Abstract
The aim of the present study was to define the mechanisms responsible for poor bioavailability of emodin by determining its metabolism using in vitro and in situ disposition models of the intestine and liver. Liver microsomes of mice, rats, guinea pigs, dogs, and humans were used along with the rat intestinal perfusion model and the rat intestinal microsomes. In the rat intestine, excretion rates of emodin-3-O-glucuronide were significantly different (p < 0.05) in four regions of the intestine and were higher in males than in females (p < 0.01). Emodin glucuronidation in liver microsomes was species-dependent, and Km values varied 5.7-fold (3.2–18.2 μM) in males and 2.8-fold (4.6–13.0 μM) in females. The male intrinsic clearance (CLint) values differed by 5-fold (27.6–138.3 mL h−1 mg−1 protein), and female CLint values differed by 4.3-fold (24.3–103.5 mL h−1 mg−1 protein). Since CLint values of emodin glucuronidation were 10-fold higher than that of isoflavones, emodin was considered rapidly glucuronidated. In contrast to the large species-dependent effects on Km and CLint values, gender had a smaller effect on these kinetic parameters (2-fold, p < 0.05). Lastly, glucuronidation rates obtained using liver microsomes from various experimental animals of the same gender correlated well with those in human liver microsomes. In conclusion, Rapid metabolism by UDP-glucuronosyltransferase is the major reason why emodin has poor bioavailability. Species and gender affected emodin metabolism to a different degree, and experimental animals are expected to be useful in predicting emodin glucuronidation in humans.
Key words: emodin glucuronidation, first-pass metabolism, gender, species, UGT
INTRODUCTION
Anthraquinones, a large family of complex naturally occurring polycyclic phenolic compounds, have a wide variety of biological activities including anticancer (1–3). There are considerable interests in developing nutraceutical and therapeutic agents from this class of compounds because anthraquinones are abundant in vegetables, teas, and fruits (4). Nutraceutical companies worldwide are eagerly marketing them as health products for an expanding range of conditions, including obesity. Pharmaceutical companies have increased their focus on these compounds because of their favorable safety profiles (2,4). Moreover, mitoxantrone, an anthraquinone derivative, is an approved anti-cancer agent (5), suggesting that this class of chemicals have attractive structure features that make them good candidates as future pharmaceuticals.
Emodin (1,3,8-trihydroxy-6-methylanthraquinone; inset in Fig. 1a) is a major active anthraquinone present in the rhubarb (rhizome of Rheum palmatum L.), aloe, leaf of senna, and root of Polygonum multiflorum. Rhubarb is known to have mild laxative properties in traditional Chinese medicine (3), which was demonstrated using traditional pharmacological studies. Hence, emodin-rich plant extracts are used in many weight-loss drugs now available from health stores because these extracts can induce mild diarrhea and reduce body weight (6).
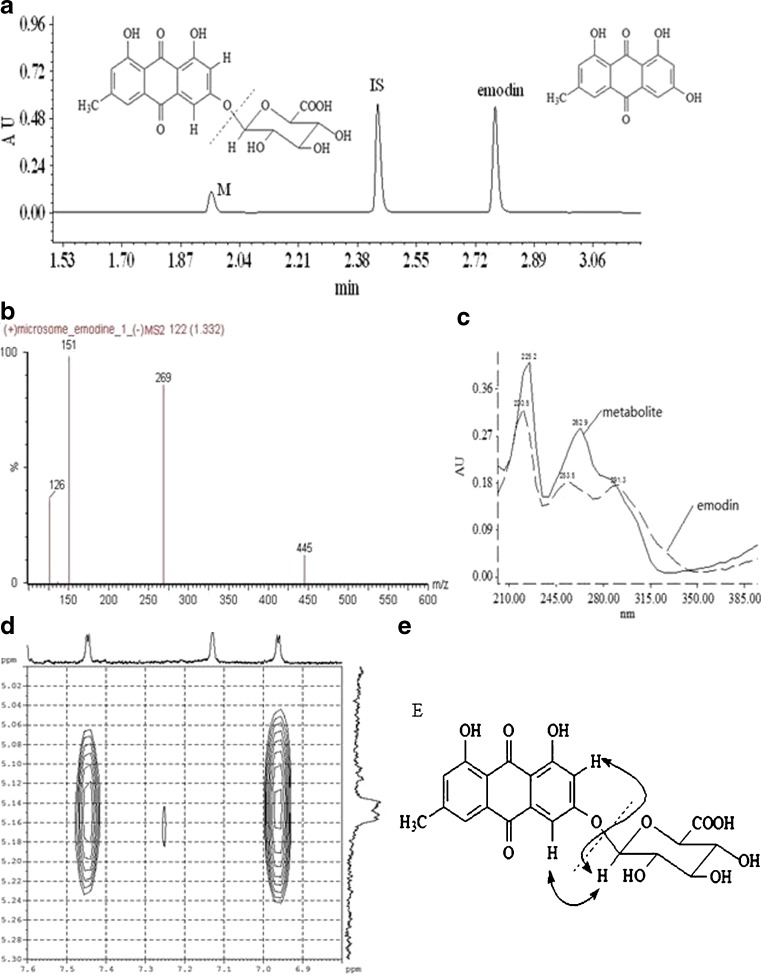
Fig. 1.
Identification of metabolite structure by UPLC, LC-MS/MS, and 1H-NMR. Emodin (final concentration, 40 μM) was incubated with liver microsomes and intestinal microsomes in phase II reaction at 37°C for 10 min as described under “MATERIALS AND METHODS.” a Metabolite (M), IS, and emodin were eluted at 1.920, 2.458, and 2.769 min, respectively. Mass spectra of the emodin glucuronide and UV spectra of emodin glucuronide and emodin were shown in b and c, respectively. d The verified location of glucuronic acid at the 3-OH position of emodin was based on an H-NMR spectrum where NOE correlations between the anomeric proton (δ 5.14) with both H-4 (δ 7.45) and H-2 (δ 6.96) in the NOESY spectrum. NOE correlations between anomeric proton and both H-2 and H-4 were shown in e
More recent studies showed that emodin induces apoptosis in several types of cancer cells (7,8) and has strong inhibitory effects on cancer cell migration (9) and invasion (10). Emodin is effective against Her2-expressing breast cancer cells (11) and other cancer cells, including prostate (12) and lung cancer cells (13). It is generally recognized that its mechanisms of action is via interruption of kinase signaling (e.g., ERK) (13). Therefore, emodin is an emerging agent for cancer chemoprevention.
There are significant obstacles to the development of emodin as a viable chemopreventive agent. First, among the numerous pharmacological activities is its reported genotoxicity (14,15). Genotoxic and mutagenic effects of emodin in vivo and in vitro have been reported in several studies (16–19). The mechanism of toxicity of emodin is reported to be the generation of reactive oxygen species, which led to lipid peroxidation, DNA oxidation, and protein damage (20). However, these toxic effects may not be very severe since the compound is not found as an intact compound in vivo (21). In fact, intact emodin was not quantifiable in rat plasma using a LC/MS method following oral doses of 20 and 40 mg/kg, and major metabolites in rat plasma were glucuronides and sulfates (21). Emodin was also found to be glucuronidated and sulfated during its absorption in Caco-2 cell model (22). In another study, emodin was found to be metabolized into six metabolites as a result of a phase I reaction (23), which was also found following i.v. but not oral administration of emodin to rats (21). Taken together, the available information appears to indicate that emodin undergoes both phase I and phase II metabolism, with glucuronidation the likely major pathway for its elimination.
Therefore, the purpose of this study was to identify the reasons for emodin’s poor bioavailability and then characterize the glucuronidation of emodin in a systematic manner by determining its metabolism in different species and investigating the effects of gender on its metabolism. Because UDP-glucuronosyltransferase (UGT) activities also tend to vary greatly depending on the first-pass organs (intestine versus liver) (24), additional studies will be conducted to compare its intestinal and liver metabolism in vitro.
MATERIALS AND METHODS
Materials
Emodin (≥98%, HPLC grade) was purchased from Chengdu Mansite Pharmaceutical Company. Female and male rat jejunal and ileal microsomes were prepared at the University of Houston (Houston, TX, USA). Ten additional types of pooled liver microsomes from five species (mouse, rat, guinea pig, dog, and human liver microsomes) of both sexes, solution A for phase I reaction (containing NADP, glucose-6-phosphate, and magnesium chloride) and solution B for phase I reaction (containing glucose-6-phosphate dehydrogenase in sodium citrate), were purchased from BD Bioscience (Woburn, MA). β-Glucuronidase, uridine diphosphate glucuronic acid (UDPGA), alamethicin, d-saccharic-1,4-lactone monohydrate, magnesium chloride, and Hank’s balanced salt solution (powder form) were purchased from Sigma-Aldrich (St Louis, MO, USA). Hydroxypropyl-β-cyclodextrin (HPβCD) was purchased from Xi’an Deli Biology & Chemical Industry Co., Ltd. (Xian, China). All other materials were typically analytical grade or better and were used as received.
Emodin Stock Solution
To improve the solubility and stability of poorly soluble emodin, emodin stock (4 mM) was prepared in 80% (w/v) HPβCD solution. The stock solution was diluted in HBSS solution before use, and emodin remained stable in the solution after dilution. The formation of emodin–HPβCD complex enhanced its equilibrium solubility, allowing us to get sufficient concentration for perfusion study. Emodin in methanol stock solution was used for studies using microsomes.
Animals
The use of animals in the present study was permitted by the Ethics Committee of Southern Medical University (1838 N. Guangzhou Ave, Guangzhou 510515, Guangdong, China). Male and female Sprague–Dawley rats weighing between 230 and 250 g were obtained from the laboratory animal center of Southern Medical University. The rats were fasted overnight with free access to water before the date of the experiment.
