Abstract
Objective
High levels of microsatellite instability (MSI-H) have been associated in many studies with improved prognosis in colon cancer. Very few studies have evaluated the effect of MSI-H on rectal cancer survival. We assessed MSI-H and other genetic and epigenetic changes on survival of 990 individuals diagnosed with first primary rectal cancer.
Methods
MSI was assessed primarily by instability in the mononucleotide repeat BAT-26. The BRAF V600E mutation was assessed by TaqMan assay. The CpG island methylator phenotype (CIMP) was determined by methylation-specific PCR of CpG islands in MLH1, methylated in tumors (MINT)1, (MINT)2, (MINT)31 and CDKN2A. KRAS2 codons 12 and 13 mutations, and TP53 mutations in exons 5–8 were determined by sequencing.
Results
Multivariate analysis revealed that MSI-H (HRR 2.47, 95% CI 1.13–5.40) and KRAS2 mutations (HRR 1.37, 95% CI 1.04–1.81) were associated with a significantly higher risk of dying of rectal cancer. Only one of 22 MSI-H tumors showed a BRAF V600E mutation. Of 15 MSI-H rectal cancers evaluated for methylation, two exhibited MLH1 methylation and four exhibited CIMP.
Conclusion
The genetic and epigenetic characteristics of MSI-H rectal cancers suggest that they are enriched for Lynch-associated tumors; adverse prognosis associated with MSI-H in these tumors may reflect the relatively high frequency of Lynch-associated cancers and/or the effect of radiation or chemotherapy on Lynch-associated rectal cancers or MSI tumors in general.
Keywords: Colorectal cancer, Microsatellite instability, Lynch syndrome, Survival, Hereditary nonpolyposis colorectal cancer
Introduction
We and others have previously reported a favorable prognosis associated with high levels of microsatellite instability (MSI-H) in colon cancer [1–4]. Since microsatellite instability, especially in sporadic colon cancer, is mostly seen in proximal tumors [5], most of these studies have not specifically evaluated the effect of microsatellite instability on prognosis in more distal tumors, especially those occurring in the rectum. We have also previously reported a relatively poor survival associated with the BRAF V600E mutation in microsatellite stable tumors, although this mutation did not appear to have an effect on the good prognosis seen in unstable tumors [6]. Again, BRAF V600E mutations were mostly seen in proximal tumors [6], and rectal cancers were not studied. Finally, we have previously reported that TP53 mutations, KRAS2 mutations, and the CpG island methylator phenotype (CIMP) were not associated with a significant impact on survival in colon tumors [6–8], but we have not evaluated the effect of these alterations on survival in rectal cancers. We, therefore, have evaluated the effect of all of these genetic and epigenetic changes on survival in a population-based series of 990 rectal adenocarcinomas.
Materials and methods
Study subjects were from a case–control study of rectal cancer conducted in the Kaiser Permanente Medical Care Program of Northern California (KPMCP) and the state of Utah. All eligible cases within these defined areas were identified and recruited for the study, which involved a detailed in-person interview and a blood draw. Case eligibility was determined by the Surveillance, Epidemiology, and End Results (SEER) Cancer Registries in Northern California and in Utah. To be eligible for these studies, participants had to be between 30 and 79 years of age at the time of diagnosis, had to be English speaking, had to be mentally competent to complete the interview, could not have had previous colorectal cancer [9], and could not have known (as indicated on the pathology report) familial adenomatous polyposis, ulcerative colitis, or Crohn’s disease.
Cases with a first primary tumor in the recto-sigmoid junction or rectum were identified between May 1997 and May 2001; tumor block ascertainment and genetic analyses were completed in 2007. Of the 1,265 eligible cases who consented to having their tissue released, we obtained DNA from 1,022 cases (81% of cases). Of the 234 cases from whom we were not able to obtain DNA, insufficient tumor for DNA extraction was present on 75 blocks, and a block was not available for 159 cases. Of the 1,022 rectal cases from whom tumor DNA was obtained, five or more years of survival data and tumor stage information was available for 990 cases.
