Abstract
A biopharmaceutics and Quality by Design (QbD) conference was held on June 10–12, 2009 in Rockville, Maryland, USA to provide a forum and identify approaches for enhancing product quality for patient benefit. Presentations concerned the current biopharmaceutical toolbox (i.e., in vitro, in silico, pre-clinical, in vivo, and statistical approaches), as well as case studies, and reflections on new paradigms. Plenary and breakout session discussions evaluated the current state and envisioned a future state that more effectively integrates QbD and biopharmaceutics. Breakout groups discussed the following four topics: Integrating Biopharmaceutical Assessment into the QbD Paradigm, Predictive Statistical Tools, Predictive Mechanistic Tools, and Predictive Analytical Tools. Nine priority areas, further described in this report, were identified for advancing integration of biopharmaceutics and support a more fundamentally based, integrated approach to setting product dissolution/release acceptance criteria. Collaboration among a broad range of disciplines and fostering a knowledge sharing environment that places the patient's needs as the focus of drug development, consistent with science- and risk-based spirit of QbD, were identified as key components of the path forward.
Electronic supplementary material
The online version of this article (doi:10.1208/s12248-010-9206-0) contains supplementary material, which is available to authorized users.
KEY WORDS: biopharmaceutics, dissolution, product performance, quality by design, quality target product profile
INTRODUCTION
The implementation of the Quality by Design (QbD) (1) paradigm is ushering in new knowledge-based approaches for product development and manufacture to better meet patient needs through clinical and safety targets in the Quality Target Product Profile (QTPP). These approaches require greater understanding of process parameters and product attributes and their impact on drug bioavailability and dissolution/release profiles. Biopharmaceutics tools including in vitro tests can guide and support development of drug products consistent with their intended clinical in vivo performance. A natural extension is setting drug dissolution/release specifications that are linked to desired biological performance of the final product.
This report provides a brief description of speaker presentations and summarizes the discussions and recommendations from the breakout sessions, from the biopharmaceutics and QbD conference in Rockville, Maryland, USA that took place on June 10–12, 2009. The meeting was co-sponsored by the Food and Drug Administration (FDA) and University of Wisconsin School of Pharmacy Extension Services and was organized in cooperation with the American Association of Pharmaceutical Scientists. There were 104 participants. Speakers, moderators, and planning committee members are listed in Table S1 (supplementary material). The agenda of workshop is listed in Table S2 (supplementary material).
The meeting objectives were (1) to establish the current state of dissolution/drug release methods utilized during various stages of development, from early stages to commercial scale manufacturing and lifecycle management of the drug product, and review the current state of biopharmaceutics in the context of the QbD paradigm and (2) to identify the tools and enablers needed to obtain a “common approach” to biopharmaceutics consistent with QbD principles (a road map) for predictive, clinically meaningful in vitro methods for dissolution/release specification setting. Presentations, plenary, and breakout session discussions were held through the 3-day conference and are also described elsewhere (2).
THE PRESENTATIONS
Helen Winkle gave the keynote address and said “the future of CMC [Chemistry, Manufacturing and Controls] review is Quality by Design (QbD) and related tools that will allow companies to build better quality into their products”. Ms. Winkle discussed the integration of biopharmaceutics and QbD to improve the regulatory process and how industry and FDA must utilize evolving science and technology and drive continuous improvement to ensure higher-quality products. Arzu Selen emphasized the value of predictive methods and dissolution test criteria that link product critical quality attributes and intended in vivo product performance. In the First Plenary Session, Anette Müllertz spoke about biorelevant in vitro methods to characterize drug release and absorption. These methods included not only various dissolution methods but also in vitro lipolysis as well as the combination of release/lipolysis with transport studies in intestinal cell cultures, e.g., Caco-2 cells. Selection of biorelevant media composition must be based on the conditions desired to simulate, e.g., prediction of release in the fed state calls for the use of media containing also monoglycerides and free fatty acids. Maria Cruañes spoke about novel miniaturized in vitro methods in early development to assess active pharmaceutical ingredient (API) characteristics that may control dissolution and to identify critical excipients and/or controls needed to enhance in vivo (pre-clinical and clinical) absorption. In this process, development scientists capture and document prior knowledge needed for subsequent developmental stages. Tahseen Mirza focused on lessons learned from in vitro and animal models and risk assessment during formulation development of low solubility compounds. He cautioned about extrapolating results from in vitro or pre-clinical studies directly to clinical human studies.
