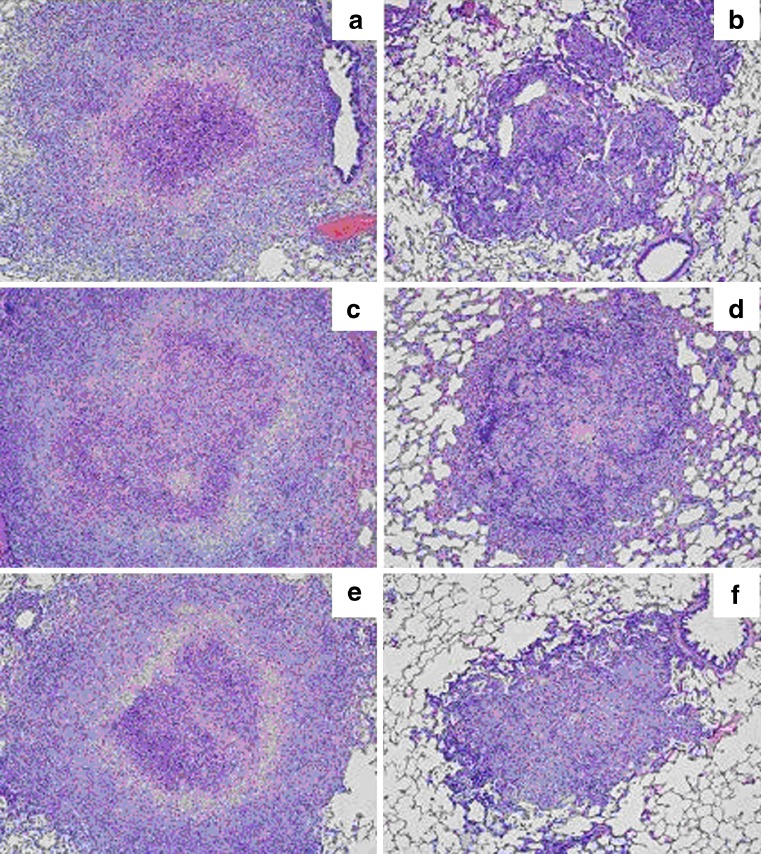
Fig. 4.
Histopathology of lungs in studied guinea pigs (n = 4). a Untreated control; b BCG solution positive control; c Single-dose P-rAg85B; d BCG–BCG; e Multiple doses of P-rAg85B; f BCG–P-rAg85B (boost administered by the pulmonary route)
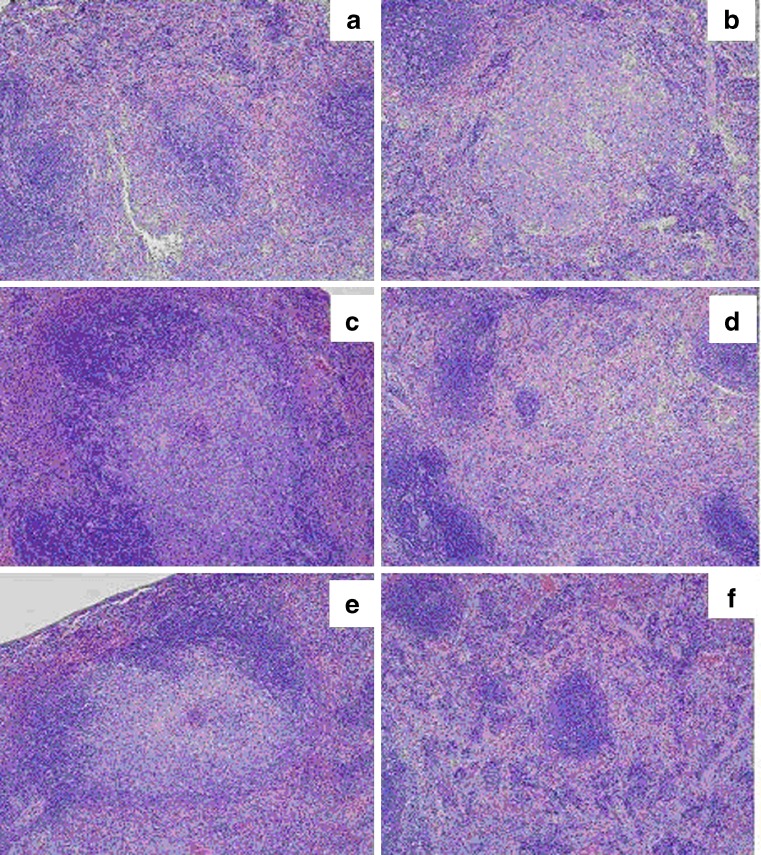
Fig. 5.
