Abstract
Novel nanoparticle-aggregate formulations containing recombinant hepatitis B surface antigen (rHBsAg) were administered to the lungs of guinea pigs and antibodies generated to this antigen evaluated. Preparations of dry powders of: (a) rHBsAg encapsulated within poly(lactic-co-glycolic acid) (PLGA)/polyethylene glycol (PEG) nanoparticles (antigen nanoparticles, AgNSD), (b) rHBsAg in a physical mixture with blank PLGA/PEG nanoparticles (antigen nanoparticle admixture (AgNASD), and (c) rHBsAg encapsulated in PLGA/PEG nanoparticles plus free rHBsAg (antigen nanoparticles and free antigen), were generated by spray drying with leucine. Control groups consisted of alum with adsorbed rHBsAg (AlumAg); reconstituted suspensions of spray-dried rHBsAg-loaded PLGA/PEG nanoparticles with leucine; and rHBsAg-loaded PLGA/PEG nanoparticles (AgN). Control preparations were administered by intramuscular injection; AgN was also spray instilled into the lungs. The IgG titers were measured in the serum for 24 weeks after the initial immunization; IgA titers were measured in the bronchio-alveolar lavage fluid. While the highest titer of serum IgG antibody was observed in guinea pigs immunized with AlumAg administered by the IM route, animals immunized with powder formulations via the pulmonary route exhibited high IgA titers. In addition, guinea pigs immunized with AgNASD via the pulmonary route exhibited IgG titers above 1,000 mIU/ml in the serum (IgG titers above 10 mIU/ml is considered protective). Thus, the disadvantages observed with the existing hepatitis B vaccine administered by the parenteral route may be overcome by administering them as novel dry powders to the lungs. In addition, these powders have the advantage of eliciting a high mucosal immune response in the lungs without traditional adjuvants.
Key words: antibody titer, dry powder formulation, hepatitis B vaccine, pulmonary delivery
INTRODUCTION
Hepatitis B virus (HBV) has infected 2 billion people worldwide, and 350 million live with chronic hepatitis B infection (1). Although an effective vaccine against hepatitis B has been available since 1982, an estimated 1 million deaths result from hepatitis B virus-related hepatocellular carcinoma each year suggesting that current strategies for vaccines are inadequate (2). Hepatitis B is a highly contagious virus transmitted by percutaneous (puncture through the skin) or per-mucosal (direct contact with mucous membrane) exposure to blood or other body fluids. It is 50-100 times more infectious than HIV and causes chronic liver diseases leading to death from cirrhosis of the liver and liver cancer (1). Southeast Asia and Sub-Saharan Africa are areas of endemic HBV, where 10–20% of the population is sero-positive for hepatitis B surface antigen (3). Modes of transmission of HBV include mother-to-infant, child-to-child, unsafe injection practices, blood transfusions, and sexual contact. Thus, there is a perceived need for a better vaccine that will enable greater coverage around the world and decrease the incidence of HBV-related chronic liver disease and hepato-cellular carcinoma.
Conventional hepatitis B vaccine is administered as an intramuscular (IM) injection along with other vaccines as part of the mass immunization program. The IM route poses at least two problems to safety and efficacy of hepatitis B immunization. First, and further discussed below, is the problem of dirty needles, a serious concern in many parts of the world where immunizations take place. Second, the probability of a local reaction at the site of injection is pronounced when multiple vaccines are administered simultaneously. For this latter reason alone, a non-injectable route of vaccine administration for the mass immunization program would likely improve safety and possibly efficacy of hepatitis B vaccination.
Hepatitis B vaccine containing alum is reported to produce nodules and erythema at the site of injection (4). Extrinsic factors such as freezing of the vaccine have been associated with decreased immune response; freezing dissociates the antigen from the alum, and thus, interferes with the vaccine’s immunogenicity. None of the currently licensed adjuvants in humans are suitable for mucosal immunization (5).
Development of mucosal immunity is critical as the existing vaccine administered by the parenteral route usually fails to induce this desirable feature that might reduce disease dissemination (6). Mucosal immunity plays an important role by preventing the attachment of virus to the mucosa (7). Mucosal vaccination does not require trained medical personnel or needles and syringes, and could be an attractive route of administration for mass vaccination in developing countries (8). Novel hepatitis B vaccines administered by the intranasal route to different species of animals has been shown to elicit a strong mucosal immune response (6,7,9–11). However, mucosal immunization caused systemic tolerance (12); the down-regulation of IgG due to systemic tolerance in the circulation may cause chronic infection. Consequently, alternative approaches should be explored.
Needle-free Immunization
As part of the mass immunization program in developing countries, billions of injections are delivered. The transmission of blood borne pathogens is thought to be a major global public health problem. Each year, an overwhelming number of infections with HBV (8-16 million) are thought to originate from the reuse of needles and syringes by health-care providers (13); this translates to 32% of HBV infections in developing countries (14). In fact, the present hepatitis B vaccine poses a greater risk for needle-based transmission of infectious diseases than through exposure to infectious blood, body fluids, or by personal contact (15,16). The disposal of waste (biologically contaminated sharps and syringes) after a mass immunization program is also a deterrent to vaccination, especially in developing countries. This has driven the Grand Challenges in Global Health to stress the development of needle-free vaccine delivery for different diseases (17).
Antigen-presenting cells such as alveolar macrophages and dendritic cells that are ideally located in the lungs for antigen sampling and subsequent presentation to T-cells, making pulmonary delivery of vaccines desirable (18–20). Increased vaccine efficacy when delivered by the pulmonary route may be explained by prolonged residence of the antigen in the alveoli and their subsequent migration to lymphoid tissues compared to vaccines administered by other routes where the antigen is transiently present before it is cleared from the body.