Animal Surgery
The rats were anesthetized with an i.p. injection of 1.33 g/kg urethane (50%, w/v). During the surgery, the body temperature was maintained at 37°C by a heating lamp or an electric blanket. The intestinal surgical procedures were essentially the same as those described previously (25,26). We perfused four segments of intestine, and each segment was 8–10 cm long. The blood circulation to the liver and intestine was not disrupted in this model. The inlet cannulate was insulated and flushed with warm emodin–HPβCD complex in HBSS, which was kept warm at 37°C by a circulating water bath.
Perfusion Experiments
Four segments of rat intestine, duodenum, upper jejunum, terminal ileum, and colon were perfused simultaneously with a perfusate containing emodin at a concentration of 40 μM using an infusion pump (model PHD2000; Harvard Apparatus, Cambridge, MA) at a flow rate of 0.1 mL/min. After a 30-min washout period, four samples were collected from each outlet cannulae every 30 min. At the end of the experiment, the length of the perfused intestinal segment was as described (25,26).
Glucuronidation of Emodin
The experimental procedures were essentially the same as those published previously (27,28). Briefly, they were as follows: (1) Microsomes (final concentration ≈ 0.005–0.026 mg, see details later), magnesium chloride (0.88 mM), saccharolactone (4.4 mM), alamethicin (0.022 mg/mL), different concentrations of substrate in a 50 mM potassium phosphate buffer (pH 7.4), and UDPGA (3.5 mM, added last) were mixed. (2) The mixture (final volume = 200 μL) was incubated at 37°C for a predetermined period of time (typically 10 min). (3) The reaction was stopped by the addition of 100 μL of 94% acetonitrile/6% glacial acetic acid containing 50 μM testosterone as the internal standard. Afterwards, the samples were centrifuged at 13,000 rpm for 15 min and the supernatant used for injection. To control the extent of metabolism to <30% parent compound, different combinations of microsomal protein amounts and incubation time were tested in preliminary studies, and 10 min was found to be the best incubation time when we used a microsomal protein concentration of 0.026 mg/mL at emodin concentrations of 30–40 µM, 0.013 mg/mL at emodin concentrations of 10–20 µM, and 0.005 mg/mL at emodin concentrations at or below 7.5 µM, respectively.
Phase I Metabolism of Emodin
The procedure for conducting phase I reaction was essentially the same as the published procedures (29). Briefly, the procedures were as follows: (1) Microsomes (20 μL, final concentration = 0.4 mg/mL) was mixed with solution A (50 μL) and solution B (10 μL) in a 50 mM potassium phosphate buffer (pH 7.4). (2) The mixture was pre-incubated at 37°C for 5 min, and emodin stock solution (20 μL, final concentration = 20 μM) was then added. (3) The final mixture (1,000 μL) was incubated for a predetermined period of time at 37°C, and the reaction was stopped by the addition of 50 μL of 94% acetonitrile/6% glacial acetic acid containing 50 μM testosterone as the internal standard. (4) CH2Cl2 (4 mL) was then added to the final solution, vortexed for 30 s, and centrifuged at 3,500 rpm for 15 min. (5) After the aqueous and protein layers were aspirated out, the CH2Cl2 layer was transferred to a clean tube and dried under nitrogen gas. (6) The residues were dissolved in 110 µL of water and methanol (1:1) and injected into UPLC for analysis. Reaction samples without NADPH-generating system served as the control. All reactions were performed at least three times in three duplicates.
Simultaneous Phase I and Glucuronidation of Emodin
Since emodin may undergo phase I oxidation and glucuronidation simultaneously, a mixed system of oxidation and glucuronidation reaction was used to determine the main pathway of metabolism of emodin in vitro. The procedures basically combined what was described earlier for separate oxidative and glucuronidated reactions, and all compounds added previously for those reactions were added for the mixed reaction as well, and therefore, both reaction systems were expected to produce the same results.
Determination of Molar Extinction Coefficients of Emodin Glucuronide
Because of the lack of emodin glucuronide standards, an emodin standard curve was used for quantitation of emodin glucuronide by using a conversion factor (K), as was done previously in our lab for isoflavones (27). The conversion factor, which is the ratio between the molar extinction coefficient of emodin glucuronide and emodin, was determined by the following procedures: (1) An aqueous sample containing emodin glucuronide and emodin was extracted three times with dichloromethane to remove emodin. (2) The extracted aqueous sample was subsequently divided into two equal parts; one part was incubated with water and then analyzed by UPLC and the other one by hydrolysis with β-glucuronidase (40 U/mL) at 37°C for 30 min and then analyzed by UPLC. (3) The difference in peak areas of metabolite and emodin obtained from the samples before and after the hydrolysis, which were represented as ΔPeak areaM and ΔPeak areaE, was calculated to be the ratio (K) 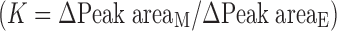 (27). Therefore, the concentration of metabolite can be estimated using emodin standard curve. The average ±SD conversion factor (K) was 1.0054 ± 0.023 at a wavelength of 254 nm, determined separately at three different concentrations (triplicates per concentration).
(27). Therefore, the concentration of metabolite can be estimated using emodin standard curve. The average ±SD conversion factor (K) was 1.0054 ± 0.023 at a wavelength of 254 nm, determined separately at three different concentrations (triplicates per concentration).
UPLC and LC-MS/MS Analysis of Emodin and its Glucuronides
The conditions used to analyze emodin and its metabolites were as follows: system, Waters (Waters Corp., Milford, MA, USA) Acquity™ UPLC with photodiode array detector and Empower software; column, BEH C18, 1.7 μm, 2.1 × 50 mm; mobile phase B, 100% acetonitrile, mobile phase A, 100% aqueous buffer (2.5 mM NH4AC, pH 7.4); flow rate, 0.4 mL/min; gradient, 0 to 0.1 min, 85%A, 0.1 to 1.8 min, 85–60%A, 1.8 to 2.2 min, 60–40%A, 2.2 to 2.8 min, 40–85%A, 2.8 to 3.2 min, 85%A, wavelength, 254 nm for emodin and its glucuronide and testosterone; and injection volume, 10 μL. The test linear response range was 0.625–100 μM for emodin. The mass spectrometer parameters were set as follows: capillary voltage, 4.5KV; ion source temperature, 350°C, desolvation temperature, 108°C; nebulizer gas (gas1), nitrogen, 40 psi; turbo gas (gas 2), argon gas, 20 psi.
Identification of Emodin and its Glucuronide Metabolite by LC-MS/MS and NMR
A mixture of reaction products in aqueous solution was extracted with dichloromethane three times. The aqueous fraction was loaded onto an ODS column (2 × 10 cm, 10 μm) and washed using pure water. The mono-glucuronide emodin (about 2 mg) was eluted using a solvent of H2O/MeOH (1:9). The structure of mono-glucuronide emodin was identified by UPLC-ESI-Q-TOF-MS (negative) and 1H-NMR. The mass spectrometer parameters were set as follows: capillary voltage, 4.5KV; ion source temperature, 350°C, desolvation temperature, 108°C; nebulizer gas (gas1), nitrogen, 40 psi; turbo gas (gas 2), argon gas, 20 psi.
Kinetic Analysis
Permeability of emodin (aglycone) was represented by P*eff, which was obtained as described previously (28). Amounts of emodin absorbed (Mab), amounts of glucuronidated emodin excreted into the intestinal lumen (Mgut), and the percentage absorbed and percentage metabolized values were calculated as described previously (28). Briefly, Mab and Mgut were expressed as Eqs. 1 and 2:
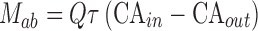 |
1 |
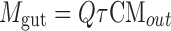 |
2 |
where Q is the flow rate of perfusion (mL/h), τ is the interval time of sampling (0.5 h), CAin and CAout are the inlet and outlet concentrations of emodin, and CMout is the concentration of emodin-3-O-glucuronide.
%Absorbed and %Metabolized were calculated as:
 |
3 |
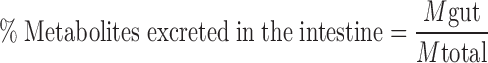 |
4 |
where Mtotal is the total amount of compound perfused over the first 30-min period.
Rates of metabolism in human liver and intestinal microsomes were expressed as amounts of metabolite (emodin-O-glucuronide) formed (nmol) per hour per milligram protein (or nmol min−1 mg−1). Kinetic parameters were then obtained based on the fit to various kinetic equations shown below based on profiles of Eadie–Hofstee plots as described previously (28). If Eadie–Hofstee plot was linear, formation rate (V) of emodin glucuronide at different substrate concentrations (C) were fit to the standard Michaelis–Menten equation:
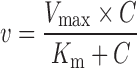 |
5 |
where Km is the Michaelis–Menten constant and Vmax is the maximum rate of glucuronidation.
If Eadie–Hofstee plots showed characteristic profiles of atypical kinetic (autoactivation and biphasic kinetics) (30,31), the data from these atypical profiles were fit to Eqs. 6 and 7, using the ADAPT II program. To confirm the best-fit model, the model candidates were discriminated using the minimum Akaike’s information criterion (AIC) value, and the rule of parsimony was employed.
The following Eq. 6 describes enzyme reactions with autoactivation:
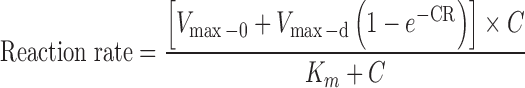 |
6 |
where Vmax-0 is the intrinsic enzyme activity and Vmax-d is maximal induction of enzyme activity. R is the rate of enzyme activity induction, C is concentration of substrate, and Km is concentration of substrate needed to achieve 50% of (Vmax-0 + Vmax-d).