Histopathology of spleens in studied guinea pigs (n = 4). a Untreated control; b BCG solution positive control; c single-dose P-rAg85B; d BCG–BCG; e multiple doses of P-rAg85B; f BCG–P-rAg85B (boost administered by the pulmonary route)
Multiple-Dose Studies
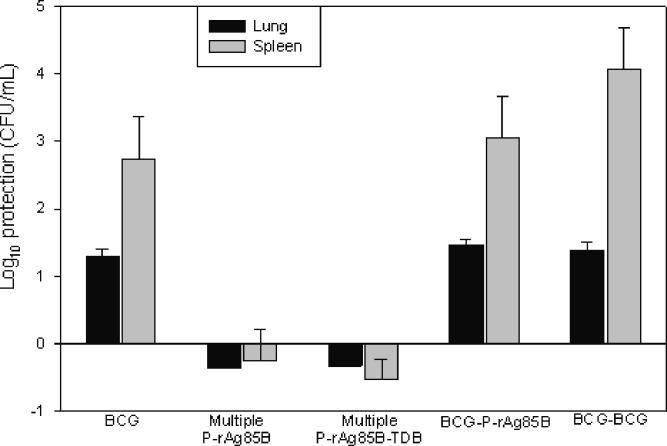
Figure 3 presents the bacteriology data for each of the treatment groups with respect to the untreated control in lungs and spleen.
Fig. 3.
Bacterial burden in lungs and spleens of guinea pigs immunized with multiple doses of the different formulations after challenge with MTB H37Rv (BCG = BCG solution; P-rAg85B = microparticles containing rAg85B; P-rAg85B-TDB = microparticles containing rAg85B and adjuvant TDB (average ± SD, n = 6)
Under the experimental conditions of the present study, vaccination strategies employing pulmonary homologous immunizations with polymeric microparticles (P-rAg85B, and P-rAg85B-TDB) were not effective in reducing bacterial burden in the lungs of animals in these groups. In contrast, regimens including BCG-prime appeared to be effective in protecting animals against virulent challenge as evidenced by bacterial counts in these groups. There was no significant difference between BCG alone (log CFU = 4.13 ± 0.35), BCG–P-rAg85B (log CFU = 3.96 ± 0.16), and BCG–BCG (log CFU = 4.04 ± 0.31), but each was significantly smaller than untreated controls (log CFU = 5.42 ± 0.32) P-rAg85B-P-rAg85B (log CFU = 5.78 ± 0.27) and P-rAg85B-TDB-P-rAg85B-TDB groups (log CFU = 5.75 ± 0.21). However, there were apparent histopathological differences among these groups (Table I), notably showing that animals in the BCG–P-rAg85B group (Fig. 4f) had a smaller proportion of the lung lobes affected by granulomas of smaller sizes than animals in the BCG (Fig. 4b) and BCG–BCG groups (Fig. 4d). Furthermore, morphometric analysis of lung tissue images confirmed that the total area of lung tissue affected by granulomas was significantly smaller in animals in the BCG–P-rAg85B group (Table II) than the BCG or BCG–BCG groups. This suggests that BCG–P-rAg85B may provide a greater protection than BCG alone or BCG–BCG. The results from the statistical analysis of bacterial burden in spleens were similar to those for lungs. There was no difference in bacterial burden between BCG alone (log CFU 2.24 ± 2.0), BCG–P-rAg85B (log CFU = 2.12 ± 1.14) and BCG–BCG (log CFU = 0.9 ± 1.47), but each of them was significantly lower than untreated controls (log CFU = 4.97 ± 0.66), P-rAg85B-P-rAg85B (log CFU = 5.23 ± 0.91), and P-rAg85B-TDB-P-rAg85B-TDB (log CFU = 5.50 ± 0.42) groups. However, the histopathology of spleens indicated that neither granulomas nor necrosis were observed in the white pulp of the BCG–P-rAg85B group (Fig. 5f), whereas in the BCG–BCG group (Fig. 5d), 30% of the white pulp was affected by coalescing granulomas groups (Table I). Conversely, no significant differences were noted by morphometric analysis of spleen tissues of these groups (Table II), possibly due to marked variations in granuloma sizes and blending with lymphoid tissue in the spleen.