The challenges of pulmonary administration include delivery of adjuvant material and the nature of formulation. Given the possibility of an immunotoxicological response associated with the delivery of traditional adjuvants (such as alum with hepatitis B vaccine) to the lungs, pulmonary formulations of vaccines must achieve adjuvancy by a more specific or localized mechanism, amplifying antigenicity while avoiding serious side effects. Liquid formulations, while easy to prepare, are frequently limited by their stability, shelf life, and requirement for expensive transport and distribution controls. Dry powder forms of vaccine for pulmonary delivery have been used in humans and animal models of disease for prevention of, for example, tuberculosis, influenza, diphtheria, and measles (21–23). The preparation of dry forms of hepatitis B vaccines requires the formulation of the vaccine in a dry state, and administration with material that can serve as an effective adjuvant in the absence of alum. One potential material is biodegradable polymer, which can be used to encapsulate the vaccine. The resulting biodegradable nanoparticles must then be formed into aggregates with a mass median aerodynamic diameter (MMAD) between 1 and 5 μm for effective delivery to the lungs, a challenge that can potentially be met using “porous nanoparticle aggregate particle” (PNAP) technology as previously developed (24,25).
Pulmonary delivery of hepatitis B vaccine as a dry powder for mass vaccination campaign has the potential to address the following critical issues: the need to increase efficacy that confers better local mucosal as well as systemic immunity; the need to demonstrate safety during administration by reducing the risk of contamination by sharps and needles; the need to eliminate powder reconstitution, thus, increasing stability during transport and administration; and the need to improve cost effectiveness.
However, there are no published clinical or experimental data for pulmonary vaccination series with rHBsAg powder. A recombinant form of HBsAg is preferred due to disadvantages associated with the use of native antigen including: the time consuming manufacturing process; the high costs of production for mass immunization, which are untenable for poor countries; the poor supply of large amounts of human plasma; and a lengthy adverse reaction test in chimpanzees for the plasma derived antigen.
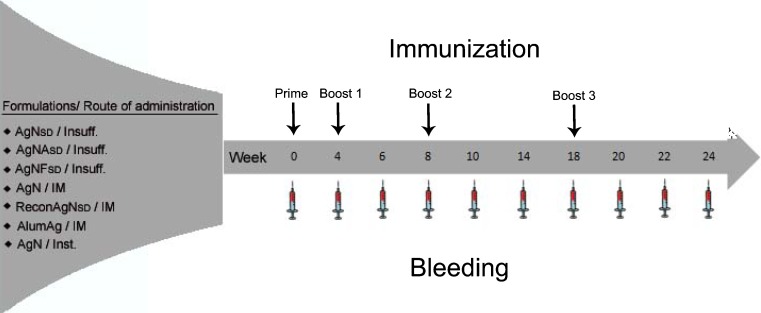
Therefore, the following study addresses the immunogenicity of pulmonary vaccination with rHBsAg, delivered as dry PNAP forms and as liquid suspensions and by different routes of administration, as determined by the serum IgG and lung IgA responses in guinea pigs. Novel dry powder formulations containing rHBsAg (Fig. 1 and Table I) were prepared and characterized with the potential for deep lung delivery. They were administered to guinea pigs at different time periods and blood was withdrawn at regular intervals to estimate the serum IgG antibody titers. Lung IgA antibody titers were evaluated at the time of sacrifice of the guinea pigs.
Fig. 1.
The study design consisting of the various vaccine formulations, their route of administration to guinea pigs and immunization and bleeding schedule (Insuff insufflation, IM intramuscular, Inst spray instillation)
Table I.
The Composition of the Dry Powder Vaccines, their Dose and Route of Administration to Guinea Pigs
| Formulation | Nomenclature | Composition | Antigen loading (µg) | Route of administration |
|---|---|---|---|---|
| AgNSD | Antigen Nanoparticles-Spray Dried | rHBsAg encapsulated within PLGA/PEG nanoparticles | 10 | Insufflation |
| AgNASD | Antigen Nanoparticle Admixture-Spray Dried | rHBsAg in a physical mixture with blank PLGA/PEG nanoparticles | 10 | Insufflation |
| AgNFSD | Antigen Nanoparticles and Free antigen-Spray Dried | rHBsAg encapsulated in PLGA/PEG nanoparticles plus free rHBsAg | 20 | Insufflation |
| AgN | Antigen Nanoparticles | rHBsAg-loaded PLGA/PEG nanoparticles | 10 | Intramuscular |
| ReconAgNSD | Reconstituted Antigen Nanoparticles-Spray Dried | Reconstituted spray dried rHBsAg loaded PLGA/PEG nanoparticles | 10 | Intramuscular |
| AlumAg | Alum with Antigen | Alum with adsorbed rHBsAg | 10 | Intramuscular |
| AgN | Antigen Nanoparticles | rHBsAg-loaded PLGA/PEG nanoparticles | 10 | Spray instillation |
MATERIALS AND METHODS
Materials
Goat anti-guinea pig IgG peroxidase conjugate was purchased from Sigma Aldrich (Sigma, Saint Louis, MO, USA) and sheep anti-guinea pig IgA peroxidase conjugate procured from ICL Inc. for the in-vivo enzyme-linked immunosorbent assay (ELISA). Anti-HBs enzyme immunoassay (EIA) and calibrator kit (Monolisa™, BIO-RAD, Redmond, WA, USA) was used for the quantification of IgG antibody (milli International Units per milliliter) to rHBsAg in guinea pig serum. All other chemicals used were of analytical grade.