In Plenary Session 2, Paul Dickinson discussed how in-depth understanding of in vivo risk and development of appropriate dissolution conditions can lead to specifications that guarantee drug exposure. In Biopharmaceutics Classification System (BCS) class 2 and 4 immediate release product case studies presented, dissolution methods were “over-discriminating” while these drug products resulted in similar exposures in humans. QbD clinical studies led to a design space for viable formulations with accompanying dissolution specifications that would ensure clinical performance (3). Gordon Muirhead discussed how clinically relevant critical quality attributes (CQAs) could be directly or indirectly linked to critical process parameters (CPPs), which in turn can be monitored by real-time testing or process analytical technology (PAT). Acceptance criteria based on the multi-dimensional relationship between CPPs and CQAs would ensure maintaining the operational criteria within the design space. Jack Cook continued the discussion of linking product performance with in vivo data and highlighted the importance of discriminatory dissolution testing. He discussed the benefits of establishing in vitro and in vivo correlations (IVIVC) and how IVIVC efforts can evolve and guide development with continuous accumulation of data and how an established IVIVC model will provide significant value, beyond regulatory need, for products reaching the market. Arzu Selen presented two examples developed from literature articles to illustrate how biopharmaceutics can link process, product, and the desired in vivo performance of the product to meet patient needs based on well-characterized CQAs and robust and predictive methods.
In Plenary Session 3, Jennifer Dressman reviewed currently available biopharmaceutics tools and emphasized the advantages of linking QbD parameters with in vivo performance. Prof. Dressman summarized that refinement of biorelevant methods, in silico modeling and greater understanding of formulation sensitivities are needed, and rational use of in vitro/in silico/in vivo approaches should lead to streamlined development programs. Christos Reppas explored whether in vitro methods/tools can adequately predict in vivo food effect and/or drug absorption under extreme conditions and also for special populations. He concluded that biorelevant media are useful for identifying solubility problems and for identifying suitable formulation strategies, including testing for robustness due to food effects. He highlighted the value of using elevated pH and transfer models for evaluating performance of the drug in GI tract.
The next three presentations focused on “Innovative, Advanced or Borrowed Tools: Pros and Cons: Tools to Move Forward”. Filippos Kesisoglou discussed the use of oral absorption modeling tools to facilitate formulation decisions through application examples on biorelevant dissolution and API properties and specifications. While these in silico tools are still evolving, the coupling of modeling with in vitro methods provides a powerful tool to facilitate QbD in the biopharmaceutics space. Modeling and IVIVC tools were further highlighted by Stefaan Rossenu who detailed the development and application of an IVIVC model to set dissolution specifications for a long-acting injectable nanosuspension. Patrick Marroum stated that the regulatory objectives are to ensure that the marketed batches have the same safety and efficacy profiles as the ones tested in clinical trials and the risk to patient is minimized by decreasing variability from batch to batch. He presented examples of in vitro and in vivo results and summarized that “understanding the relationship between the critical manufacturing variables and their impact on bioavailability will aid in future manufacturing changes, and potentially alleviate the regulatory burden”.
In Plenary Session 4, Jobst Limberg discussed the importance of IVIVC and emphasized the value of providing release rate information in product labels, particularly for extended release products. He concluded with a novel specification setting approach, such as using PAT tools and multivariate analysis for detecting differences by using principal component analysis and building clusters of acceptable and not acceptable batches. Chikako Yomota focused on bioequivalence guidelines for oral dosage forms in Japan and regulatory perspective on QbD. She indicated that if dissolution tests were going to be used to develop design space, discriminative conditions should be selected so that differences in bioavailability can be detected. She also gave an example for formulation design space and concluded by identifying the multivariate relationship as formulation design space for the product.
Alan Royce provided a pharmaceutical industry perspective and described QTPP as a collection of CQAs which include biopharmaceutical properties for ensuring product quality and that it would typically resemble the product specifications (dosage form, strength, drug release: dissolution, disintegration time) and that QTPP would evolve with new knowledge. He presented risk assessment examples and summarized that QbD provides multivariate understanding of biopharmaceutics, CQAs, and, ultimately, improvement in biopharmaceutics tools that will empower the QbD development process.