Table II.
Morphometric Analysis of Granulomas Affecting Lung and Spleen Tissues of Guinea Pigs in Different Vaccination Groups (average ± standard deviation, n = 3–4)
| Treatment Group | Total tissue area affected by granulomas (mm2) | |
|---|---|---|
| Lung | Spleen | |
| BCG | 2.140 ± 0.356 | 0.255 ± 0.357 |
| BCG–P-rAg85B | 1.102 ± 0.412a | 0.009 ± 0.011 |
| BCG–BCG | 2.102 ± 0.581 | 0.007 ± 0.010 |
aSignificantly smaller than other vaccination groups
DISCUSSION
The proteins of the Antigen 85 complex have been reported to be strongly recognized, during the course of natural infection, in the same animal model in which they promote a protective immune response if administered as a pre-infection vaccine (36,37). In previous studies, Ag85B showed to afford protection in mice (38) and guinea pigs (13) when administered by parenteral routes. Pulmonary immunization capitalizes on the location and capacity for targeting the antigen presenting cells (mainly alveolar macrophages) in the lungs by engulfing the particles in appropriate size ranges. Thus, it was hypothesized that pulmonary immunization with P-rAg85B may result in the enhancement of the immune response elicited in the lungs of vaccinated animals.
In the present study, a single-dose immunization with P-rAg85B or P-rAg85B-TDB did not provide protection against virulent challenge in guinea pigs. Given the complex design of the present studies, there may be several reasons for this outcome, including the dose of the antigen that was delivered to the lungs of immunized animals, the immunization regimen, and the animal species employed in the evaluation of this vaccine. Studies conducted by Horwitz et al. demonstrated protection in guinea pigs immunized with Ag85(13) when compared to untreated animals, but it is important to note that a control group of animals vaccinated with BCG was not included in this study. Nevertheless, the protective effect shown in Horwitz’s study may indicate that the dose of Ag85B delivered in a single pulmonary immunization was insufficient to elicit a protective immune response. Therefore, multiple doses of Ag85B microparticles and different immunization regimens were assessed, including BCG controls.
CD4 Th1 immunity is an important factor in protection from tuberculosis. Memory CD4 T cells require restimulation before acting on target cells to elicit an immune response (39). Multiple homologous vaccinations should repeatedly stimulate CD4 T memory cells specific for Ag85B epitopes. However, in the present studies, no protection was observed in the lungs or spleens by homologous strategies with multiple doses of P-rAg85B and P-r-Ag85B-TDB, suggesting that the limited number of epitopes from this antigen, compared to a whole BCG bacterium, is insufficient alone to generate an appropriate immune response. This was surprising, given the results from a previous in vitro study in which P-rAg85B microparticles elicited the production of IL2 that was two orders of magnitude than the response elicited by soluble rAg85B (14). These two results together suggest that the ability of the immune system T cells to recognize antigens in an in vitro bioassay and the ability to induce protection following immunization in vivo do not necessarily correlate. It may also indicate that the production of one cytokine alone, IL2 in this case, is not a sufficient indicator, and the response of other cytokines should also be considered. Majlessi et al. (40) also arrived to the same conclusion when they observed that a high level of IFN-γ, a relevant cytokine marker of CD4 Th1 immunity, in the lung parenchyma of mice immunized with Ag85A in an adenylate cyclase toxoid vector did not allow induction or improvement of protection against MTB infection. These studies also generated experimental evidence that despite large IFN-γ and IL-2 cytokine responses with respect to IL-4 and IL-5, protective effects were not seen in mice after virulent MTB challenge (40).