Methods
Poly(lactic-co-glycolic acid) (PLGA)/polyethylene glycol (PEG) nanoparticles composed of a PLGA core and a PEG shell, with or without rHBsAg, were prepared by the double emulsion method. The particle size, polydispersity index, and zeta potential of PLGA/PEG nanoparticles as measured by photon correlation spectroscopy and laser Doppler anemometry (Zetasizer, Malvern instruments, UK) were 160 ± 22 nm, 0.16 and −20 mV, respectively. The entrapment efficiency of the antigen within the PLGA/PEG nanoparticles taking into account a theoretical loading of 2% was 52.2 ± 5.1% as determined by ELISA.
Dry powder formulations with excellent aerosolization properties for pulmonary delivery were obtained by spray drying. Aqueous suspensions of rHBsAg encapsulated PLGA/PEG nanoparticles or PLGA/PEG nanoparticles alone were spray dried (Niro atomizer, Columbia, MD, USA) with a solution of L-leucine. The ratio of nanoparticles to leucine was kept at 10/90. The inlet and outlet temperatures of the spray drier were maintained at 80°C and 33°C, respectively. The feed rate of the solution was 30 ml/min, and the air flow rate 98 kg/h.
The different forms of rHBsAg spray dried with leucine were: (1) free rHBsAg with PLGA/PEG nanoparticles alone (AgNASD), (2) rHBsAg encapsulated in PLGA/PEG nanoparticles (AgNSD), (3) rHBsAg encapsulated PLGA/PEG nanoparticles and free rHBsAg in a physical mixture (AgNFSD). The theoretical loading of rHBsAg in the first two formulations was 10 µg/mg dry powder-1, whereas it was 20 µg for the third powder (Table I and Fig. 1). All the dry powder formulations showed good properties for deep lung delivery based on the geometric standard deviation (GSD), MMAD, density, and water content.
The geometric diameter of the spray-dried powders as measured by laser light diffraction (Sympatec, Germany) with powder dispersion (Rodos, Sympatec, Germany) was around 7.0 µm. The MMAD and fine particle fractions (FPF) determined by the Andersen eight stage non-viable cascade impactor (ACI) was around 4.80 µm and 50%, respectively, based on a particle size cut-off of <5.8 µm. Tap density was obtained using a Varian Tap Density machine by measuring the volume of a known amount of powder after tapping it for 500 times and was 0.048 g/ml. Water content of the spray-dried powders as measured by Karl Fisher method was less than 1%.
SEM was used to elucidate the structures of the PLGA/PEG nanoparticles after spray drying with leucine. Hollow and low density particles with a rough inner surface probably due to the presence of intact nanoparticles were obtained when PLGA/PEG nanoparticles were spray dried at a low outlet temperature of 33°C.
Vaccination and Immunization Schedule
All animal procedures were approved by the University of North Carolina, Chapel Hill Institutional Animal Care and Use Committee. Male guinea pigs weighing 350-400 g (Hilltop Laboratory Animals, Inc., Scottsdale, PA, USA) were housed in a 12-h light/12-h dark cycle and constant temperature of 22°C. A standard diet and water were supplied ad libitum. Animals were randomly assigned to seven different groups (n = 6 each) and immunized with novel rHBsAg formulations and administered as dry powders/suspensions as shown in Fig. 1 and Table I. Figure 1 also summarizes the time intervals between the four immunizations. Table I shows the various formulations administered to guinea pigs, their composition, antigen loading, and route of administration. Guinea pigs were anesthetized with ketamine, xylazine and acepromazine (3:1:0.5) administered by the subcutaneous route in the nape of the neck. Untreated animals were used as negative controls. Dry powder formulations containing rHBsAg were administered to anesthetized animals by insufflation. Approximately 10 mg of the powder, corresponding to 10/20 µg of rHBsAg, were administered with a DP-4 insufflator (Penn Century, Inc., Philadelphia, PA, USA). Briefly, anesthetized guinea pigs were placed on their abdomen on a flat surface and the tracheal opening located by inserting a fiber optic laryngoscope (Fiber-Lite system 181-1 Dolan-Jenner Industries, Inc., Woburn, MA, USA) into the mouth of the animal. The DP-4 insufflator was loaded with the required amount of powder and inserted into the tracheal opening until the insufflator reached a few millimeters from the carina of the lung for proper aerosolization of the powder. The dry powder was delivered into the lungs of the guinea pigs by pumping air with a 5-ml disposable plastic syringe attached to the other end of the insufflator. The delivered dose was determined by subtracting the weight of the insufflator after powder administration from that of the insufflator before administration. The loss of powder adhering inside the insufflator was not greater than 1 mg; consequently, the dose +1 mg was loaded to compensate this loss.
For spray liquid instillation, the Microsprayer® (Penn Century, Inc., Philadelphia, PA, USA) connected to a prefilled Hamilton gas-tight syringe (1,000 cc) was inserted into the oro-pharyngeal tube. Subsequently, 100 µl of the vaccine suspension containing 10 µg of the rHBsAg in saline was instilled. Intramuscular immunizations were performed in the calf muscle of anesthetized guinea pigs with the antigens suspended in sterile phosphate-buffered saline; a volume of 100 µl was injected. In all the cases, the immune responses were followed for 6 months after the prime immunization.
Measurement of In-vivo Antibody Response
ELISA
Blood was collected at intervals, as shown in Fig. 1, from anesthetized animals by the saphenous vein and serum recovered by centrifugation; final bleeding was carried out at 24 week after the first immunization. After the final bleed, bronchio-alveolar lavage was performed on the guinea pigs. Sera and lavage fluids were stored in aliquots at −80°C prior to analysis and frozen samples thawed only once before analysis.
