INTRODUCTION
Using inhalable nanoparticles (NPs) to treat pulmonary diseases is an emerging field (1). Lungs as part of the mononuclear phagocyte system (MPS) are extensively rich with alveolar macrophages (2). These alveolar macrophages are part of the body’s immune system and responsible for clearing inhaled foreign objects from the alveolar spaces (3), which cause a substantial portion of the inhaled NPs to be cleared after inhalation once NPs are settled in the alveolar spaces (4). Clearance of NPs by macrophages is not exclusive to inhalable NPs; as it is associated with other routes of administrations, such as intravenous i.v. (5). In fact, the failure of early attempts of using NPs for cancer targeting was mainly attributed to the interference of macrophages of the MPS. The early work showed that after i.v. injection, NPs were cleared immediately by phagocytotic cells, specifically by macrophages and Kuppfer cells of the liver (6). Thus, in order to evade macrophages and increase NPs circulation, a new strategy was implemented by changing the NPs surface properties (5). This was accomplished by using hydrophilic surfactants, such as polysorbate 80 to coat the NPs surface (7). Hydrophilic coating of NPs changes the physicochemical characteristics of NPs. This inhibits opsonization, which is the precipitation of antibodies and other proteins on the surface of NPs (8). This strategy was shown to successfully prolong the circulation time of NPs and also enhanced the distribution of NPs to some organs such as the brain (9). Using coated NPs for pulmonary delivery may be associated with increased bioavailability since inhaled NPs may have a higher chance to evade alveolar macrophages. However, the delicate composition of the alveolar space should stay unchanged and kept intact after delivery of any inhalable treatment including NPs. In the alveolar space, a thin film of lung surfactant covers the epithelium. It is composed of phospholipids and proteins (10). The main component of the film is 1,2-Dipalmitoyl-sn-Glycero-3-Phosphocholine (DPPC), a phosphatidylcholine with two saturated 16-carbon long fatty acyl chains (11). This phospholipid is a key factor for controlling the surface tension and the gas exchange process in the alveolar sacs (12). Moreover, the ability of the lungs to function properly depends on the integrity of this surfactant layer (13). In vitro, lung-surfactant model systems have been developed to study the impact of NPs on the surfactant layer (14). Changes in the surface pressure-area isotherms induced by NPs were recorded and considered as an indicator for interactions of NPs and components of the surfactant model. Previous studies investigated the effect of non-coated NPs on the integrity of the lung surfactant film in vitro (10,11,14). Azarmi et al. showed in vivo that non-coated NPs are well tolerated by Balb/c nude mice after pulmonary administration (15). However, the impact of coated NPs on the integrity of the surfactant film is yet unknown. Therefore, the aim of this study was to investigate the biophysical interactions of polysorbate-80-coated NPs with DPPC monolayer film in vitro. The results were compared with in vivo observations.
METHODS
Preparation and Characterization of NPs
Please refer to the supplementary files.
Surface Pressure-Area Isotherm
Experiments were carried out using a custom built small-scale Langmuir trough from Accurion (Göttingen, Germany), changes in surface pressure was measured using a pressure sensor (model PS4, Nima, Coventry, UK) based on the Wilhelmy principle at the air-water interface using the Nima517 software from Nima (Coventry, UK). In the lungs, the surfactant layer exists at the gaseous-surfactant interface with an aqueous subphase (10). Therefore, in order to simulate this situation, this study was designed to have NPs suspended in the aqueous subphase of the trough with DPPC monolayer film spread on top at the air-water interface. NPs concentration was determined using an evaporation method; a known volume of NPs suspension was taken, the solvent was totally evaporated, the residues were weighted, and the value was used to calculate NPs concentration. Dilutions of NPs dispersion were made to obtain a subphase solution of 1 mg/mL of NPs in purified water. Calculated amount of DPPC (1,2-Dipalmitoyl-sn-Glycero-3-Phosphocholine) from Avanti Polar Lipids (Alabaster, AL, USA) was dissolved in chloroform (ACS grade, Thermo Fisher, Toronto, Canada) to obtain a concentration of 0.75 mg/mL. Drops of DPPC solution were added to the top of the NPs-containing subphase and the solvent was allowed to evaporate for 10 min before recording isotherms. All experiments were performed at 5 cm2/min compression rate. The trough was cleaned between experiments with acetone, methanol, hexane, and chloroform followed by purified water for several times. Data was transferred to Microsoft Excel and plotted as area (Å2/molecule × 103) versus surface pressure milli-Newton per meter (mN/m) (14). The surface pressure-area isotherms of DPPC on the top of subphase of purified water alone or purified water containing polysorbate 80 at concentration of 0.01 mg/ml were recorded and considered as controls for further comparison. Each experiment was repeated five times and average values were reported.