In contrast, other studies have reported a modest success with homologous vaccination employing Ag85B. Pioneer studies by Pal et al. (41) reported a 1.1 and 1.2 log reduction in bacterial burden of lungs and spleens, respectively, in guinea pigs immunized subcutaneously with 120 μg of extracellular proteins of MTB, twice or four times, 3 weeks apart. In a subsequent study (13), the same group reported a 0.8 log reduction in lung bacterial burden after two or three subsequent intradermal immunizations, 3 weeks apart, with 100 µg of Ag85B. However, neither of these studies included control animals vaccinated with BCG for comparison. Although Sugawara et al. (42) used a gene-based vaccine instead of a peptide-based vaccine used in the present study, they reported a 0.5 log reduction in bacterial burden after three dermal administrations, 3 weeks apart, of 50 µg of Ag85A DNA. Even though it may not have influenced the outcome, another difference between the above described studies and the present study is the MTB strain used for bacterial challenge. While we employed H37Rv strain, Pal et al. (41) and Horwitz et al. (13) employed Erdmand strain, and Sugawara et al. (42) employed Kurono strain. Given the results of studies performed by others described above, despite the fact that multiple doses were administered, it is likely that the dose of antigen delivered in microparticles by pulmonary administration may not have been sufficient to elicit an immune response. Whereas by parenteral administration, the entire dose is delivered to the animal, several factors influence the pulmonary delivery of particles, including encapsulation efficiency of the antigen, the site of lung deposition, and potential for mucociliary clearance in the lung. The 91.3% encapsulation efficiency for rAg85B in PLGA particles was acceptable, but the amount of antigen loaded in the microparticles is only 0.57–2.28% (w/w). Thus, if considering the amount of microparticles delivered and the percentage of antigen loaded in particles, 166.5 µg of rAg85B in total would have been delivered to the lungs of animals with the priming and boosting doses, which is a smaller dose compared with those administered in the studies described above (240–480 μg (41) and 200–300 µg (13), respectively). An additional factor that may have influenced the protection in immunized animals is the rate at which the antigen was released from the polymeric particles. We have previously shown that antigen release from microspheres was not instantaneous with high initial burst of 58% of the rAg85B in the first day, a cumulative 66% on day 3, 80% by day 20, and completed 100% release by day 31 (14). Consequently, it is likely that at any time point, a lower dose of antigen is available to elicit an immune response in comparison with other literature citations.
Besides the dose of antigen delivered to the lungs and its rate of release, another factor that may have played a role in the outcome of the present study is the animal model. Compared to the existing mouse models, the guinea pig model is known for being more “demanding” in the evaluation of TB vaccines (43). A DNA vaccine encoding the Ag85A protein was reported to yield protection comparable to BCG in BALB/c and B6 mice (44) whereas it failed to induce significant protection in Hartley guinea pigs under conditions in which BCG was highly protective (34). The susceptibility of each species to MTB may be a possible explanation for this difference. The mouse is more resistant to MTB infection, and different mouse strains have shown different susceptibilities to MTB, whereas the guinea pig is extremely susceptible to MTB and can be infected with one or two virulent organisms (45).
Primary immunization with BCG establishes a favorable immune response (balanced Th1/Th2 immunity) (46) that is necessary for protection against tuberculosis. From the existing reports in literature, it appears that in the stringent guinea pig model, subunit vaccines have not been able to compete with the protection provided by BCG (47), and some recombinant vaccines have shown only a modest improvement compared to BCG (43). Since the great majority of people living in TB-endemic areas of the world are individuals already vaccinated with BCG, a boosting strategy in this population would be a better approach. However, homologous BCG boosting has shown little or decreased efficacy (19), and a study has also reported decreased survival in guinea pigs (48). Deaths in the animals were attributed to disseminated lesions in several organs, fibrosis, inflammation, and necrosis. These deleterious effects have also been observed in animals after a booster dose with MTB auxotrophs, which induced a stronger inflammatory response and worsening of lung pathology (49). In contrast, guinea pigs receiving the BCG–BCG prime-boost in the present study appeared to be not adversely affected within the limits of the experimental design.