Formalin-fixed paraffin-embedded blocks were retrieved from biopsies as well as from resections. In some instances, because of radiation and/or chemotherapy prior to resection for rectal cancer, little or no tumor was present in the resection; in those instances, biopsy specimens were used for making tumor DNA. In Utah, blocks were requested for all cases except from those who refused release of blocks. For those who were not interviewed and had not signed a medical record release, the Utah Cancer Registry retrieved the blocks and released them to the study without key identifiers of name, address, and complete date of birth (year and month of birth were released). At the KPMCP, samples were retrieved from persons who signed a consent form or who had died. Detailed methods for collection of tissue have been described [10]. All aspects of this study were approved by the University of Utah and Kaiser Permanente Medical Care Program institutional review boards.
Genetic analysis
Tumor DNA was obtained from paraffin-embedded tissue as described previously [11]. Mutations in exons 5–8 of the TP53 gene and in codons 12 and 13 of KRAS2 gene were determined by sequencing as described previously [7, 8]. Methylation of MLH1, p16, and methylated in tumors (MINTs) 1, 2, and 31 was determined by methylation-specific PCR of sodium bisulfite-modified DNA as described previously [12]. As before, tumors with two or more methylated CpG islands were scored as CIMP positive. At this time, there is no “consensus” as to the appropriate CpG island panel or method of detection to determine CIMP. However, we have used our panel to demonstrate significant relationships between CIMP and numerous clinicopathologic variables, including cigarette smoking and the BRAF V600E mutation, which were independent of microsatellite instability [12, 13]. This work has also helped to support the legitimacy of the CIMP concept [14]. The BRAF V600E mutation was determined by a TaqMan assay as described previously [15]. Microsatellite instability (MSI-H) was determined by instability in the mononucleotide repeat BAT-26 (for approximately 95% of the tumors) and by a coding mononucleotide repeat in TGFβRII for the small fraction of tumors in which BAT-26 failed. BAT-26 by itself is a very good measure of generalized instability [16], and we have shown high correlations between instability in BAT-26 and TGFβRII and the Bethesda consensus panel [17, 18].
Immunohistochemical analysis
Immunohistochemical analysis for mismatch repair proteins was performed after the other analyses in this study. At that time, blocks from six of the 22 MSI-H rectal cancers were available for immunostaining. Immunohistochemical analysis for MLH1, MSH2, MSH6, and PMS2 was performed as previously described [19].
Statistical methods
Survival information and tumor stage information were available for 990 individuals with rectal tumors and DNA available for analysis; of these, MSI status was determined for 979 subjects. Survival was evaluated using log-rank statistic p values for differences in 5-year survival based on Kaplan-Meier estimates for mortality due to rectal cancer. Median follow-up time was 68 months. Associations between TP53, KRAS2, MSI, CIMP, and BRAF V600E and risk of dying of rectal cancer were evaluated using Cox proportional hazards models to provide unadjusted and multivariate adjusted hazard rate ratios (HRRs) and 95% confidence intervals (CIs) for age at diagnosis, American Joint Committee on Cancer (AJCC) stage, and other tumor alterations. All data analyses were done using SAS® 9.1.2 (SAS Institute, Cary, NC). AJCC stage was categorized as I, II (including IIA and IIB), III (including IIIA, IIIB, and IIIC), or IV.
Results
The effect of stage, MSI, and CIMP and mutations in TP53, KRAS2, and BRAF on survival in rectal cancer is presented in Table 1. In univariate and multivariate analyses, increasing AJCC stage was associated with decreased survival. In a univariate analysis, KRAS2 mutations and CIMP were associated with a significantly worse percentage 5-year survival and HRR for the risk of dying of rectal cancer. There was also a trend toward a decreased survival associated with MSI and BRAF mutations. With adjustment for age and stage, MSI-H (HRR 3.03, 95% CI 1.64– 5.60) and CIMP (HRR 1.45, 95% CI 1.02–2.07) were associated with a significantly higher risk of dying, with KRAS2 showing a trend toward an increased risk (HRR 1.26, 95% CI 0.99–1.61). In an analysis further adjusted for the various genetic and epigenetic alterations, MSI-H (HRR 2.47, 95% CI 1.13–5.40) and KRAS2 mutations (HRR 1.37, 95% CI 1.04–1.81) were associated with a significantly higher risk of dying of rectal cancer. The stage-specific effect of microsatellite instability on survival is shown in Fig. 1. Survival appears worse for stages III and IV MSI-H tumors combined; this was statistically significant for stage III (p values for stage I–IV: 0.45, 0.43, 0.0058, and 0.72, respectively).
