Abstract
The purpose of this study was to evaluate, in vitro and in vivo, doxorubicin-loaded poly (vinyl alcohol-sodium acrylate) copolymer microspheres [QuadraSphere microspheres (QSMs)] for transcatheter arterial delivery in an animal model of liver cancer. Doxorubicin loading efficiency and release profile were first tested in vitro. In vivo, 15 rabbits, implanted with a Vx-2 tumor in the liver, were divided into three groups of five rabbits each, based on the time of euthanasia. Twenty-five milligrams of QSMs was diluted in 10 ml of a 10 mg/ml doxorubicin solution and 10 ml of nonionic contrast medium for a total volume of 20 ml. One milliliter of a drug-loaded QSM solution containing 5 mg of doxorubicin was injected into the tumor feeding artery. Plasma doxorubicin and doxorubicinol concentrations, and intratumoral and peritumoral doxorubicin tissue concentrations, were measured. Tumor specimens were pathologically evaluated to record tumor necrosis. As a control, one animal was blandly embolized with plain QSMs in each group. In vitro testing of QSM doxorubicin loadability and release over time showed 82–94% doxorubicin loadability within 2 h and 6% release within the first 6 h after loading, followed by a slow release pattern. In vivo, the doxorubicin plasma concentration declined at 40 min. The peak doxorubicin intratumoral concentration was observed at 3 days and remained detectable till the study’s end point (7 days). Mean percentage tumor cell death in the doxorubicin QSM group was 90% at 7 days and 60% in the bland QSM embolization group. In conclusion, QSMs can be efficiently loaded with doxorubicin. Initial experiments with doxorubicin-loaded QSMs show a safe pharmacokinetic profile and effective tumor killing in an animal model of liver cancer.
Keywords: Drug-eluting microspheres, VX-2 tumor, Liver tumo, Hepatic artery chemoembolization, Superabsorbent microspheres
Introduction
Recently, microspheres loaded with chemotherapeutic agents, such as doxorubicin and irinotecan, have been introduced into clinical practice for transcatheter delivery and palliative treatment of liver cancer. These microspheres contain anionic groups which may interact with positively charged groups of doxorubicin or irinotecan by an ion-exchange mechanism, leading to controlled and sustained intratumoral drug deposition, as well as decreased systemic toxicity [1–5]. There is preliminary preclinical and clinical evidence that drug-loaded microspheres are a highly efficient method for drug delivery, as shown by the high intratumoral concentrations and low doxorubicin plasma levels [3].
Poly(vinyl alcohol-sodium acrylate) copolymer microspheres [QuadraSpheres microspheres (QSMs); BioSphere Medical, Inc., Rockland, MA, USA] are one type of commercially available spherical and calibrated microspheres. Hori and his colleagues first reported the successful utilization of these polycopolymer microspheres composed of vinyl alcohol and sodium acrylate in 1996 [6]. Hepaspheremicrospheres are identical in all respects to QSMs and are marketed in the European Union and Japan. Translational and clinical studies have reported their application for embolization of hepatic tumors and vascular malformations in Europe and Japan [7–10]. Preliminary clinical studies in Europe have shown evidence that these microspheres can be successfully loaded with doxorubicin, epirubicin, or oxaliplatin. However, the pharmacokinetic profile of drug-loaded QSMs has never been prospectively tested in a preclinical setting.
In this study, our aim was to evaluate, in vitro and in vivo, the pharmacokinetic profile of doxorubicin-loaded QSMs as well as their tumoricidal effect when delivered intra-arterially into the Vx-2 liver tumor model in rabbit.