Animal Study
The animal study was approved by the Animal Care Committee at the Cross Cancer Institute. Coated and non-coated inhalable nanoparticle (NP) powders were administered to 6-7 week-old Balb/c nude mice. The animals were divided into two different treatment groups and one control group, each treatment group received either:
1 mg non-coated inhalable NPs powder (n = 4)
1 mg polysorbate-80-coated inhalable NPs powder (n = 4)
Control group mice (n = 4) received 0.25 mL of air which is the same volume used for powder insufflation in the treatment groups for each mouse. Animals were anesthetized with intraperitoneal injections of 0.15 ml of a mixture of katemine/acepromazine/saline. After being anesthetized, each animal was positioned against an angled restraining stand and 1 mg of the designated inhalable NP powder, coated or non-coated, was administered using a DP-4M insufflator (Penn-Century Inc., Philadelphia, PA, USA). In order to accurately determine the amount of the inhalable NPs powder delivered, the insufflator was weighed before and after powder filling, as well as after powder administration. With the help of a fiber optic light, the tip of the insufflator was positioned near the carina (first bifurcation) so that the delivered dose of powder could penetrate deep into the lung. DP-4M device was calibrated to use 0.25 mL of air for each puff. Each mouse was assigned a number according to the group and the type of treatment used. After the administration of inhalable NPs, animal morbidity was closely monitored using a morbidity score that focuses on changes in breathing pattern. The animals were observed continuously for the first 2 h after administration, then every hour for the following 6 h, then every 8 h for the following day and daily thereafter. Animals were sacrificed when the compiled morbidity score reached a value higher than 10 or any individual item scored a value higher than 4. All other animals were sacrificed after 14 days.
Histopathological Study
The sacrificed mice were dissected, lungs were collected and inflated with 1 ml of 10% neutral buffered formalin and fixed for a minimum period of 24 h. Following fixation, lung tissues were trimmed with a scalpel to a thickness of 2-3 mm and a section of the lung was placed in tissue cassette. These tissues were processed into paraffin, embedded in a paraffin block, sectioned on a microtome to a thickness of 5 μm, placed on a microscope slide, and stained with hematoxylin and eosin stain. A board-certified veterinary pathologist examined the slides and each tissue section was either recorded as normal, or a description was made of any abnormalities. The pathologist was blinded in term of the type of treatment administered previously to each mouse.
Statistical Analysis
Data are presented as mean ± standard error of the mean. A one-way ANOVA was used to test for differences among the collapse pressure values of five duplicates for each experiment. The collapse pressure values were compared using Student’s t test. NPs characteristics such as size, zeta potential, and mass median aerodynamic diameter (MMAD) were compared using Student’s t test. A result was considered statistically significant at a value p < 0.05.
RESULTS
NPs Characteristics
No significant difference was observed between polysorbate-80-coated NPs and non-coated NPs in terms of size and zeta potential. The average size of non-coated NPs was 137.2 ± 1.5 nm, the polydispersity index was 0.12 ± 0.03 and the zeta potential was −23.5 ± 0.4 mV. Coated NPs showed almost the same values for average size and zeta potential, 145.4 ± 3.2 and −24.7 ± 0.2 mV, respectively. The MMAD of the inhalable microparticles loaded with coated and non-coated NPs was 3.2 ± 0.2 and 3.3 ± 0.7 µm, respectively, with 60% as fine powder fraction. These results were similar to those reported previously (16).