Table 1.
Rectal cancer survival and hazard rate ratios for risk of dying of rectal cancer
| Na | 5-year Survival % | Pb | Deaths Person-yc | Unadjusted HRR | 95% CI | Age-stage Adjusted HRR | 95% CI | Multivariate Adjusted HRRd | 95% CI | |
|---|---|---|---|---|---|---|---|---|---|---|
| AJCC stage | ||||||||||
| I | 448 | 89.1 | 46/1,997 | 0.25 | 0.18–0.36 | 0.26 | 0.18–0.36 | 0.25 | 0.17–0.36 | |
| II and III | 422 | 63.6 | 140/1,550 | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | |
| IV | 120 | 11.0 | < .001 | 101/209 | 5.47 | 4.21–7.12 | 5.80 | 4.43–7.58 | 5.83 | 4.29–7.93 |
| TP53− | 495 | 67.9 | 149/1,838 | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | |
| TP53+ | 431 | 71.6 | 0.19 | 115/1,686 | 0.87 | 0.68–1.10 | 0.82 | 0.64–1.04 | 0.84 | 0.65–1.10 |
| KRAS2− | 686 | 71.3 | 186/2,653 | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | |
| KRAS2+ | 294 | 63.3 | 0.02 | 100/1,059 | 1.33 | 1.05–1.68 | 1.26 | 0.99–1.61 | 1.37 | 1.04 –1.81 |
| MSS | 957 | 69.5 | 274/3,631 | 1.00 | Ref. | 1.00 | Ref | 1.00 | Ref. | |
| MSI-H | 22 | 50.0 | 0.05 | 11/80 | 1.78 | 0.98–3.25 | 3.03 | 1.64–5.60 | 2.47 | 1.13–5.40 |
| CIMP− | 761 | 72.0 | 200/2,966 | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | |
| CIMP+ | 103 | 63.2 | 0.04 | 36/371 | 1.43 | 1.01–2.03 | 1.45 | 1.02–2.07 | 1.32 | 0.88–1.97 |
| BRAF− | 931 | 69.7 | 264/3,556 | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | |
| BRAF+ | 27 | 58.0 | 0.07 | 11/84 | 1.72 | 0.94–3.13 | 1.39 | 0.76–2.55 | 1.32 | 0.65–2.70 |
HRR hazard rate ratio, AJCC American Joint Committee on Cancer, MSS microsatellite stable
N differs slightly from Slattery et al. [29] Table 1 due to missing data for stage or survival
P is a log-rank statistic for differences in 5-year survival based on Kaplan-Meier estimates
Person-y is the sum of each person’s time at risk in years; maximum of 5 years
Adjusted for status of other mutations/alterations, in addition to age and stage
Fig. 1.