Materials and Methods
Biophysical and Biochemical Properties of QuadraSphere Microspheres
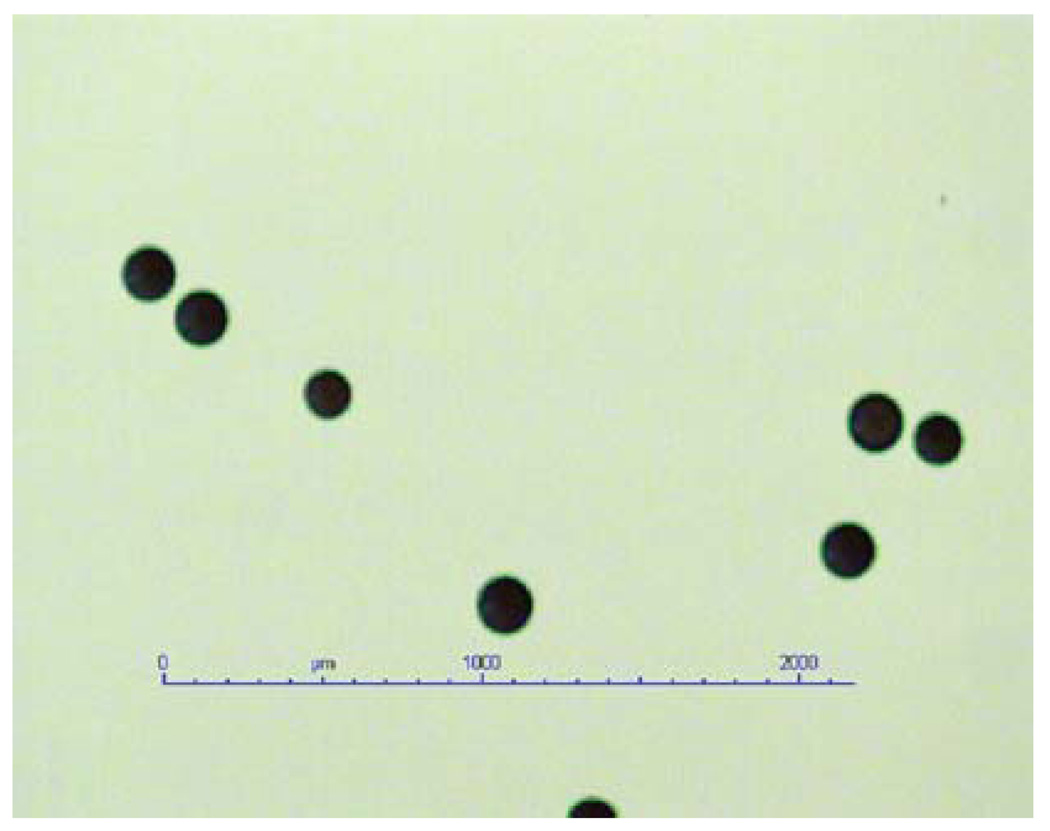
Most of the basic biological and biophysical properties of QSMs, such as biocompatibility, hydrophilia, and nonresorbability, have been demonstrated to be similar to these of other commercially available micropsheres. However, QSMs possess the unique feature of expanding in size when exposed to an aqueous solution, with predictable and homogeneous size ranges. Once the dry form of poly(vinyl alcohol-sodium acrylate) copolymer microspheres is exposed to aqueous solution (0.9% NaCl, blood, and nonionic contrast medium), it swells to about four times the size of its diameter and 64 times its volume within 10 min (Fig. 1). The diameter of the swollen form is quite invariable and predictably proportional to its dry form size. For instance, 50–100 µm in dry form may expand up to 200–400 µm, 100–150 µm may expand to 400–600 µm, and 150–200 µm may expand to 600–800 µm when hydrated. A 50- to 100-µm-sized dry form was used in this study, resulting in 200- to 400-µm sized hydrated microspheres. The hydrated microspheres are compressible and this results in good transcatheter injectability as well as intravascular distribution according to the shape of vasculature [10].
Fig. 1.
Dry form of QuadraSphere microspheres
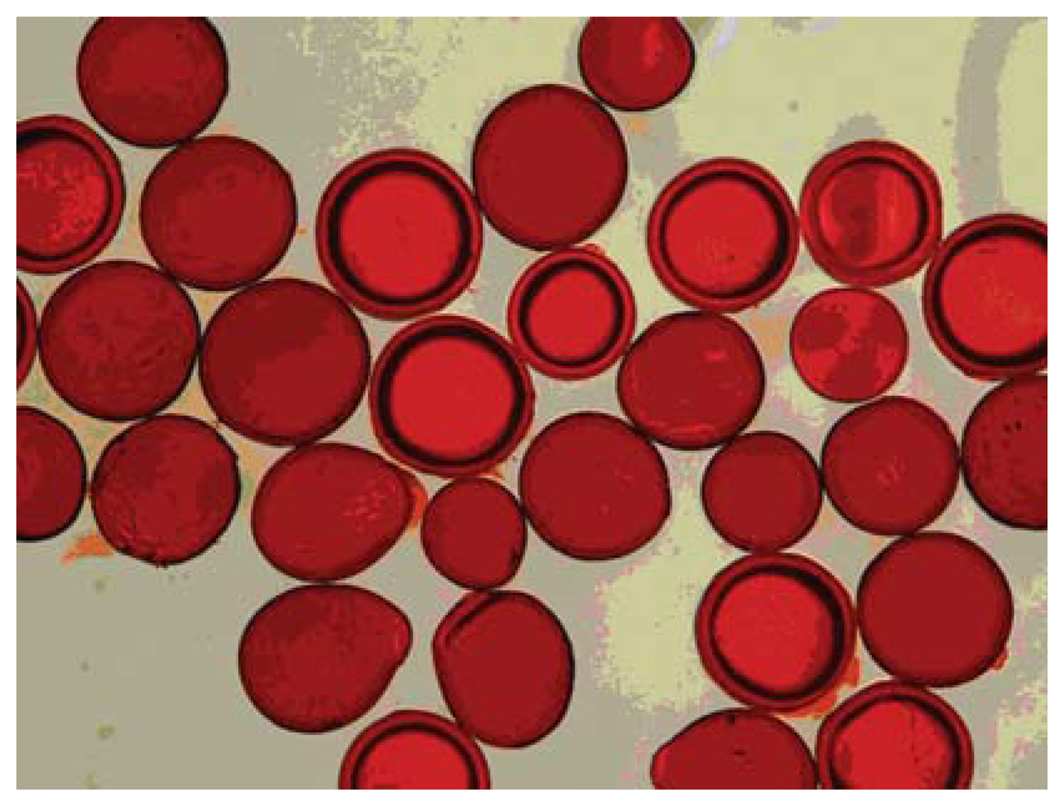
Another feature of QSM is that their negatively charged acrylate groups of QSMs may interact with the positively charged protonated amine group of the doxorubicin hydrochloride, leading to a microscopic change of color (Fig. 2).