Surface Pressure-Area Isotherm of DPPC Film over Different Subphases
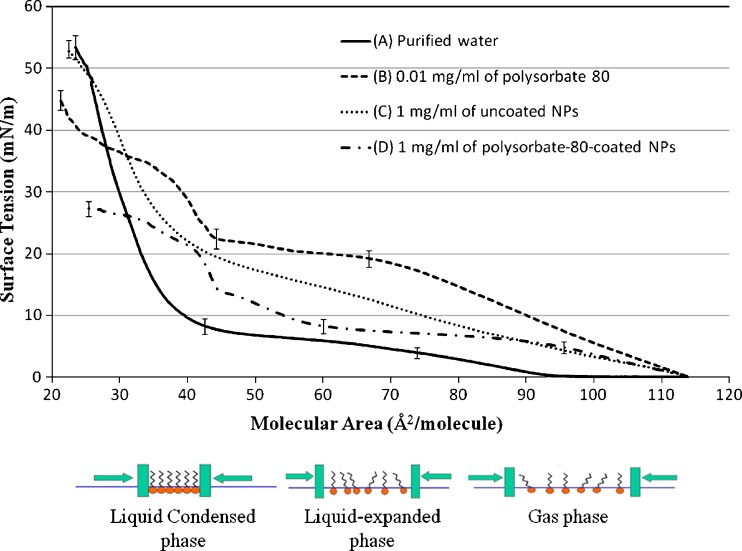
Figure 1 shows the surface pressure-area isotherms of DPPC monolayers spread on top of different subphases. The subphase varied between purified water, purified water containing 0.01 mg/ml polysorbate 80, or purified water containing1 mg/ml of polysorbate 80 coated or non-coated NPs. The reported data are the average of five duplicates. The surface tension values at different points were statistically compared. Those points were chosen at the beginning and end of different phases of the isotherm. All experiments were reproducible with no significant difference between five runs.
Fig. 1.
Surface pressure-area isotherms for 0.75 mg/mL 1,2-Dipalmitoyl-sn-Glycero-3-Phosphocholine (DPPC) on the top of subphase containing a purified water, b 0.01 mg/mL of polysorbate-80, c 1 mg/ml of uncoated nanoparticles, and d1 mg/ml of polysorbate 80-coated NPs
The surface pressure-area isotherm of DPPC using purified water as subphase (Fig. 1a) shows that as compression increases, the monofilm stays in the gas phase until around 95 Å2/molecules. This is indicated by the zero surface pressure increase recorded as the molecular area decreased from around 115 to 95 Å2/molecules. The increase in the surface pressure detected after 95 Å2/molecules is correlated to the rearrangement of DPPC molecules to from a so-called liquid expanded phase. A plateau region was seen approximately at 4 mN/m with molecular area of 75 Å2/molecule. This plateau region indicates the coexistence of both a liquid expanded and a liquid condensed phase. Further compression shows a steep increase in the pressure as the lipid molecules get packed tightly to an area of about 25 Å2/molecule whereby the maximum pressure is reached around 53.4 ± 3.7 mN/m, after which the DPPC monolayer collapsed. Adding polysorbate 80 to the subphase changed the onset of DPPC monolayer phase formation under progressive compression. As shown in Fig. 1b, a gradual increase in the surface pressure was recorded directly until a value of 20 mN/M was reached. This immediate response in the surface pressure to the compression indicates that the DPPC molecules are not in a gaseous status but they shift directly upon compression to the liquid expanded phase. Moreover, the addition of polysorbate 80 resulted in a less distinguished plateau region which was also formed at much higher surface pressure (18 and 22 mN/m), when compared to the of additive-free DPPC isotherm. After the plateau phase, the surfaced pressure started to increase sharply in a similar way to what was observed with the DPPC isotherm. The collapse pressure was 45.3 ± 3.1 mN/m. The addition of polysorbate 80 resulted in small difference in the collapse pressure when compared with the DPPC alone, 45.3 ± 3.1 and 53.4 ± 3.7 mN/m, respectively. The