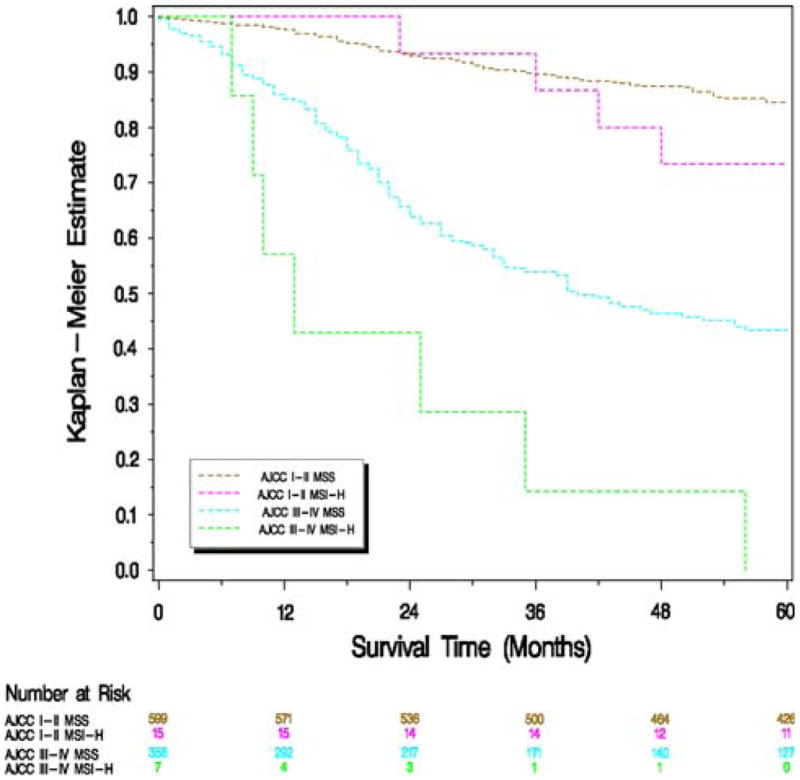
Kaplan-Meier survival estimates of individuals with rectal cancer stratified by the microsatellite instability status of the tumor
One of 22 MSI-H rectal cancers showed a BRAF V600E mutation. Of 15 MSI-H rectal cancers evaluable for methylation, four exhibited CIMP; two of these four CIMP + tumors exhibited MLH1 methylation. Formalin-fixed paraffin-embedded blocks from six tumors were available for immunohistochemical staining of mismatch repair proteins. Four tumors showed loss of expression of MSH2 and MSH6, one showed loss of MLH1 and PMS2 (but did not show MLH1 methylation, CIMP, or BRAF mutation), and one showed intact expression of all four proteins.
Discussion
In this population-based study of 990 rectal cancers, a multivariate analysis adjusting for age, stage, and tumor genetic and epigenetic alterations revealed that MSI-H (HRR 2.47, 95% CI 1.13– 5.40) and KRAS2 mutations (HRR 1.37, 95% CI 1.04–1.81) were associated with a significantly higher risk of dying of rectal cancer. Kaplan-Meier survival estimates showed an adverse effect of MSI on survival for stages III and IV combined, although this was only statistically significant for stage III (p = 0.0058). Interestingly, we and others have previously shown a survival advantage associated with MSI [1, 4]. Since most MSI-H cancers are located in the proximal colon [5], previous studies of MSI and survival probably evaluated relatively few rectal cancers; indeed, our previous study [1] specifically excluded rectal cancers. One previous study did focus exclusively on the relationship between MSI and survival in rectal cancers, and an improved prognosis associated with MSI was reported [20]. In contrast to the current investigation, this study was not population based, and most of the individuals with microsatellite unstable tumors were from the island of Sardinia, a region of extensive inbreeding. These individuals were also treated with adjuvant, rather than neoadjuvant, chemoradiation [20].
One important difference between rectal and colonic tumors with MSI-H is that they appear to differ with respect to the frequency of BRAF mutations, MLH1 methylation, and CIMP. Using our previous study for comparison [12], MSI-H rectal cancers were much less likely to harbor BRAF mutations (4% vs. 56%), MLH1 methylation (13% vs. 73%), and CIMP (26% vs. 82%) than MSI-H colonic cancers. These genetic and epigenetic alterations are associated with sporadic as opposed to Lynch-associated MSI-H cancers, and our results, therefore, suggest that rectal cancers with MSI-H are probably enriched with Lynch-associated tumors. This also is supported by the limited immunohistochemical results, although tissue for this purpose was only available on a minority of rectal cancers with MSI-H. Of the six tumors, MSI-H cancers (out of a total of 22) assessed by immunohistochemical testing, four showed loss of MSH2 and MSH6, an immunohistochemical profile strongly suggestive of Lynch syndrome. One showed intact expression of all four proteins, a result which is also not typical for sporadic unstable tumors. The only tumor, which did show the immunohistochemical profile most commonly associated with sporadic MSI-H tumors, namely loss of MLH1 and PMS2, did not show BRAF mutations, MLH1 methylation, or CIMP, again more suggestive of Lynch-associated disease rather than a sporadic MSI-H cancer. The notion that Lynch-associated cancers might be over-represented in rectal MSI-H cancers is not surprising, as while both sporadic and Lynch-associated MSI-H tumors are predominantly proximal, it has long been observed that sporadic unstable tumors are more uniformly right-sided [5].