Fig. 2.
Microscopic images of doxorubicin-loaded hydrated Quadra-Sphere microspheres. Note that the microspheres acquire a red color after doxorubicin loading (photographic evidence of doxorubicin loading) as well as increase in diameter (swell after hydration; compare with Fig. 1) (Color figure online)
In Vitro Drug Loadability and Controlled Release
Doxorubicin [Yick-Vic Chemicals & Pharmaceuticals (HK) Ltd.] was provided in dry form and prepared by solubilizing the drug with 0.9% NaC in a total of 10 ml. Twenty-five milligrams (±2 mg) of dry QSMs was weighted and introduced into a 10-ml vial containing 100 mg of doxorubicin. The solution was then gently agitated and let to reconstitute for at least 10 min. In vitro measurement of the doxorubicin concentration in the supernatant solution was performed by drawing 150 µl of a solution containing microspheres in a solution. The concentration of doxorubicin was analyzed by reverse-phase high-performance liquid chromatography (Uptisphere C18 column; 150 × 4.6 mm). The elution phase consisted inof 30% (v/v) acetonitrile and 0.1% (v/v) trifluoroacetic acid in water. UV detection was at λmax 480 nm. The loading efficiency was calculated using the following rule:
The Bio-Rad apparatus was then used as a means of simulating the in vivo blood flow to evaluate the release of doxorubicin from the QSMs. The microspheres were then weighed and placed in a column to which 10 ml of the doxorubicin solution was added. They were let reconstitute for 10 min. The solution flow rate was 2 ml/min. The doxorubicin concentration over time was analyzed with high-performance liquid chromatography by extracting 500 µl from the solution reservoir at each time point. The mean diameters of the three tested size ranges were 90, 121, and 170 µm.
In Vitro Doxorubicin Loading into QuadraSphere Microspheres
One hundred milligrams of doxorubicin powder was solubilized in 10 ml of nonionic contrast medium (Visipaque 270; Iodixanol, Nicomed Inc., Princeton, NJ). Twenty-five milligrams of QSMs was added to the solution and the mixture let reconstitute for 2 h. The solution was then aspirated in a 30-ml syringe, where another 10 ml of the same contrast medim was added to a total volume of 20 ml. Finally, 1 ml of the above solution containing QSMs in a 5 mg/ml concentration of doxorubicin was injected into the tumor feeding artery.
Animal Groups
Our institution’s Animal Care and Use Committee approved the study, and all animal care and procedures were performed under institutional guidelines. Our study was carried out between March and August 2008. Fifteen adult New Zealand white male rabbits weighing 3.8– 4.3 kg (Myrtle’s Rabbitry, Thompson Station, TN) were used for this study. The rabbits were divided into three groups of five rabbits each, based on selected time points of euthanasia (1, 3, and 7 days after the treatment). Three more rabbits were blandly embolized and used as controls.
Anesthesia and Analgesia
As a premedication, a mixture of acepromazine (2.5 mg/kg; Phoenix, St. Joseph, MO) and ketamine hydrochloride (44 mg/kg; Phoenix, St. Joseph, MO) was injected intramuscularly. Intravenous access was secured via a marginal ear vein, and 0.1–0.2 ml (2.5–5 mg) of sodium pentobarbital (Hospira, Inc., Lake Forest, IL) was administered intravenously to maintain anesthesia. During each hepatic artery intervention, all rabbits were intubated with a 3.0-mm endotracheal tube (Mallinckrodt Medical, St. Louis, MO) to measure end-tidal CO2 and respiration rate. After surgery, analgesic buprenorphine (0.02–0.05 mg/kg) was injected intramuscularly for pain relief.
Tumor Implantation
To obtain solid tumor for the implantation, 125 µl of a Vx-2 carcinoma cell suspension was injected into each thigh muscle of a carrier rabbit. One week later, distinct solid tumors that had grown in each thigh muscle were harvested from a carrier rabbit and put into 0.9% sodium chloride.