Another possible vaccination strategy in the protection against TB is to boost the waning memory immune response from BCG with a fundamentally different vaccine, i.e., heterologous immunization (19,36). When the boost immunization, different from the priming vaccine, is administered, the same effector cells are quickly recruited, and the response is augmented, while a different subset of effector cells may also be stimulated to enhance the immunity. A non-replicating vaccine capable of protecting as well as BCG, but without the overall variability seen with BCG, would be of considerable value (50). An advantage of delivering the vaccine directly to the lungs is that the respiratory mucosal boost immunization can lead to rapid activation and retention of T cells located primarily within the airway lumen, which play an important role in protection from pulmonary MTB challenge (51).
In the past decade, several investigators have suggested that relaying on “protection” alone may not be the best criteria to screen potential TB vaccines and that survival/pathology data in the guinea pig model may give a better idea of the long-term effectiveness of a vaccine (34,49,50,52). Studies have shown that bacillary burden at early time points do not necessarily correlate with animal survival and that the extent of pathology in involved organs may be a better predictor of long-term disease outcome following MTB infection (34,49,52). For example, an Ag85A-DNA vaccine administered to guinea pigs did not induce a significant reduction in the day-30 bacterial-load assay but showed excellent long-term survival in immunized animals (52). Baldwin et al. (34) suggested that the survival data in the guinea pig model is model is influenced by the type and severity of lesion produced in involved organs and the degree of lymphocytic infiltration. Therefore, based on histopathological findings and morphometric analysis in the present study, immunization with BCG–P-rAg85B appeared to enhance the protection afforded by BCG alone and BCG–BCG, even though the bacterial burden in the lungs and spleens of animals receiving the vaccination regimen of BCG–P-rAg85B was not statistically different from that of animals receiving BCG and BCG–BCG vaccinations (possibly due to a smaller dose of rAg85B delivered).
Although different administration routes were employed and a smaller dose was delivered, the protective effect in lungs with P-rAg85B pulmonary boosting immunization in our study was comparable to that observed by Horowitz et al. in response to 100 μg rAg85B administered intradermally as a boost following BCG priming (19). Perhaps the most successful study of protection with the BCG prime was followed by boosting with poxviruses expressing Ag85 (53). Guinea pigs vaccinated with this regimen had a 100% survival rate at 26 weeks compared to 40% after BCG alone and 0% in saline controls.
In summary, polymeric microparticles containing rAg85B were prepared in sizes suitable for delivery by inhalation. Based on histopathological and morphometric analysis, direct pulmonary immunization using these particles enhanced the protection afforded by primary immunization with BCG against TB infection in guinea pigs. Since a large proportion of the population of the world has been immunized with BCG as infants, an aerosol boost would be a promising strategy to enhance and prolong the protective immunity based on BCG-initiated immunity.
Acknowledgments
This study was funded by the National Heart Lung and Blood Institute, grant number HL67221, and a PhRMA Foundation Fellowship to Dongmei Lu.
References
- 1.WHO. Tuberculosis Fact Sheet. WHO 2007.
- 2.Brewer TF. Preventing tuberculosis with bacillus Calmette-Guérin vaccine: a meta-analysis of the literature. Clin Infect Dis. 2000;31(Suppl 3):S64–7. doi: 10.1086/314072. [DOI] [PubMed] [Google Scholar]
- 3.Lu D, Hickey AJ. Pulmonary vaccine delivery. Expert Rev Vaccines. 2007;6(2):213–26. doi: 10.1586/14760584.6.2.213. [DOI] [PubMed] [Google Scholar]