Anti-HBs IgG titer was measured in pooled and individual serum samples by indirect ELISA. Briefly, 96 well flat-bottom immuno plates (MaxiSorp, Nalge NUNC International, Rochester, NY, USA) were coated with rHBsAg in 50 µl/well of coating buffer (50 mM carbonate buffer, pH 9.6) at a concentration of 2.5 µg/ml at 4°C overnight. The plates were washed four times with wash buffer (phosphate-buffered saline (PBS), 0.05% Tween 20 (Sigma, St. Louis, MO, USA)) and blocked to prevent non-specific binding at 37°C for 2 h with 150 µl/well blocking buffer (PBS, 0.05% Tween 20, 1% bovine serum albumin). The blocking buffer was aspirated and the plates washed four times with wash buffer. Pooled and individual serum samples diluted in blocking buffer were added to the plates at a starting dilution of 1:200 and further diluted twofold across the plates. The plates were incubated at 37°C for 1 h with 50 µl/well of each serum dilution. After aspirating the serum dilutions and washing four times, the plates were incubated for another 1 h at 37°C with 50 µl/well with anti-guinea pig IgG peroxidase conjugate diluted 1/8,000 in blocking buffer. After aspirating the conjugate and the final four washes, the plates were developed with the substrate o-phenylenediamine (OPD, Sigma, Saint Louis, MO, USA) for 30 min at 37°C in the dark. The substrate consisted of one tablet (10 mg) of OPD, 20 ml citrate buffer (50 mM, pH 4.9), and 15 µl of 30% H2O2. The reaction was stopped after 30 min by adding 50 µl/well of 3 M HCL solution to the plates and optical density measured at 485 nm with 570 nm as the reference wavelength (Multi-detection microplate reader, Synergy HT; Bio-Tek). Relative antibody titers were determined by comparison to a curve generated with sera from rHBsAg-alum immunized guinea pig and expressed as the reciprocal of the endpoint dilution which yielded an absorbance value at least three times background levels. All samples were analyzed in duplicate.
In addition, to compare IgG titers with clinically relevant values, anti-HBs IgG were also measured (in triplicate) using the commercial Monolisa™ Anti-HBs EIA Diagnostic Kit and quantification in milli International Units per milliliter was performed following instructions provided by the manufacturer.
IgA antibody titers were evaluated in the bronchio-alveolar lavage fluid at the end of the study when the guinea pigs were sacrificed. Guinea pigs were anesthetized as described earlier and the trachea isolated by a midline incision. Animals were tracheostomized, and a 14-gage stainless steel catheter was secured with a suture. The animals were exsanguinated, and the lungs gently perfused three times with 3 ml of saline at room temperature. Recovery volume was ≥80%. The lavage fluid was centrifuged and the saline supernatant was assayed for IgA antibody titers by ELISA. The protocol followed was same as for the evaluation of IgG titers except that the anti-guinea pig IgG secondary antibody was replaced with sheep anti-guinea pigs IgA peroxidase conjugate at a dilution of 1/2,000 in blocking buffer. Non-diluted BAL samples from individual guinea pigs were added to the plates, and further diluted twofold across the plates.
Statistical Analysis
One-way ANOVA was used to compare the data. Post-hoc pair wise comparisons were conducted using Holm-Sidak multiple comparison method when the differences in the means were significant (Sigmaplot version 11, Systat Software, Inc., California, USA). P values of less than 0.05 were considered statistically significant.
RESULTS
Humoral Antibody Response
Various dry powder vaccine formulations containing rHBsAg were administered to guinea pigs by the pulmonary route and the immune response elicited, based on serum IgG and lungs IgA, assessed. Anti-HBs IgG and IgA titers were measured using indirect ELISA (Figs. 2 and 3) and quantified (in milli International Unit per milliliter) using the Monolisa™ kit (Fig. 4). Significant increases in IgG antibody titers were observed in all groups after the third boost immunization except the insufflated AgNSD group. Only a slight increase in titer levels was detected in this latter case after three boosts. In contrast, the same dry powder formulation administered by the IM route elicited high antibody titers especially after the third boost. In the AgNASD administered group by the pulmonary route, an increase in antibody titers was observed after the third boost. Peak levels of titers were achieved 15 days after the third boost and remained unchanged till the end of the study (45 days after the last boosting, Fig. 2a). Peak expressions of antibodies were observed at 15 days following the third boost in groups of guinea pigs receiving antigen encapsulated in nanoparticles by injection and spray instillation, but antibody levels were not sustained beyond 30 days. A higher antibody response was elicited in guinea pigs when AgN were administered by the IM route rather than by spray instillation (Fig. 2b).
Fig. 2.
Anti-hepatitis B surface antigen specific serum IgG titers in guinea pigs after one prime and three boost immunizations with dry powder formulations administered by pulmonary route (a) and with suspension formulations by IM route (b). Responses are compared to the surface antigen adsorbed alum control administered IM. Error bars represent the mean ± standard error. Arrows indicate the immunization schedule which corresponds to 0, 4, 8 and 18 weeks. Antibody titers are expressed as the reciprocal of the sample dilution corresponding to at least three times the background level
Fig. 3.
The last time point (week 24) anti-hepatitis B surface antigen specific serum IgG (a) and bronchio-alveolar lavage IgA (b) antibody titers in guinea pigs after one prime and three boost immunizations with different novel formulations administrated by the pulmonary and intramuscular routes. Error bars represent the mean ± standard error. Antibody titers are expressed as the reciprocal of the sample dilution corresponding to at least three times the background level. a *P < 0.001, results are significantly different than all other treatment groups; b *P < 0.002, significant differences compared to #
Fig. 4.