It is not clear, however, why a difference in frequency of sporadic vs. Lynch-associated tumors in colonic and rectal MSI-H cancers should lead to the apparent survival difference. Lynch-associated cancers have also been reported to be associated with an improved survival, although this was recently questioned [21]. One possible explanation involves the fact that rectal cancers are often treated with pre-operative chemotherapy and radiation. Although only limited treatment information is available from this retrospective study, we do know that at least eleven of the 22 individuals with MSI-H rectal cancers received either pre-operative radiation or chemotherapy, and that seven of these individuals received both. It is possible that Lynch-associated cancers do not respond as well to one or both of these treatment modalities.
It is also possible that MSI-H tumors in general do not respond well to radiation; since this treatment is rarely used in nonrectal cancers, the use of radiation could explain the apparent survival difference in these locations. Studies on cell lines with MSI, however, have suggested an increased sensitivity to radiation [22, 23]. An MLH1 knockout mouse model did show increased tumor growth as a late effect of radiation, suggesting the possibility of an increased risk of secondary cancers in individuals with Lynch syndrome treated with radiation [24].
There is also evidence that the traditional chemotherapeutic agent for colorectal cancer, 5-flourouracil, may not be effective (and may even be harmful) in MSI-H cancers [25–27], although this would not be expected to lead to a difference in survival between rectal and colonic MSI-H cancers.
We and others have previously reported a decreased survival associated with BRAF mutations; in our study, this was limited to microsatellite stable cancers [6, 28]. This decrease in survival was not seen in rectal cancer, although power may have been a limiting factor. A small increase in rectal cancer death was associated with KRAS2 mutations, an association which was not seen in colon tumors [8]. TP53 mutations, as in colon cancer, did not have an effect on survival in rectal cancer [7]. Strengths of this study are its large size, and the fact that it is population based. A potential weakness is the relatively small number of rectal MSI-H cancers available for investigation; although the entire study of nearly 990 rectal cancers is quite large, MSI in these tumors is rare. Studies in other populations are therefore necessary to assess the reproducibility of these results.
In summary, MSI-H in rectal cancers, in contrast to colonic MSI-H cancers, appears to be an adverse prognostic factor. This may be related to a relatively high frequency of Lynch-associated cancers in rectal MSI-H tumors and/or the effect of radiation or chemotherapy on Lynch-associated cancers or MSI-H tumors in general.
Acknowledgments
We would like to acknowledge the contributions of Sandra Edwards, Leslie Palmer, and Judy Morse to the data collection and management efforts of this study and to Michael Hoffman and Erica Wolff for mutational analysis.Funding: This study was funded by NIH grants R01 CA48998 and CA61757 to Dr. Slattery. The Utah Cancer Registry is funded by Contract #N01-PC-67000 from the National Cancer Institute, with additional support from the State of Utah Department of Health and the University of Utah, the Northern California Cancer Registry, and the Sacramento Tumor Registry. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official view of the National Cancer Institute.
Abbreviations
- CIMP
CpG island methylator phenotype
- HNPCC
Hereditary nonpolyposis colon cancer
- PCR
Polymerase chain reaction
- OR
Odds ratio
- CI
Confidence interval
Footnotes
Competing interests The authors have no conflicts of interests to disclose.
Contributor Information
Wade S. Samowitz, Email: wade.samowitz@aruplab.com, Department of Pathology, University of Utah Health Sciences Center, Salt Lake City, UT 84132, USA
Karen Curtin, Department of Internal Medicine, University of Utah Health Sciences Center, Salt Lake City, UT 84132, USA.