All rabbits were shaved in the thoracoabdominal area before tumor implantation. The site of implantation was identified using percutaneous ultrasonography (Aloka Co., Ltd., Tokyo; 7.5-MHz probe) via a low intercostal or subcostal sonic window. Both the probe- and the ultrasound-inspected skin surface were sterile. A small (2- to 3-mm) skin incision was made with a scalpel at the decided point for percutaneous puncture. The target site for implantation was punctured by percutaneous ultrasound guidance with a 16-G, 2-in.-long angiocath. After the needle tip location was confirmed, the minced tumor cells were inserted using a 0.035-in. guidewire.
Hepatic Artery Intervention
Three weeks after the tumor implantation, selective hepatic arterial delivery of doxorubicin-loaded QSMs was performed. Under intravenous anesthesia and intubation as described above, intervention was performed with a digital subtraction angiographic machine (Toshiba, Tokyo). Surgical cutdown of the right-side femoral artery and insertion of 4-Fr sheath (Cook, Inc., Bloomington, IN) were performed to gain access into the abdominal aorta and select hepatic artery. A 2-Fr JB1 catheter (Cook, Bloomington, IN) was manipulated into the celiac trunk and common hepatic artery. By performing a common hepatic arteriogram, hepatic arterial anatomy, tumor staining and vascularity, size, and location were verified. The JB1 catheter was first exchanged for a fiber-braided hydrophilic 2.5-Fr microcatheter (Renegade; Boston Scientific, Natick, MA) over a 0.014–in. hydrophilic guidewire (Transend; Boston Scientific/Medi-tech, Miami, FL), the tumor feeding artery was then selected and the doxorubicin-loaded or plain QSM solution was injected. After the procedure, the common femoral artery was ligated using absorbable suture material.
Plasma Concentration of Doxorubicin/Doxorubicinol
After each transcatheter arterial delivery of doxorubicin-loaded QSMs, whole-blood samples (5 ml) were collected to measure the plasma concentration of doxorubicin and doxorubicinol at various time points (20, 40, 60, and 120 min). According to previous experience with testing drug-loaded microspheres in the VX-2 rabbit model of liver cancer, the plasma doxorubicin levels beyond 120 min were very low or beyond the level of detection, and therefore, we decided that the end point for the pharmacokinetic study would be the 120-min time point. Whole-blood samples were placed on ice and centrifuged within 3.5 h at 2000 rpm for 10 min at room temperature. Isolated plasma was frozen at −20°C refrigerator until the time of analysis.
Tumor Doxorubicin Concentration and Histopathology
At each time point, rabbits were euthanized under deep anesthesia by slow intravenous injection of a lethal dose (100 mg/ml) of sodium pentobarbital. Samples from the tumor, peritumoral liver parenchyma, and nontargeted liver tissues in the left and right lobe were obtained. These tissue samples were placed in a dry ice container immediately after preparation and frozen at −80°C until the time of analysis. Doxorubicin concentration analysis was performed via atomic absorption spectroscopy. Pieces from the tumor core, tumor periphery, and peritumoral surrounding liver parenchyma were stained with H&E and sent for pathologic analysis. Tumor necrosis as seen with H&E on pathology slides was estimated using a freeware image analysis program (Image J; NIH, Bethesda, MD).
Results
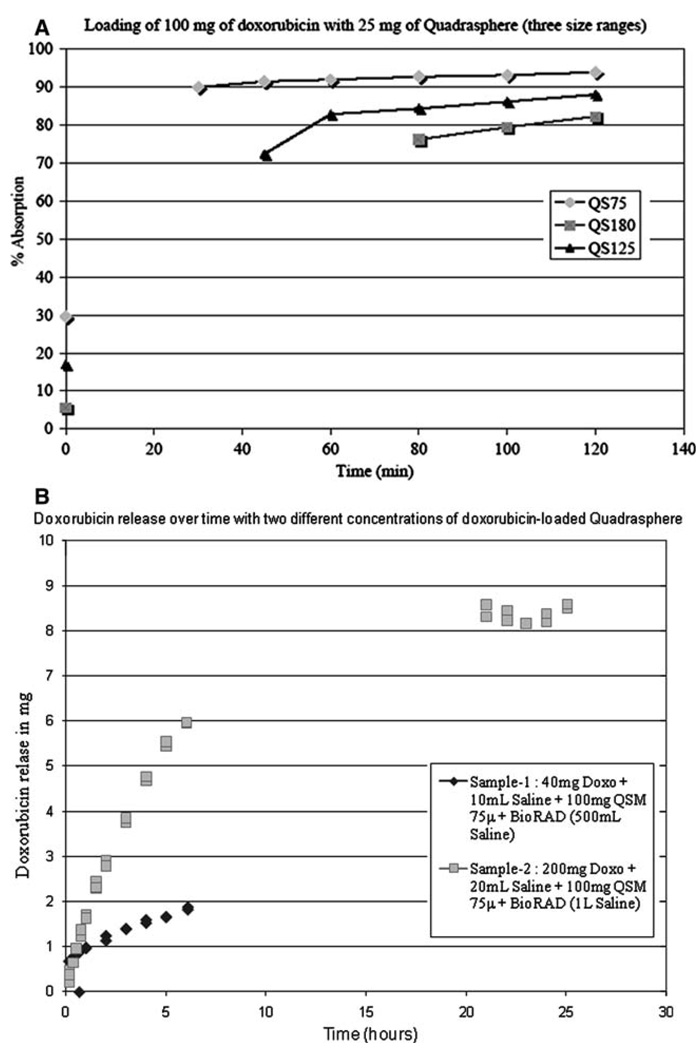
The in vitro experiment showed 82–94% maximal doxorubicin loadability into the QSMs at 2 h and 6% doxorubicin release within the first 6 h, followed by a slow drug release pattern (Fig. 3A, B).