isotherm of DPPC monolayer spread on top of a subphase containing non-coated NPs showed different behavior (Fig. 1c). Neither gas phase nor plateau region were detected which is in agreement with previously published reports (14). More specifically, an initial increase in the surface pressure was recorded between 0 and 20 mN/m as the molecular area decreased from 115 to 43 Å2/molecule. This reflects the formation of a liquid-expanded phase. The initial increase of the surface pressure was followed by a second, but sharper increase to a liquid-condensed phase before a collapse pressure of 53.1 ± 2.4 mN/m was reached. The collapse pressure was not significantly affected by the addition of non-coated NPs at concentration of 1 mg/ml. Similar to what was observed with both polysorbate 80 and non-coated NPs, the addition of polysorbate-80-coated NPs resulted in a missing gas phase (Fig. 1d). In contrast to polysorbate 80 and non-coated NPs, the addition of polysorbate-80-coated NPs resulted in a more distinct plateau region of the surface pressure. This plateau region was similar to what was observed with the DPPC isotherm. The plateau region expanded over molecular area values of 95 and 60 Å2/molecule with a surface pressure between 7 and 9 mN/m. The coated NPs caused a huge decrease in the collapse pressure as shown in Fig. 1d. The recorded collapse pressure value of the DPPC mono-film after the addition of coated NPs was 27.3 ± 2.2 mN/m, which is almost half of what recorded with DPPC film alone. This value was significantly lower than all other recorded collapse pressure values.
In vivo Pulmonary Toxicity
According to the approved animal protocol, compiled morbidity scored higher than 10 or any individual morbidity score of 4 requires that the animal will be instantly euthanized to avoid unnecessary suffering. Three mice, out of four, treated with polysorbate80-coated NPs reached the critical value of 4 within 1 h of administration, and after 2 h for the fourth mouse. These mice exhibited very shallow breathing pattern with slower than 50% of the normal breathing rate. The controlled group and the group treated with uncoated NPs experienced slight changes in the breathing pattern for a very short time and they totally recovered within 3 h, none of these animals had an individual score higher than 2. These mice had no other significant changes in their morbidity scores, e.g., body weight, food intake, or behavioral signs over the duration of the study.
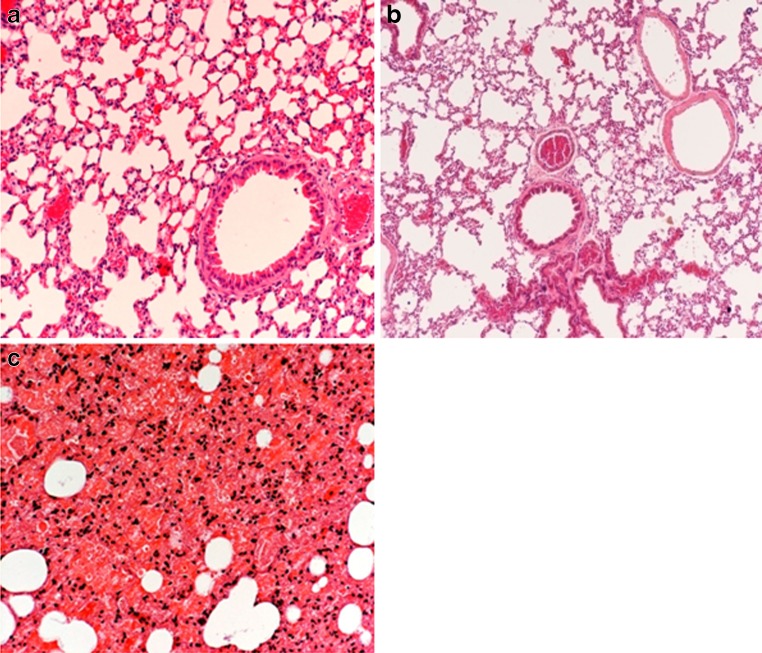
Figure 2a shows a tissue section taken form a lung of a control animal and examined under the microscope at ×200 magnification. The section did not exhibit any abnormalities. The alveolar sacs are well defined with no signs of hemorrhage, flooding, or collapse of the alveolar spaces which all can be defined as a manifestation of pulmonary toxicity.