Roger K. Wolff, Department of Internal Medicine, University of Utah Health Sciences Center, Salt Lake City, UT 84132, USA
Sheryl R. Tripp, Department of Pathology, University of Utah Health Sciences Center, Salt Lake City, UT 84132, USA
Bette J. Caan, Division of Research, Kaiser Permanente Medical Care Program, Oakland, CA, USA
Martha L. Slattery, Department of Internal Medicine, University of Utah Health Sciences Center, Salt Lake City, UT 84132, USA
References
- 1.Samowitz WS, Curtin K, Ma KN, et al. Microsatellite instability in sporadic colon cancer is associated with an improved prognosis at the population level. Cancer Epidemiol Biomarkers Prev. 2001;10:917–923. [PubMed] [Google Scholar]
- 2.Lothe RA, Peltomäki P, Meling GI, et al. Genomic instability in colorectal cancer: relationship to clinicopathological variables and family history. Cancer Res. 1993;53:5849–5852. [PubMed] [Google Scholar]
- 3.Halling KC, French AJ, McDonnell SK, et al. Microsatellite instability and 8p allelic imbalance in stage B2 and C colorectal cancers. J Natl Cancer Inst. 1999;91:1295–1303. doi: 10.1093/jnci/91.15.1295. [DOI] [PubMed] [Google Scholar]
- 4.Popat S, Houlston RS. A systematic review and meta-analysis of the relationship between chromosome 18q genotype, DCC status and colorectal cancer prognosis. Eur J Cancer. 2005;41:2060–2070. doi: 10.1016/j.ejca.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 5.Smyrk TC. Colon cancer connections: cancer syndrome meets molecular biology meets histopathology. Am J Pathol. 1994;145:1–6. [PMC free article] [PubMed] [Google Scholar]
- 6.Samowitz WS, Sweeney C, Herrick J, et al. Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers. Cancer Res. 2005;65:6063–6069. doi: 10.1158/0008-5472.CAN-05-0404. [DOI] [PubMed] [Google Scholar]
- 7.Samowitz WS, Curtin K, Ma KN, et al. Prognostic significance of p53 mutations in colon cancer at the population level. Int J Cancer. 2002;99:597–602. doi: 10.1002/ijc.10405. [DOI] [PubMed] [Google Scholar]
- 8.Samowitz WS, Curtin K, Schaffer D, et al. Relationship of Ki-ras mutations in colon cancers to tumor location, stage, and survival: a population-based study. Cancer Epidemiol Biomarkers Prev. 2000;9:1193–1197. [PubMed] [Google Scholar]
- 9.Slattery ML, Potter J, Caan B, et al. Energy balance and colon cancer–beyond physical activity. Cancer Res. 1997;57:75–80. [PubMed] [Google Scholar]
- 10.Slattery ML, Edwards SL, Palmer L, et al. Use of archival tissue in epidemiologic studies: collection procedures and assessment of potential sources of bias. Mutat Res. 2000;432:7–14. doi: 10.1016/s1383-5726(99)00010-2. [DOI] [PubMed] [Google Scholar]
- 11.Spirio LN, Samowitz W, Robertson J, et al. Alleles of APC modulate the frequency and classes of mutations that lead to colon polyps. Nat Genet. 1998;20:385–388. doi: 10.1038/3865. [DOI] [PubMed] [Google Scholar]
- 12.Samowitz WS, Albertsen H, Herrick J, et al. Evaluation of a large, population-based sample supports a CpG island methylator phenotype in colon cancer. Gastroenterology. 2005;129:837–845. doi: 10.1053/j.gastro.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 13.Samowitz WS, Albertsen H, Sweeney C, et al. Association of smoking, CpG Island methylator phenotype, and V600E BRAF mutations in colon cancer. J Natl Cancer Inst. 2006;98:1731–1738. doi: 10.1093/jnci/djj468. [DOI] [PubMed] [Google Scholar]