Fig. 3.
In vitro doxorubicin loading and release profiles of QuadraSphere microspheres. The three size ranges tested, HS75, HS125, and HS180, have a mean diameter of 90, 121, and 170 µm, respectively. A Doxorubicin loadability profile. B Doxorubicin release profile
All implanted Vx-2 tumors were grown successfully in the liver, with a mean axial diameter of 3.0 cm (SD, ±0.3 cm), measured on pathology. A sufficient tumor size and hypertrophic tumor feeding artery allowed the selective arteriography in all rabbits, and selective delivery of the whole amount of doxorubicin-loaded QSM (5 mg doxorubicin/1 ml) was possible.
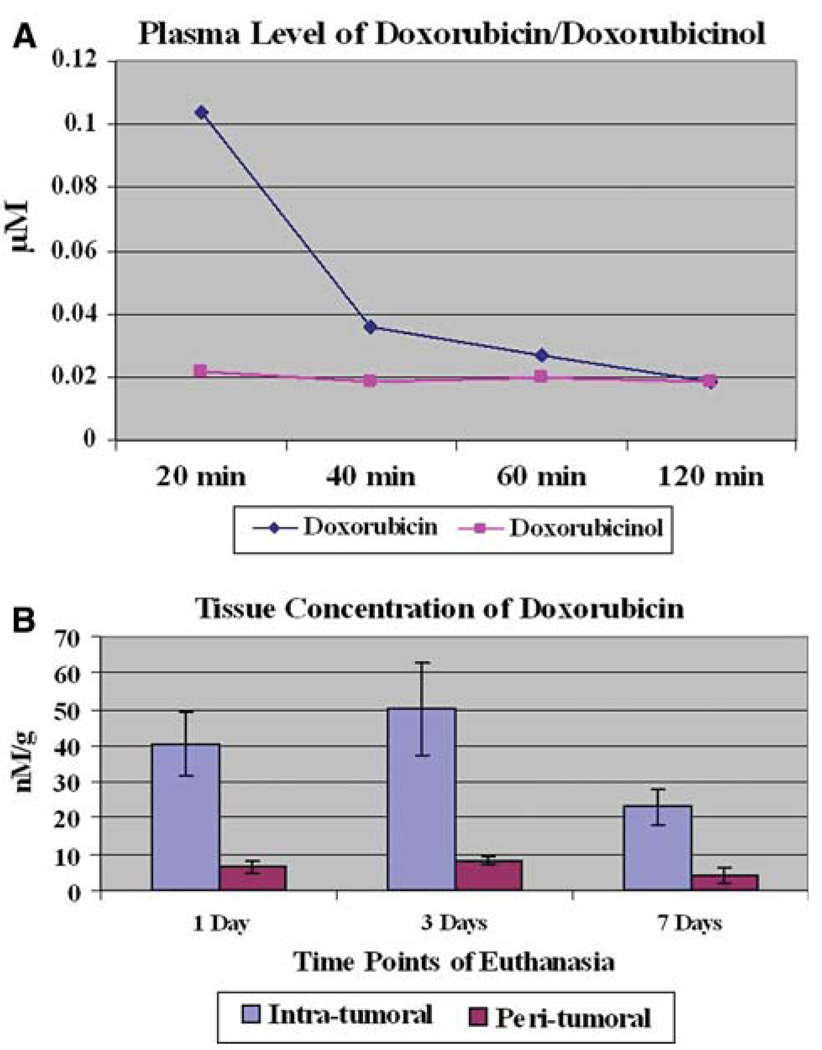
In our study, the highest doxorubicin plasma concentration was noted at 20 min after treatment (mean: 0.1041 µM), which subsequently dropped over time (Fig. 4A). Of note, doxorubicin levels were not measured between 0 and 19 min after injection, since the 20-min time point was our initial one.