The last time point (week 24) IgG antibody titers in milli International Unit per milliliter in the serum guinea pig after administration of dry powder formulations by the pulmonary and intramuscular routes. Error bars represent the mean ± standard error. *P < 0.001, results are significantly different than all other treatment groups
IgG antibody titers increased after AgNSD or AgNFSD powders were administered by the pulmonary route. A significant increase in antibody titers was observed 15 days after the third boost, which was similar to the response observed to AgNASD administered by insufflation. However, antibody levels began to wane after 30 days (Fig. 2a).
Comparing Fig. 4 with Fig. 3a, it is apparent that the IgG values in milli International Unit per milliliter were proportional to the values obtained by indirect ELISA for the samples collected at the last time point (week 24). The AlumAg group administered by the IM route elicited a significantly higher IgG immune response compared to other formulations administered by IM and pulmonary route (P < 0.001).
Although IgG titers generated by administering antigen via the pulmonary route to guinea pigs were below those of AlumAg administered IM, the values were sufficient to provide protection (IgG ≥ 1,000 mIU/ml in all insufflated groups, Fig. 4).
Interestingly, the IgA antibody titers elicited in the lungs of guinea pigs were higher in the group receiving the novel dry powder formulations by the pulmonary route compared to the group administered by the IM route (Fig. 3b). AgNFSD elicited a significantly higher IgA response 24 weeks after the first immunization compared to reconAgNSD and AlumAg administered by the IM route (P < 0.002). An immunological advantage is clearly afforded by pulmonary administration with respect to eliciting mucosal immunity.
DISCUSSION
Immunization by the pulmonary route in the prophylaxis and therapy of infectious diseases is still in its early stages (26). The purpose of this study was to investigate the possibility of inducing anti-HBs IgG in the serum and IgA titers in the lung of guinea pigs after administering novel dry powder formulations by the pulmonary route.
Nanoparticles have been pursued in drug and vaccine delivery because of their ability for enhanced absorption and better migration through tissues and cells. Since nanoparticles have the tendency to agglomerate due to their high surface-to-volume ratio, they have been incorporated into porous microparticles through spray drying to form structures that may contain varying proportion of nanoparticles. Once delivered to the deep lung, these appropriately sized formulations will collapse to release the nanoparticles in the alveolar region.
In the present study, dry powders intended for vaccine delivery were based on the PNAP technology (24,25). To better understand the effect of formulation type, PLGA/PEG nanoparticles containing rHBsAg and spray dried with leucine were used to obtain a dry powder aerosol formulation. The selection of PLGA/PEG copolymer was based on its extensive application in the biomedical and tissue engineering fields. In the form of nanoparticles, it was feasible to entrap a significant amount of rHBsAg thus preserving its structural integrity.
After spray drying PLGA/PEG nanoparticles either containing the antigen within the structure, free as a physical mixture or both (encapsulated and free), the nanoparticles were able to stabilize rHBsAg, thus preserving its antigenicity as determined by ELISA. The geometric diameter, density, and aerodynamic diameter of the powders were adequate to allow their deposition in the deep lung. In addition, these formulations disperse easier than other dense particles allowing for efficient delivery of large quantities of powder from any inhaler device. These dry powder formulations were administered to guinea pigs by the pulmonary route and the extent of the immune response generated in the systemic circulation and in the lungs was evaluated. Where humoral immunity is thought to be important in preventing disease, the most frequently measured parameter associated with vaccine performance is the antigen-specific IgG serum antibodies present. In addition to measuring the serum IgG, the antigen-specific IgA antibodies, an indicator of mucosal immunity, were measured in the bronchio-alveolar lavage fluid of the lung in the present studies.
Various animal models (e.g., primate, tree shrew, woodchuck, squirrel, and several avian species) have been used to study the pathogenesis of hepatitis B; most of these animals are not easily available or are difficult to handle in captivity (27). The hepatitis B transgenic mouse model has been extensively used in the study of viral hepatitis B (27), but mouse strains have demonstrated intra-species variations to vaccine and adjuvants (28). The guinea pig, on the other hand, has been used in previous hepatitis B vaccine studies to investigate the antibody response to novel vaccines (29–32). These studies describe guinea pigs as highly sensitive to HBsAg and that they produce a significant antibody response to both novel and the standard human hepatitis B vaccine. Furthermore, it is suggested that the guinea pig is a good model for predicting human responses to candidate hepatitis B vaccines.
The current vaccines against hepatitis B consist of either plasma-derived HBV S protein or recombinant HBsAg, both given a minimum of three times with the alum adjuvant; two doses given 1 month apart and a third dose given 6 months after the second dose. Increasing the interval between the first and second dose of the vaccine has an insignificant effect on the final antibody titer. Longer intervals between the last two doses in humans result in higher final antibody levels (33). This pattern was also observed in our study after administering the formulations by the pulmonary route, as well as by the intramuscular route. The antibody titers appeared to increase significantly after the third boost in all the immunized groups irrespective of the route of administration; the spacing between the last two boost was 75 days compared to 30 days between the prime, first, and the second boost. In addition, after the third and final boost, the antibody response observed in most of the immunized animals was not only more rapid but also reached higher and more persistent serum levels (Fig. 2).
Since a dry powder vaccine against hepatitis B has never been examined in an animal model, the initial study design considered having one prime and three booster doses. However, Fig. 2a demonstrates that one prime and two booster doses are sufficient to provide as well as maintain high IgG titers (>10 mIU/ml) in the systemic circulation for 60 days after the second booster (prior to the last immunization). A long-term study is required to examine the effect of number of doses on sustaining the antibody levels above 10 mIU/ml.