- 14.Issa JP, Shen L, Toyota M. CIMP, at last. Gastroenterology. 2005;129:1121–1124. doi: 10.1053/j.gastro.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 15.Benlloch S, Paya A, Alenda C, et al. Detection of BRAF V600E mutation in colorectal cancer: comparison of automatic sequencing and real-time chemistry methodology. J Mol Diagn. 2006;8:540–543. doi: 10.2353/jmoldx.2006.060070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoang J-M, Cottu PH, Thuille B, et al. BAT-26, an indicator of the replication error phenotype in colorectal cancers and cell lines. Cancer Res. 1997;57:300–303. [PubMed] [Google Scholar]
- 17.Samowitz WS, Holden JA, Curtin K, et al. Inverse relationship between microsatellite instability and K-ras and p53 gene alterations in colon cancer. Am J Pathol. 2001;158:1517–1524. doi: 10.1016/S0002-9440(10)64102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samowitz WS, Slattery ML. Transforming growth factor-beta receptor type 2 mutations and microsatellite instability in sporadic colorectal adenomas and carcinomas. Am J Pathol. 1997;151:33–35. [PMC free article] [PubMed] [Google Scholar]
- 19.Tripp SR, Gedge FEH, Lyon E, et al. Microsatellite instability testing by immunohistochemistry: initial evaluation of hereditary non-polyposis colon cancer and potential prognostic and therapeutic information. J Histotechnol. 2004;27:169–172. [Google Scholar]
- 20.Colombino M, Cossu A, Manca A, et al. Prevalence and prognostic role of microsatellite instability in patients with rectal carcinoma. Ann Oncol. 2002;13:1447–1453. doi: 10.1093/annonc/mdf240. [DOI] [PubMed] [Google Scholar]
- 21.Barnetson RA, Tenesa A, Farrington SM, et al. Identification and survival of carriers of mutations in DNA mismatch-repair genes in colon cancer. N Engl J Med. 2006;354:2751–2763. doi: 10.1056/NEJMoa053493. [DOI] [PubMed] [Google Scholar]
- 22.Franchitto A, Pichierri P, Piergentili R, et al. The mammalian mismatch repair protein MSH2 is required for correct MRE11 and RAD51 relocalization and for efficient cell cycle arrest induced by ionizing radiation in G2 phase. Oncogene. 2003;22:2110–2120. doi: 10.1038/sj.onc.1206254. [DOI] [PubMed] [Google Scholar]
- 23.Barwell J, Pangon L, Hodgson S, et al. Biallelic mutation of MSH2 in primary human cells is associated with sensitivity to irradiation and altered RAD51 foci kinetics. J Med Genet. 2007;44:516–520. doi: 10.1136/jmg.2006.048660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tokairin Y, Kakinuma S, Arai M, et al. Accelerated growth of intestinal tumours after radiation exposure in Mlh1-knockout mice: evaluation of the late effect of radiation on a mouse model of HNPCC. Int J Exp Pathol. 2006;87:89–99. doi: 10.1111/j.0959-9673.2006.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carethers JM, Smith EJ, Behling CA, et al. Use of 5-fluorouracil and survival in patients with microsatellite-unstable colorectal cancer. Gastroenterology. 2004;126:394–401. doi: 10.1053/j.gastro.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 26.Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349:247–257. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jover R, Zapater P, Castells A, et al. Mismatch repair status in the prediction of benefit from adjuvant fluorouracil chemotherapy in colorectal cancer. Gut. 2006;55:848–855. doi: 10.1136/gut.2005.073015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogino S, Nosho K, Kirkner GJ, et al. CpG island methylator phenotype, microsatellite instability, BRAF mutation and clinical outcome in colon cancer. Gut. 2009;58(1):90–96. doi: 10.1136/gut.2008.155473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slattery ML, Curtin K, Wolff RK, et al. A comparison of colon and rectal somatic DNA alterations. Dis Colon Rectum. 2009;52(7):1304–1311. doi: 10.1007/DCR.0b013e3181a0e5df. [DOI] [PMC free article] [PubMed] [Google Scholar]