Christine Moore provided the US regulatory perspective and gave an example of a QbD approach for dissolution specification consistent with ICH Q8 R1. She concluded that QbD approaches can lead to a fundamental shift in development and manufacturing of pharmaceutical products with clear links between patient, product, and process and result in a more scientific and risk-based regulatory oversight and that “much progress has been made but scientific, organizational and regulatory challenges remain”.
THE BREAKOUT SESSIONS
Four moderating teams led breakout session discussions on four topics: Integrating Biopharmaceutical Assessment into the QbD Paradigm, Predictive Statistical Tools, Predictive Mechanistic Tools, and Predictive Analytical Tools. Discussion was framed to answer four questions about the tools and approaches needed to move from the current state to the desired future state (Fig. 1).
Fig. 1.
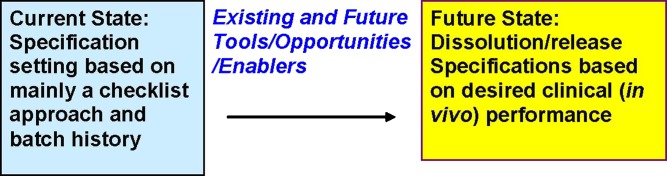
Illustration of current and future state. Existing and future tools, opportunities, and enablers will result in dissolution/release specifications based on desired clinical (in vivo) performance
The four questions were: (1) What are current tools and enablers? (2) What are desired tools and enablers? (3) How to develop better tools and how to better integrate efforts? (4) What are existing and potential opportunities?
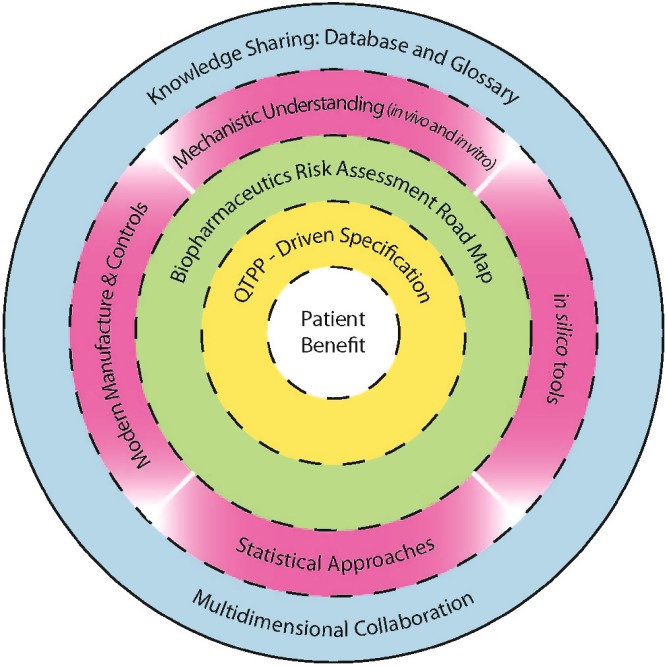
An executive coach facilitated the moderating teams before and after the breakout sessions. Participants’ responses were collected and displayed. Participants voted to assign priority to the recommendations. The nine priority areas, identified as having the highest potential for advancing effective integration of biopharmaceutics into the QbD paradigm for patient benefit, were presented in draft form to all attendees on the last morning of the meeting and in the 2-week update (http://www.pharmacy.wisc.edu/esp/prog/fdauwqbdfiles/Bio%20and%20QbD%20conference-two%20week%20update.pdf). The inter-relationships between these nine areas are illustrated in Fig. 2.
Fig. 2.
Nine areas and their inter-relationships identified in the workshop to integrate biopharmaceutics and QbD for patient benefit
Table I further organizes these nine priority areas into four broad categories: (1) QTPP-driven specifications to guide development of the product, consistent with its intended use (i.e., clinical targets for the intended population); (2) science- and risk-based biopharmaceutical road map for product development and manufacturing that utilizes available knowledge, models, and technologies to guide product design and control strategies; (3) concerted advancement and leveraging of science and technology to enable more predictive tools, more robust processes, and more rigorous application of statistics; and (4) knowledge sharing and collaborations across disciplines and organizations which are necessary to leverage prior knowledge and generate new knowledge.