According to Fig. 2a, groups administered with dry powder vaccines by the pulmonary route required more time to induce IgG titers similar to AlumAg after the first immunization. However, the IgG titers raised by the dry powder vaccines at the first blood sampling point should be sufficient to provide protection (titers >10 mIU/ml). Besides, based on the IgA titer values at week 24 (Fig. 3b) for the dry powder vaccines compared to the control AlumAg/IM, we can infer that the former may have had a higher IgA titer in the lungs at early time points; this may be critical for hepatitis B infections entering through the mucosal route. However, future studies needs to be performed to establish the IgA response in the lungs at various time points after administering each immunization dose.
Incorporation of L-leucine in the aerosol formulation prior to spray drying affects the surface properties of PLGA/PEG particles leading to improvement in powder flowability and aerosolization properties (34,35). This would in turn increase the respirable fraction of the total dose. Surface antigen encapsulated in nanoparticles and spray dried with leucine were sticky and exhibited strong inter-particulate cohesion leading to poor flow properties. These formulations administered as dry powder to the lungs elicited a smaller IgG immune response compared to those immunized by the IM route, at all sampling time points; spray-dried nanoparticles containing antigen delivered by the IM route may rapidly release antigen. When administered as aggregated dry powders, the antigen may be deposited in the upper airways leading to mucociliary clearance.
The significantly higher IgG response elicited by the AlumAg group could not be matched by other formulations irrespective of administering them by the IM or pulmonary route primarily due to the presence of alum; it was shown that when HBsAg was administered with alum to mice, it increased the anti-HBs titers nearly sevenfold compared to administering antigen without alum (36). Free and nanoparticle encapsulated rHBsAg spray dried with leucine (AgNFSD) elicited an IgG immune response in the systemic circulation which was slightly smaller than AgNASD when administered by the pulmonary route (Figs. 3a and 4). The antibody response observed in the former group may be explained by the action of the free rHBsAg present in the dry powder formulation rather than the antigen being present inside the nanoparticles.
Persistence of antibodies against rHBsAg is dependent upon the peak antibody response after the full vaccination course (37). There is a rapid decline in protective antibody in the first 12 months in humans after the last dose and a more gradual decline over time. In 30-60% of adult vaccinees, the levels decline to less than 10 mIU/ml by 10 years after vaccination (38).
Individual serum samples from each group were analyzed to determine the level of seroprotection conferred to single animals and to ascertain the variability in response to dry powder administration to the lungs. This study demonstrated that vaccination by the pulmonary route in guinea pigs generated an IgG antibody response to values much higher than 10 mIU/ml after the primary course of vaccination. This response is considered to be protective in nature.
Unlike serum IgG titers, IgA antibodies generated in the lungs were higher for guinea pigs receiving the novel dry powder formulations by the pulmonary route compared to those subjected to IM delivery (Fig. 3b). AgNFSD administered guinea pigs yielded a significantly higher IgA response than the AlumAg group, establishing the fact that alum containing vaccines are poor generators of mucosal immune response (39). Indeed, guinea pigs receiving alumAg by the IM route, which elicited a high systemic IgG response, had low IgA titers in the BAL fluid in the lungs at week 24 after first immunization. Thus, the administration of parenteral vaccines containing alum has the propensity to elicit high IgG titers in the serum but may not be sufficient to evoke an IgA mucosal response in the lungs which is desirable to prevent dissemination of disease. We did not examine the IgG titers in the BAL fluid though they are elicited significantly in response to infection or vaccination. The IgG in BAL fluid approximates the serum value, with the majority of it coming from transudation from the plasma compartment.
Therefore, the lungs may prove a promising alternative route for administration of novel hepatitis B vaccines. The requirement for adjuvancy, whether by incorporation of a known adjuvant or through reformulation and dosing considerations, was indicated by the absence of significant IgG titers in the serum of guinea pigs immunized by the pulmonary route with the dry powder formulations in the absence, compared to that in the presence of adjuvant. Interestingly, IgA titers in the lungs were higher when these powder formulations were administered by the pulmonary route compared to hepatitis B surface antigen administered by the IM route along with alum. Currently, alum is the only approved adjuvant for human vaccines including multiple HBsAg vaccine though it is yet to be approved for use in the lungs. Protective efficacy studies could not be performed in guinea pigs due to the species specificity of HBV.
The mechanism by which sufficient serum antibody titers are developed after delivering antigens to the lungs is not clearly understood, but may be due to the presence of antigen presenting cells and the proximity to the mediastinal lymph nodes. However, other factors may be involved. Undoubtedly, further studies are required to explore the mechanisms involved.
Hepatitis B vaccine aerosols delivered to the lungs show promise for future use in humans. However, there are a few limitations with the delivery of vaccines to the lungs. High variability in the delivered dose was observed for dry powder formulations administered by the pulmonary route in our study; this was evident by the high standard error observed in groups receiving this treatment. The lungs as an organ may also be more sensitive to foreign antigens which may lead to more adverse reactions.
CONCLUSION
The present studies demonstrate that pulmonary immunization with dry powder formulations of hepatitis B antigen is possible. An immune response, as measured in terms of IgG levels, in the systemic circulation equivalent to that elicited following parenteral administration was demonstrated. In addition, a significant response, in terms of local IgA levels, was achieved in the lungs, following aerosol administration. It may be concluded that the lungs would be a suitable route of administration that offer not only clinical advantages but also the pharmaceutical advantages of absence of needles and cold chain for delivery globally.