The following Eq. 7 describes enzyme reactions with biphasic kinetics:
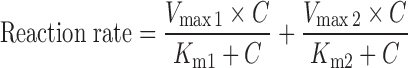 |
7 |
where Vmax1 is the maximum enzyme velocity of the high-affinity phase, Vmax2 is the maximum velocity of the low-affinity phase, Km1 is concentration of substrate to achieve half of Vmax1 for high-affinity phase, and Km2 is concentration of substrate to achieve half of Vmax2 for low-affinity phase.
Statistical Analysis
One-way ANOVAs with or without Tukey–Kramer multiple comparison (post hoc) tests were used to evaluate statistical difference. Additional test used was the Student’s t test. Differences were considered significant when p values were <0.05.
RESULTS
Determination of Emodin and Emodin Glucuronide by UPLC, UPLC-ESI-Q-TOF-MS, and 1H-NMR
The UPLC method developed for emodin had a run time of 4 min (Fig. 1) and a linear calibration curve over the concentration range of 0.6125–40 μM (the equation of the regression line through the origin was y = 0.0605x, r2 = 0.9997). The intra- and inter-day variabilities at 1.25, 10, and 40 μM of emodin were less than 4.2% and 3.8%, respectively.
In microsomal incubation samples, one new peak eluted at 1.92 min (Fig. 1a). A UPLC ESI-Q-TOF-MS running at a negative ion mode was used to determine the MS spectrum of the metabolite. The mass spectra of this metabolite exhibited a molecular ion (emodin glucuronide-H−) at m/z 445.0780, calculated as C21H17O11: 445.0776, which corresponded to the molecular weight of emodin glucuronide, and the major fragment ion (emodin) at m/z 269.0462, which corresponded to the molecular weight of emodin (Fig. 1b). LC-MS/MS study also indicated that all metabolites generated from various microsomes of different species showed identical mono-glucuronide of emodin (not shown). The UV spectra of emodin glucuronide and emodin (Fig. 1c) were similar, which were supportive of the notion that the new eluted peak is closely related to emodin. 1H-NMR spectra of the metabolite displayed very similar signals with those of emodin except for the signals derived from an additional sugar moiety which was determined to be β-glucuronide group from its H-1′ signal at δ 5.14 (1H, d, J = 8 Hz) and H-5′ signal at δ 4.21 (1H, d, J = 8 Hz). The location of glucuronide group was confirmed to be at 3-OH by the observation of NOE correlations between the anomeric proton (δ 5.14) with both H-4 (δ 7.45) and H-2 (δ 6.96) in the NOESY spectrum shown in Fig. 1d. Based on the above evidences, the metabolite was identified as emodin 3-O-β-D-glucuronide (Fig. 1e). Since the same glucuronide was found in all glucuronidation reactions using liver microsomes of any species or gender, emodin 3-O-β-D-glucuronide was the only glucuronide formed in the present study.
Glucuronidation of Emodin by Rat Liver Microsomes
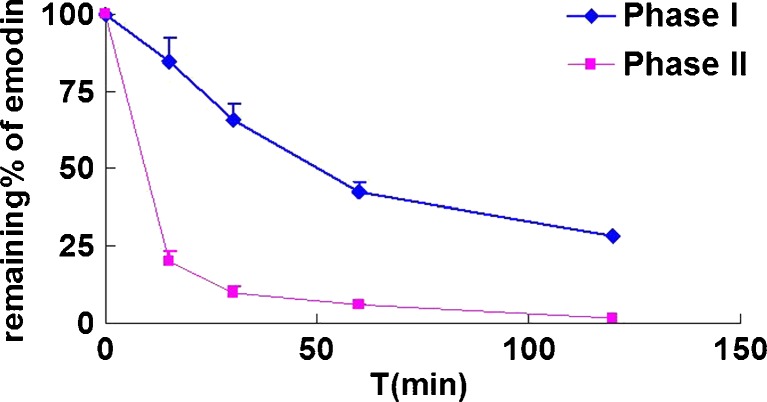
Emodin (40 μM) was rapidly glucuronidated by rat liver microsomes (final concentration 0.4 mg/mL). After 15 min, only 20% of emodin was left (Fig. 2). After incubation times of 30 min, 1 h, and 2 h, percent remaining were 9.73%, 5.73%, and 1.87%, respectively.
Fig. 2.
Comparison of phase I oxidation and glucuronidation metabolisms of 40 μM emodin. Using different protein concentration (1 mg/mL for phase I oxidation reaction and 0.053 mg/mL for glucuronidation reaction), amounts of emodin remained in the reaction mixture (expressed as percent remaining) was monitored and compared after phase I (a CYP reaction system) or phase II (a UGT reaction system) metabolism of emodin was allowed to proceed as a function of time at 37°C. The experimental conditions were described in details in “MATERIALS AND METHODS”. Each point represents the average of three determinations and error bar are the standard deviation of the means (n = 3)
Phase I Metabolism of Emodin by Rat Liver Microsomes
For phase I oxidation reaction conducted using identical concentration of rat liver microsomes, the percent emodin remaining was 84.81% after 15 min of reaction time. After reaction times of 0.5, 1, and 2 h, the percent remaining were 65.53%, 42.53%, and 28.35%, respectively (Fig. 2). Therefore, it was clear that oxidative metabolism was at least five times slower than glucuronidation. In oxidative metabolism, one main metabolite was found, which was eluted at the retention time of 2.07 min and a molecular ion at 285.16 Da, 16 more than that of emodin (m/z 269→285), indicating that the compound is a hydroxylated metabolite of emodin (Fig. 3a). The MS/MS spectrum of product ion at m/z 255 ([M-H-HCHO]−) and m/z 268([M-H-OH]) suggested that the metabolite should be hydroxyemodin, as reported previously (23). The MS2 profile of the hydroxyemodin is seen in Fig. 2a, but we were unable to assign the position of the hydroxylation.
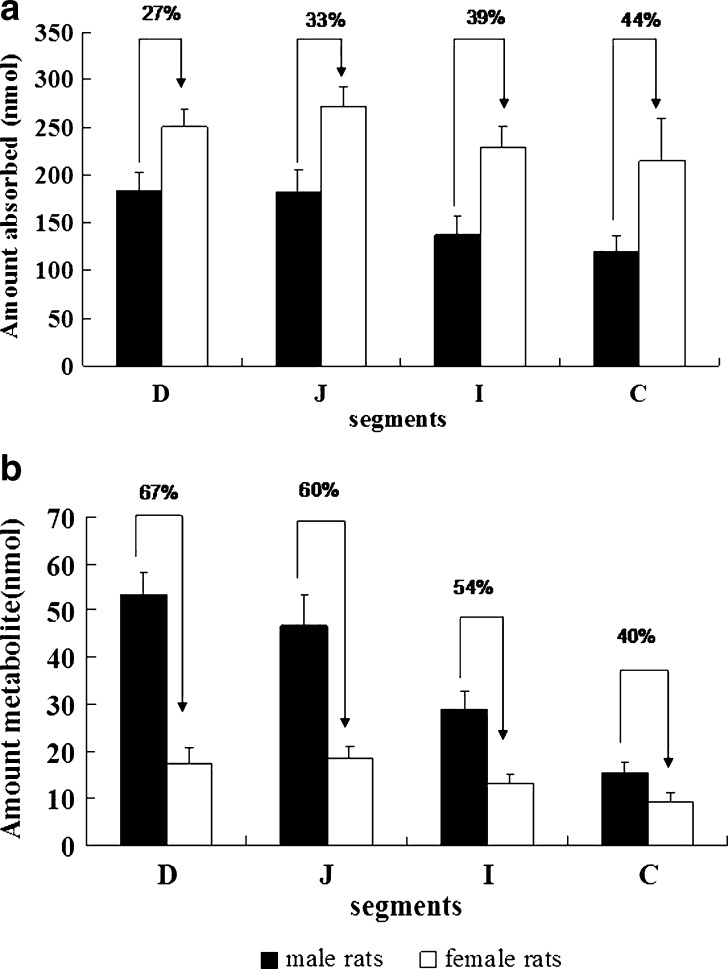
Fig. 3.
Effect of gender differences on the absorption and metabolism of emodin in rats. a Amount of emodin glucuronide excreted and b amounts of emodin absorbed in four segments of male (solid bars) and female (open bars) rat intestine using a single-pass perfusion model (n = 4). Perfusate was HBSS containing 40 μM of emodin. Four segments of intestine were perfused at a flow rate of 0.1 mL/min. The rates, normalized to 10-cm intestinal length, were calculated using Eqs. 1–3. Each bar represents the average of four determinations, and error bars are the standard deviation of the means (n = 4). The symbol (arrow with percent difference) indicates a statistically significant difference in glucuronidation rates between genders
Metabolism of Emodin in a Mixed Oxidation and Glucuronidation Reaction System
The mixed system of oxidation and glucuronidation reaction was used to determine the main pathway of metabolism of emodin by using male rat liver microsomes at 1.67 mg/mL with both oxidation and glucuronidation reaction cofactors. Detectable amount of emodin glucuronide was observed within 6 min of incubation, and emodin was metabolized nearly completely within 1 h. The metabolite was confirmed to be emodin-3-O-β-D-glucuronide by LC-MS/MS, which was the only metabolite found in the mixed reaction system. There were no detectable amounts of hydroxyemodin found in the mixed reaction system, confirming earlier observation that glucuronidation reaction was much more rapid than oxidation reaction.