- 4.Gupta PK, Hickey AJ. Contemporary approaches in aerosolized drug delivery to the lung. J Controlled Releas. 1991;17(2):127–47. doi: 10.1016/0168-3659(91)90053-G. [DOI] [Google Scholar]
- 5.Gehr P, Geiser M, ImHof V, Schurch S, Waber U, Baumann M. Surfactant and inhaled particles in the conducting airways: structural, stereological, and biophysical aspects. Microsc Res Tech. 1993;26(5):423–36. doi: 10.1002/jemt.1070260510. [DOI] [PubMed] [Google Scholar]
- 6.Raychaudhuri A, Rock KL. Fully mobilizing host defense: building better vaccines. Nat Biotechnol. 1998;16:1025–31. doi: 10.1038/3469. [DOI] [PubMed] [Google Scholar]
- 7.Jiang W, Gupta RK, Deshpande MC, Schwendeman SP. Biodegradable poly (lactic-co-glycolic acid) microparticles for injectable delivery of vaccine antigens. Adv Drug Deliv Rev. 2005;57:391–410. doi: 10.1016/j.addr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Alpar HO, Somavarapu S, Atuah KN, Bramwell VW. Biodegradable mucoadhesive particulates for nasal and pulmonary antigen and DNA delivery. Adv Drug Deliv Rev. 2005;57:411–30. doi: 10.1016/j.addr.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Audran R, Peter K, Dannull J, Men Y, Scandella E, Groettrup M, et al. Encapsulation of peptides in biodegrable microspheres prolongs their MHC class I presentation by dendritic cells and macrophages in vitro. Vaccine. 2003;21:1250–5. doi: 10.1016/S0264-410X(02)00521-2. [DOI] [PubMed] [Google Scholar]
- 10.Vordermeier HM, Coombes AGA, Jenkins P, McGee JP, O'Hagan DT, Davis SS, et al. Synthetic delivery system for tuberculosis vaccines: immunological evaluation of the M. tuberculosis 38kDa protein entrapped in biodegrable PLG microspheres. Vaccine. 1995;13:1576–82. doi: 10.1016/0264-410X(95)00084-E. [DOI] [PubMed] [Google Scholar]
- 11.Daffe M. The mycobacterial antigen 85 complex—from structure to function and beyond. Trends Microbiol. 2000;8:438–40. doi: 10.1016/S0966-842X(00)01844-8. [DOI] [PubMed] [Google Scholar]
- 12.Wiker HG, Harboe M. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol Rev. 1992;56:648–61. doi: 10.1128/mr.56.4.648-661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horwitz MA, Lee BW, Dillon BJ, Harth G. Protective immunity against tuberculosis induced by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1995;92(5):1530–4. doi: 10.1073/pnas.92.5.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu D, Garcia-Contreras L, Xu D, Kurtz SL, Liu J, Braunstein M, et al. Poly (lactide-co-glycolide) microspheres in respirable sizes enhance an in vitro T cell response to recombinant Mycobacterium tuberculosis antigen 85B. Pharm Res. 2007;24(10):1834–43. doi: 10.1007/s11095-007-9302-8. [DOI] [PubMed] [Google Scholar]
- 15.Lakey DL, Voladri RKR, Edwards KM, Hager C, Samten B, Wallis RS, et al. Enhanced production of recombinant Mycobacterium tuberculosis antigens in E coli by replacement of low-usage codons. Infect Immun. 2000;68(1):233–8. doi: 10.1128/IAI.68.1.233-238.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McShane H, Hill AJ. Prime-boost immunization strategies for tuberculosis. Microbes Infect. 2005;7:962–7. doi: 10.1016/j.micinf.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Ritzinger GS, Meredith SC, Takayama K, Hunter RL, Kezdy FJ. The role of surface in the biological activities of trehalose 6,6′-dimycolate. J Biol Chem. 1981;256(15):8208–16. [PubMed] [Google Scholar]
- 18.Cai H, Yu DH, Hu XD, Li SX, Zhu YX. A combined DNA vaccine-prime, BCG-boost strategy results in better protection against Mycobacterium bovis challenge. DNA Cell Biol. 2006;25(8):438–47. doi: 10.1089/dna.2006.25.438. [DOI] [PubMed] [Google Scholar]