Acknowledgments
Shantha Biotechnics Limited (Hyderabad, India) for providing the hepatitis B surface antigen.
CP acknowledges a fellowship from Angeles Alvariño Program (Xunta de Galicia).
References
- 1.World Health Organization . Hepatitis B Factsheet No. 204 (revised August 2008) Geneva: World Health Organization; 2008. [Google Scholar]
- 2.Fontana RJ. Management of patients with decompensated HBV cirrhosis. Semin Liver Dis. 2003;23(1):89–100. doi: 10.1055/s-2003-37591. [DOI] [PubMed] [Google Scholar]
- 3.Monto A, Wright TL. The epidemiology and prevention of hepatocellular carcinoma. Semin Oncol. 2001;28(5):441–9. doi: 10.1016/S0093-7754(01)90137-X. [DOI] [PubMed] [Google Scholar]
- 4.Chong H, Brady K, Metze D, Calonje E. Persistent nodules at injection sites (aluminium granuloma)—clinicopathological study of 14 cases with a diverse range of histological reaction patterns. Histopathology. 2006;48(2):182–8. doi: 10.1111/j.1365-2559.2005.02312.x. [DOI] [PubMed] [Google Scholar]
- 5.Moschos SA, Bramwell VW, Somavarapu S, Alpar HO. Adjuvant synergy: the effects of nasal coadministration of adjuvants. Immunol Cell Biol. 2004;82(6):628–37. doi: 10.1111/j.0818-9641.2004.01280.x. [DOI] [PubMed] [Google Scholar]
- 6.McCluskie MJ, Wen YM, Di Q, Davis HL. Immunization against hepatitis B virus by mucosal administration of antigen-antibody complexes. Viral Immunol. 1998;11(4):245–52. doi: 10.1089/vim.1998.11.245. [DOI] [PubMed] [Google Scholar]
- 7.Jaganathan KS, Vyas SP. Strong systemic and mucosal immune responses to surface-modified PLGA microspheres containing recombinant hepatitis B antigen administered intranasally. Vaccine. 2006;24(19):4201–11. doi: 10.1016/j.vaccine.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Olszewska W, Openshaw PJM. Mucosal vaccination. In: Kaufmann SHE, editor. Novel vaccination strategies. Weinheim: Wiley-VCH GmbH & Co. KGaA; 2004. pp. 343–64. [Google Scholar]
- 9.Isaka M, Yasuda Y, Mizokami M, Kozuka S, Taniguchi T, Matano K, et al. Mucosal immunization against hepatitis B virus by intranasal co-administration of recombinant hepatitis B surface antigen and recombinant cholera toxin B subunit as an adjuvant. Vaccine. 2001;19(11–12):1460–6. doi: 10.1016/S0264-410X(00)00348-0. [DOI] [PubMed] [Google Scholar]
- 10.McCluskie MJ, Brazolot Millan CL, Gramzinski RA, Robinson HL, Santoro JC, Fuller JT, et al. Route and method of delivery of DNA vaccine influence immune responses in mice and non-human primates. Mol Med. 1999;5(5):287–300. [PMC free article] [PubMed] [Google Scholar]
- 11.Joseph A, Louria-Hayon I, Plis-Finarov A, Zeira E, Zakay-Rones Z, Raz E, et al. Liposomal immunostimulatory DNA sequence (ISS-ODN): an efficient parenteral and mucosal adjuvant for influenza and hepatitis B vaccines. Vaccine. 2002;20(27–28):3342–54. doi: 10.1016/S0264-410X(02)00295-5. [DOI] [PubMed] [Google Scholar]
- 12.Eriksson K, Holmgren J. Recent advances in mucosal vaccines and adjuvants. Curr Opin Immunol. 2002;14(5):666–72. doi: 10.1016/S0952-7915(02)00384-9. [DOI] [PubMed] [Google Scholar]
- 13.Kane A, Lloyd J, Zaffran M, Simonsen L, Kane M. Transmission of hepatitis B, hepatitis C and human immunodeficiency viruses through unsafe injections in the developing world: model-based regional estimates. Bull World Health Organ. 1999;77(10):801–7. [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization . Safety of injections: global facts and figures. Geneva: World Health Organization; 2004. [Google Scholar]
- 15.Lai CL, Ratziu V, Yuen MF, Poynard T. Viral hepatitis B. Lancet. 2003;362(9401):2089–94. doi: 10.1016/S0140-6736(03)15108-2. [DOI] [PubMed] [Google Scholar]
- 16.Zuckerman JN, Zuckerman AJ. Current topics in hepatitis B. J Infect. 2000;41(2):130–6. doi: 10.1053/jinf.2000.0720. [DOI] [PubMed] [Google Scholar]
- 17.Varmus H, Klausner R, Zerhouni E, Acharya T, Daar AS, Singer PA. Public health. Grand challenges in global health. Science. 2003;302(5644):398–9. doi: 10.1126/science.1091769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambrecht BN, Prins JB, Hoogsteden HC. Lung dendritic cells and host immunity to infection. Eur Respir J. 2001;18(4):692–704. [PubMed] [Google Scholar]
- 19.von Garnier C, Filgueira L, Wikstrom M, Smith M, Thomas JA, Strickland DH, et al. Anatomical location determines the distribution and function of dendritic cells and other APCs in the respiratory tract. J Immunol. 2005;175(3):1609–18. doi: 10.4049/jimmunol.175.3.1609. [DOI] [PubMed] [Google Scholar]