Intestinal Absorption and Metabolism of Emodin
Absorption of emodin displayed regional difference in male but not in female rats (Fig. 3a). On the other hand, excretion of emodin glucuronide displayed region dependence (p < 0.05) in both male and female rats (Fig. 3b). The amounts of emodin glucuronide excreted in duodenum were significant higher (p < 0.05) than that in jejunum, followed by ileum and colon in male rats (p < 0.05, Fig. 3b). In female rats, the rank order of amounts of metabolite excreted was jejunum≈duodenum>ileum>colon (p < 0.05, Fig. 3b). The amounts of emodin absorbed in each of the four regions of female rat intestine were higher than that in the male rats (p < 0.01), and range of the increase was 27–44% (Fig. 3a). In contrast, amounts of emodin glucuronide excreted were higher in each of the four segments of intestine in the male rats than the female rats (p < 0.01), and the range of the increase was 40–67%, indicating somewhat larger difference in metabolism than in excretion.
Concentration-Dependent Glucuronidation of Emodin by Rat Intestinal Microsomes
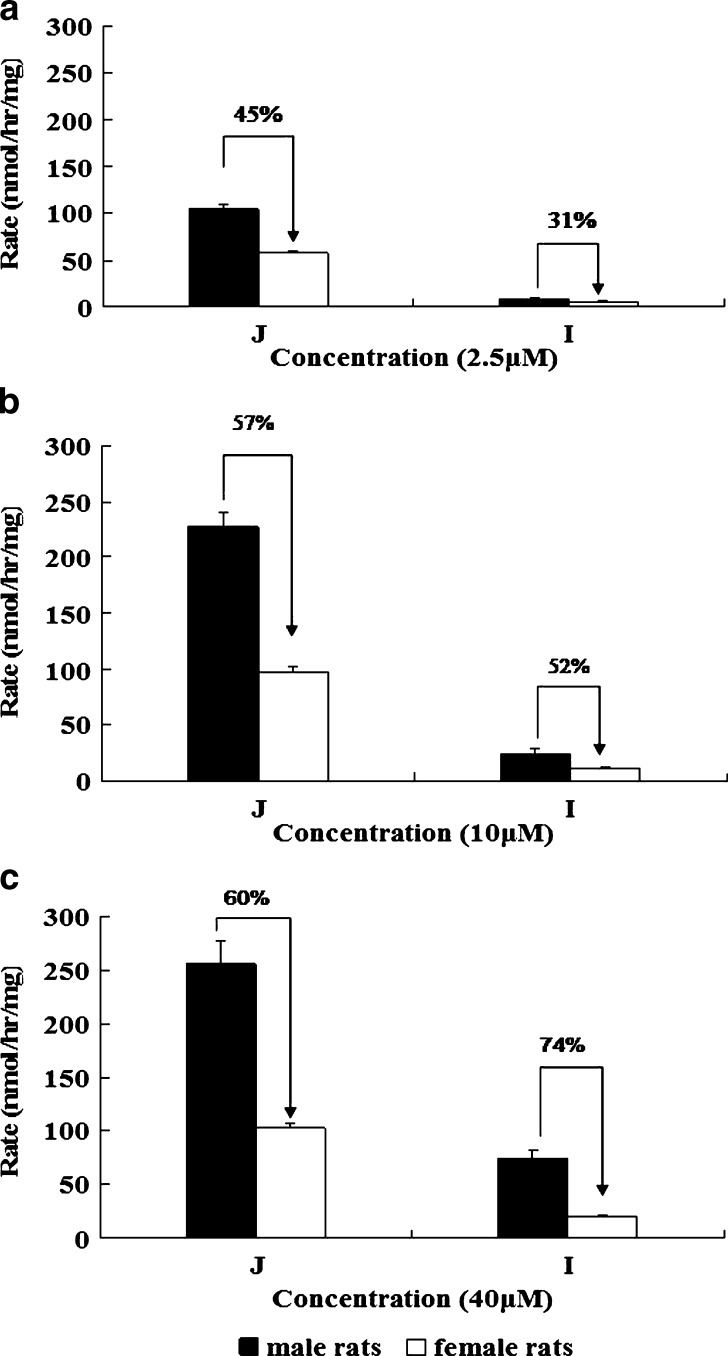
To determine if the above observed pattern of metabolite excretion is the result of difference in UGT activities, we measured glucuronidation rates of emodin in jejunal and ileal microsomes of male and female rats at 2.5, 10, and 40 μM. The result showed that emodin was glucuronidated faster in rat jejunal microsomes than in ileal microsomes regardless of gender (p < 0.05), and the extent of the difference was larger at a lower concentration (∼11-fold at 10 μM) than at a higher concentration (∼5-fold at 40 μM). Furthermore, emodin was metabolized faster in male than in female rats at all tested concentrations (p < 0.01, Fig. 4), and the range of difference was smaller at a lower concentration (31–45% at 2.5 μM) than at a higher concentration (60–74% at 40 μM). These results are consistent with intestinal perfusion data where glucuronide excretion was faster in male than female.
Fig. 4.
Effects of gender on the jejunal and ileal glucuronidation of emodin. Glucuronidation of emodin by jejunal and ileal microsomes of male (solid bars) and female (open bars) rats were measured at three concentrations of a 2.5 μM, b 10 μM, and c 40 μM. Rates of glucuronidation were expressed as nanomoles per hour per milligram. Different concentrations of microsomal protein (0.1 mg/mL for 2.5 μM of emodin; 0.25 mg/mL for 10 μM of emodin; and 0.5 mg/mL for 40 μM of substrate) were used to limit the extent of metabolism to <30%. Each bar is the average of three determinations, and the error bars are the standard deviations of the mean (n = 3). The symbol (arrow with percent difference) indicates a statistically significant difference in glucuronidation rates between genders
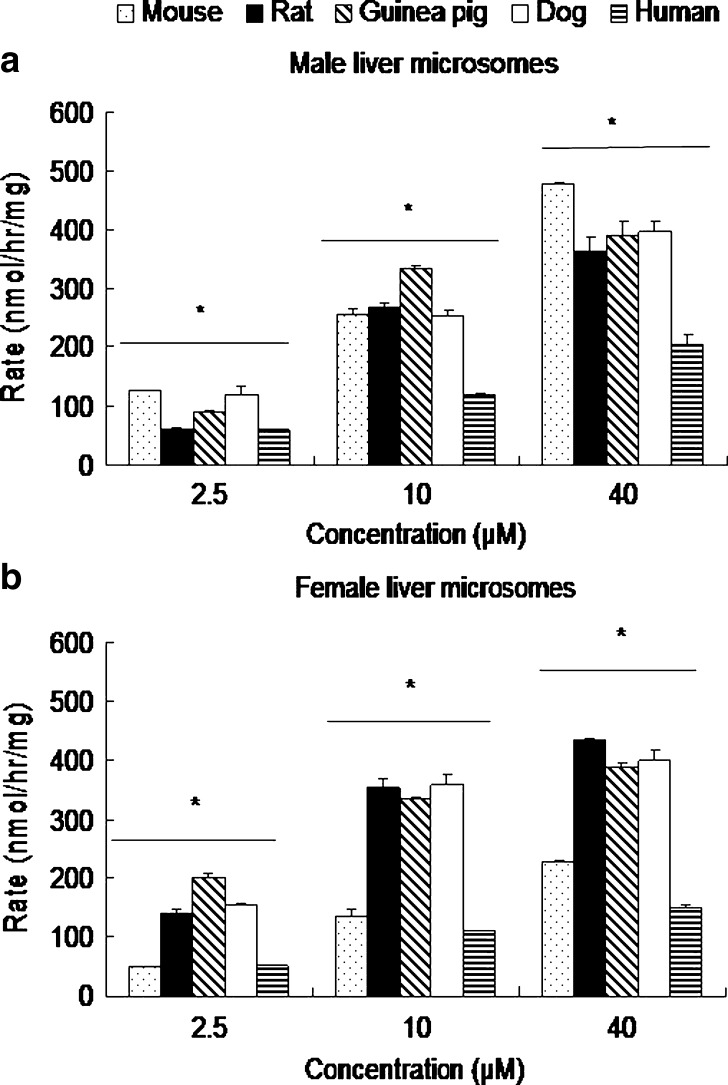
Species-Dependent Glucuronidation of Emodin by Liver Microsomes
Glucuronidation of emodin in different species has not been determined, but is expected to be different since different species expressed different UGTs. Therefore, glucuronidation rates of emodin at three different concentrations (2.5, 10, and 40 μM) were measured using mouse, rat, guinea pig, dog, and human liver microsomes (Fig. 5a, b). We first compared the glucuronidation in male liver microsomes (Fig. 5a) and then did the same for female liver microsomes (Fig. 5b). In the male group, glucuronidation rates of emodin in liver microsomes displayed significant species effects (p < 0.05, one-way ANOVA). At 2.5 μM, the rank order of emodin glucuronidation in males was: mouse (127.51 ± 0.50 nmol h−1 mg−1) ≈ dog (119.32 ± 12.87 nmol h−1 mg−1) > guinea pig (89.50 ± 12.55 nmol h−1 mg−1) > rat (59.33 ± 1.48 nmol h−1 mg−1) ≈ man (59.17 ± 1.47 nmol h−1 mg−1). But at 10 μM substrate concentration, the trend changed slightly, and the rank order was: guinea pig (333.95 ± 2.67 nmol h−1 mg−1) > rat (268.82 ± 5.4 nmol h−1 mg−1) ≈ mouse (256.45 ± 9.20 nmol h−1 mg−1) ≈ dog (252.26 ± 10.11 nmol h−1 mg−1) > men (119.90 ± 7.00 nmol h−1 mg−1). At 40 μM substrate concentration, the trend was generally the same as those at 2.5 μM, although the magnitude of the differences was slightly different.