Fig. 4.
Pharmacokinetic profile of doxorubicin-loaded QuadraSphere microspheres. A Doxorubicin and doxorubicinol plasma concentrations over time. B Intratumoral and peritumoral tissue concentrations of doxorubicin at selected time points of euthanasia
High intratumoral doxorubicin concentrations were recorded during the first 3 days following treatment (mean ranges, 40.632–50.052 nM/g) (Fig. 4B). At 7 days following treatment (the latest time point of our study), intratumoral doxorubicin concentration dropped to 23.1372 nM/g. The percentage drug concentration in the peritumoral liver parenchyma ranged from 5.6% to 6.2% of the intratumoral concentration. Doxorubicin concentrations in the nontargeted left and right lobe of the liver were undetectable (<0.6 nM/g).
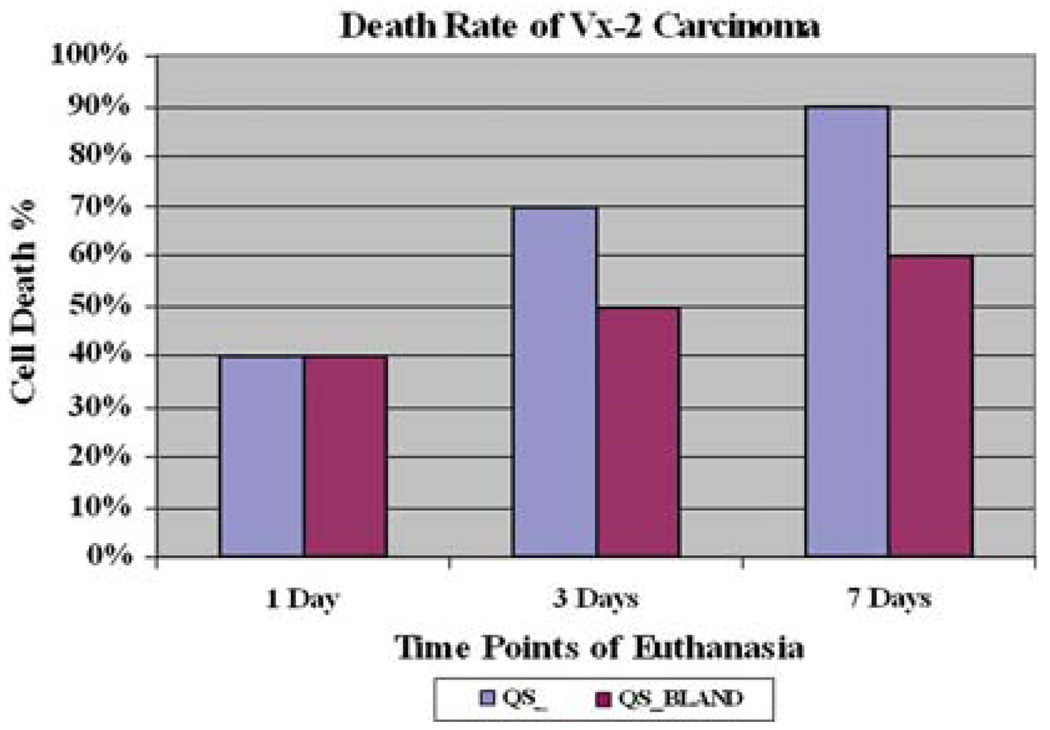
Upon histopathology, an initial burst of tumor necrosis was observed at 3 days and a pronounced 90% tumor killing effect was achieved at 7 days after treatment with doxorubicin-loaded QSMs. At 7 days, the control group achieved 60% tumor necrosis (Fig. 5). Of note, the Vx-2 tumor model is notorious for being necrotic at baseline, and according to our experience, a 40% tumor necrosis was expected and taken into account when comparing groups [3]. The intratumoral presence of doxorubicin-loaded QSMs was well demonstrated in all rabbits (Fig. 6A, B).
Fig. 5.