Fig. 2.
Lung tissue section taken from a control mouse treated with 0.25 ml air, b mice treated with non-coated inhalable NPs, c mice treated with polysorbate-80-coated inhalable NPs. (×200 magnification)
Tissue sections taken from animal treated with non-coated inhalable NPs (group 1) appeared very similar to what was seen in the control group (Fig. 2b). Again, the histological examination indicated the absence of pulmonary toxicity in this group.
All mice that received coated inhalable NPs had to be sacrificed with 1 h of the administration time due to breathing difficulties. These animals expressed very shallow breathing pattern followed by 50% reduction in the breathing rate. Moreover, the histopathological results associated with polysorbate-80-coated inhalable NPs powder were totally different from what was seen in all other groups. Figure 2c shows that alveolar sacs were deflated and there was an acute terminal microhemorrhage from the alveolar capillaries accompanied by local flooding of many alveoli with a protein-rich fluid. An increased number of neutrophils were present in the fluids of the affected alveoli. There were also large perivascular sacs filled with edema fluid around some of the large pulmonary vessels. These changes were consistently associated with all animals treated with polysorbate-80-coated NPs. Pathology reports along with the observed high morbidity scores prove the connection between polysorbate-80-coated NPs and the observed pulomary toxicity.
DISCUSSION
Colloidal drug delivery systems are one of the most promising contemporary drug delivery approaches designed to treat lung diseases such as cancer (17). Polymeric NPs have been extensively studied for this purpose. Different types of polymers are being used to formulate NPs that exert most of the desired properties in terms of size, physiochemical characteristics, loading efficiency and, ultimately, in terms of efficacy (18). During the past years, different approaches have been investigated for increasing the circulation time of NPs in the body including the use of surfactants to coat the NPs surface (5). Studies showed that inhalable NPs were associated with pulmonary side effects depending mainly on their size (19). However, the interactions of coating materials with the lung surfactant film were not investigated till now. Monolayer systems as an in vitro lung surfactant model have been previously used to investigate the biophysical interaction of nano-materials with lung surfactants especially for inorganic NPs and nano-crystals (10). In the present study, we investigated the effect of polysorbate 80 as NP coating material on the integrity of the lung surfactant layer using an in vitro model. The result showed that polysorbate-80-coated NPs affected the integrity and the ability of a DPPC monofilm to compensate for compression forces, resulting in a significantly reduced collapse pressures. These results were associated with higher pulmonary toxicity in vivo as shown by histological examinations.
The recorded surface pressure-area isotherm of DPPC over a subphase of purified water was close to previously reported data. The differences in values emerge from the different dimensions of the mini-trough used for this study (20). The addition of free polysorbate 80 to the subphase influenced the phases of the isotherm differently (Fig. 1b). These changes indicate an interaction of polysorbate 80 and DPPC mono-films upon compression. Molecular interactions were facilitated indicated by the fact that the gas phase was abolished. Although polysorbate 80 influenced the onset and formation of the different monolayer phases, it is important to notice that the collapse pressure of the DPPC mono-film was not significantly affected. This observation suggests that the film was intact and its integrity was not compromised. Isotherms of DPPC monolayer spread on top of subphase containing 1 mg/ml of non-coated NPs showed also interaction between NPs and film components (Fig. 1c). These interactions were manifested mainly by the absence of the gas phase. Previous studies have shown that particle size is also a factor which influences the penetration of NP into the DPPC monofilms (11). The presence of NPs between DPPC molecules in monofilms is expected to change the packing of these molecules under compression. Nevertheless, it is important that the collapse pressure was very close to that observed with DPPC alone. Similar to previously observed non-coated NPs, polysorbate-80-coated NPs abolished the gas phase. The plateau region in the liquid expanded phase was comparable to the DPPC isotherm. However, the collapse pressure was almost one half of those recorded in other experiments. This indicates that polysorbate-80-coated NPs significantly reduce the stability of the DPPC monolayer film and its ability to compensate for high compression forces. The fact that polysorbate 80 alone has no significant effect on the collapse pressure supports the theory that coated nanoparticles enhanced the interaction between NPs and the DPPC monolayer.