Fig. 5.
Species-dependent glucuronidation of emodin in liver microsomes prepared from five different a male and b female species at low (2.5 μM), medium (10 μM), and high (40 μM) concentrations. Species were mouse (dot bars), rat (black bars), guinea pig (slashed bars), dog (open bars), and human (ladder bars). Rates of glucuronidation are expressed as nanomoles per hour per milligram. Each bar is the average of three determinations, and the error bars are the standard deviations of the mean (n = 3). Significant differences (p < 0.05) as determined by one-way ANOVA are marked by an asterisk
Among the female species, differences in glucuronidation rates via liver microsomes were also significant (p < 0.05, one-way ANOVA; Fig. 5b). At 2.5 μM substrate concentration, the rank order of emodin glucuronidation rates in female species was: guinea pig (199.67 ± 9.46 nmol h−1 mg−1) > dog (153.78 ± 3.76 nmol h−1 mg−1) ≈ rat (142.23 ± 3.15 nmol h−1 mg−1) > women (50.90 ± 1.99 nmol h−1 mg−1) ≈ mouse (48.90 ± 1.25 nmol h−1 mg−1). But at 10 μM substrate concentration, the trend was obviously different, and the rank order was dog (357.24 ± 15.57 nmol h−1 mg−1) ≈ rat (352.4 ± 16.02 nmol h−1 mg−1) ≈ guinea pig liver microsomes (332.77 ± 4.18 nmol h−1 mg−1), all three of which were much faster than mouse (136.8 ± 9.14 nmol h−1 mg−1) and women (111.62 ± 0.58 nmol h−1 mg−1). At 40 μM substrate concentration, the trend was essentially the same as those observed at 10 μM concentration (Fig. 5b).
Effects of Gender on Glucuronidation of Emodin by Liver Microsomes of Different Species
We contrasted the effects of gender on the rates of glucuronidation in liver microsomes (see Fig. 5a, b) and found that at 2.5 μM, rates in male were greater than that in female mouse liver microsomes. Rates in human male and female microsomes were the same, whereas the metabolism rates were faster in females than in males for the other three species. The same trend was maintained at 10 μM concentration for all species except guinea pig, which had the same rates in male and female guinea pigs. At 40 μM concentration, the trend again changed from that at 10 μM in that the rates were the same for both guinea pig and dog, but became higher for men (p < 0.05). In general, the extent of difference in glucuronidation rates was larger at lower concentration, but gender effects on human microsomal activities were small.
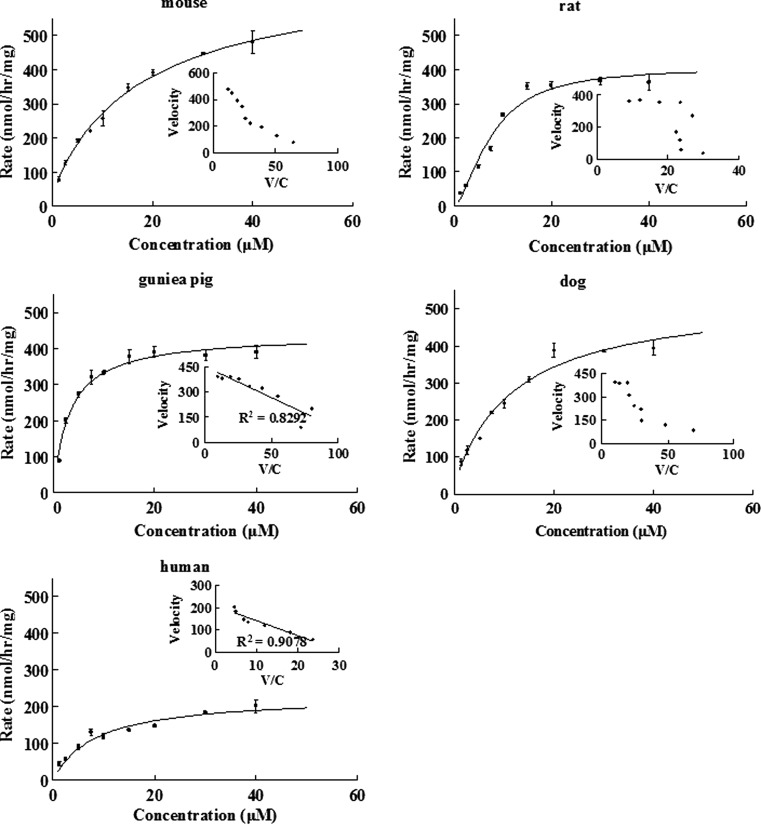
Kinetic of Emodin Glucuronidation Using Male Liver Microsomes from Five Species
Kinetics of emodin glucuronidation were determined in liver microsomes of male species (Fig. 6), and the results indicated that metabolism of emodin was saturable at higher concentrations. Among the five male species, glucuronidation in guinea pig and human liver microsomes followed the classical Michaelis–Menten equation, whereas the others did not. The apparent kinetic parameters are listed in Table I. Using intrinsic clearance as the most important criterion to compare metabolism, we found that a larger intrinsic clearance value was associated with a small Km value and a large Vmax value (Table I), though both values varied less than 3-fold.
Fig. 6.
Kinetics of emodin glucuronidation via liver microsomes in five different male species: mouse, rat, guinea pig, dog, and human. Rates of glucuronidation were expressed as nanomoles per hour per milligram. Different concentrations of microsomal protein (0.1 mg/mL for 1.25–7.5 μM of emodin; 0.25 mg/mL for 10–20 μM of emodin; and 0.5 mg/mL for 30–40 μM of substrate, respectively) were used to limit the extent of metabolism to <30%. Each point is the average of three determinations, and the error bars are the standard deviations of the mean (n = 3). In each figure, the insert is the Eadie–Hofstee plot for the rates versus rates over concentration, indicative of the mechanism of reaction kinetics
Table I.
Apparent Kinetic Parameters of Metabolism of Emodin by Liver Microsomes Prepared from Male Mice (MMLM), Rats (MRLM), Guinea Pigs (MGLM), Dog (MDLM), and Humans (MHLM)
| Kinetic parameters | MMLM | MRLM | MGLM | MDLM | MHLM |
|---|---|---|---|---|---|
| K m1 (μM) | 18.2 | 3.40 | 3.18 | 12.3 | 8.40 |
| V max1 (nmol h−1 mg−1) | 634.7 | 400.0 | 439.2 | 507.3 | 232.0 |
| V max1/K m1 (mL h−1 mg−1) | 34.8 | 117.6 | 138.3 | 51.3 | 27.6 |
| V max2 (biphasic, nmol h−1 mg−1) | 49.4 | – | – | 29.9 | – |
| K m2 (μM) | 0.22 | – | – | 4.0 × 10−6 | – |
| V max2/K m2(mL h−1 mg−1) | 224.8 | – | – | 7.4 × 106 | – |
| R (autoactivation only) | – | 0.160 | – | – | – |
| V max-0 (auto, nmol h−1 mg−1) | – | 6.2 × 10−5 | – | – | – |
| V max-d (auto, nmol h−1 mg−1) | – | 419.4 | – | – | – |
| R 2 | 0.995 | 0.969 | 0.976 | 0.971 | 0.970 |
| AIC | 68.7 | 84.0 | 73.7 | 81.3 | 57.5 |
Kinetic parameters were obtained from using simple Michaelis–Menten, autoactivation (auto), and biphasic enzyme kinetics models as described under “MATERIALS AND METHODS”
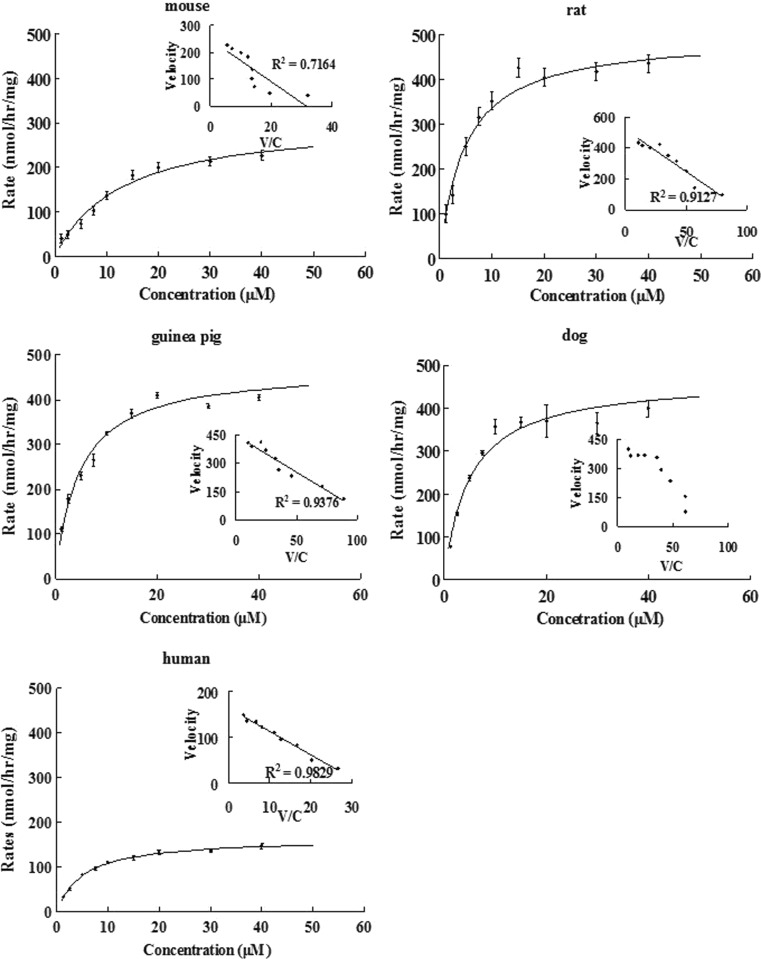
Kinetic of Emodin Glucuronidation Using Female Liver Microsomes from Five Species
Kinetics of emodin glucuronidation were determined in liver microsomes of female species (Fig. 7), and the results indicated that metabolism of emodin was also saturable at higher concentrations. Among the five species, glucuronidation of emodin in the liver microsomes of mouse, rat, guinea pig and human all followed simple Michaelis–Menten equation, whereas glucuronidation in the dog followed autoactivation equation. The apparent kinetic parameters are listed in Table II. In general, compounds with higher intrinsic clearance values had lower Km values (difference of ∼3-fold) or large Vmax values (difference of ∼3-fold) or a combination of smaller Km and large Vmax values. The observed kinetic phenomenon is not due to procedural limitation but rather involvement of multiple enzyme isoforms responsible for metabolism of emodin in microsome studies. Therefore, these metabolism parameters can be considered as apparent kinetic parameters and not necessarily the UGT enzyme isoform-specific parameters.