- 20.Holt PG. Pulmonary dendritic cells in local immunity to inert and pathogenic antigens in the respiratory tract. Proc Am Thorac Soc. 2005;2(2):116–20. doi: 10.1513/pats.200502-017AW. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Contreras L, Wong YL, Muttil P, Padilla D, Sadoff J, Derousse J, et al. Immunization by a bacterial aerosol. Proc Natl Acad Sci U S A. 2008;105(12):4656–60. doi: 10.1073/pnas.0800043105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amidi M, Pellikaan HC, Hirschberg H, de Boer AH, Crommelin DJ, Hennink WE, et al. Diphtheria toxoid-containing microparticulate powder formulations for pulmonary vaccination: preparation, characterization and evaluation in guinea pigs. Vaccine. 2007;25(37–38):6818–29. doi: 10.1016/j.vaccine.2007.05.064. [DOI] [PubMed] [Google Scholar]
- 23.Cutts FT, Clements CJ, Bennett JV. Alternative routes of measles immunization: a review. Biologicals. 1997;25(3):323–38. doi: 10.1006/biol.1997.0103. [DOI] [PubMed] [Google Scholar]
- 24.Sung JC, Pulliam BL, Edwards DA. Nanoparticles for drug delivery to the lungs. Trends Biotechnol. 2007;25(12):563–70. doi: 10.1016/j.tibtech.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Pulliam B, Sung JC, Edwards DA. Design of nanoparticle-based dry powder pulmonary vaccines. Expert Opin Drug Deliv. 2007;4(6):651–63. doi: 10.1517/17425247.4.6.651. [DOI] [PubMed] [Google Scholar]
- 26.Lu D, Hickey AJ. Pulmonary vaccine delivery. Expert Rev Vaccin. 2007;6(2):213–26. doi: 10.1586/14760584.6.2.213. [DOI] [PubMed] [Google Scholar]
- 27.Dandri M, Volz TK, Lutgehetmann M, Petersen J. Animal models for the study of HBV replication and its variants. J Clin Virol. 2005;34(Suppl 1):S54–62. doi: 10.1016/S1386-6532(05)80011-3. [DOI] [PubMed] [Google Scholar]
- 28.Gupta RK, Siber GR. Adjuvants for human vaccines—current status, problems and future prospects. Vaccine. 1995;13(14):1263–76. doi: 10.1016/0264-410X(95)00011-O. [DOI] [PubMed] [Google Scholar]
- 29.Valinger Z, Trescec A, Tomasic J. Comparison of immunogenicity of recombinant and plasma-derived hepatitis B antigen in guinea pigs. Vaccine. 1990;8(6):585–9. doi: 10.1016/0264-410X(90)90014-D. [DOI] [PubMed] [Google Scholar]
- 30.Kamiyama T, Sato H, Takahara T, Kageyama S, Shiraki K. Novel immunogenicity of Oka varicella vaccine vector expressing hepatitis B surface antigen. J Infect Dis. 2000;181(3):1158–61. doi: 10.1086/315336. [DOI] [PubMed] [Google Scholar]
- 31.Moynihan JS, D'Mello FI, Howard CR. 48-mer synthetic peptide analogue of the hepatitis B virus "a" determinant induces an anti-HBs antibody response after a single injection. J Med Virol. 2000;62(2):159–66. doi: 10.1002/1096-9071(200010)62:2<159::AID-JMV6>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 32.Phumiamorn S, Sato H, Kamiyama T, Kurokawa M, Shiraki K. Induction of humoral and cell-mediated immunity to hepatitis B surface antigen by a novel adjuvant activity of Oka varicella vaccine. J Gen Virol. 2003;84(Pt 2):287–91. doi: 10.1099/vir.0.18692-0. [DOI] [PubMed] [Google Scholar]
- 33.Broderick A, Jonas MM. Hepatitis B and D viruses. In: Feigin RD, Demmler GJ, Cherry JD, Kaplan SL, editors. Textbook of pediatric infectious diseases. 5. Foster City: W. B. Saunders; 2003. pp. 1863–83. [Google Scholar]
- 34.Li HY, Neill H, Innocent R, Seville P, Williamson I, Birchall JC. Enhanced dispersibility and deposition of spray-dried powders for pulmonary gene therapy. J Drug Target. 2003;11(7):425–32. doi: 10.1080/10611860410001659786. [DOI] [PubMed] [Google Scholar]
- 35.Najafabadi AR, Gilani K, Barghi M, Rafiee-Tehrani M. The effect of vehicle on physical properties and aerosolisation behaviour of disodium cromoglycate microparticles spray dried alone or with L-leucine. Int J Pharm. 2004;285(1–2):97–108. doi: 10.1016/j.ijpharm.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 36.Davis HL, Weeratna R, Waldschmidt TJ, Tygrett L, Schorr J, Krieg AM. CpG DNA is a potent enhancer of specific immunity in mice immunized with recombinant hepatitis B surface antigen. J Immunol. 1998;160(2):870–6. [PubMed] [Google Scholar]
- 37.Jilg W, Schmidt M, Deinhardt F. Persistence of specific antibodies after hepatitis B vaccination. J Hepatol. 1988;6(2):201–7. doi: 10.1016/S0168-8278(88)80032-1. [DOI] [PubMed] [Google Scholar]
- 38.Mahoney FJ, Kane M. Hepatitis B vaccine. In: Plotkin SA, Orenstein WA, editors. Vaccines. 3. Philadelphia: W.B. Saunders Company; 1999. pp. 158–82. [Google Scholar]
- 39.Singh M, O' Hagan DT. Microparticles as vaccine adjuvants and delivery systems. In: Kaufmann SHE, editor. Novel vaccination strategies. Weinheim: Wiley-VCH; 2004. pp. 148–72. [Google Scholar]