The in vivo results confirmed the in vitro observations. As seen in the tissue sections taken from lungs of mice treated with polysorbate-80-coated NPs (Fig. 2c), their anatomical structure was compromised. This indicates that the surfactant film lining the alveolar sacs was not able to tolerate the compression forces caused by breathing and the alveolar sacs eventually collapsed. In order to keep the lung functions, the surfactant layer must have the ability to spread as well as getting compressed during inhalation and exhalation. The surfactant layer must stay in a balance between fluidity and rigidity to stabilize the alveolar sacs. Decreasing the collapse pressure of the surfactant monolayer caused by polysorbate-80-coated NPs disturbed the naturally occurring balance of the surfactant and caused the lungs to collapse. Even though non-coated NPs induced some changes in the DPPC monolayer, the collapse pressure was only slightly affected. No histological differences were observed between lung slices taken from a control group and the group treated with non-coated inhalable NPs. This observation supports the in vitro results in which the collapse pressure of a DPPC film was not affected by the addition of non-coated NPs to the subphase.
This study demonstrated that an in vitro model measuring the collapse pressure of a model lung surfactant film correlated well with observed in vivo pulmonary toxicity of polysorbate-80-coated NPs.
CONCLUSION
The presented in vitro model for studying the surface pressure-area isotherms is an early screening tool to assess the biophysical compatibility of selected drug carriers with lung surfactant films. The decrease in the collapse pressure of the monolayer film caused by coated NPs, in vitro, was associated with an acute pulmonary toxicity in vivo. This in vivo toxicity was not observed when uncoated nanoparticles were used. Therefore, the dosage from toxicity of colloidal carriers intended for pulmonary delivery is mainly determined by their final composition rather than their individual components. More investigations are required to set different cut-off points for the collapse pressure to correlate them with different stages of pulomary toxicity in vivo. The outcomes of this study should not be generalized for all surfactants or bi-block polymers. Other surfactants with different hydrophilic-lipophilic properties might interact differently with lung surfactant films. This method may be useful to establish upper deposition limits for inhalable dry powders.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOC 35 kb)
Acknowledgment
This study was supported by a Grant of Alberta Cancer Board. MHD Kamal Al-Hallak acknowledges the receipt of Damascus University scholarship. Shirzad Azarmi acknowledges the receipt of TRTC fellowship from the Alberta Cancer Board.
References
- 1.Azarmi S, Roa WH, Lobenberg R. Targeted delivery of nanoparticles for the treatment of lung diseases. Adv Drug Deliv Rev. 2008;60(8):863–875. doi: 10.1016/j.addr.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Oberdorster G. Pulmonary effects of inhaled ultrafine particles. Int Arch Occup Environ Health. 2001;74(1):1–8. doi: 10.1007/s004200000185. [DOI] [PubMed] [Google Scholar]
- 3.Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113(7):823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muhlfeld C, Rothen-Rutishauser B, Blank F, Vanhecke D, Ochs M, Gehr P. Interactions of nanoparticles with pulmonary structures and cellular responses. Am J Physiol Lung Cell Mol Physiol. 2008;294(5):817–829. doi: 10.1152/ajplung.00442.2007. [DOI] [PubMed] [Google Scholar]
- 5.Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol Rev. 2001;53(2):283–318. [PubMed] [Google Scholar]