Fig. 7.
Kinetics of emodin glucuronidation via liver microsomes in five different female species: mouse, rat, guinea pig, dog, and human. Rates of glucuronidation, expressed as nanomoles per hour per milligram, were determined at different concentrations of protein as described as Fig. 6. Each point is the average of three determinations, and the error bars are the standard deviations of the mean (n = 3). In each figure, the insert is the Eadie–Hofstee plot that is indicative of the mechanism of reaction kinetics
Table II.
Apparent Kinetic Parameters of Metabolism of Emodin by Liver Microsomes Prepared from Female Mice (FMLM), Rats (FRLM), Guinea Pigs (FGLM), Dog (FDLM), and Humans (FHLM)
| Kinetic parameters | FMLM | FRLM | FGLM | FDLM | FHLM |
|---|---|---|---|---|---|
| K m1 (μM) | 13.0 | 4.81 | 4.62 | 4.91 | 5.15 |
| V max1 (nmol h−1 mg−1) | 310.8 | 497.7 | 471.0 | 468.7 | 164.1 |
| V max1/Km1 (mL h−1 mg−1) | 24.3 | 103.5 | 102.0 | 95.5 | 31.9 |
| V max2 (biphasic, nmol h−1 mg−1) | – | – | – | – | – |
| K m2 (μM) | – | – | – | – | – |
| V max2/K m2(mL h−1 mg−1) | – | – | – | – | |
| R (autoactivation only) | – | – | – | 1.8 × 10−4 | – |
| V max-0 (nmol h−1 mg−1) | – | – | – | 468.7 | – |
| V max-d (nmol h−1 mg−1) | – | – | – | 1.15 × 103 | – |
| R 2 | 0.978 | 0.988 | 0.973 | 0.967 | 0.995 |
| AIC | 65.4 | 62.1 | 66.0 | 72.8 | 41.6 |
Kinetic parameters were obtained from using simple Michaelis–Menten, autoactivation (auto), and biphasic enzyme kinetics models as described under “MATERIALS AND METHODS”
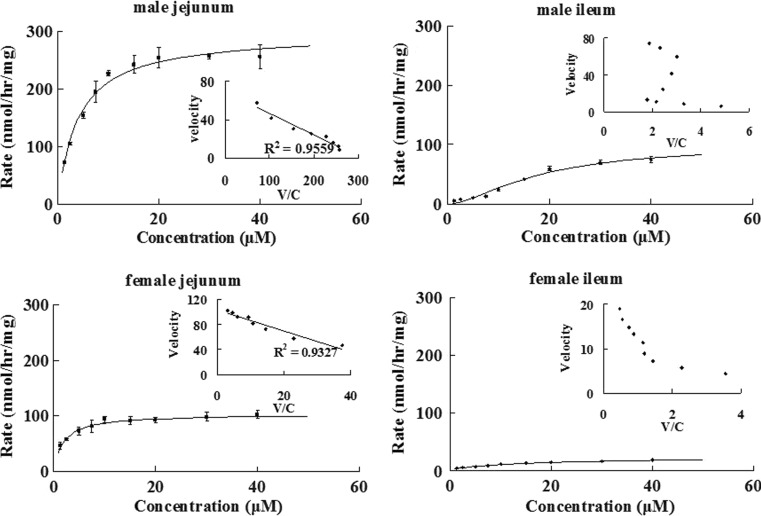
Kinetics of Emodin Glucuronidation by Rat Intestinal Microsomes
To compare the relative importance of liver versus intestine in the metabolism of emodin, its glucuronidation was also investigated using male rat intestinal microsomes (Fig. 8). Emodin glucuronidation in jejunal microsomes showed the classical Michaelis–Menten pattern, whereas its glucuronidation in ileal microsomes followed the autoactivation pattern. In female rat intestine, emodin glucuronidation in jejunal microsomes also showed a classical Michaelis–Menten pattern, whereas glucuronidation in ileal microsomes followed a biphasic pattern (inset in Fig. 8). The apparent kinetic parameters describing various intestinal glucuronidation were listed in Table III. We also compared intestinal versus liver glucuronidation of emodin and found that liver microsomes had much higher Vmax values than intestinal microsomes regardless of the gender (p < 0.05). On the other hand, male rat intestinal microsomes had higher Vmax values than corresponding female intestinal microsomes, although the Vmax values of liver microsomes were similar.
Fig. 8.
Kinetics of emodin glucuronidation via jejunal and ileal microsomes of male and female rats. Rates of glucuronidation, expressed as nanomoles per hour per milligram, were determined at different concentrations of protein as described as Fig. 6. Each point is the average of three determinations, and the error bars are the standard deviations of the mean (n = 3). In each figure, the insert is the Eadie–Hofstee plot that is indicative the mechanism of reaction kinetics
Table III.
Apparent Kinetic Parameters of Metabolism of Emodin by Intestinal Microsomes Prepared from Male Jejunum (MRJM) and Ileum (MRIM) and Female Jejunum (FRJM) and Ileum (FRIM)
| Kinetic parameters | MRJM | MRIM | MRLM | FRJM | FRIM | FRLM |
|---|---|---|---|---|---|---|
| K m1 (μM) | 4.00 | 14.43 | 3.40 | 1.89 | 2.90 | 4.81 |
| V max1 (nmol h−1 mg−1) | 296.4 | 100.0 | 400.0 | 103.0 | 23.6 | 497.7 |
| V max1/K m1(mL h−1 mg−1) | 74.5 | 6.90 | 117.6 | 54.6 | 8.10 | 103.5 |
| V max2 (biphasic, nmol h−1 mg−1) | – | – | – | – | 2.46 × 10−7 | – |
| K m2 (μM) | – | – | – | – | 19.9 | – |
| V max1/K m1 (m h−1 mg−1) | – | – | – | – | 1.24 × 10−8 | – |
| R (autoactivation only) | – | 0.095 | 0.161 | – | – | – |
| V max-0(nmol h−1 mg−1) | – | 0.836 | 0 | – | – | – |
| V max-d (nmol h−1 mg−1) | – | 107.2 | 419.4 | – | – | – |
| R 2 | 0.981 | 0.979 | 0.969 | 0.98 | 0.994 | 0.988 |
| AIC | 63.5 | 52.5 | 84.0 | 36.6 | 10.4 | 62.1 |
Metabolism of emodin by liver microsomes prepared from male rats (MRLM) and female rats (FRLM) were also shown for the comparison purpose. The kinetic parameters were obtained based on curve fitting using simple Michaelis–Menten, autoactivation (auto), or biphasic enzyme kinetics models as described under “MATERIALS AND METHODS”
DISCUSSION
Understanding the disposition of emodin would represent the first step toward solving a major challenge associated with the development of emodin: poor bioavailability. Because the bioavailability of emodin was nearly zero in one study (21), we had hypothesized that first-pass metabolism was the main reason why intact emodin was not quantifiable in rat plasma in vivo, although substantial amount of emodin glucuronide was found in the plasma (21). Since liver is considered to be a major site of metabolism as more than 50% of orally administered emodin was found in the bile (32), the focus of our study was on liver metabolism along with some disposition studies in the rat intestine. The latter is important since it was found that orally administered emodin did not result in the formation of ω-hydroxyemodin (a phase I metabolite), whereas the i.v. administered emodin did (21).
The results of this study clearly showed that the rate of emodin’s glucuronidation was rapid via the liver and intestinal microsomes of male rats as its intrinsic clearance values (Table III) were much higher (at least 10-fold) than isoflavones (28,33), a class of compounds with bioavailabilities <8% (34). This difference in intrinsic clearance values was the result of large difference in Vmax values (Tables III) (28,33). Therefore, it appeared to us that UGTs were able to turnover emodin much faster than isoflavones. Since metabolism rates and intrinsic clearance values showed small gender effects (Fig. 5 and Table III), poor bioavailabilities were expected in both male and female rats. Moreover, since intestinal metabolism of emodin was very rapid with intrinsic clearance close to that of the liver (Table III and Fig. 8), much of the absorbed emodin was expected to be metabolized first in intestine, with smaller amounts reaching the liver for phase I transformation. The latter is consistent with in vivo oral dosing study that showed no phase I metabolite in rat plasma at a detectable level (21). This is not entirely surprising since intestinal concentration of emodin is expected to be much higher than plasma concentration and, hence, the more rapid rate of glucuronidation in intestine.