Table I.
Four Broad Categories and the Identified Nine Priority Areas
| Quality target product profile-driven specifications | |
| Specifications are set to meet performance criteria consistent with a medication’s intended purpose and patient population needs, as defined by quality target product profile | |
| Biopharmaceutics risk assessment road map | |
| Biopharmaceutics risk assessment employs a workstream that leverages knowledge from areas below to assess risk and guide product development, manufacture, and control strategies with the goal of insuring that the product meets pre-specified criteria | |
| Advancing and leveraging science and technologies | |
| Mechanistic understanding | Experimental work to obtain understanding of in vivo drug release and drug absorption complemented by better understanding of in vitro models of drug release |
| In silico tools | For supporting research and hypothesis testing, capturing mechanistic knowledge and as an objective tool to probe hypothesis arising from the mechanistic work |
| Statistical approaches | More rigorous application of statistics to understand and incorporate variability and uncertainty in developing risk models during product design, development, and manufacturing |
| Manufacturing and controls | Based on modern pharmaceutical unit operations and analytical methods to insure dosage form consistency linked to desired product performance |
| Knowledge sharing and collaborations | |
| Multi-dimensional collaborations | Involves multidisciplinary interactions across industry, academia, and regulatory agencies |
| Database | To capture knowledge, access to tools, and facilitate links across all technical areas |
| Glossary | To have a common language that facilitates communication across diverse skill disciplines |
More detailed description and recommendations for each of the nine areas are given below. For ease of reference, “QTPP-driven specification” is used in Fig. 2 and in Table I and later discussed in detail under the heading “Specification (Dissolution/Release Acceptance Criteria) Driven by Quality Target Product Profile”.
Knowledge Sharing and Collaborations
Multi-dimensional Collaboration
A key enabler to link the pharmaceutical process to patient outcomes is collaboration among all stakeholders. It was agreed that no one entity had sufficient knowledge and insight to understand all factors, from manufacturing issues to individual patient outcomes. There was a strong desire of all attendees to better understand the perspectives of other stakeholders.
Stakeholders derive from several dimensions. One dimension is from the pharmaceutical development process such as industry, worldwide regulatory authorities, and academia. Another dimension is the non-pharmaceutical disciplines from which pharmaceutical scientists can learn from their well-characterized and developed automated technologies, such as continuous manufacturing in food industry. Hence, stakeholders include formulation scientists, analytical scientists, clinical scientists, pharmaceutical scientists, process engineers, programmers, regulatory specialists, statisticians, biologists, and chemists. Stakeholders should organize and interact to expedite development and communicate both forward and backward throughout the development process.
Recommendation
Meeting participants clearly enunciated the need for multi-dimensional collaboration and practiced it through thoughtful and passionate dialogue. The intention will be to develop several mechanisms that foster collaboration; primarily, this will be via formation of a regulatory/industry/academia focus group populated by functional experts drawn from all the necessary disciplines. Further facilitation of multi-dimensional collaboration could be achieved via conferences organized to cover this topic, annual prizes for collaboration or best collaborative publication, and potentially through the award of research grants based on collaborative criteria. Conferences (specifically AAPS) could be utilized to further engage individuals, vet ideas/proposals made by the focus group, or gather more information.
Database
The identified need denoted notionally as “Database” encompasses a greater resource of knowledge, tools, and lessons learned than a typical database. It is envisioned as a platform that can connect and facilitate knowledge and information sharing across multiple sources and areas.
The pharmaceutical industry generates vast amount of data during drug development. Academic institutions collectively carry out significant biopharmaceutical research. However, these data are fragmented and reside on disparate systems making it difficult to mine relevant data for patterns and trends that yield insight and knowledge. Each scientist is limited to the small fraction of data to which his or her institution has access. Each institution must generate their own set of data when trying to answer similar questions, resulting in an enormous cost in time and money.