- 19.Horwitz MA, Harth G, Dillon BJ, Masleša-Galic S. Enhancing the protective efficacy of Mycobacterium bovis BCG vaccination against tuberculosis by boosting with the Mycobacterium tuberculosis major secretory protein. Infect Immun. 2005;73(8):4676–83. doi: 10.1128/IAI.73.8.4676-4683.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hart PD. Efficacy and applicability of mass BCG vaccination in tuberculosis control. Br Med J. 1967;1:587. doi: 10.1136/bmj.1.5540.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO . The WHO standard tuberculin test. Geneva: World Health Organization; 1963. [Google Scholar]
- 22.Koch R. Uber neue Tuberkulinpraparate. Dtsch Med Wochenschr. 1897;14:209. doi: 10.1055/s-0029-1204932. [DOI] [Google Scholar]
- 23.Ly LH, Russell MI, McMurray DN. Cytokine profiles in primary and secondary pulmonary granulomas of guinea pigs with tuberculosis. Am J Respir Cell Mol Biol. 2008;38(4):455–62. doi: 10.1165/rcmb.2007-0326OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White AM, Yoshimura T, Smith AW, Westwisck J, Watson ML. Airway inflammation induced by recombinant guinea pig tumor necrosis factor-alpha. Am J Physiol. 1997;273:L524–30. doi: 10.1152/ajplung.1997.273.3.L524. [DOI] [PubMed] [Google Scholar]
- 25.Yoshimura T. cDNA cloning of guinea pig monocyte chemoattractant protein-1 and expression of the recombinant protein. J Immunol. 1993;150:5025–32. [PubMed] [Google Scholar]
- 26.Yoshimura T, Johnson DG. cDNA cloning and expression of guinea pig neutrophil attractant protein-1 (NAP-1) J Immunol. 1993;151:6225–36. [PubMed] [Google Scholar]
- 27.Wiegeshaus EH, McMurray DN, Grover AA, et al. Host-parasite relationships in experimental airborne tuberculosis. III. Relevance of microbial enumeration to acquired resistance in guinea pigs. Am Rev Respir Dis. 1970;102:422–9. doi: 10.1164/arrd.1970.102.3.422. [DOI] [PubMed] [Google Scholar]
- 28.Grover AA, Kim HK, Wiegeshaus EH, Smith DW. Host-parasite relationships in experimental airborne tuberculosis: II. Reproducible infection by means of an inoculum preserved at −70°C. J Bacteriol. 1967;94:832–5. doi: 10.1128/jb.94.4.832-835.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mainali ES, McMurray DN. Protein deficiency induces alterations in the distribution of T-cell subsets in experimental pulmonary tuberculosis. Infect Immun. 1998;66(3):927–31. doi: 10.1128/iai.66.3.927-931.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiegeshaus EH, McMurray DN, Grover AA, Harding GE, DW S. relationships in experimental airborne tuberculosis. 3. Relevance of microbial enumeration to acquired resistance in guinea pigs. Am Rev Respir Dis. 1970;102(3):422–8. doi: 10.1164/arrd.1970.102.3.422. [DOI] [PubMed] [Google Scholar]
- 31.McMurray DN. Guinea pig model of tuberculosis. In: Bloom B, editor. Tuberculosis: pathogenesis, protection and control. Washington: ASM; 1994. pp. 135–48. [Google Scholar]
- 32.Hickey AJ, Concessio NM, Van Ort MM, et al. Factors influencing the dispersion of dry powders as aerosols. Pharm Technol. 1994;18:58–64. [Google Scholar]
- 33.Fine PEM, Stern JAC, Ponnighaus JM, Rees RJW. Delayed-type hypersensitivity, mycobacterial vaccines and protective immunity. Lancet. 1994;344:1245. doi: 10.1016/S0140-6736(94)90748-X. [DOI] [PubMed] [Google Scholar]
- 34.Baldwin SL, D'Souza C, Roberts AD, Kelly BP, Frank AA, Lui MA, et al. Evaluation of new vaccines in the mouse and guinea pig model of tuberculosis. Infect Immun. 1998;66(6):2951–9. doi: 10.1128/iai.66.6.2951-2959.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pais TF, Silva RA, Smedegaard B, Appelberg R, Andersen P. Analysis of T cells recruited during delayed-type hypersensitivity to purified protein derivative (PPD) versus challenge with tuberculosis infection. Immunology. 1998;95:69–75. doi: 10.1046/j.1365-2567.1998.00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andersen P. TB vaccines: progress and problems. Trends Immunol. 2001;22(3):160–8. doi: 10.1016/S1471-4906(01)01865-8. [DOI] [PubMed] [Google Scholar]