- 6.Illum L, Jones PD, Baldwin RW, Davis SS. Tissue distribution of poly(hexyl 2-cyanoacrylate) nanoparticles coated with monoclonal antibodies in mice bearing human tumor xenografts. J Pharmacol Exp Ther. 1984;230(3):733–736. [PubMed] [Google Scholar]
- 7.Araujo L, Lobenberg R, Kreuter J. Influence of the surfactant concentration on the body distribution of nanoparticles. J Drug Target. 1999;6(5):373–385. doi: 10.3109/10611869908996844. [DOI] [PubMed] [Google Scholar]
- 8.Moghimi SM, Hunter AC. Capture of stealth nanoparticles by the body's defences. Crit Rev Ther Drug Carrier Syst. 2001;18(6):527–550. [PubMed] [Google Scholar]
- 9.Alyaudtin RN, Reichel A, Lobenberg R, Ramge P, Kreuter J, Begley DJ. Interaction of poly(butylcyanoacrylate) nanoparticles with the blood-brain barrier in vivo and in vitro. J Drug Target. 2001;9(3):209–221. doi: 10.3109/10611860108997929. [DOI] [PubMed] [Google Scholar]
- 10.Ku T, Gill S, Lobenberg R, Azarmi S, Roa W, Prenner EJ. Size dependent interactions of nanoparticles with lung surfactant model systems and the significant impact on surface potential. J Nanosci Nanotechnol. 2008;8(6):2971–2978. doi: 10.1166/jnn.2008.171. [DOI] [PubMed] [Google Scholar]
- 11.Gill S, Lobenberg R, Ku T, Azarmi S, Roa W, Prenner EJ. Nanoparticles: characteristics, mechanisms of action, and toxicity in pulmonary drug delivery—a review. J Biomedl Nanotech. 2007;3(2):107–119. doi: 10.1166/jbn.2007.015. [DOI] [Google Scholar]
- 12.Smith FB. Role of the pulmonary surfactant system in lung diseases of adults. N Y State J Med. 1983;83(6):851–856. [PubMed] [Google Scholar]
- 13.Schief WR, Antia M, Discher BM, Hall SB, Vogel V. Liquid crystalline collapse of pulmonary surfactant monolayers. Biophys J. 2003;84(6):3792–3806. doi: 10.1016/S0006-3495(03)75107-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stuart D, Lobenberg R, Ku T, Azarmi S, Ely L, Roa W, et al. Biophysical investigation of nanoparticle interactions with lung surfactant model systems. J Biomed Nanotech. 2006;2(2–3):245–252. doi: 10.1166/jbn.2006.031. [DOI] [Google Scholar]
- 15.Azarmi S, Lobenberg R, Roa WH, Tai S, Finlay WH. Formulation and in vivo evaluation of effervescent inhalable carrier particles for pulmonary delivery of nanoparticles. Drug Dev Ind Pharm. 2008;34(9):943–947. doi: 10.1080/03639040802149079. [DOI] [PubMed] [Google Scholar]
- 16.Sham JO, Zhang Y, Finlay WH, Roa WH, Lobenberg R. Formulation and characterization of spray-dried powders containing nanoparticles for aerosol delivery to the lung. Int J Pharm. 2004;269(2):457–467. doi: 10.1016/j.ijpharm.2003.09.041. [DOI] [PubMed] [Google Scholar]
- 17.Azarmi S, Tao X, Chen H, Wang Z, Finlay WH, Lobenberg R, et al. Formulation and cytotoxicity of doxorubicin nanoparticles carried by dry powder aerosol particles. Int J Pharm. 2006;319(1–2):155–161. doi: 10.1016/j.ijpharm.2006.03.052. [DOI] [PubMed] [Google Scholar]
- 18.Vauthier C, Labarre D, Ponchel G. Design aspects of poly(alkylcyanoacrylate) nanoparticles for drug delivery. J Drug Target. 2007;15(10):641–663. doi: 10.1080/10611860701603372. [DOI] [PubMed] [Google Scholar]
- 19.Brown DM, Wilson MR, MacNee W, Stone V, Donaldson K. Size-dependent proinflammatory effects of ultrafine polystyrene particles: a role for surface area and oxidative stress in the enhanced activity of ultrafines. Toxicol Appl Pharmacol. 2001;175(3):191–199. doi: 10.1006/taap.2001.9240. [DOI] [PubMed] [Google Scholar]
- 20.Kodama M, Shibata O, Nakamura S, Lee S, Sugihara G. A monolayer study on three binary mixed systems of dipalmitoyl phosphatidyl choline with cholesterol, cholestanol and stigmasterol. Colloids Surf B Biointerfaces. 2004;33(3–4):211–226. doi: 10.1016/j.colsurfb.2003.10.008. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 35 kb)