The comments in the brainstorming sessions highlighted a wide range of expectations from the “database”. While it would be a structured resource (containing information such as formulations, dissolution data/methods, pharmacokinetic properties of drugs, stability, and other product attributes), it was also expected to have “tool-like” interactive capabilities to allow data visualization and mining as well as providing access to tools to enable data analyses (including pharmacokinetic, statistical, and simulation tools). Ideally, such an interface might provide standardization in approaches (when standardization would be helpful). The “database” would include historical data from both successful and unsuccessful projects and thus facilitate exploration of different data analyses approaches and ways to test new hypotheses.
Recommendation
The need identified by the group supports the role of interactive and integrated systems that go beyond the typical deliverables of a “smart” database, serving as a connecting platform that is flexible to handle multiple data sources and programs. The incorporation of tool-like capabilities may be accomplished by links to a variety of programs and to the necessary training to support the applications.
It was also felt that there should be little concern regarding the cross institutional sharing of such data. The vast majority of intellectual property concerns pharmacologic drug properties rather biopharmaceutical characteristics. Thus, it should be possible to design a database to protect intellectual property and still provide useful data.
Glossary
A biopharmaceutical glossary is recommended. It was suggested that agreed-upon terminology is needed to integrate multiple disciplines and achieve multi-dimensional collaborations. A glossary accessible to all would facilitate clarity and communication. One approach that is under consideration is described below. It is envisioned that, at one point, the glossary and the database would be linked.
Recommendation
A website (final name TBD) would be developed as an online glossary of terms used in the pharmaceutical industry. Glossary contributions would come from pharmaceutical community members who maintain and police its content. Interested parties may suggest additions or corrections. An editorial board identifies experts in the field to help define terms. Contributions are subject to independent review. The glossary will take advantage of state-of-the-art technology and be linked to other initiatives that involve glossary terms. The glossary will serve as an authoritative nomenclature focal point to collate information from diverse sources to facilitate development and approval of drug products.
Advancing and Leveraging Science and Technology
Mechanistic Understanding
There is a wealth of work in the area of in vitro models of drug release and a plethora of choices of compendial and non-compendial apparatuses and media (4) available to the development and regulatory scientist. Not all of these methods are necessarily predictive of (or correlate to) in vivo drug release and those that have potential predictive or correlative capabilities are not fully leveraged. It was recognized that there is a need to improve our understanding of in vitro methodologies and to consolidate this knowledge in guidance documents or decision trees that provide a harmonized rationale for method selection. It was also recognized that further development and more effective utilization of in vitro models would only be possible if based on a more fundamental understanding of in vivo events occurring during drug absorption.
Immediate focus should be placed on two key areas for advancing mechanistic understanding: (1) how and where the drug is released from the dosage form in vivo and (2) the role formulation excipients may play in drug release. Increased knowledge of in vivo drug release and involved mechanisms are expected to lead to methodologies with higher probability of predicting in vivo performance and thus enabling more efficient development of products with optimal efficacy and increased safety.
Recommendation
A stepwise procedure for obtaining mechanistic understanding is recommended as follows:
- Place priority on in vivo studies with model compounds/dosage forms.
- Clinical studies to elucidate the absorption profiles of a given class of compounds in formulations of various modes and rates of dissolution/release so as to test hypotheses probing the mechanism of drug release and absorption
- Imaging of dosage form behavior in GI tract and using various formulation types to understand the role of functional excipients that may impact drug release
- Understand physiology in relation to drug release and absorption, for example, by characterizing human GI fluids under different conditions such as in fed/fasted state, disease state, or in infants or elderly patients
Use in vivo data and physiology knowledge generated with model molecules/dosage forms above to guide development and validation of in vitro (and in silico) models that better mimic in vivo release characteristics, i.e., choice of media, media volume, “sink”, and hydrodynamics. In vitro methods could also potentially include novel detection techniques to probe drug–excipient interactions. These methods could then be applied with more confidence to other molecules of similar properties, to other formulations of the same drug molecule, or to the same formulations of different molecules.
It is an ultimate goal that the resulting in vitro models should be grouped in harmonized categories that are suitable for specific development purposes. The categorization criteria could include (a) characteristics of the drug molecule and dose (e.g., solubility, permeability, pKa, etc.), (b) intended or actual mechanism of release (the latter from in vivo studies), and (c) type of dosage form and the functional role of excipients.