Tumor cell death rates. At 7 days after treatment, a 90% mean tumor necrosis rate was observed in the doxorubicin QSM group, compared to a mean tumor necrosis of 60% seen in the control bland embolization group
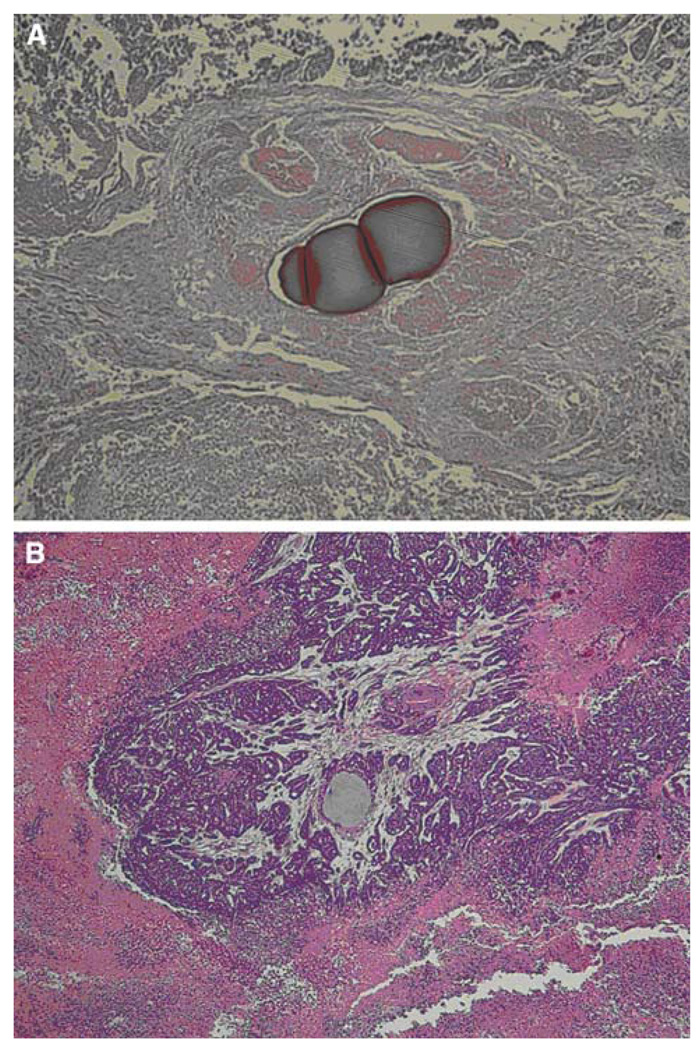
Fig. 6.
Pathologic images obtained at 7 days after treatment according to the treatment modalities. A A case treated with doxorubicin-loaded QuadraSphere microspheres shows near complete tumor cell death, whereas B a case treated with bland embolization shows residual viable cells around the embolized area. Note that the pink color of the QuadraSphere microspheres containing doxorubicin at 7 days after treatment indicates that doxorubicin is still present in the micropshere (A), in contrast to the gray color of plain QuadraSphere microspheres (B) (Color figure online)
Discussion
In this animal study, we utilized poly(vinyl alcohol-sodium acrylate) copolymer microspheres (QSMs), which have the unique feature of proportionally expanding in size when in aqueous solution. Moreover, this material is a negatively charged polymer and may interact with positively charged drugs, such as doxorubicin. Our in vitro experiment demonstrated a high doxorubicin loadability and sustained drug release over time. Our in vivo study showed a safe pharmacokinetic profile and sustained doxorubicin release over time, with detectable intratumoral drug concentrations and high tumoricidal effects at 7 days after treatment. Moreover, the remarkable difference in doxorubicin concentration between intratumoral and peritumoral tissues suggested that hepatic arterial delivery of doxorubicin-loaded QSMs was done selectively. Histopathological tumor necrosis at 7 days was more prominent in the group treated with doxorubicin-loaded QSMs than in the bland embolization group.
In our study, the highest doxorubicin plasma concentration, which was noted at 20 min after treatment, was 0.1041 µM and subsequently dropped overtime. This value is higher than the one measured at 20 min in the initial rabbit study testing the efficacy of LC Beads [3]. This difference could be attributed to the different biochemical and physical properties of the two microspheres and subsequent different drug loading and release patterns. In our study, tumor necrosis at 7 days was high and comparable to that observed at the same time point in the LC Beads study (90% and 100%, respectively).
Our study has several limitations. We chose not to directly compare our microspheres to the commercially available drug-eluting beads, since we detected a stable pharmacokinetic drug profile, with tumor killing comparable to that reported in the rabbit LC Bead (Biocompatibles, Inc., UK) study performed by our group [2]. We also chose not to include comparable numbers in a conventional TACE control arm, since the superiority of doxorubicin-loaded microspheres over chemoembolization was also shown in the aforementioned study [2].
In summary, both in vitro and in vivo studies showed a high drug loadability and sustained drug release over time, high intratumoral doxorubicin concentrations at each time point, and, on histopathology, increased tumor necrosis.
Acknowledgments
This work was supported by a grant from BioSphere Medical and by the Charles W. Pratt Fund for Liver Cancer Research (J.F.G. and K.H.L).
Contributor Information
Kwang-Hun Lee, Division of Vascular and Interventional Radiology, The Russell H. Morgan Department of Radiology, The Johns Hopkins University School of Medicine, 600 North Wolfe Street, Baltimore, MD 21287, USA; Division of Interventional Radiology, Department of Radiology & Research Institute of Radiological Science, Severance Hospital, Yonsei University College of Medicine, Seoul, Republic of Korea.