Whereas the glucuronidation metabolism via glucuronidation appears to be one of the main reasons that emodin has very poor to zero oral bioavailability, another reason is its very poor solubility. Poor solubility was the reason that HPβCD was used to increase the solubility of emodin so that a perfusate solution can be prepared. Without the use of HPβCD, the solubility of emodin was <1 μM (not shown), insufficient for our perfusion studies. It is unknown if HPβCD would have increased the bioavailability of emodin in rats, but without it, its bioavailability was very poor (21).
In contrast to extensive metabolism, poor permeability was not the reason for emodin’s poor bioavailability. This was because more than 100 nmol of emodin was absorbed over a 30-min time period (Fig. 2a), corresponding to an effective wall permeability (P*w) of >2 (not shown). A P*w value of 1 and greater was correlated with percent absorption of better than 75% (35). Taken together, the results of our studies clearly showed that extensive metabolism via glucuronidation in rats were the main contributors to emodin’s poor bioavailability in vivo.
To further characterize emodin’s disposition behaviors, its metabolism via glucuronidation was determined in liver microsomes derived from four additional species (mice, guinea pigs, dogs, and humans). As expected, there were substantial and significant differences between species in the metabolism of emodin via glucuronidation (Figs. 5, 6, and 7 and Tables I and II), although the magnitude of the differences was surprisingly small. For example, the difference in intrinsic clearance and Km values was <5-fold in male and even less in female (4-fold).
Lastly, comparison was made between glucuronidation of emodin in male and female liver microsomes in an attempt to understand if the gender-dependent metabolism has the same general trend across species. The results clearly showed that gender-dependent metabolism was species-dependent. In liver microsomes, the rates were faster or similar in the females than in the males with the exception that the glucuronidation rates were substantially faster in male mice than in female mice. The observed species-dependent glucuronidation was not entirely surprising since each species expresses different UGT isoforms, and UGT isoforms from different species have different substrate specificities. For example, UGT1a7 is the major rat UGT isoform responsible for the metabolism of isoflavones (36), but UGT1A7 was not one of the major human UGT isoforms responsible for the metabolism of isoflavones (27). Nevertheless, it is rather surprising that male mouse intestine was able to metabolize emodin much more efficiently than female mice. This result could be due to the much higher expression level of UGT2b1 (2-fold) in male mouse liver, which was the only mouse UGT isoform with a higher mRNA level in the liver of male mice than in female mice (37). It could also explain why the gender effect was reversed in rats where UGT2b1 is much highly expressed in females than in males (38). On the other hand, human does not express UGT2B1, which could be one of the reasons why there is a lack of major gender effect in emodin glucuronidation in humans.
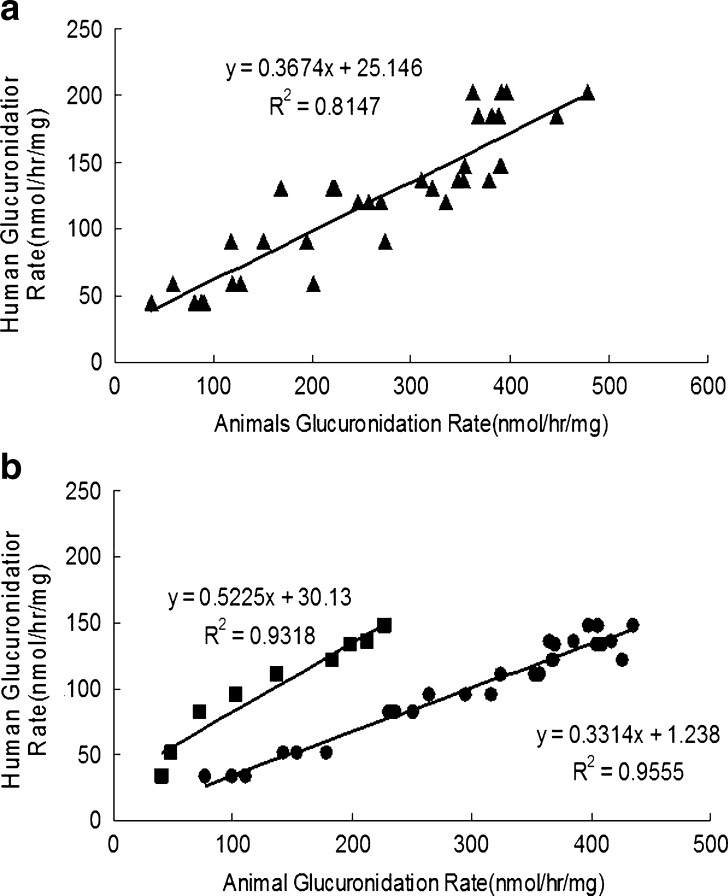
In addition to determine the reasons for poor bioavailabilities, our investigation is the first study that determined systemically microsomal glucuronidation of emodin across several species of different body sizes including humans. This study has the potential for us to understand which species to use for pharmacokinetic studies that will mimic humans. We found, rather surprisingly, that the rates of glucuronidation in all male animal species correlated well with those in human males (Fig. 9a). For females, the correlation was also rather good, but we had to separate female mice from the other animal species (Fig. 9b). The latter might be necessary due to the unique UGT2b1 expression pattern that favors male mice as discussed earlier (glucuronidation in all other animals usually favor females). In all the correlations, the slope was close to or near 0.5, suggesting that glucuronidation in the small animals was always faster than humans, which is expected. Taken together, we believe that human glucuronidation of emodin can be predicted from various commonly available experimental animal species.
Fig. 9.
Correlations between glucuronidation rates in human liver microsomes and four microsomes derived from experimental animal species (mice, rats, guinea pigs, and dogs). a Rates in liver microsome (triangles) from man were plotted against male animal species. b On the other hand, rates in liver microsome (circles) from women were plotted against female animal species. In a, all species were used. In b, mice data (diamonds) were separated out from other animals. Each bar is the average of three determinations, and the error bars are the standard deviations of the mean (n = 3)
In conclusion, this systemic metabolic characterization study showed for the first time that rapid metabolism of emodin via glucuronidation to emodin 3-O-β-D-glucuronide in intestine and liver is a major reason why this compound has very low bioavailability in rats. Similarly, rapid metabolism in liver microsomes of mice, guinea pigs, dogs, and humans would indicate that emodin would have extensive metabolism in those four species as well. Because of the good correlation between glucuronidation rates in human liver microsomes and animal liver microsomes, the use of small experimental animal species such as rats and guinea pigs is expected to be able to provide relevant information about the pharmacokinetic behaviors of emodin in humans, although the latter has to be verified experimentally. Assuming glucuronidation is shown to be the reason for poor emodin bioavailability in humans, future studies should focus on decreasing emodin glucuronidation to improve its bioavailability.
Acknowledgments
This work was mainly supported by Key Project of Chinese National Programs for Fundamental Research and Development (973 program) 2009CB522800, and the Grant of Science and Technology of Guangzhou 2006Z1-E6021, both to ZL. MH was also supported by NIH GM070737.
Footnotes
WL and LT contributed equally to this paper.
Contributor Information
Ming Hu, Phone: +1-713-7958320, Email: mhu@uh.edu.
Zhongqiu Liu, Phone: +86-20-61648596, Email: liuzq@smu.edu.cn.