As an overall objective, knowledge and understanding gained from the mechanistic studies should be integrated into the “Biopharmaceutics Risk Assessment Road Map” section described below. It was noted that the fundamental nature of the proposed mechanistic research will require multidisciplinary collaborations that bring separate fields of expertise together, in particular, pharmacokinetics, biopharmaceutics, chemistry, analytical instrumentation, imaging, and physiology.
In Silico Tools
Utilization of in silico tools to link in vitro observations to clinical formulation performance should span the drug development process. Greater modeling and simulation capabilities in the biopharmaceutical roadmap further facilitates the leveraging of knowledge of in vitro and in vivo behavior of the compound, leading to a more rational clinical study design and relevant specifications on either product attributes (e.g., API particle size, formulation excipients, manufacturing processes) or in vitro release methods (e.g., dissolution). The use of in silico tools to link in vitro observations to clinical performance will also add a level of understanding to aid in the decision-making process. It will be important to develop in silico models that predict not only an outcome but also the likelihood of the outcome occurring and the error associated in the prediction.
While absorption modeling tools exist linking formulation properties to clinical performance, e.g., pharmacokinetics or pharmacodynamics, extensive assessment of their accuracy to predict these outcomes in the public domain is lacking. Current models largely focus on simulating the absorption of drugs from conventional immediate, delayed, and sustained release solid dosage forms. While these represent the majority of marketed drugs, recently, there has been an increase in the number of poorly soluble compounds in development (up to 90% of new chemical entities estimated as BCS II/IV) (5), and a more extensive assessment of application of the available software tools for modeling insoluble compounds and their related novel dosage forms (e.g., amorphous systems, nano-suspensions, liquid-filled capsules) is highly desirable. While these efforts could be initiated within each pharmaceutical company, collaborative efforts could leverage cross-organization databases to test models against a diverse set of compounds.
Recommendation
Through proactive implementation and continuous improvement of in silico tools, modeling and simulation centering on biopharmaceutical properties can become an integral part of the formulation and drug development process, facilitating QbD from the early stages of development through registration.
While efforts around model improvement can take place within individual organizations, cross-organizational exploration of these tools would provide the greatest value with respect to both improvement of the “models” and “tools”. This will be accomplished through:
The use of model compounds in close synergy with the above database to model and simulate PK/PD and release patterns of novel and conventional dosage forms
Broadening the application of existing or novel in silico tools for supporting research and hypothesis testing, capturing mechanistic knowledge, and to probe hypothesis arising from the mechanistic work
Statistical Approaches and Appreciation of Variability
A key enabler for developing high-quality products is rigorous application of statistics and statistical modeling for appreciation of variability. Better appreciation of variability leads to more informed risk assessment and management. Appreciation of variability includes (1) understanding variability, its sources, and its impact, (2) managing variability through design and control, and (3) using variability to facilitate the decision-making process.
Efforts to manage variability are influenced by appreciated risk. Highly variable processes yield product which may be unfit for use, resulting in failure to achieve a given benefit to the patient. Highly variable assays mask the true signal and hinder their use as decision-making tools. Highly variable experimental results hinder decision making, creating a risk of drawing the wrong conclusion. New technologies coupled with statistical tools help manage variability and the consequent risks. Multifactor design of experiments, multivariable analysis, and other statistical modeling tools provide a mathematical framework for the assessment of variability. User-friendly software to implement these tools can facilitate an appreciation of variability and assist the practitioner in reducing risk.
The importance of robust statistical approaches was expressed throughout the breakout sessions of the workshop. Many felt the need to identify sources of variability in product manufacture, patient outcomes, analytical measurement, and supply. The need to decrease variability both in vivo and in vitro was identified as key to implementing in vitro and in vivo relationships and supporting sound decisions. It was further acknowledged that uncertainty need be incorporated into specifications, to reduce the risks to the patient population, while similar sensitivity to the manufacturer’s risk should be brought to bear. Manufacturing control through feedback mechanisms was likewise highlighted.
Recommendation
The appropriate use of statistical tools and collaboration between scientists and statisticians was recommended with particular focus on the following: (1) work with the statistical community to help define the problem(s), design efficient study paradigms to effectively address the question(s), and incorporate uncertainty into our models and decisions in order to manage and communicate risks; (2) in conjunction with database, make sure measures of uncertainty accompany key data in order to weigh the results across multiple experiences; (3) educate pharmaceutical scientists in elements of statistical thinking, including recognition of variability, elements of experimental design, statistical measures of outcomes, etc.; and (4) engage software vendors in development of practical user-friendly statistical tools to address common biopharmaceutics problems.