- 37.Andersen P, Askgaard D, Ljungqvist L, Bentzon MW, Heron I. T-cell proliferative response to antigens secreted by Mycobacterium tuberculosis. Infect Immun. 1991;59(4):1558–63. doi: 10.1128/iai.59.4.1558-1563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andersen P. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect Immun. 1994;62(6):2536–44. doi: 10.1128/iai.62.6.2536-2544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janeway CA, Travers P, Walport M, Shlochik M. Immunobiology. 5. New York: Garland Publishing; 2001. [Google Scholar]
- 40.Majlessi L, Simsova M, Jarvis Z, Brodin P, Rojas MJ, Bauche C, et al. An increase in antimycobacterial Th1-cell responses by prime-boost protocols of immunization does not enhance protection against tuberculosis. Infect Immun. 2006;74(4):2128–37. doi: 10.1128/IAI.74.4.2128-2137.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pal PG, Horwitz MA. Immunization with extracellular proteins of Mycobacterium tuberculosis induces cell-mediated immune responses and substantial protective immunity in a guinea pig model of pulmonary tuberculosis. Infect Immun. 1992;60(11):4781–92. doi: 10.1128/iai.60.11.4781-4792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sugawara I, Yamada H, Udagawa T, Huygen K. Vaccination of guinea pigs with DNA encoding Ag85A by gene gun bombardment. Tuberculosis. 2003;83(6):331–7. doi: 10.1016/S1472-9792(03)00054-4. [DOI] [PubMed] [Google Scholar]
- 43.Horwitz MA, Harth G, Dillon BJ, Maslea-Gali S. Recombinant bacillus Calmette–Guérin (BCG) vaccines expressing the Mycobacterium tuberculosis 30-kDa major secretory protein induce greater protective immunity against tuberculosis than conventional BCG vaccines in a highly susceptible animal model. Proc Natl Acad Sci USA. 2000;97:13853–8. doi: 10.1073/pnas.250480397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huygen K, Content J, Denis O, Montgomery DL, Yawman AM, Deck RR, et al. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat Med. 1996;2(8):893–8. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 45.Kaufmann SH. Is the development of a new tuberculosis vaccine possible? Nat Med. 2000;6(9):955–60. doi: 10.1038/79631. [DOI] [PubMed] [Google Scholar]
- 46.Sable SB, Goyal D, Verma I, Behera D, Khuller GK. Lung and blood mononuclear cell responses of tuberculosis patients to mycobacterial proteins. Eur Respir J. 2007;29(2):337–46. doi: 10.1183/09031936.00111205. [DOI] [PubMed] [Google Scholar]
- 47.Olsen AW, Williams A, Okkels LM, Hatch G, Andersen P. Protective effect of a tuberculosis subunit vaccine based on a fusion of antigen 85B and ESAT-6 in the aerosol guinea pig model. Infect Immun. 2004;72(10):6148–50. doi: 10.1128/IAI.72.10.6148-6150.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Basaraba RJ, Izzo AA, Brandt L, Orme IM. Decreased survival of guinea pigs infected with Mycobacterium tuberculosis after multiple BCG vaccinations. Vaccine. 2006;24(3):280–6. doi: 10.1016/j.vaccine.2005.07.103. [DOI] [PubMed] [Google Scholar]
- 49.Sampson SL, Dascher CC, Sambandamurthy VK, Russell RG, Jacobs WR BRB, et al. Protection elicited by a double leucine and pantothenate auxotroph of Mycobacterium tuberculosis in guinea pigs. Infect Immun. 2004;72(5):3031–7. doi: 10.1128/IAI.72.5.3031-3037.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reed SG, Alderson MR, Dalemans W, Lobet Y, Skeiky YA. Prospects for a better vaccine against tuberculosis. Tuberculosis (Edinb) 2003;83(1–3):213–9. doi: 10.1016/S1472-9792(02)00080-X. [DOI] [PubMed] [Google Scholar]
- 51.Xing Z, Caters TJ. Heterologous boost vaccines for bacillus Calmette–Guérin prime immunization against tuberculosis. Expert Rev Vaccines. 2007;6(4):539–46. doi: 10.1586/14760584.6.4.539. [DOI] [PubMed] [Google Scholar]
- 52.Orme IM. The search for new vaccines against tuberculosis. J Leukoc Biol. 2001;70(1):1–10. [PubMed] [Google Scholar]
- 53.Williams A, Goonetilleke NP, McShane H, Clark SO, Hatch G, Gilbert SC, et al. Boosting with poxviruses enhances Mycobacterium bovis BCG efficacy against tuberculosis in guinea pigs. Infect Immun. 2005;73(6):3814–6. doi: 10.1128/IAI.73.6.3814-3816.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]