Eleni A. Liapi, Division of Vascular and Interventional Radiology, The Russell H. Morgan Department of Radiology, The Johns Hopkins University School of Medicine, 600 North Wolfe Street, Baltimore, MD 21287, USA
Curt Cornell, BioSphere Medical, Rockland, MA 02370, USA.
Philippe Reb, Research and Development Department, Biosphere Medical, Roissy Charles de Gaulle, France.
Manon Buijs, Division of Vascular and Interventional Radiology, The Russell H. Morgan Department of Radiology, The Johns Hopkins University School of Medicine, 600 North Wolfe Street, Baltimore, MD 21287, USA.
Josephina A. Vossen, Division of Vascular and Interventional Radiology, The Russell H. Morgan Department of Radiology, The Johns Hopkins University School of Medicine, 600 North Wolfe Street, Baltimore, MD 21287, USA
Veronica Prieto Ventura, Division of Vascular and Interventional Radiology, The Russell H. Morgan Department of Radiology, The Johns Hopkins University School of Medicine, 600 North Wolfe Street, Baltimore, MD 21287, USA.
Jean-Francois H. Geschwind, Division of Vascular and Interventional Radiology, The Russell H. Morgan Department of Radiology, The Johns Hopkins University School of Medicine, 600 North Wolfe Street, Baltimore, MD 21287, USA Division of Vascular and Interventional Radiology, The Johns Hopkins Hospital, 600 North Wolfe Street, Blalock 545, Baltimore, MD 21287, USA, jfg@jhmi.edu.
References
- 1.de Luis E, Bilbao JI, de Ciercoles JA, Martinez-Cuesta A, de Martino Rodriguez A, Lozano MD. In vivo evaluation of a new embolic spherical particle (HepaSphere) in a kidney animal model. CardioVasc Interv Radiol. 2008;31(2):367–376. doi: 10.1007/s00270-007-9240-1. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez MV, Tang Y, Phillips GJ, et al. Doxorubicin eluting beads-2: methods for evaluating drug elution and in-vitro: in-vivo correlation. J Mater Sci Mater Med. 2008;19(2):767–775. doi: 10.1007/s10856-006-0040-y. [DOI] [PubMed] [Google Scholar]
- 3.Hong K, Khwaja A, Liapi E, Torbenson MS, Georgiades CS, Geschwind JF. New intra-arterial drug delivery system for the treatment of liver cancer: preclinical assessment in a rabbit model of liver cancer. Clin Cancer Res. 2006;12(8):2563–2567. doi: 10.1158/1078-0432.CCR-05-2225. [DOI] [PubMed] [Google Scholar]
- 4.Lewis AL, Gonzalez MV, Leppard SW, et al. Doxorubicin eluting beads 1: effects of drug loading on bead characteristics and drug distribution. J Mater Sci Mater Med. 2007;18(9):1691–1699. doi: 10.1007/s10856-007-3068-8. [DOI] [PubMed] [Google Scholar]
- 5.Lewis AL, Taylor RR, Hall B, Gonzalez MV, Willis SL, Stratford PW. Pharmacokinetic and safety study of doxorubicineluting beads in a porcine model of hepatic arterial embolization. J Vasc Interv Radiol. 2006;17(8):1335–1343. doi: 10.1097/01.RVI.0000228416.21560.7F. [DOI] [PubMed] [Google Scholar]
- 6.Jiaqi Y, Hori S, Minamitani K, et al. Anew embolic material: super absorbent polymer (SAP) microsphere and its embolic effects. Nippon Igaku Hoshasen Gakkai Zasshi. 1996;56(1):19–24. [PubMed] [Google Scholar]
- 7.Osuga K, Hori S, Kitayoshi H, et al. Embolization of high flow arteriovenous malformations: experience with use of superabsorbent polymer microspheres. J Vasc Interv Radiol. 2002;13(11):1125–1133. doi: 10.1016/s1051-0443(07)61954-x. [DOI] [PubMed] [Google Scholar]
- 8.Osuga K, Khankan AA, Hori S, et al. Transarterial embolization for large hepatocellular carcinoma with use of superabsorbent polymer microspheres: initial experience. J Vasc Interv Radiol. 2002;13(9; Pt 1):929–934. doi: 10.1016/s1051-0443(07)61777-1. [DOI] [PubMed] [Google Scholar]
- 9.Osuga K, Murakami T, Nakamura H, Hori S. A new embolic material: SAP-microsphere. Nippon Rinsho. 2001;59 Suppl 6:534–538. [PubMed] [Google Scholar]
- 10.Khankan AA, Osuga K, Hori S, Morii E, Murakami T, Nakamura H. Embolic effects of superabsorbent polymer microspheres in rabbit renal model: comparison with tris-acryl gelatin microspheres and polyvinyl alcohol. Radiat Med. 2004;22(6):384–390. [PubMed] [Google Scholar]