References
- 1.Garg AK, Buchholz TA, Aggarwal BB. Chemosensitization and radiosensitization of tumors by plant polyphenols. Antioxid Redox Signal. 2005;7:1630–47. doi: 10.1089/ars.2005.7.1630. [DOI] [PubMed] [Google Scholar]
- 2.Huang Q, Lu G, Shen HM, Chung MC, Ong CN. Anti-cancer properties of anthraquinones from rhubarb. Med Res Rev. 2007;27:609–30. doi: 10.1002/med.20094. [DOI] [PubMed] [Google Scholar]
- 3.Srinivas G, Babykutty S, Sathiadevan PP, Srinivas P. Molecular mechanism of emodin action: transition from laxative ingredient to an antitumor agent. Med Res Rev. 2007;27:591–608. doi: 10.1002/med.20095. [DOI] [PubMed] [Google Scholar]
- 4.Sakakibara H, Honda Y, Nakagawa S, Ashida H, Kanazawa K. Simultaneous determination of all polyphenols in vegetables, fruits, and teas. J Agric Food Chem. 2003;51:571–81. doi: 10.1021/jf020926l. [DOI] [PubMed] [Google Scholar]
- 5.Riahi S, Reza Ganjali M, Dinarvand R, Karamdoust S, Bagherzadeh K, Norouzi P. A theoretical study on interactions between mitoxantrone as an anticancer drug and DNA: application in drug design. Chem Biol Drug Des. 2008;71:474–82. doi: 10.1111/j.1747-0285.2008.00653.x. [DOI] [PubMed] [Google Scholar]
- 6.Matsuda Y, Yokohira M, Suzuki S, Hosokawa K, Yamakawa K, Zeng Y, Ninomiya F, Saoo K, Kuno T, Imaida K. One-year chronic toxicity study of Aloe arborescens Miller var. natalensis Berger in Wistar Hannover rats. A pilot study. Food Chem Toxicol. 2008;46:733–9. doi: 10.1016/j.fct.2007.09.107. [DOI] [PubMed] [Google Scholar]
- 7.Srinivas G, Anto RJ, Srinivas P, Vidhyalakshmi S, Senan VP, Karunagaran D. Emodin induces apoptosis of human cervical cancer cells through poly(ADP-ribose) polymerase cleavage and activation of caspase-9. Eur J Pharmacol. 2003;473:117–25. doi: 10.1016/S0014-2999(03)01976-9. [DOI] [PubMed] [Google Scholar]
- 8.Yi J, Yang J, He R, Gao F, Sang H, Tang X, Ye RD. Emodin enhances arsenic trioxide-induced apoptosis via generation of reactive oxygen species and inhibition of survival signaling. Cancer Res. 2004;64:108–16. doi: 10.1158/0008-5472.CAN-2820-2. [DOI] [PubMed] [Google Scholar]
- 9.Huang Q, Shen HM, Ong CN. Emodin inhibits tumor cell migration through suppression of the phosphatidylinositol 3-kinase-Cdc42/Rac1 pathway. Cell Mol Life Sci. 2005;62:1167–75. doi: 10.1007/s00018-005-5050-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Q, Shen HM, Ong CN. Inhibitory effect of emodin on tumor invasion through suppression of activator protein-1 and nuclear factor-kappaB. Biochem Pharmacol. 2004;68:361–71. doi: 10.1016/j.bcp.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 11.Wang SC, Zhang L, Hortobagyi GN, Hung MC. Targeting HER2: recent developments and future directions for breast cancer patients. Semin Oncol. 2001;28:21–9. doi: 10.1053/sonc.2001.29724. [DOI] [PubMed] [Google Scholar]
- 12.Cha TL, Qiu L, Chen CT, Wen Y, Hung MC. Emodin down-regulates androgen receptor and inhibits prostate cancer cell growth. Cancer Res. 2005;65:2287–95. doi: 10.1158/0008-5472.CAN-04-3250. [DOI] [PubMed] [Google Scholar]
- 13.Su YT, Chang HL, Shyue SK, Hsu SL. Emodin induces apoptosis in human lung adenocarcinoma cells through a reactive oxygen species-dependent mitochondrial signaling pathway. Biochem Pharmacol. 2005;70:229–41. doi: 10.1016/j.bcp.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 14.Muller SO, Eckert I, Lutz WK, Stopper H. Genotoxicity of the laxative drug components emodin, aloe-emodin and danthron in mammalian cells: topoisomerase II mediated? Mutat Res. 1996;371:165–73. doi: 10.1016/S0165-1218(96)90105-6. [DOI] [PubMed] [Google Scholar]
- 15.Huang XZ, Wang J, Huang C, Chen YY, Shi GY, Hu QS, Yi J. Emodin enhances cytotoxicity of chemotherapeutic drugs in prostate cancer cells: the mechanisms involve ROS-mediated suppression of multidrug resistance and hypoxia inducible factor-1. Cancer Biol Ther. 2008;7:468–75. doi: 10.4161/cbt.7.3.5457. [DOI] [PubMed] [Google Scholar]
- 16.Masuda T, Ueno Y. Microsomal transformation of emodin into a direct mutagen. Mutat Res. 1984;125:135–44. doi: 10.1016/0027-5107(84)90065-4. [DOI] [PubMed] [Google Scholar]
- 17.Masuda T, Haraikawa K, Morooka N, Nakano S, Ueno Y. 2-Hydroxyemodin, an active metabolite of emodin in the hepatic microsomes of rats. Mutat Res. 1985;149:327–32. doi: 10.1016/0027-5107(85)90148-4. [DOI] [PubMed] [Google Scholar]
- 18.Morita H, Umeda M, Masuda T, Ueno Y. Cytotoxic and mutagenic effects of emodin on cultured mouse carcinoma FM3A cells. Mutat Res. 1988;204:329–32. doi: 10.1016/0165-1218(88)90107-3. [DOI] [PubMed] [Google Scholar]
- 19.Krivobok S, Seigle-Murandi F, Steiman R, Marzin DR, Betina V. Mutagenicity of substituted anthraquinones in the Ames/Salmonella microsome system. Mutat Res. 1992;279:1–8. doi: 10.1016/0165-1218(92)90259-3. [DOI] [PubMed] [Google Scholar]
- 20.Huang HC, Chang JH, Tung SF, Wu RT, Foegh ML, Chu SH. Immunosuppressive effect of emodin, a free radical generator. Eur J Pharmacol. 1992;211:359–64. doi: 10.1016/0014-2999(92)90393-I. [DOI] [PubMed] [Google Scholar]
- 21.Shia CS, Hou YC, Tsai SY, Huieh PH, Leu YL, Chao PD. Differences in pharmacokinetics and ex vivo antioxidant activity following intravenous and oral administrations of emodin to rats. J Pharm Sci. 2010;99:2185–95. doi: 10.1002/jps.21978. [DOI] [PubMed] [Google Scholar]
- 22.Teng ZH, Zhou SY, Ran YH, Liu XY, Yang RT, Yang X, Yuan CJ, Mei QB. Cellular absorption of anthraquinones emodin and chrysophanol in human intestinal Caco-2 cells. Biosci Biotechnol Biochem. 2007;71:1636–43. doi: 10.1271/bbb.70025. [DOI] [PubMed] [Google Scholar]
- 23.Song R, Xu F, Zhang Z, Liu Y, Dong H, Tian Y. Structural elucidation of in vitro metabolites of emodin by liquid chromatography–tandem mass spectrometry. Biomed Chromatogr. 2008;22:1230–6. doi: 10.1002/bmc.1050. [DOI] [PubMed] [Google Scholar]
- 24.Le Guellec C, Lacarelle B, Villard PH, Point H, Catalin J, Durand A. Glucuronidation of propofol in microsomal fractions from various tissues and species including humans: effect of different drugs. Anesth Analg. 1995;81:855–61. doi: 10.1097/00000539-199510000-00034. [DOI] [PubMed] [Google Scholar]
- 25.Hu M, Roland K, Ge L, Chen J, Li Y, Tyle P, Roy S. Determination of absorption characteristics of AG337, a novel thymidylate synthase inhibitor, using a perfused rat intestinal model. J Pharm Sci. 1998;87:886–90. doi: 10.1021/js970251e. [DOI] [PubMed] [Google Scholar]
- 26.Hu M, Sinko PJ, deMeere AL, Johnson DA, Amidon GL. Membrane permeability parameters for some amino acids and beta-lactam antibiotics: application of the boundary layer approach. J Theor Biol. 1988;131:107–14. doi: 10.1016/S0022-5193(88)80124-3. [DOI] [PubMed] [Google Scholar]
- 27.Tang L, Singh R, Liu Z, Hu M. Structure and concentration changes affect characterization of UGT isoform-specific metabolism of isoflavones. Mol Pharm. 2009;6:1466–82. doi: 10.1021/mp8002557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang SW, Chen J, Jia X, Tam VH, Hu M. Disposition of flavonoids via enteric recycling: structural effects and lack of correlations between in vitro and in situ metabolic properties. Drug Metab Dispos. 2006;34:1837–48. doi: 10.1124/dmd.106.009910. [DOI] [PubMed] [Google Scholar]
- 29.Hu M, Krausz K, Chen J, Ge X, Li J, Gelboin HL, Gonzalez FJ. Identification of CYP1A2 as the main isoform for the phase I hydroxylated metabolism of genistein and a prodrug converting enzyme of methylated isoflavones. Drug Metab Dispos. 2003;31:924–31. doi: 10.1124/dmd.31.7.924. [DOI] [PubMed] [Google Scholar]
- 30.Houston JB, Kenworthy KE. In vitro–in vivo scaling of CYP kinetic data not consistent with the classical Michaelis–Menten model. Drug Metab Dispos. 2000;28:246–54. [PubMed] [Google Scholar]
- 31.Hutzler JM, Tracy TS. Atypical kinetic profiles in drug metabolism reactions. Drug Metab Dispos. 2002;30:355–62. doi: 10.1124/dmd.30.4.355. [DOI] [PubMed] [Google Scholar]
- 32.Bachmann M, Schlatter C. Metabolism of [14C]emodin in the rat. Xenobiotica. 1981;11:217–25. doi: 10.3109/00498258109045294. [DOI] [PubMed] [Google Scholar]
- 33.Chen J, Wang S, Jia X, Bajimaya S, Lin H, Tam VH, Hu M. Disposition of flavonoids via recycling: comparison of intestinal versus hepatic disposition. Drug Metab Dispos. 2005;33:1777–84. doi: 10.1124/dmd.105.003673. [DOI] [PubMed] [Google Scholar]
- 34.Coldham NG, Sauer MJ. Pharmacokinetics of [(14)C]Genistein in the rat: gender-related differences, potential mechanisms of biological action, and implications for human health. Toxicol Appl Pharmacol. 2000;164:206–15. doi: 10.1006/taap.2000.8902. [DOI] [PubMed] [Google Scholar]
- 35.Amidon GL, Sinko PJ, Fleisher D. Estimating human oral fraction dose absorbed: a correlation using rat intestinal membrane permeability for passive and carrier-mediated compounds. Pharm Res. 1988;5:651–54. doi: 10.1023/A:1015927004752. [DOI] [PubMed] [Google Scholar]
- 36.Wang SW, Kulkarni KH, Tang L, Wang JR, Yin T, Daidoji T, Yokota H, Hu M. Disposition of flavonoids via enteric recycling: UDP-glucuronosyltransferase (UGT) 1As deficiency in Gunn rats is compensated by increases in UGT2Bs activities. J Pharmacol Exp Ther. 2009;329:1023–31. doi: 10.1124/jpet.108.147371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buckley DB, Klaassen CD. Mechanism of gender-divergent UDP-glucuronosyltransferase mRNA expression in mouse liver and kidney. Drug Metab Dispos. 2009;37:834–40. doi: 10.1124/dmd.108.024224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takeuchi T, Tsutsumi O, Nakamura N, Ikezuki Y, Takai Y, Yano T, Taketani Y. Gender difference in serum bisphenol A levels may be caused by liver UDP-glucuronosyltransferase activity in rats. Biochem Biophys Res Commun. 2004;325:549–54. doi: 10.1016/j.bbrc.2004.10.073. [DOI] [PubMed] [Google Scholar]