Modern Manufacture and Controls
One purpose of dissolution testing is to monitor the consistency of the manufacturing process across single and multiple batches over time. It was recognized at the breakout session discussions that such end-product test does not necessarily insure that the product meets the desired quality as defined by biopharmaceutical performance targets. Quality can be built into the product by means of modernized unit operations that afford greater control of individual dosage units and lend themselves to real-time measurements of critical process parameters or material attributes.
Recommendation
Modernization of pharmaceutical manufacturing was identified as having great potential to enable the production and characterization of solid dosage forms that consistently deliver the biopharmaceutical performance indicated by the QTPP. Recommended areas of focus for advancement of pharmaceutical manufacturing are continuous operations and process analytical tools for real-time monitoring and release.
Biopharmaceutics Risk Assessment Road Map
Biopharmaceutic studies play a significant role in drug development, for example, in supporting drug candidate selection, formulation optimization, and interpretation of impact of changes such as in formulation, manufacturing process, and manufacturing site, on in vivo performance of the drug product. As described under mechanistic understanding, following conducting in vitro, in vivo, and in silico studies, data may be modeled to identify contributing factors and for predicting outcomes under various conditions. In a general sense, the “Biopharmaceutics Risk Assessment Road Map” is a document for leveraging knowledge from mechanistic and supportive studies, capturing specific product characteristics (such as based on BCS and/or Biopharmaceutics Drug Disposition Classification System classifications (5)) and key decision points, to assess risk on in vivo performance of the drug product. In an academic organization, the function of such a risk assessment approach would be to guide to the necessary studies for further characterizing the product and provide direction for assessing risk and guiding efficient product development, manufacture, and control strategies. The ultimate goal, as applicable, would be that the product meets pre-specified patient centric quality criteria. It is expected that the following would be helpful in a biopharmaceutics risk assessment road map: (1) in vitro methods for characterizing in vitro and in vivo performance of the product, (2) the product attributes critical for intended therapeutic effect of the drug product, and (3) risk assessment methodology for decision making.
Recommendation
This workstream will develop a document that facilitates decision making by identifying the experiments that are necessary and maps the development routes to result in a dissolution test (or alternative) and, if applicable, specification based on intended clinical (in vivo) performance. The document will:
Consist of approaches and methodology that are current “state-of-the-art” knowledge with emphasis on leveraging existing knowledge (e.g., BCS classification, regional absorption, etc.)
Likely present the knowledge in a question based format and assist with decision trees providing options and logic for development (e.g., if drug has a particular property, consider step A or B; if step A is selected, then steps C and D are subsequent options)
Suggest presentation formats of data and knowledge for ease of communication of main focus
Elucidate decision points and rationale in method development (i.e., identify red flag(s) for a dissolution method and/or indicate when more extensive efforts are required) and criteria for deciding on method suitability such as the method being consistent with in vivo rank order results, discriminating, etc.
- Provide guidance on how to perform quality risk assessments (QRA) related to drug bioavailability (rate and exposure) and in vitro dissolution/release profiles and describe potential outcomes for each step and the impact on the development plan
- API, product, and processes risk profiling is a fundamental foundation for defining a successful development pathway (e.g., QRA allows prioritization of the process parameters and quality attributes to be investigated experimentally to further quantify the risks to exposure). Subsequent risk assessments based on the increased knowledge will inform what controls need to be placed on which process parameters and quality attributes
- Appropriate selection and application of biopharmaceutical tools can facilitate better estimation of the probability of specific failure modes as identified in the quality risk assessment (ICH Q9; e.g., in silico modeling to anticipate API particle size impact)
Once complete, if applicable, this document can serve to describe the biopharmaceutic information/knowledge that can be found in regulatory documents and facilitate better understanding of the rationale for the proposed methodology (dissolution testing or an alternative approach), related acceptance criteria, and/or other key product development decisions.
The purpose of such a biopharmaceutics risk assessment road map will be for generating and leveraging knowledge and communicating decision points and rationale. The contents are expected to be dependent on organizational needs.
Specification (Dissolution/Release Acceptance Criteria) Driven by Quality Target Product Profile
A primary objective of the workshop was to explore how best to integrate biopharmaceutics and QbD principles for ensuring product quality by linking process and product knowledge with in vivo product performance and establish the most informative and critical boundaries as product specification. Biopharmaceutics tools and approaches have long been used to develop drug dissolution/release acceptance criteria for specification setting, and in some cases, in vitro methods are shown to be highly predictive of in vivo performance of drug products.
The QTPP presents predefined objectives that guide the development of a product from its beginning. For example, the desired in vivo drug release profile guides the design of a dosage form, i.e., formulation composition and manufacturing process to achieve the therapeutic effect of the drug. The acceptance criteria for drug dissolution/release (or alternate methods) derived with the QTPP in mind aim to insure the relationship between the product characteristics and the desired in vivo performance of the drug product.
Feedback from the breakout sessions emphasized linking product specification to in vivo performance, based on a sound mechanistic understanding of the product (such as its critical process parameters and critical quality attributes), its intended use and unique characteristics that need to be taken into account (such as dosage form, route of administration, and the patient population). While it was raised that “personalized” product development should not be a direction for advancement, flexibility in the dosage form, tailoring to therapeutic area/patient population (including age groups), and the specifications connected to the intended use are greatly desirable.
Effectively, drug product specification reflecting such a link between QTPP and product component and manufacturing process parameters will ensure pharmaceutical quality (an acceptable low risk of failing to achieve the desired clinical attributes).
Recommendation
The leaders for this priority area will develop a core group and explore collaborations with groups involved in specification setting in pharmaceutical industry as well as in other areas. The core group will explore and broaden likely scenarios and develop examples for illustrating impact of specification setting in the QbD paradigm.
CONCLUSION
The defined objectives were met through the participants’ development of nine priority areas that were identified as having the highest potential for advancing effective integration of biopharmaceutics into the QbD paradigm for patient benefit. The importance of these nine areas was further recognized as recommendations were made by the participants for implementing these QbD principles into daily product development practice. As part of the scope of these recommendations, key components of the path forward were identified and future involvement of regulatory, industry, and academic leaders will provide the necessary input to develop and establish guidelines for implementing QbD practices that place the patient's needs as the focus of drug development.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Speakers, moderators, and planning committee (*) (DOC 51 kb)
The workshop agenda (DOC 47 kb)
Acknowledgments
• Leanne Cusumano Roque, Esq. ACC (Executive Coach)
• School of Pharmacy, Extension Services, University of Wisconsin, Madison, Wisconsin, USA
• Meetings Management Staff, American Association of Pharmaceutical Sciences, Arlington, Virginia, USA
• The 2009 Conference Participants
Disclosure
The views expressed in this article are those of the authors and do not necessarily reflect the opinions of their companies/institutions or the official policy of the US Food and Drug Administration (FDA). No official endorsement by the FDA is intended or should be inferred.
References
- 1.Guidance for Industry Q8(R2) Pharmaceutical development, 2009 (ICH Q8 (R2)). http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm073507.pdf.
- 2.Gray VA. Meeting report: University of Wisconsin/AAPS/FDA Workshop Applied Biopharmaceutics and Quality by Design for Dissolution/Release Specification Setting: Product Quality for Patient Benefit. Dissolution Technologies. 2009;16(4):35–39. [Google Scholar]
- 3.Dickinson PA, Lee WW, Stott PW, Townsend AI, Smart JP, Ghahramani P, et al. Clinical relevance of dissolution testing in quality by design. AAPS J. 2008;10(2):380–390. doi: 10.1208/s12248-008-9034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Müllertz A. Biorelevant dissolution media, biotechnology. Pharmaceutical aspects. Arlington: AAPS; 2007. pp. 151–177. [Google Scholar]
- 5.Benet LZ, Wu CY. Using a biopharmaceutics drug disposition classification system to predict bioavailability and elimination characteristics of new molecular entities. Somerset: NJDMDG; 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Speakers, moderators, and planning committee (*) (DOC 51 kb)
The workshop agenda (DOC 47 kb)